Graphical abstract
Keywords: Covid-19, SARS-CoV-2, Seasonality, Seasonal variation, Temperature
2022 MSC: 00-01, 99-00
Abstract
From march 2020 to march 2022 covid-19 has shown a consistent pattern of increasing infections during the Winter and low infection numbers during the Summer. Understanding the effects of seasonal variation on covid-19 spread is crucial for future epidemic modelling and management. In this study, seasonal variation in the transmission rate of covid-19, was estimated based on an epidemic population model of covid-19 in Denmark, which included changes in national restrictions and introduction of the -variant covid-19 strain, in the period March 2020 - March 2021. Seasonal variation was implemented as a logistic temperature dependent scaling of the transmission rate, and parameters for the logistic relationship was estimated through rejection-based approximate bayesian computation (ABC). The likelihoods used in the ABC were based on national hospital admission data and seroprevalence data stratified into nine and two age groups, respectively. The seasonally induced reduction in the transmission rate of covid-19 in Denmark was estimated to be , (95% CI [; ]), when moving from peak Winter to peak Summer. The reducing effect of seasonality on transmission rate per C in daily average temperature were shown to vary based on temperature, and were estimated to be pr. 1 C around C; pr. 1 C around C; and pr. 1 C around a daily average temperature of 11 C.
1. Introduction
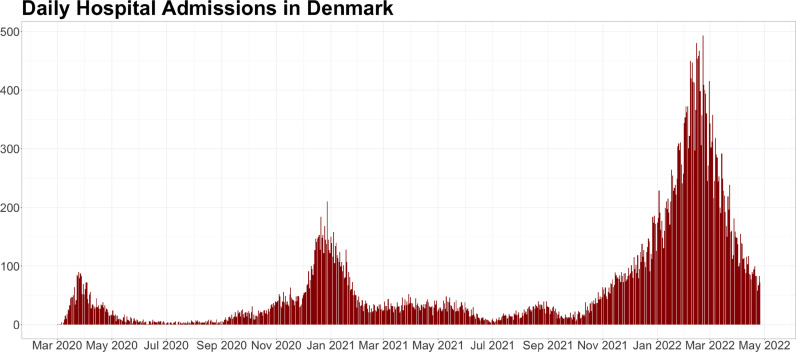
The first cases of coronavirus disease 2019 (covid-19) in Denmark were registered in the late Winter and early Spring of 2020 and a national lockdown ensued on the 11th of March 2020 (Statsministeriet, 2020). Since then, a distinct pattern of covid-19 surges during the Winter and diminished infections during Summer have been evident (Fig. 1 ).
Fig. 1.
New covid-19 hospital admissions pr. day in Denmark.
Identification and quantification of factors that impact infection rates, is central for building reliable covid-19 epidemic models, which have been and continues to be valuable tools for understanding the epidemic development and ensuring public health during the pandemic. Several studies have reported seasonal variation in the transmission rate of covid-19 to act in concert with especially the level of non-pharmaceutical interventions (NPI) (Smith, Flaxman, Gallinat, Kinosian, Stemkovski, Unwin, Watson, Whittaker, Cattarino, Dorigatti, Tristem, Pearse, 2021, Baker, Yang, Vecchi, Metcalf, Grenfell, 2021) and vaccination status (D’Amico et al., 2022) as explanation for annual fluctuations in infection numbers. Especially temperature and secondarily also humidity are described as central meteorological parameters for describing seasonality in the transmission rate of covid-19 (Byun, Heo, Jo, Kim, Kim, Lee, Park, Baek, 2021, Mecenas, Bastos, Vallinoto, Normando, 2020, Gavenciak et al., 2021). This study exclusively focus on temperature as explanatory variable for the seasonal changes in transmission rate and use a novel approach to estimate the seasonality of covid-19 through an estimation of the relationship between temperature and transmission rate. This contribute to: 1) Easy, reliable implementation of seasonality in covid-19 epidemic models; 2) Better understanding of the role of seasonality through calculation of a an estimate of the seasonal variation for a given annual temperature interval and insight into the relative impact of temperature changes on transmission rate. We focus exclusively on Danish data, and use an extended SEIRHD epidemic model - with age-stratification and exhaustive implementation of national NPIs - during a period where immunity and vaccinations are not yet relevant, to fit a logistic average daily temperature dependent seasonality-function to the observed hospital admissions and covid-19 antibody seroprevalence. For viral infections in general, seasonality is caused by environmental parameters and human behaviour (Moriyama et al., 2020), as such, the results from this study can be extrapolated to concern countries in temperate climate (same environmental parameters) and of similar social structure (same social behaviour) as Denmark.
2. Methods
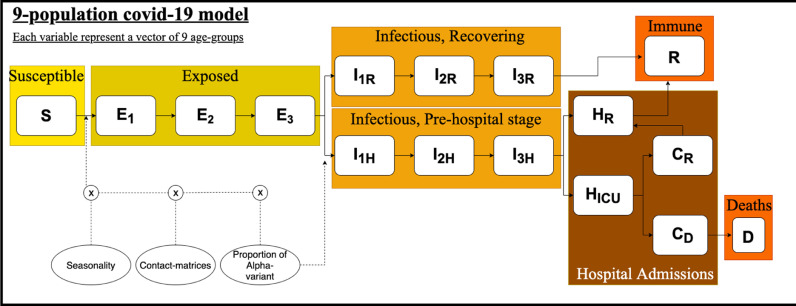
The used model is an version of the classic SIR epidemic population model. Additionally, each disease state in the model is stratified into nine age-populations; [1: 0–9, 2: 10–19, 3: 20–29, 4: 30–39, 5: 40–49, 6: 50–59, 7: 60–69, 8: 70–79, 9: 80+]. The transmission rates between and within these age groups change over time as a function of three elements seen in Eq. (1): 1) : Changes in restrictions 2) : Introduction and dissemination of the -variant 3) : Seasonality. The dissemination of the -variant, , also affected the risk of hospital admission as seen in Eq. (8) and Eq. (5).
Each variable in the differential equation system (Eqs. (1)–16) is a vector (bold font) indicating the used age-stratification. In Eq. (1)-(2) describe a matrix with the amount of susceptible individuals, S, repeated nine times as columns (One age-group in each row). The matrix is a matrix, with transmission rates ‘from’ (columns) each age group ‘to’ (rows) each of the other age groups. In Eqs. (1)-(2) and refer to the infectious disease stages seen in Eqs. (5)–(10). and are scaling factors and implement the effect of the dissemination of the -variant over time on infections and hospital admissions, respectively. produce a temperature dependent scaling factor and implement the seasonal variation in transmission rate (Fig. 2 ).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
Fig. 2.
Flowdiagram of the system of the differential equations of this study, with the addition of seasonality. The calculation of the new exposed individuals is a function of the restriction-based contact-matrices, seasonality, amount of infectious individuals and amount of susceptible individuals, this is indicated by a dotted line. Each variable represent a vector of nine age-groups. S: Susceptible, E: Exposed, I: Infectious, H: Hospitalized, R: Recovered, C: ICU admitted, D: dead. lowercase letters: R indicates Recovering. H indicates ”Going to Hospital”. ICU indicates ”Going to ICU”, D is the proportion of ICU admitted which is estimated to die as a consequence of their infection.
S: Susceptible, E: Exposed, : Infectious recovering, : Infectious moving to the hospital, : Hospitalized and recovering, : Hospitalized and going to ICU, : Admitted to ICU and recovering, : Admitted to ICU and will die of the infection. Parameter values are the medians of the sampling intervals published by Statens Serum Institut (SSI) on 20th of May 2020 (SSI, 2020a) and can be found in Table (A.3 ) in the appendix).
Table A.3.
Parameter values used in the differential equation model.
| Value | Units | |
|---|---|---|
| Changes over time with restrictions | ||
| Estimated through maximum likelihood parameter estimation | 1 | |
| [] | 1 | |
| [] | 1 | |
| [] | ||
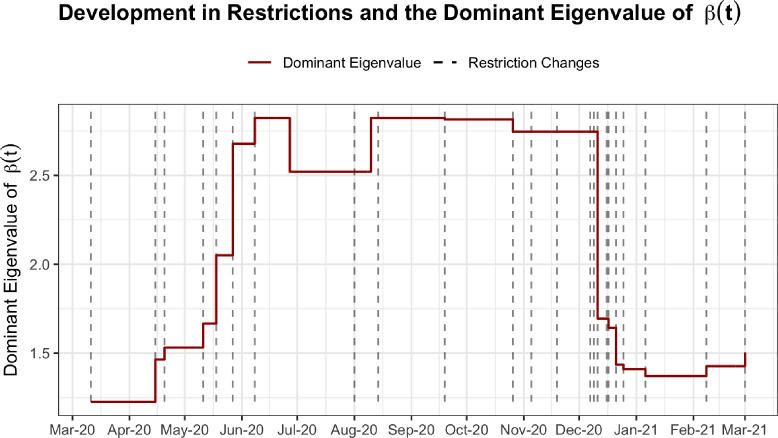
The population model is an extension of the epidemic population model published by SSI on May 20th 2020 (SSI, 2020a), and the structure of the differential equations and -matrices have thus been described in previous publications by SSI (2020b). -matrices for the Spring and Summer of 2020 originated from the script published by SSI in the Spring of 2020 (SSI, 2020a). These -matrices were adapted to reflect the observed reopening timeline of the Danish society in the period March-July 2020. The used timeline adaptation for this period and the following period from 1st of August to 26th of October 2020 has previously been described by Johnsen et al. (2020). As part of this study, contact matrices for the period 26th of October to 31st of December 2020 were created. This period include two important dates or periods with national changes in restrictions: 26th of October (Regeringen, 2020) and the sequential shutdowns in December (Kulturministeriet, Statsministeriet, Sundhedsministeriet (c), Sundhedsministeriet (b), Sundhedsministeriet (a)). The regional/municipal lockdowns on the 3rd, 9th, 11th and 16th of December were aggregated and implemented on the 11th of December. -matrices for 2021 are based on the matrices used by SSI for reports in the Spring of 2021 (SSI, 2020 and 2021b) which describe a regionally stratified development in contacts in response to restrictions - these matrices have not yet been published by SSI. Based on these matrices, the national effect of regional restrictions were calculated as a weighted average based on regional population size. The development in the dominant eigenvalue of over time can be seen in Fig. 3 alongside dates with changes in national restrictions. Restrictions involving changes in e.g. limits on gatherings and opening hours of bars are excluded from the -matrices (SSI, 2020b). Additionally, tradition specific behaviour changes during Christmas were not included in the model - except for indirectly through the seasonal variation.
Fig. 3.
Development in the dominant eigenvalue of over time compared to dates with restriction changes in the Danish society.
In the model, the -variant (previously called B.1.1.7) is associated with an increased risk of hospital admission by 42% and a 55% increase in transmission (SSI, Peter Bager, Jan Wohlfahrt, Jannik Fonager, Morten Rasmussen, Mads Albertsen, Thomas Yssing Michaelsen, Camilla Holten Mller, Steen Ethelberg, Rebecca Legarth, Mia Sarah Fischer Button, Sophie Gubbels, Marianne Voldstedlund, Prof Kre Mlbak, Robert Leo Skov, Anders Fomsgaard, Tyra Grove Krause). This is implemented by multiplying these increases onto the existing rates according to the proportion of observed -variant cases over time in Eqs. (1), (2), (5), (8) (Danish Covid-19 Genome Consortium, 2021). The initiation of the Danish vaccination programme in December 2020 (Sundhedsstyrelsen, 2021) and its effect on infections and hospital admissions were not included in the population model.
2.1. Implementation of temperature dependent seasonality
The fundamental assumption, which enable estimation of seasonality, is that the structure of the system of differential equations, and the implementation of restrictions through changes in contact-matrices, is a sufficient approximation of reality, to such degree, that the remaining quantitative discrepancy between model and observed data, is primarily caused by lack of implementation of seasonal variation. This assumption is justified through the fact that the model corrects for NPIs; new covid-19 strains; proportions of registered against actual covid-19 cases; and that immunity numbers - whether induced by infection or vaccinations - are still low and thus, not yet relevant during the optimization period from 01-03-2020 to 01-03-2021.
In order to estimate the seasonal effect on the transmission rate of covid-19 in Denmark, a logistic temperature dependency Eq. (17) was used to calculate a scaling factor of the transmission rate, . T(t) indicates the observed national average daily temperature in Denmark ([dataset] DMI, 2021).
| (17) |
The logistic temperature dependent model in Eq. (17) contains four parameters; a, b, c & d. Initially, 500 Maximum likelihood parameter estimations of these parameters were performed. This method of estimation turned out to show poor convergence due to local minima and saddle points and therefore, the parameters were instead estimated through rejection based approximate bayesian computation (ABC-rejection). The 500 maximum likelihood parameter estimations (MLPE) which were performed prior to the ABC-rejection, were instead used to establish informed prior intervals to for the ABC-rejection algorithm. This was done by accepting a broad interval around the 5% best models from the MLPE as uniform priors. The likelihood evaluation used in both methods were based on two data sources from the period 1st of March 2020 to 1st of March 2021:
-
1.The recorded new daily covid-19 related hospital admissions: A registered covid-19 hospital admission in Denmark is defined as a covid-19 positive PCR test 14 days prior to or during hospital admission (SSI, 2021c). Two different age stratifications (see below) were used for different time periods as the ten-year age stratified data were not available before 1st of April 2020. The summer period 1st of June to 31st of August 2020, were excluded from the likelihood evaluation, due to presence of a large amount of zeros in the age-stratified observed data during this period.
-
i.Two age groups ([dataset] Christiansen, 2020): [0–59,60+] from 11th of March to 1st of April 2020.
-
ii.Nine age groups ([dataset] SSI, 2020 and 2021a): Ten year intervals from 0 - 80+; [0–9,10-19,...,70-79,80+] from 1st of April 2020 to 1st of March 2021.
-
i.
-
2.
Covid-19 antibody seroprevalence ([dataset] SSI (2020 and 2021)): The amount of tested and covid-19 antibody positive blood samples for the two age groups 12–59 years and 60+ years. Weeks with less than 5 tested subjects were removed from the dataset. In Denmark the vaccination programme was initiated in December 2020, and therefore seroprevalence data beyond this point were not available.
In addition to the seasonality parameters, a constant common scaling factor of the observed risks of hospital admission for each age group was also estimated. This ensured that the model could fit to the seroprevalence data as well, in order to avoid e.g. premature depletion of susceptible individuals if the risk of hospital admission had been underestimated in this long-term simulation. The observed hospital admission risks were taken from [dataset] SSI (2020 and 2021a) during the Summer of 2021. This common scaling factor of the admission risks represents the proportion of registered cases out of total covid-19 cases. Initial infections on the 11th of March originated from SSI (2020a) and were adjusted according to these new estimated risks of admission. Estimation of all five parameters (four seasonality and one admission risk parameters) were based on likelihood evaluations based on a negative binomial distribution, with concomitant optimization of the dispersion parameter. 50.000 parameter combinations were sampled from the uniform prior distributions and were used in the ABC-Rejection algorithm. The rejection criteria in the algorithm was set as the 0.25% quantile (125 observations). The tolerance level was chosen by iteratively lowering the tolerance to exclude local minima from the posterior distribution. After the parameter estimation, the estimated magnitude of the annual seasonal variation in the transmission rate of covid-19 was calculated based on the estimated parameters and the average monthly temperatures in Denmark in the last 30 years ([dataset] DMI, 2022). For each monthly average a seasonal scaling of transmission rate was calculated, and from this value, the seasonal variation in the transmission rate was defined as the relative reduction in covid-19 transmission rate going from February (coldest month on average) to July (hottest month on average), based on averaged historic temperature data. This ensured a general estimate of the seasonal variation for a ‘typical year’.
3. Results & discussion
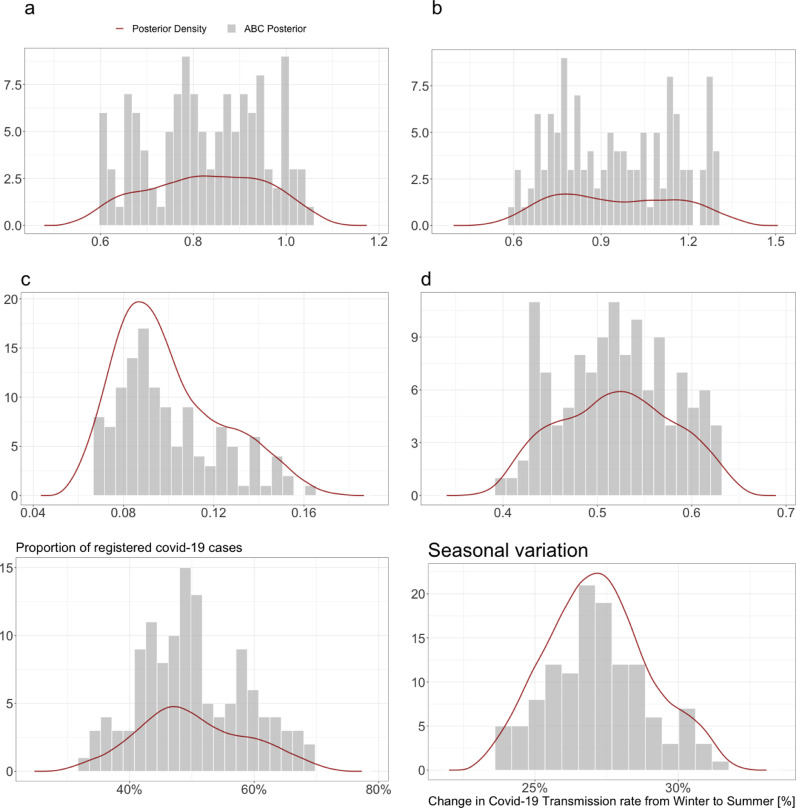
Table 1 shows the informed prior intervals alongside the mode and the 95% credible interval (CI) from the ABC-rejection posterior. Figure 4 show the generated posterior distributions for the five estimated parameters and the seasonal variation.
Table 1.
Priors and posteriors for the seasonality function parameters, proportion of registered cases and the estimated seasonal variation. Seasonality indicate the estimated relative change in transmission rate when transitioning from Winter to Summer.
| Parameter | Prior | Posterior mode | 95% Credible Interval |
|---|---|---|---|
| a | U [0.6 ; 1.05] | 0.79 | [0.61; 1.0] |
| b | U [0.6 ; 1.3] | 0.77 | [0.62; 1.3] |
| c | U [0.05 ; 0.2] | 0.09 | [0.07; 0.15] |
| d | U [0.4 ; 0.68] | 0.5 | [0.42; 0.62] |
| Proportion of registered cases | U [30% ; 70%] | 49% | [35%; 67%] |
| Seasonality: | [11%; 52%] | 27% | [24%; 31%] |
Fig. 4.
Posterior distribution (grey histogram) for each parameter (a, b, c, d) in Eq. (17), and the resulting estimated seasonal variation associated with these parameter values (Eq. (18)). The posterior distribution for the estimated proportion of registered out of total cases is also shown. The red line indicate the estimated density (using biweight kernel density estimation (KDE)) of the posterior distributions. Here we see that the posterior distribution for the seasonal variation is well-defined, and yield a seasonal variation estimate of 27% (95% CI: [24%; 31%]). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The posterior distribution for c and to some degree also d show well defined informed distributions. However, a and b show uninformed distributions with plateaus and multimodality which is likely a consequence of both remaining local minima and parameter correlations. The key result from these posterior distributions is the fact that the correlations between the parameters ensure, that when they are combined in the calculation of the posterior distribution of the seasonality (Eq. (18)), then an informed posterior distribution with a well-defined mode of seasonality is achieved - in spite of multimodality in some of the individual temperature function parameters.
| (18) |
Here, is the average temperature during July during the last 30 years, and is the average temperature during February in the same period.
3.1. Model fit
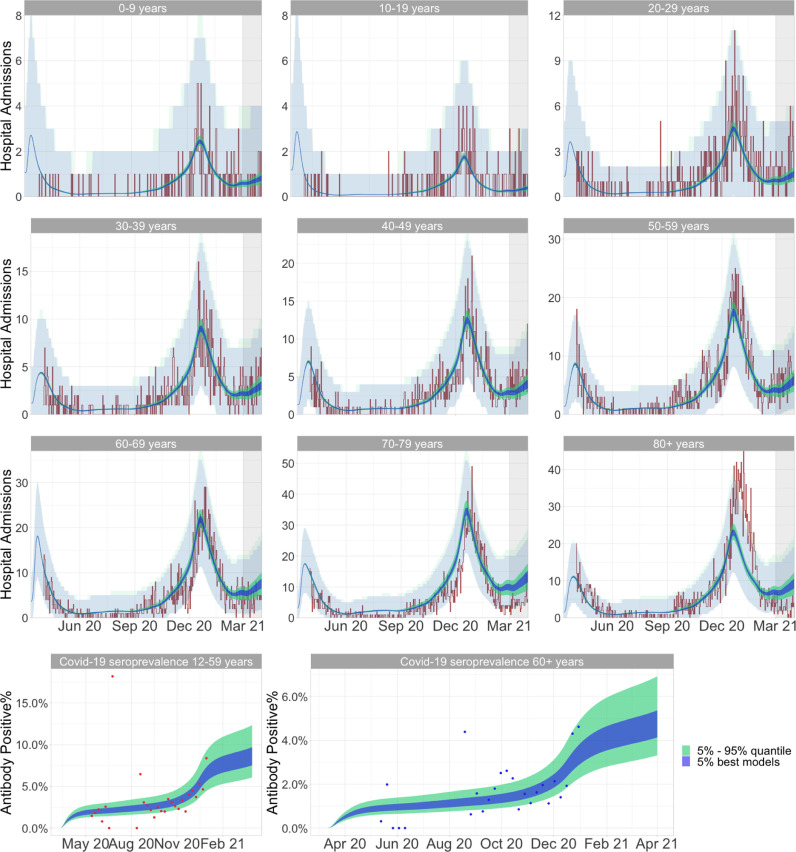
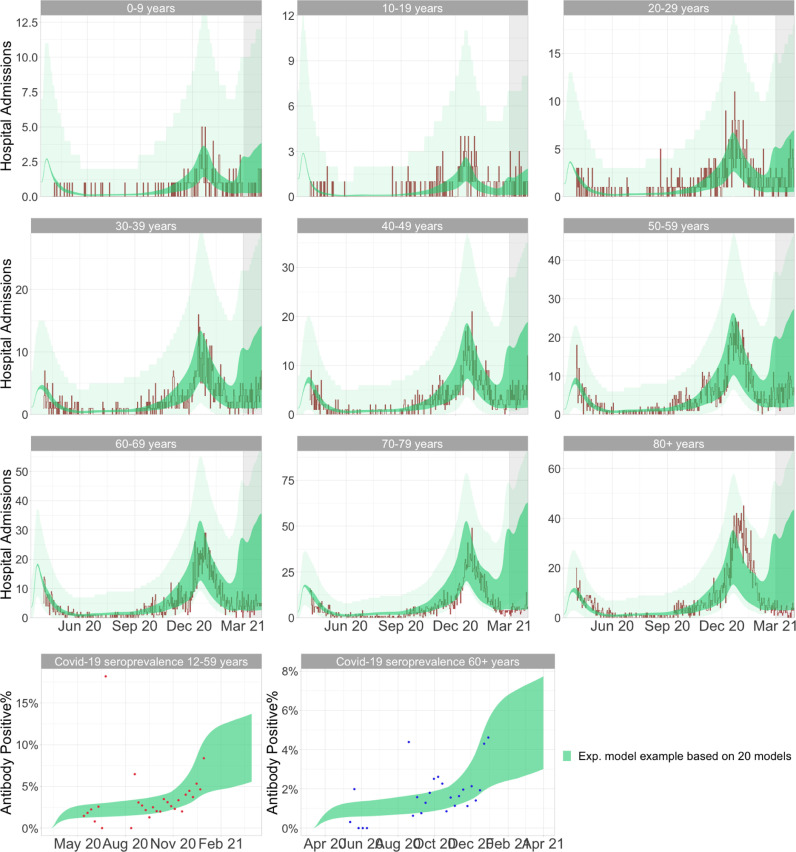
Figure 5 shows the observed data compared to the model output of the models included in the 95% seasonality CI in Table 1. The first nine figures show age stratified hospital admissions and the bottom two figures show seroprevalence for the two age groups; 12–59 and 60+ years.
Fig. 5.
New admissions and seroprevalence fit of the population models from the ABC-rejection algorithm. The shaded area for new hospital admissions indicate the 95% negative binomial distribution confidence interval which were used for the likelihood evaluation of the model. The vaccination programme in Denmark was initiated in December 2020 in the older age groups, and thus the risk of hospital admission in these age groups were reduced compared to predicted during the Spring. Additionally, as a consequence of the vaccination programme initiation, seroprevalence data was not available beyond December 2020. The displayed 5% best models spans seasonality estimates of 27%-29%; and the 5%-95% quantile estimates spans seasonality magnitudes from 24%–31% (i.e. the 95% CI for seasonality).
The population model in Fig. 5 produces deterministic outputs of the rate of hospital admission. Thus, the observed data points are expected to present as daily stochastic fluctuations around the rate presented by the population model. The addition of the 95% negative binomial confidence interval (the transparent shaded area around new hospital admissions), serve as an extension of the hospital admission rate from the deterministic model, and thus extend the model to expect the new daily hospital admissions - with stochastic fluctuations - to lie within this interval rather than on a single line.
Figure 5 shows that the population model with implemented seasonal variation, is highly capable of capturing the long term development in the age stratified observed hospital admissions and seroprevalence for all age groups - especially for the age groups 0–69 years (See appendix Figs. A.1 & A.2 for the simulation without seasonal effects). The two oldest age-groups (70–79 and 80+), present two discrepancies between model and the observed data.
-
1.
The hospital admissions in the oldest age group 80+, was underestimated during December, which secondarily leads to an underestimated admission rate in January 2021. This underestimation can be caused by several different factors. One explanation could be the fact that the population model does not include any Christmas-specific scaling of contacts. Here, it is likely that especially the oldest generation - grandparents and great-grandparents - experience the greatest relative increase in contacts during the holidays, through spending increased amounts of time together with their children and grandchildren. This would cause a relatively large increase in the amount of cross generational infection transmissible contacts, and thus enable the epidemic to jump from the younger population to the elderly population and subsequently flourish in the elderly population e.g. in nursery homes (Fig. 1 from SSI, 2021b). This would lead to an increase in hospital admissions in especially the oldest age group, which would not be captured by the model - This is supported by data on the behaviour of the population 70+ years of age during Christmas, where there is an increase in close family contacts during 1st and 2nd advent (governmental restrictions were introduced shortly after 2nd advent) and around Christmas eve (HOPE project, 2021). This also emphasize the fact, that the estimated seasonal variation of , might be higher for the 80+ population.
-
2.
The rates of hospital admissions for the two oldest age groups (70–79 and 80+ years of age) from the 1st of February 2021 and onward, underline the fact that the population model does not include the Danish vaccination programme. The vaccination programme was excluded from the model as it would drastically increase the complexity of the model through requirement of age-stratified estimates for the effect - of both one and two vaccine doses and for each type of vaccine - on both infectiousness, risk of infection and risk of hospital admission. This would compromise the precision of the model, through a drastic increase in model-parameter uncertainties. Instead, the optimization period of the model is terminated on 1st of March 2021, thus only including a period where vaccine induced immunity is small and can be assumed not to have a large impact on seasonality estimates, whilst still including the second wave of hospital admissions, which is required to get the best evaluation of the seasonality model, which should be able to capture both the Autumn/Winter increase and the subsequent Spring reduction in admissions. In Denmark the first vaccinations were given on the 27th of December and the roll out of the vaccination programme was originally prioritized in six target groups which were to initiate vaccinations before March 2021. These target groups were vaccinated sequentially as vaccines became available (Sundhedsstyrelsen, Sundhedsstyrelsen). The six target groups can be seen in Table 2 and generally consist of the older age groups and high risk individuals or individuals with daily activities related to these two categories. Thus, the population model - which doesn’t include vaccinations - overestimate hospital admissions for the age groups from 70 to 79 years of age and especially for 80+ years in the Spring of 2021. This overestimation increase over time as more individuals are vaccinated and the difference between the population model structure and the real world increase (Fig. 5); 33.1% (93459), 4.5% (26036) and 4.8% (31976) had finished their second vaccination and 51.5% (145321), 12.1% (69569) and 12.6% (84293) had received their first vaccine in mid March 2021 in the age groups 80+, 70–79 and 60–69 respectively ([dataset] SSI, 2022).
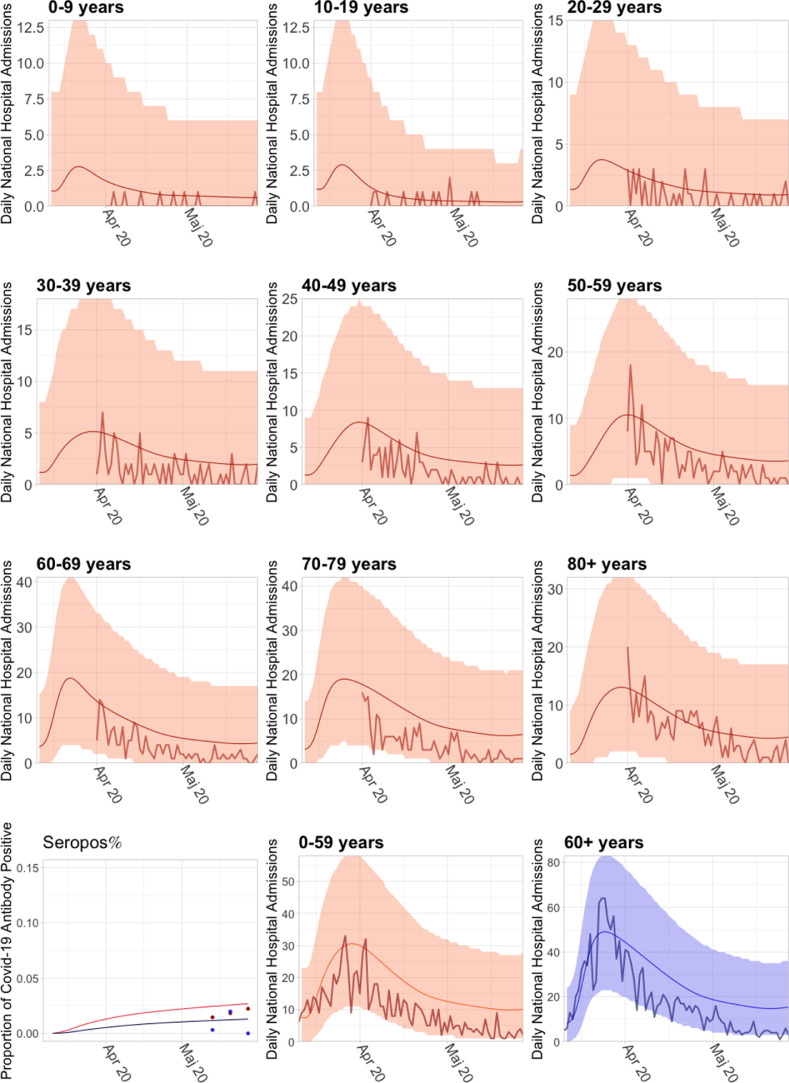
Fig. A.1.
The population model without implementation of seasonal effect, but with the optimised hospital admission risk.
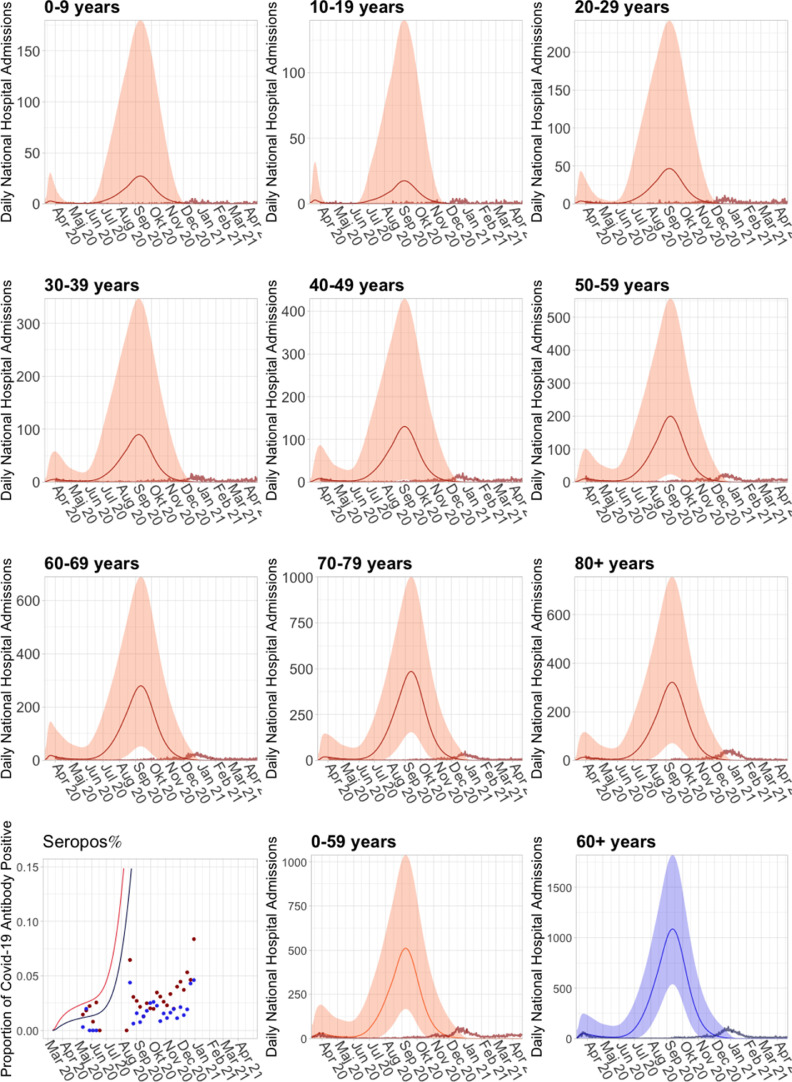
Fig. A.2.
Long term simulation of the population model, without implementation of seasonal variation. Although this population model is based on the population model published by SSI in May 2020 SSI (2020a), this figure cannot in any way be considered as a long term prediction from May 2020, as it was well-known at that time, that a seasonal effect was present, which would cause a decrease in infections during the summer, and the model was at that time not suited for, nor capable of long term simulations. Thus the inclusion of this figure is for the sole purpose of showing the drastic effects of introduction of a seasonal variation, and how it contributed to reducing the amount of infections in the Spring of 2020.
Table 2.
| Target Group | Description |
|---|---|
| 1 | Residents at nursery homes. |
| 2 | 65 years old with requirements for practical help and personal care. |
| 3 | Citizens 85+ years old. |
| 4 | High risk of exposure personnel in the health care system, elderly care system and parts of social services. |
| 5 | Certain patients with increased risk of severe infection. |
| 6 | Select relatives to persons in high risk of severe infections. |
3.2. Logistic relationship between temperature and transmission rate
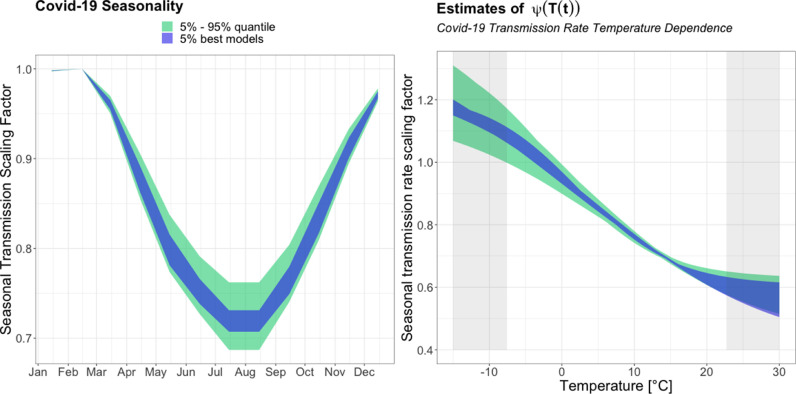
Figure 6 presents the relative seasonal development in the transmission rate of covid-19 (left) and the logistic relationship between transmission rate and daily average temperature (right) for the models in the 95% seasonality CI from the ABC-rejection posteriors (Table 1). The grey areas in the right-most figure (Fig. 6) indicate temperatures outside the observed temperature interval in the period March 2020 - March 2021. The uncertainties in the figure on the left are exclusively due to uncertainties in model fit, not in the yearly variation of temperature.
Fig. 6.
Estimated monthly development in transmission rate of covid-19 and the used seasonal scaling as a function of temperature. The displayed 5% best models spans seasonality estimates of 27%–29%; and the 5%–95% quantile estimates spans seasonality magnitudes from 24%-31% (i.e. the 95% CI for seasonality).
Figure 6 (left) shows that the seasonal variation causes a rapid decline in transmission rate during the Spring and early Summer in March-June, then reach a plateau in July-August, and from September-December there’s a rapid increase in transmission rate. During December-February the seasonally induced changes in transmission rate are estimated to be small. The seasonality estimate is a general estimate based on the average annual temperature behaviour over 30 years, thus the temperature fluctuations - and thus also the magnitude of the seasonal variation - in a given year might deviate slightly from what is seen in this general estimate. A hot Spring (early Summer) one year, might lead to a left shift of the Spring transition period, and induce a reduction in infections earlier than expected. A cold Winter might lead to higher magnitude of the seasonal variation, and a cold Spring/late Summer, might lead to a slight right shift of the Spring transition, which could lead to a delayed seasonally induced decrease in covid-19 cases.
Within the observed temperature interval (non-shaded area in Fig. 6 (right)) the temperature dependent transmission rate in the best models are starting visibly to plateau above C and the beginning of an upper scaling boundary is visible for temperatures lower than C. The presence of plateaus for extreme temperature values, is a direct consequence of the choice of using a logistic temperature dependent function. The logistic temperature structure in Eq. (17) allow the influence of temperature changes on the transmission rate to vary as a function of current temperature. During this study an exponential temperature dependence was also examined (results not shown in the article, however examples of the exponential model fits can be seen in appendix Fig. A.3). This revealed that the logistic temperature dependency - because of the presence of both an upper and lower boundary - provided a better fit (evaluated quantitatively on likelihood and qualitatively on visual fit) when estimating seasonality through the Winter of 2020/2021. The use of a logistic temperature relationship is also supported by its physical interpretation; As with other viral respiratory infections, the seasonal changes in transmission rate of covid-19 are caused by changes in environmental factors and human behaviour (Moriyama et al., 2020). Here, we hypothesize the logistic relationship between temperature and transmission rate excels because it captures that there exists an upper (and lower) limit to the extent of temperature dependent human behaviour changes - meeting inside versus outside, airing etc.) - and possibly also to the physical effects of temperature and correlated parameters on stability and infectiousness of the virus.
Fig. A.3.
An example of the quantitative and qualitative behaviour of the exponential temperature models. Here, it is evident that the exponential models generally are unable to accurately capture the decrease in admissions during the late Winter and Spring of 2021 due to a too drastic response to low temperatures. Additionally, it can be seen that some of the models underestimate the admissions during this period. This is due to the fact that these exponential models aren’t sensitive enough to temperature changes, which enables them to avoid the unrealistic rise in admissions, but simultaneously also cause them to underestimate the influence of seasonality. Consequently, the logistic models shows a more realistic description of the relationship between temperature and covid-19 transmission rate.
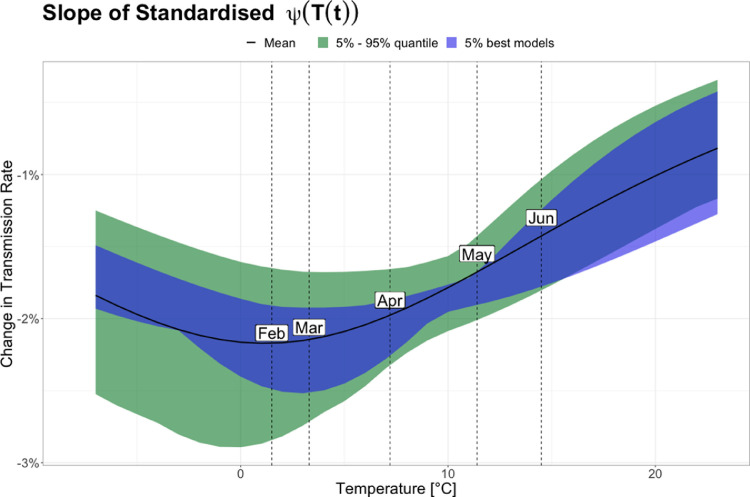
The influence of temperature changes on transmission rate at certain temperature levels, can be estimated by calculating the relative effect of C temperature increase based on Fig. 6 (right). In order to do so, Fig. 6 (right) were standardized at 1.5 C and the slope of the curve was subsequently calculated. 1.5 C is the lowest average monthly temperature in Denmark based on climate normal data ([dataset] DMI, 2022). In Fig. 7 can be seen the slope of this standardized curve, which show that the impact of temperature changes on transmission rate for the 5% best seasonality models are highest around 0–7.5 C and decrease as temperatures move further away from this interval. Figure 7 also shows the average temperatures for the five months February, March, April, May and June. Thus, it can be seen that around 2 C average daily temperature - corresponding to around February and March in Denmark - the reduction in transmission rate is pr. C increase; around C (around April in Denmark) the change is pr. C increase and at C (around mid May in Denmark) the impact on transmission rate is pr. C increase.
Fig. 7.
Slope of standardised logistic temperature dependency. Shows the impact of temperature on the transmission rate pr. 1 Celsius change. Standardisation is based on the averaged coldest monthly temperature through the last thirty years: February, C. The displayed 5% best models spans seasonality estimates of 27%-29%; and the 5%-95% quantile estimates spans seasonality magnitudes from 24%-31% (i.e. the 95% CI for seasonality).
A recent study on the seasonal effect on SARS-CoV 2 in temperate climates (Gavenciak et al., 2021) estimated a seasonal effect of 42.1% CI [24.7%; 53.4%] in the reproductive number , from Winter to Summer, the study was from June 2021 and included data from 143 temperate European regions. Their estimate for the seasonal variation is wider and higher than then estimated seasonal effect of (95% CI ) which was found in this study. However, the two 95% CIs overlap and show a similar scale of seasonality. Another study from August 2020, found a 3.08% (95% CI [1.53%; 4.63%]) reduction in daily infections and a 1.19% (95% CI [0.44%; 1.95%]) reduction in daily deaths pr. C increase in temperature (Wu et al., 2020). These results are of similar scale as the estimates of the effect of temperature changes on daily hospital admissions found in our study (Fig. 7). The novel contribution of our study to current literature on seasonality is threefold and based on the fact that we are using a model-based approach to contribute to a more holistic insight into covid-19 seasonality modelling through the estimation of the relationship between temperature and transmission rate. Firstly, rather than describing the peak change in transmission rate or the effect of C increase at one time point, we robustly estimated a logistic relationship between transmission rate and temperature in Denmark. This facilitate an improved general understanding of the boundaries of seasonality and easy temperature dependent implementation of seasonality in epidemic models for countries of similar (temperate) climate and social structure. Secondly, this relationship, facilitate calculation of both a temperature dependent estimate of the magnitude of covid-19 seasonality for a given year, and the impact of a C temperature change on transmission rate, and how this impact varies depending on current temperature through the year. Lastly, the focus on a single country facilitate the exhaustive implementation of NPIs; the -strain; and age stratified hospital admission risks which alongside the good fits to both hospital admissions and immunity (seroprevalence) data reduce the risk of spurious seasonality results and thus facilitate an accurate and robust estimation of the seasonality.
3.2.1. Limitations
The social and environmental interactions with the transmission rate of covid-19 are extremely complex, and as such it’s important to bear in mind that the results in this study should not be considered globally applicable and definitive. Under the limitations of this study, the results should rather be considered a contribution to the existing, growing literature on covid-19 seasonality, its relationship with temperature as a proxy parameter and how it can be reliably implemented in epidemic covid-19 population models.
There are three main limitations in this study. The first being the fact that the model is exclusively based on Danish data, and thus the results are accurate for Denmark, and serve as estimates for countries of similar climate conditions and social behaviour. The degree of transferability of the results to other temperate countries depends heavily on the nature of social and meteorological similarities between Denmark the given country.
Secondly, this study does not attempt to and is unable to infer any causality between temperature and the seasonal changes in covid-19 transmission rate. It exclusively found that temperature can serve as a proxy for the implementation of seasonality in a mathematical population model of covid-19 in the Danish climate and social setting. Thus, increasing or decreasing temperatures can yield different outcomes in countries which are not comparable to Denmark on these two parameters. The different effects of temperature on covid-19 have been addressed in a review article by Byun et al. (2021), where mostly negative - especially for Europe - but also positive and no effects of increasing temperature on transmission rate have been found depending on the given country and method used in the study.
Thirdly, it is not possible, based on this study to infer the effect of temperature changes and seasonality on emerging strains (variants emerging after the -variant) of covid-19, which might show variance in transmission patterns in response to temperature changes e.g. Smith et al. (2021a) (pre-print) found evidence of the -variant having better transmission at warmer temperatures than the previous strains.
3.2.2. Social implications
Excluding seasonality from future models and considerations regarding the spread of covid-19, can lead to an underestimation of covid-19 infections in the Fall and Winter in temperate countries. This underestimation could potentially lead local governments and decision-makers to delay the implementation of precautions - such as NPIs or vaccine booster doses, which are essential for saving lives during periods of rapid community spread. Notwithstanding the aforementioned limitations, the results from this study shows how temperature can be used as a proxy in a logistic model to reliably implement covid-19 seasonality in mathematical models. This knowledge enable better anticipation of the timing and magnitude of coming Winter surges and enable better covid-19 management through e.g. timed implementations of preliminary precautions such as governmentally implemented restrictions and administration of vaccine booster doses. Equally important, having these reliable models of seasonality also aid in the timing of removal of NPIs thus, e.g. avoiding periods of futile maintained implementation of NPIs.
3.3. Conclusion
This study shows that implementation of seasonal variation in the transmission rate of covid-19 in a temperate climate can be implemented in epidemic population models by using daily average temperature as a proxy for seasonal changes in transmission rate. The seasonal variation in the transmission rate of covid-19 in Denmark (temperate climate) from March 2020 to March 2021 is - through this study - estimated to be (95% CI [24%; 31%]) reduction from February to July. This estimate is a general estimate based on the average monthly temperatures through the last 30 years, and fluctuations in temperatures in given years will create fluctuations in the magnitude of seasonality around this estimate. Additionally it is shown that the impact of increasing temperature on transmission rate is greatest for daily average temperatures around 0–7.5C, and decreases for both lower daily temperatures and increasing daily temperature. The highest impact of seasonality is thus seen during early Spring, late Autumn and Winter were temperatures typically are within this interval. The temperature dependent reduction in covid-19 transmission rate pr. C increase is estimated to pr. C increase around C; pr. C increase around C; and pr. C increase at C. In Danish climate these temperatures correspond to February-March; April and May, respectively.
Declaration of Competing Interest
Whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Appendix A. No seasonal effect
Exponential model example
Parameters for the differential equation system
References
- Baker R.E., Yang W., Vecchi G.A., Metcalf C.J.E., Grenfell B.T. Assessing the influence of climate on wintertime SARS-CoV-2 outbreaks. Nat. Commun. 2021;12(846) doi: 10.1038/s41467-021-20991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun W.S., Heo S.W., Jo G., Kim J.W., Kim S., Lee S., Park H.E., Baek J.-H. Is coronavirus disease (covid-19) seasonal? a critical analysis of empirical and epidemiological studies at global and local scales. Environ. Res. 2021;196:110972. doi: 10.1016/j.envres.2021.110972. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://www.sciencedirect.com/science/article/pii/S0013935121002668.
- Christiansen, L. E., 2020. linelist_snapshotċsv. https://github.com/laecdtu/C19DK/blob/master/SSEIH_model/linelist_snapshot.csv.
- D’Amico F., Marmiere M., Righetti B., Scquizzato T., Zangrillo A., Puglisi R., Landoni G. Covid-19 seasonality in temperate countries. Environ. Res. 2022;206:112614. doi: 10.1016/j.envres.2021.112614. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://www.sciencedirect.com/science/article/pii/S0013935121019150.
- Danish Covid-19 Genome Consortium, 2021. Genomic overview of SARS-CoV-2 in Denmark. https://www.covid19genomics.dk/statistics.
- DMI, 2021. Vejrarkiv. https://www.dmi.dk/vejrarkiv/.
- DMI, 2022. Klimanormaler Danmark. https://www.dmi.dk/vejrarkiv/normaler-danmark/.
- HOPE project, 2021. Estimating local protective behavior in denmark with dynamic MRP. https://hope-project.dk/#/reports/estimating_local_protective_behaviour/versions/11-01-2021.
- Johnsen, M., Grsbll, K., Christiansen, L., Kirkeby, C., 2020. Validering af den danske covid-19 populationsmodelhttps://findit.dtu.dk/en/catalog/2691321304.
- Kulturministeriet. Coronaudbrud i nordjylland medfrer nedlukning i syv kommuner. https://kum.dk/aktuelt/nyheder/coronaudbrud-i-nordjylland-medfoerer-nedlukning-i-syv-kommuner.
- Gavenciak T., Monrad J.T., Leech G., Sharma M., Mindermann S., Brauner J.M., Bhatt S., Kulveit J. Seasonal variation in SARS-CoV-2 transmission in temperate climates. 2021. https://www.medrxiv.org/content/10.1101/2021.06.10.21258647v3 [DOI] [PMC free article] [PubMed]
- Mecenas P., Bastos R.T.d.R.M., Vallinoto A.C.R., Normando D. Effects of temperature and humidity on the spread of covid-19: a systematic review. PLoS ONE. 2020;15(9):1–21. doi: 10.1371/journal.pone.0238339. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://doi.org/10.1371/journal.pone.0238339.
- Moriyama, M., Hugentobler, W. J., Iwasaki, A., 2020. Seasonality of respiratory viral infections10.1146/annurev-virology-012420-022445. [DOI] [PubMed]
- Peter Bager, P., Jan Wohlfahrt, D., Jannik Fonager, P., Morten Rasmussen, P., Mads Albertsen, P., Thomas Yssing Michaelsen, M., Camilla Holten Mller, Steen Ethelberg, P., Rebecca Legarth, P., Mia Sarah Fischer Button, M., Sophie Gubbels, P., Marianne Voldstedlund, P., Prof Kre Mlbak, D., Robert Leo Skov, M., Anders Fomsgaard, P., Tyra Grove Krause, P., 2021. Risk of hospitalisation associated with infection with SARS-CoV-2 lineage b.1.1.7 in Denmark: an observational cohort study 10.1016/S1473-3099(21)00290-5. [DOI] [PMC free article] [PubMed]
- Regeringen, 2020. Restriktioner fra 26. oktober 2020. https://www.regeringen.dk/media/10077/restriktioner_uge44_final.pdf.
- Smith T.P., Dorigatti I., Mishra S., Volz E., Walker P.G.T., Ragonnet-Cronin M., Tristem M., Pearse W.D. Environmental drivers of SARS-CoV-2 lineage b.1.1.7 transmission intensity. medRxiv. 2021 doi: 10.1101/2021.03.09.21253242. [DOI] [Google Scholar]; https://www.medrxiv.org/content/early/2021/03/19/2021.03.09.21253242.
- Smith T.P., Flaxman S., Gallinat A.S., Kinosian S.P., Stemkovski M., Unwin H.J.T., Watson O.J., Whittaker C., Cattarino L., Dorigatti I., Tristem M., Pearse W.D. Temperature and population density influence SARS-CoV-2 transmission in the absence of nonpharmaceutical interventions. Proc. Natl. Acad. Sci. 2021;118(25) doi: 10.1073/pnas.2019284118. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://www.pnas.org/doi/abs/10.1073/pnas.2019284118, e2019284118
- SSI, 2020a. Covid-19 models used by the SSI modelling group. https://github.com/laecdtu/C19DK.
- SSI, 2020b. Teknisk gennemgang af modellernehttps://files.ssi.dk/teknisk-gennemgang-af-modellerne-10062020.
- SSI, 2020 and 2021a. Download file: Filer med covid-19-opgrelser fra dashboardet (zip-csv), den 8. marts 2021 og frem. https://covid19.ssi.dk/overvagningsdata/download-fil-med-overvaagningdata.
- SSI, 2020 and 2021b. Modelberegninger ifm. covid-19https://covid19.ssi.dk/analyser-og-prognoser/modelberegninger.
- SSI, 2020 and 2021. Prvalensundersgelse af covid-19. https://covid19.ssi.dk/overvagningsdata/undersoegelser/praevalensundersogelsen.
- SSI, 2021a. Beskrivelse af udvalgte virusvarianter. https://covid19.ssi.dk/virusvarianter/virusvariantbeskrivelser.
- SSI, 2021b. Covid-19 i danmark - fokusrapport: Plejehjem. https://www.ssi.dk/-/media/arkiv/subsites/covid19/fokusrapporter/covid19-p-plejehjem-fokusrapport-30092021.pdf?la=da.
- SSI, 2021c. Dashboard: Statens serum institut - covid-19 - danmark (kommune) - opdateres hverdage kl. 14. https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d/page/page_0/.
- SSI, 2022. Download fil med vaccinationsdata for covid-19. https://covid19.ssi.dk/overvagningsdata/download-fil-med-vaccinationsdata.
- Statsministeriet,. Pressemde den 7. december 2020. https://www.stm.dk/presse/pressemoedearkiv/pressemoede-den-7-december-2020/.
- Statsministeriet, 2020. Pressemde om covid-19 den 11. marts 2020. https://www.stm.dk/presse/pressemoedearkiv/pressemoede-om-covid-19-den-11-marts-2020/.
- Sundhedsministeriet, a. Covid-19-nedlukning hen over jul og nytr. https://sum.dk/nyheder/2020/december/covid-19-nedlukning-hen-over-jul-og-nytaar.
- Sundhedsministeriet, b. Udvidelse af restriktioner til hele landet. https://sum.dk/nyheder/2020/december/udvidelse-af-restriktioner-til-hele-landet.
- Sundhedsministeriet, c. Udvidelse af skrpede tiltag til i alt 69 kommuner. https://sum.dk/nyheder/2020/december/udvidelse-af-skaerpede-tiltag-til-i-alt-69-kommuner.
- Sundhedsstyrelsen, 2020. S er vi i gang - i dag bliver de frste vaccineret mod covid-19. https://www.sst.dk/da/nyheder/2020/saa-er-vi-i-gang---i-dag-bliver-de-foerste-vaccineret-mod-covid-19.
- Sundhedsstyrelsen, 2021. Vaccinationskalender, 1. juli 2021. https://www.sst.dk/-/media/Udgivelser/2021/Corona/Vaccination/Kalender/Specificeret-vaccinationskalender-01072021.ashx?la=da&hash=236BE3849A49F85892527D9F803845DC5E85C3D8.
- Wu Y., Jing W., Liu J., Ma Q., Yuan J., Wang Y., Du M., Liu M. Effects of temperature and humidity on the daily new cases and new deaths of covid-19 in 166 countries. Sci. Total Environ. 2020;729:139051. doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://www.sciencedirect.com/science/article/pii/S0048969720325687.