To the Editor,
In this journal, Yang and colleagues recently reviewed effectiveness of monoclonal antibody therapy in organ transplant recipients with COVID-19.1 Pre-exposure prophylaxis (PrEP) of COVID-19 is essential for immunocompromised patients who do not respond to SARS-CoV-2 vaccines. Prior to the spread of Omicron variants, a single 300 mg IM dose of AZD7442 (Tixagevimab/Cilgavimab, Evusheld) was 76.7% effective in preventing symptomatic COVID-19.2 In vitro studies showed that AZD7442 has lost through various degrees part of its efficacy against all Omicron sublineages, including BA.4 and BA.53 which are currently becoming predominant in some parts of the world with a surge in COVID-19 cases.4 The neutralizing activity of sera from AZD7442-treated patients against all Omicron sublineages remains poorly characterized.
The ANRS-0166s PRECOVIM prospective cohort study included severely immunocompromised patients not responding to vaccination and receiving AZD7442 300 mg IM as PrEP (NCT05216588). Here we present the first results, namely the neutralizing capacity of patients' sera one month after treatment against the Omicron variants BA.1, BA.2 and BA.5 compared to the European ancestral variant D614G.
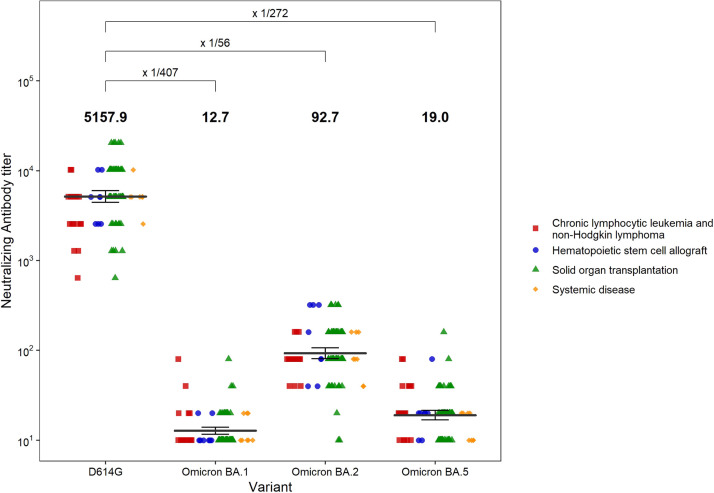
One hundred patients (94 analyzable) from 15 French centers were included between 1/31/22 and 2/24/22 (58 solid organ transplant recipients, 20 chronic lymphocytic leukemia or non-Hodgkin lymphoma, 8 allogenic stem cell transplant recipients and 8 chronic inflammatory disorders under immunosuppressive drugs). Median age was 58 years (19–87). Using clinical replicative strains of the ancestral D614G European variant and the Omicron BA.1, BA.2 and BA.5 sublineages, we showed that the geometric mean neutralizing titers of sera from the 94 analyzable patients were respectively 5157.9, 12.7, 92.7 and 19.0 (Fig. 1 ). Neutralization titers were >10 (and considered positive) in 100%, 27%, 98% and 66% of patients’ sera, respectively. The in vitro half-maximal effective concentrations (EC50) of AZD7442 against the same strains were 13.36, 580.87, 27.04 and 56.56 ng/mL, respectively.
Fig. 1.
Sera neutralizing titers of 94 patients one month after 150 mg Tixagevimab and 150 mg Cilgavimab administration against authentic live viruses from the D614G historical lineage and the Omicron BA.1, BA.2 and BA.5 sublineages.
Dots indicate individual samples. The serum geometric mean titers are shown with black bars and in bold characters at the top of the plot- I bars represent its 95% confidence intervals. Geometric means of individual ratio between viral strains neutralization are indicated in the upper part of the figure.
Median anti-SARS-CoV-2 spike protein IgG antibody concentrations one month after AZD7442 administration were 2996.3 (876.1-13566.7) BAU/mL.
In this prospective cohort study including severely immunocompromised patients non-responding to SARS-CoV-2 vaccines, neutralization activity of patients’ sera one month after administration of 300 mg of AZD7442 was low against both the Omicron BA.1 and BA.5 sublineages. As the serum-neutralizing capacity against SARS-CoV-2 is associated with protection against COVID-19,5 the decreased AZD7442 in vitro activity against BA.16 had already prompted numerous regulatory agencies (FDA, MHRA, ANSM) to recommend doubling the AZD7442 dosing4. Our findings of low in vivo anti-BA.1 and -BA.5 neutralizing activity in treated patients' sera re-inforce the importance of continuous optimization of the AZD7442 dosing according to current and emerging SARS-CoV-2 variants.
Declaration of Competing Interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
Financial disclosures
Authors have no financial disclosure to declare.
Ethical approval
This study received the ethical approval of the Comité de Protection des Personnes Sud-Ouest et Outre-Mer II.
Transparency declaration
VL (the manuscript's guarantor) affirm that the manuscript is an honest. accurate. and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Funding
The ANRS0166s PRECOVIM cohort is conducted with the support of ANRS│MIE and funded by French ministries: Ministère des Solidarités et de la Santé and Ministère de l'Enseignement Supérieur. de la Recherche et de l'Innovation
Acknowledgments
We thank Pr Yazdan Yazdanpanah and all the ANRS-MIE team for their invaluable support and help.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.10.006.
Appendix. Supplementary materials
References
- 1.Yang M., Li A., Wang Y., Tran C., Zhao S., Ao G. Monoclonal antibody therapy improves severity and mortality of COVID-19 in organ transplant recipients: a meta-analysis. J Infect. 2022 doi: 10.1016/j.jinf.2022.06.027. S0163-4453(22)00384-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin M.J., Ustianowski A., De Wit S., et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuekprakhon A, Huo J, Nutalai R, et al. Further antibody escape by Omicron BA.4 and BA.5 vaccine and BA.1 serum. bioRxvid 2022. doi: 10.1101/2022.05.21.492554. [DOI] [PMC free article] [PubMed]
- 4.Callaway E. What Omicron's BA.4 and BA.5 variants mean for the pandemic. Nature. 2022;606:848–849. doi: 10.1038/d41586-022-01730-y. [DOI] [PubMed] [Google Scholar]
- 5.Cromer D., Steain M., Reynaldi A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta- analysis. Lancet Microbe. 2022;3:e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruel T., Hadjadj J., Maes P., et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med. 2022;28:1297–1302. doi: 10.1038/s41591-022-01792-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.