Abstract
Lipocalin-2 (LCN2) is becoming recognized as a pleiotropic mediator of metabolic disorders. However, the relationship between LCN2 and gestational diabetes mellitus (GDM) is not well understood. We performed a systematic review and meta-analysis to explore it. A systematic search of Cochrane Library, PubMed, Embase, Scopus, Web of Science, Chinese National Knowledge Infrastructure, and Wan-fang Database was done for relevant articles published up to September 29, 2021. Standardized mean difference (SMD) with 95% confidence intervals (CI) was calculated to explore the association of LCN2 levels with GDM using Revman 5.3 and Stata 15.1. Fifteen case-control studies were included in this meta-analysis. The patients with GDM had significantly higher levels of blood LCN2 than parturients with normal glucose tolerance (SMD=3.41, 95% CI=2.24 to 4.58). Meta-regression and subgroup analysis were conducted to investigate the source of heterogeneity. Likely sources of heterogeneity were age and testing methods. This study found that GDM showed higher blood LCN2 levels than controls. However, caution is warranted on the interpretation of these findings. Standardized LCN2 measurement methods and longitudinal studies are required to disentangle and better understand the relationships observed.
Key words: lipocalin-2, gestational diabetes mellitus, meta-analysis, insulin resistance , adipokines
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance that begins or is initially noticed during pregnancy 1 . Being the most common pregnancy complication, GDM may complicate 15–20% pregnancies, and its prevalence has increased by 27% in the last 20 years in all ethnic groups 2 . GDM induces both maternal and fetal complications and gives rise to poor pregnancy outcomes, including preterm delivery, pre-eclampsia, macrosomia, and perinatal death 3 . The pathogenesis of GDM has not been completely clarified, though insulin resistance plays a fundamental role in its development 4 5 . Increased insulin resistance, sterile inflammation, and endothelial cell dysfunction are the three central features that contribute to hyperglycemia in patients with GDM 6 . Recently, accumulating evidence indicated that adipokines are involved in the pathogenesis of GDM 7 8 .
Lipocalin-2 (LCN2), also known as neutrophil gelatinase-associated lipocalin, is a 25-kDa secreted adipokine and a member of the lipocalin superfamily 9 10 . LCN2 was first discovered in human neutrophils 11 , and is expressed in many tissues such as the liver, kidneys, and adipose tissue 12 . It was identified as a critical regulator of energy metabolism, glucose and lipid homeostasis, and insulin function 9 13 . Metabolic disorders, as observed in diabetes and obesity, have been closely associated with the upregulation of LCN2 in blood and several other tissues 9 . There have been reports of increasing circulating LCN2 levels during pregnancy 14 . Recent evidence points to the role of LCN2 in the pathophysiology of GDM. Although a previous study proposed that blood LCN2 were higher in patients with GDM compared with parturients with normal glucose tolerance (NGT) 15 , subsequent studies showed inconsistent results 16 17 . Since the existing results from accessible studies are conflicting, evidence-based quantitative analyses evaluating the correlation between circulating LCN2 levels and GDM are indispensable.
Materials and Methods
Search strategy
We systematically searched the following 7 electronic databases: Cochrane Library, PubMed, EMBASE, Scopus, Web of Science, Chinese National Knowledge Infrastructure (CNKI) Database, and Wan-fang Database. The final literature search was performed on September 29, 2021. Only articles published in English and Chinese were used. The search strategy included key terms were listed as follows: (1) “Diabetes, Gestational”, “Diabetes, Pregnancy-Induced”, “Diabetes, Pregnancy-Induced”, “Pregnancy-Induced Diabetes”, “Gestational Diabetes”, “Diabetes Mellitus, Gestational”, “Gestational Diabetes Mellitus” and “GDM” and (2) “Lipocalin-2”, “Lipocalin 2”, “NGAL Protein”, “Oncogene 24p3 Protein”, “Siderocalin Protein”, “Neutrophil Gelatinase-Associated Lipocalin”, “Neutrophil Gelatinase-Associated Lipocalin”, “Lipocalin-2 Protein”, “Lipocalin 2 Protein” and “NGAL”. The reference lists of related original and review articles were also analyzed using a manual approach. The protocol has been registered on the INPLASY website as INPLASY202190097 (https://inplasy.com/inplasy-2021–9–0097/).
Eligibility criteria
Publications were considered eligible if the following criteria were met: (1) included pregnant women with GDM as participants; (2) circulating LCN2 levels were detected; (3) controls were pregnant women with normal glucose tolerance; (4) evaluate the association of LCN2 levels with GDM; and (5) observational study design. The exclusion criteria were: (1) review, editorial, abstract, case report, or unpublished article; (2) studies with insufficient data to calculate the mean and standard deviation (SD); (3) studies that worked on animals or cells. Studies with Newcastle-Ottawa Scale (NOS) score<7 were of not good quality 18 and excluded in the quantitative analysis (see Quality Assessment below).
Data extraction and quality assessment
Two independent authors performed the literature search, data extraction, and quality assessment of the included studies, according to the predefined inclusion criteria. Discrepancies were resolved by discussion with a third investigator. The following data were extracted: first author of the study, publication year, study country, study design, sample size, LCN2 levels, mean age, mean pre-pregnancy body mass index (BMI), testing method, measurement trimester, and applied GDM criteria. The data were presented as median with interquartile range transformed into mean±SD 19 20 21 . The quality of the studies was assessed by the NOS for case-control studies 22 . A system of points was given to the eligible categories: (I) selection (II) comparability, and (III) exposure. A study could be awarded a maximum of one point for each numbered item within the Selection and Exposure categories. A maximum of two points could be given for comparability. For case-control studies, points ranged from 0 to 9 points with≥7 points classified as good quality 18 .
Statistical analysis
Data were analyzed using Review Manager (RevMan 5.3, The Cochrane Collaboration, Oxford, UK) and Stata software (Stata version 15.1, STATA Corporation, College Station, TX, USA). Blood LCN2 level is a continuous variable and expressed using different units depending on individual studies, therefore we used standardized mean difference (SMD) and 95% confidence intervals (CIs) for analysis. The significance of the pooled SMD was evaluated by the Z-test. Heterogeneity across the studies was evaluated using Cochrane’s Q-test and I 2 metric with a range of 0–100%. A random-effects model was used if I 2 >50%; otherwise, the fixed-effects model was used. Sensitivity analyses, by omitting one individual study at a time, were performed to test the robustness of the results. To explore the potential cause of heterogeneity and determine whether various items affected the heterogeneity across studies, meta-regression and subgroup analysis were performed. Finally, funnel plots were used initially to evaluate visually publication bias while Egger’s regression test and Begg’s test were performed to inferentially evaluate publication bias. Substantial publication bias was final determined by the trim-and-fill technique. The statistical significance was defined as p-values less than 0.05.
Results
Search results
The literature search yielded 145 relevant articles, of which 12 were from PubMed, 25 from Embase, 16 from Web of Science, 11 from Scopus, 48 from CNKI, and 33 from Wan-fang. No relevant publications were identified in the Cochrane Library. After removing duplicates, and further eliminating unrelated articles, reviews or editorials and animal studies, 40 articles were assessed for eligibility by full-text screening. From these, two articles had no control group, nine articles did not use peripheral blood sample, and three articles had overlapped data with other studies. Of the remaining 26 articles, data presented as “Median (first quartile- third quartile)” in 1 article was significantly skewed away from normality, and not recommended to data transformation. Hence, mean and SD were unavailable in this article. Twelve articles that scored<7 according to the NOS quality assessment criteria were not of good quality. These 12 articles were therefore also excluded. This left 13 articles (containing 15 studies), which were eventually analyzed in this meta-analysis, among which LCN2 levels in one article were tested in three pregnancy trimesters 23 . The flow chart of the study selection is shown in Fig. 1 .
Fig. 1.
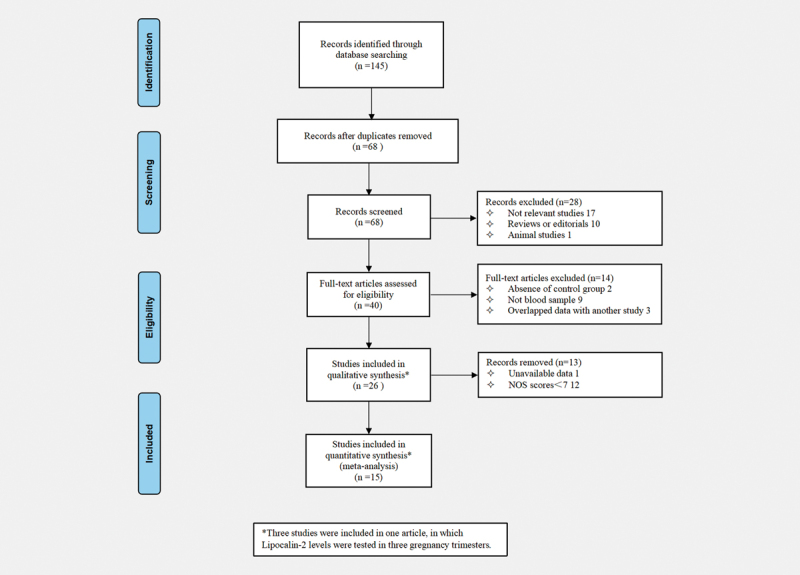
Flowchart of database search and study identification.
Study characteristics are described in Table 1 . Overall, 15 studies with 1882 individuals were included. The studies were performed in Denmark 16 , Italy 15 , Mexico 17 , Poland 24 , and China 4 12 23 25 26 27 28 29 30 . All included studies were case-control studies and five were nested case-control studies. Blood samples were collected in different trimesters of pregnancy. Thirteen studies used enzyme-linked immunosorbent assay (ELISA) for measurements, and mass spectrometer and Magpix technology were used in the other studies. ADA and IADPSG diagnostic criteria for GDM were used in most studies, and other diagnostic criteria were supported by references in original articles. The NOS scores of the included studies ranged 7–9, indicating generally good study quality.
Table 1 Characteristics of the included articles.
| Study [Ref] | Country | Study Design | Sample Size (GDM/controls) | Mean Age | Mean BMI | Fasting | Methods | Measurement trimesters | GDM criteria | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| D’Anna et al. (2009) 15 | Italy | NCC | 41/82 | 27.2 | 26.7 | Yes | ELISA | First | C & C | 9 |
| Duan et al. (2012) 25 | China | CC | 77/77 | 29.5 | 22.7 | Yes | ELISA | Third | ADA | 7 |
| Wang et al. (2013) 29 | China | CC | 26/66 | NA | NA | Yes | ELISA | Third | IADPSG | 9 |
| Lou et al. (2014) 12 | China | CC | 84/96 | 28.31 | 20.43 | Yes | ELISA | Third | ADA | 7 |
| Guo (2014) 26 | China | CC | 28/21 | 28.5 | NA | Yes | ELISA | Third | CSOG | 7 |
| Ma et al. (2015) 23 | China | NCC | 101/100 | 29.92 | 22.78 | Yes | ELISA | First, Second, third | IADPSG | 9 |
| Ravnsborg et al. (2016) 16 | Denmark | NCC | 101/104 | 32.35 | ≥27 | No | MRMMS | First | EDPSG | 7 |
| He et al. (2018) 30 | China | CC | 37/34 | 31.6 | 22.9 | Yes | ELISA | Second | ADA | 7 |
| Kang (2018) 28 | China | NCC | 107/110 | 28.8 | 25.81 | Yes | ELISA | First | CSOG | 8 |
| Guo et al. (2019) 27 | China | CC | 85/50 | 29.12 | 22.54 | Yes | ELISA | Third | ADA | 7 |
| Yin et al. (2020) 4 | China | CC | 49/39 | 32.47 | 23.15 | Yes | ELISA | Third | IADPSG | 7 |
| Mierzynski et al. (2021) 24 | Poland | NCC | 153/84 | 27.59 | 23.71 | Yes | ELISA | Second | WHO | 8 |
| Saucedo et al. (2021) 17 | Mexico | CC | 65/65 | 31.65 | 32.36 | Yes | Magpix | Third | IADPSG | 7 |
BMI: Body Mass Index; NCC: Nested case-control; CC: Case-control; ELISA: Enzyme Linked Immuno Sorbent Assay; MRM-MS: Multiple Reaction Monitoring- Mass Spectrometry; C & C: Carpenter and Coustan’s criteria; EDPSG: European Diabetic Pregnancy Study Group; ADA: American Diabetes Association; IADPSG: International Association of Diabetes and Pregnancy Study Group; CSOG: Chinese Society of Obstetrics and Gynecology; WHO: World Health Organization; NOS: Newcastle-Ottawa Scale; NA: Not available.
Meta-Analysis of Association of Lipocalin-2 Levels and GDM
Fifteen studies (1882 participants included in 13 articles) compared the LCN2 levels between patients with GDM and controls. Patients with GDM had significantly higher levels of blood LCN2 than parturients with NGT (SMD=3.41; 95% CI 2.24–4.58; p<0.001; Fig. 2 ). Random-effects models were used due to significant heterogeneity among the studies (I 2 =98.9%; p<0.001).
Fig. 2.
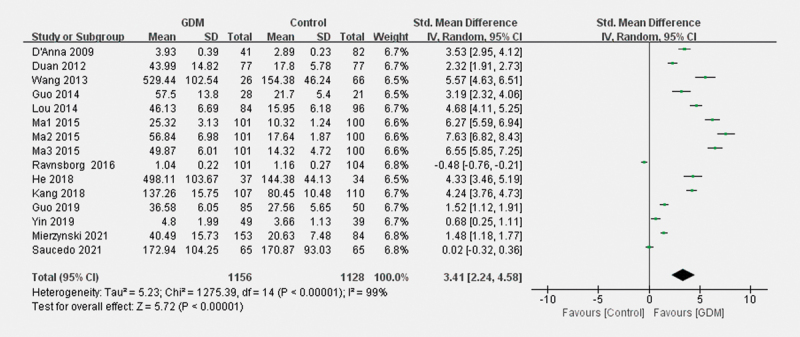
The forest plot about the association of blood LCN2 levels with GDM.
Meta-regression and subgroup analysis
Since significant heterogeneity was observed among studies (I 2 = 98.9%; p<0.001), meta-regression was performed to find the sources of heterogeneity, such as mean pre-pregnancy BMI, measurement trimesters, testing methods, mean age, and fasting sample. Testing methods and mean age were found to moderate the results (p=0.018 and p=0.035, Table 2 ), While measurement trimesters, mean pre-pregnancy BMI, and fasting sample did not affect the findings.
Table 2 The results of Meta-regression analysis.
| Coef. | t-Value | p | 95% CI | |
|---|---|---|---|---|
| Mean pre-pregnancy BMI | –2.10 | –1.40 | 0.190 | –5.409, 1.209 |
| Sampling trimesters | –0.25 | –0.33 | 0.749 | –1.930, 1.423 |
| Detecting methods | –4.21 | –2.71 | 0.018 | –7.570, –0.850 |
| Mean age | –3.02 | –2.38 | 0.035 | –5.783, –0.253 |
| Fasting sample | –4.18 | –1.75 | 0.103 | –9.333, 0.973 |
BMI: Body mass index; Coef: Coefficient; CI: Confidence interval.
As shown in Fig. 3 , subgroup analyses revealed that compared to pregnant women with NGT, significantly higher blood LCN2 concentrations in patients in GDM were detected using ELISA (SMD=3.98; 95% CI: 2.86–5.09, p<0.001). No significant difference was exhibited using other testing methods (SMD=–0.24; 95% CI: –0.74–0.25, p=0.34). As shown in Fig. 4 , for women aged≤30 years, women with GDM showed higher circulating LCN2 levels (SMD=4.12; 95% CI: 2.87–5.37, p<0.001). However, for women aged>30 years, the difference was not significant (SMD=1.06; 95% CI: −0.20–2.32, p = 0.10).
Fig. 3.
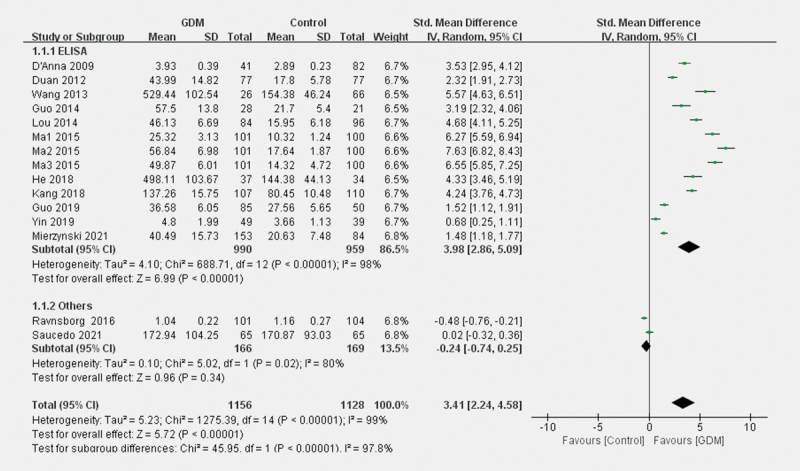
Forest plots for the subgroup analysis for the difference of blood LCN2 level between patients with GDM and parturients with NGT according to testing methods.
Fig. 4.
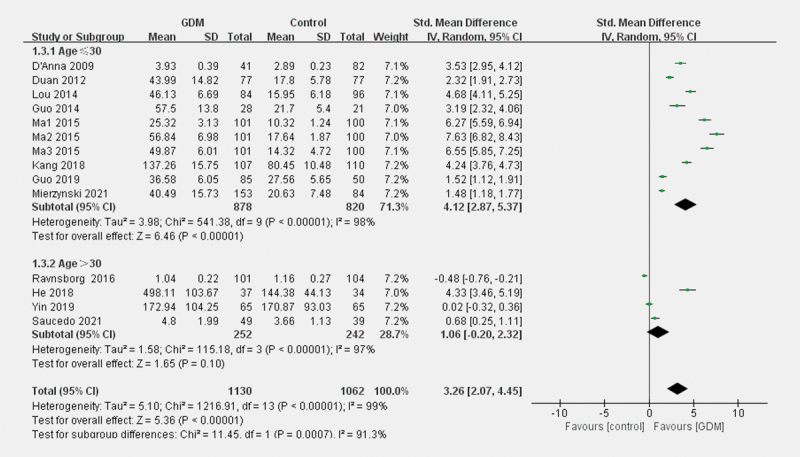
Forest plots for the subgroup analysis for the difference of blood LCN2 level between patients with GDM and parturients with NGT according to age.
Sensitivity analysis and publication bias
Sensitivity analyses showed that none of the studies were extremely outliers ( Fig. 5 ). Sensitivity analysis results indicated that the results were stable and credible by omitting one study at a time.
Fig. 5.
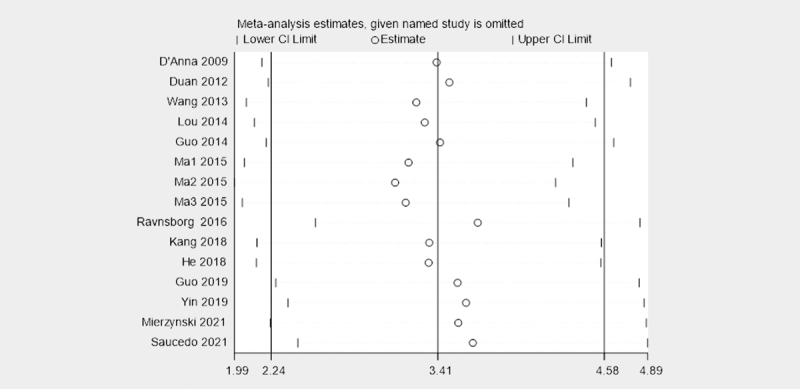
The plot of sensitivity analysis about the association of blood LCN2 levels with GDM.
Publication bias was measured by funnel plots and quantified by Begg’s and Egger’s tests. Asymmetry was observed by visual inspection of funnel plots ( Fig. 6 ). Begg’s and Egger’s tests revealed the existence of potential publication bias (p=0.008 and p<0.001, respectively). We further used the trim-and-fill method to evaluate the publication bias. There were no indications of publication bias with the trim-and-fill method (no new studies added).
Fig. 6.
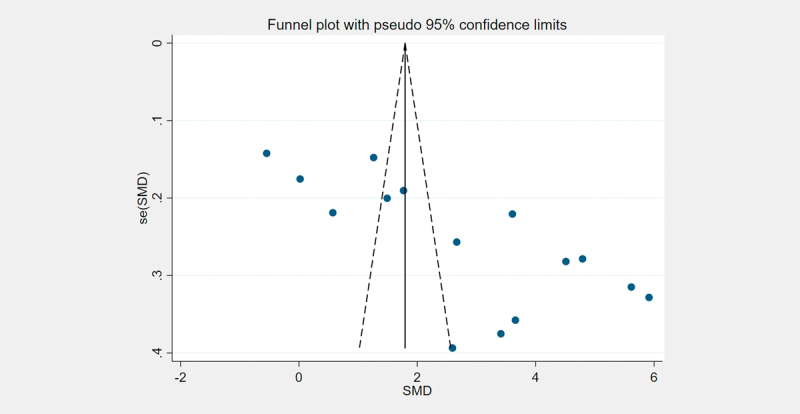
Funnel plots for the publication bias underlying the meta-analysis of the association of blood LCN2 levels with GDM.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to summarize the association of blood LCN2 with GDM. The main findings of the meta-analysis were as follows: Patients with GDM had significantly higher levels of blood LCN2 than parturients with NGT. Sensitivity analyses revealed that these pooled results were stable. Meta-regression analysis suggested that the testing methods and age were the sources of heterogeneity. Subgroup analyses showed that, compared to pregnant women with NGT, significantly higher LCN2 levels were observed in GDM samples using the ELISA. For women aged<30, the same trend was found. Taken together, these results indicated that LCN2 might be involved in the pathogenesis of GDM.
As a common pregnancy complication, GDM affects about 1 in 7 pregnancies worldwide, and is associated with adverse outcomes for both mothers and babies. Although the exact mechanisms of GDM are still unclarified, insulin resistance was considered as the main mechanism elaborated in the pathogenesis of GDM 31 . During normal pregnancy, more insulin was produced to meet the progressive demands of fetus development, which caused insulin resistance occurring at the start of the second trimester of pregnancy 32 33 . The dysfunction of insulin signaling in peripheral tissues made GDM mediated insulin resistance further exacerbated by nearly 56% 6 . Maternal and placental hormones, such as estrogen, placental lactogen, and placental growth hormone, also participated in pregnancy insulin resistance 34 . Moreover, inflammation and endothelial cell dysfunction also played essential roles in the pathogenesis of GDM.
A lot of evidence emphasized the association between LCN2 and metabolic diseases, such as obesity, type 2 diabetes, and nonalcoholic fatty liver disease, which partly shared the same underlying pathogenesis with GDM 35 36 . Above all, a positive correlation between LCN2 and pregnancy had been recognized from very early 14 . It was widely recognized that LCN2 is involved in insulin resistance, and the potential mechanisms were multifaceted 37 38 . LCN2 deficiency in mice caused an improvement in insulin resistance and inhibited gluconeogenesis in mice, which was probably mediated by decreasing AMP-activated protein kinase activity, and regulating forkhead transcription factor O1 and its downstream genes phosphoenolpyruvate carboxykinase/glucose-6-phosphatase, a hepatic gluconeogenesis moderator 39 40 . In H9c2 cells, LCN2 aggravated insulin resistance by inhibition of autophagy 41 . LCN2 probably also caused mitochondrial dysfunction through inhibiting the estrogen receptor α-polymerase gamma 1 axis 42 . Moreover, insulin itself is an inducer of LCN2, and LCN2 interfered with insulin signaling 43 . Glucose transporter (GLUT) 1 and GLUT4, the primary transporter responsible for glucose uptake, were declined in isolated human subcutaneous adipocytes after treating with recombinant LCN2 44 . Furthermore, LCN2 played a role in GDM via inflammation and endothelial cell dysfunction. LCN2 induced many pro-inflammatory mediators, such as interleukin-6, matrix metalloproteinase 2 (MMP2), and MMP9 45 46 . Interestingly, LCN2 induced the expression of tumor necrosis factor α, a critical insulin resistance–inducing factor, in fat tissue 40 . LCN2 was critically involved in diet-induced endothelial dysfunction by modulating cytochrome P450 2 C9 activity 47 .
Given the substantial heterogeneity among studies, meta-regression and subgroup analyses were carried out to investigate possible reasons for this heterogeneity. The plausible sources of heterogeneity were detecting method and age. Human LCN2 was originally purified from the supernatants of phorbol myristate acetate-stimulated neutrophils. Due to ligand-binding, post-translational modifications, and protein–protein interactions, different variants carried out different functions. The unknown sources and structural diversity of the variants of LCN2 lead to significant variabilities for clinical assessment 48 . LCN2 levels in most studies included were tested by ELISA, the difference between subjects with GDM and subjects in the control group were significant, whereas two studies 16 17 using other methods did not show the same trend. According to recent reviews, there is currently no “gold standard” method for detecting and measuring LCN2. ELISA was the first analytical procedure set up for measurement of LCN2 in blood samples and is currently the most widely used method 48 49 . It seems that other methods are still far from being extensively production ready. Standardization and quality control of LCN2 measurement are needed in future studies. For other testing methods, the number of studies was insufficient to draw clear conclusions. Age was considered as another factor leading to heterogeneity. Many studies have confirmed blood LCN2 correlated with age in both humans and mice. Previous studies have noticed the positive correlation between blood LCN2 levels and age in patients with GDM, while the explanations of mechanisms were not presented due to lack of evidence 24 . The increase of maternal age is paralleled by the progressive increase of maternal adipose tissue deposition, which means more adipocytokines produced 50 . In our study, younger patients with GDM showed significantly higher LCN2 levels than pregnant women with NGT. The recent study revealed that LCN2 may play a protective role in younger mice 51 . Nonetheless, the relationship between LCN2 and age is ambiguous, and research on age fertility demand further attention.
The heterogeneity did not decrease significantly, which suggests that not all heterogeneity sources could be found. Additional factors influencing LCN2 levels like exogenous insulin dosages and diet style might contribute to heterogeneity. Insulin can upregulate circulating LCN2 levels in humans, an effect that was mediated by phosphatidylinositol 3-kinase and mitogen-activated protein 52 . Diet lifestyle had been confirmed as a regulatory factor in blood LCN2 levels. The intake of saturated fatty acids contributed to changes in LCN2 levels after weight loss in obese subjects 53 . These factors are rarely mentioned in studies included; therefore subgroup analyses are unavailable and future research should take these variables into consideration.
There are limitations to our study. First, only case-control studies were included and heterogeneity between studies was high 54 . Existing uncontrolled factors in original studies might influence the heterogeneity of the meta-analysis. Though the trend of the relationship between LCN2 and GDM from these studies was clear, longitudinal cohort studies are optimal for further research. The evolution of LCN2 levels in GDM and their causality can be further confirmed if longitudinal cohort studies are conducted. Second, LCN2 testing methods in different laboratories lack standardization. Hemolysis, storage temperature, and manual measurement impact assay outcome and reduce comparability between different laboratories 49 . Third, most of the studies were from China, with limited data from other countries, and thus the regional effect must be considered. Therefore, there were strict quality control measures for studies included and quantitative analyses were conducted with a NOS score subject of≥7.
Conclusion
In conclusion, our study found that GDM is associated with higher LCN2 levels. However, caution should be taken in the generalization of the conclusions of this study. Large scale longitudinal studies covering more influencing factors with a unified standard detection method are needed to validate this finding, and whether LCN2 changes are causative or a consequence of GDM may be further investigated.
Funding Statement
Funding This study was funded by Changzhou Health Commission Young Talents Project (QN202018), Natural Science Foundation of China (81900768), and General Project of Maternal and Child Health Research Project of Jiangsu Province (F201803).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Metzger B E, Gabbe S G, Persson B et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30:S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigo N, Glastras S J. The emerging role of biomarkers in the diagnosis of gestational diabetes mellitus. J Clin Med. 2018;7:120. doi: 10.3390/jcm7060120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin X, Huo Y, Liu L et al. Serum levels and placental expression of NGAL in gestational diabetes mellitus. Int J Endocrinol. 2020:8.760563E6. doi: 10.1155/2020/8760563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre H D, Catalano P, Zhang C et al. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen-Ngo C, Jayabalan N, Salomon C et al. Molecular pathways disrupted by gestational diabetes mellitus. J Mol Endocrinol. 2019;63:R51–R72. doi: 10.1530/JME-18-0274. [DOI] [PubMed] [Google Scholar]
- 7.de Gennaro G, Palla G, Battini L et al. The role of adipokines in the pathogenesis of gestational diabetes mellitus. Gynecol Endocrinol. 2019;35:737–751. doi: 10.1080/09513590.2019.1597346. [DOI] [PubMed] [Google Scholar]
- 8.Gutaj P, Sibiak R, Jankowski M et al. The role of the adipokines in the most common gestational complications. Int J Mol Sci. 2020;21:9408. doi: 10.3390/ijms21249408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhusal A, Rahman M H, Lee W H et al. Paradoxical role of lipocalin-2 in metabolic disorders and neurological complications. Biochem Pharmacol. 2019;169:113626. doi: 10.1016/j.bcp.2019.113626. [DOI] [PubMed] [Google Scholar]
- 10.Xiao X, Yeoh B S, Vijay-Kumar M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu Rev Nutr. 2017;37:103–130. doi: 10.1146/annurev-nutr-071816-064559. [DOI] [PubMed] [Google Scholar]
- 11.Kjeldsen L, Bainton D F, Sengeløv H et al. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83:799–807. [PubMed] [Google Scholar]
- 12.Lou Y, Wu C, Wu M et al. The changes of neutrophil gelatinase-associated lipocalin in plasma and its expression in adipose tissue in pregnant women with gestational diabetes. Diabetes Res Clin Pract. 2014;104:136–142. doi: 10.1016/j.diabres.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Bhusal A, Lee W H, Suk K. Lipocalin-2 in diabetic complications of the nervous system: physiology, pathology, and beyond. Front Physiol. 2021;12:638112. doi: 10.3389/fphys.2021.638112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesur S, Yucel A, Noyan V et al. Plasma lipocalin-2 levels in pregnancy. Acta Obstet Gynecol Scand. 2012;91:112–116. doi: 10.1111/j.1600-0412.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- 15.D’Anna R, Baviera G, Corrado F et al. First trimester serum neutrophil gelatinase-associated lipocalin in gestational diabetes. Diabet Med. 2009;26:1293–1295. doi: 10.1111/j.1464-5491.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- 16.Ravnsborg T, Andersen LL T, Trabjerg N D et al. First-trimester multimarker prediction of gestational diabetes mellitus using targeted mass spectrometry. Diabetologia. 2016;59:970–979. doi: 10.1007/s00125-016-3869-8. [DOI] [PubMed] [Google Scholar]
- 17.Saucedo R, Valencia J, Moreno-Gonzalez L E et al. Maternal serum adipokines and inflammatory markers at late gestation and newborn weight in mothers with and without gestational diabetes mellitus. Ginekol Polska. 2021 doi: 10.5603/GP.a2021.0083. [DOI] [PubMed] [Google Scholar]
- 18.Komici K, Dello Iacono A, De Luca A et al. Adiponectin and sarcopenia: a systematic review with meta-analysis. Front Endocrinol (Lausanne) 2021;12:576619. doi: 10.3389/fendo.2021.576619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi J, Luo D, Weng H et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Meth. 2020;11:641–654. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 20.Luo D, Wan X, Liu J et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 21.Wan X, Wang W, Liu J et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo C K, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Q, Chen Y. Study on the expression of serum level of NGAL and its relationship with gestational diabetes in early pregnancy. J Clin Exp Med (Chinese) 2015:930–933. doi: 10.3969/j.issn.1671-4695.2015.011.022. [DOI] [Google Scholar]
- 24.Mierzynski R, Poniedzialek-Czajkowska E, Dluski D et al. The potential role of chemerin, lipocalin 2, and apelin in the diagnosis and pathophysiology of gestational diabetes mellitus. J Diabetes Res. 2021;5547228 doi: 10.1155/2021/5547228.eCollection2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan D, Niu J, Zhu B et al. Serum level of neutrophii gelatinase-assoeiated lipocalin in preeclampsia with gestatiunal diabetes mellitus. Chin J Perinat Med (Chinese) 2012;15:222–227. [Google Scholar]
- 26.Guo J.Relationship between neutrophil gelatinase-associated lipocalin and insulin resistance in diabetic gravida Modern Prevent Med (Chinese) 2014411599–1601.1604 [Google Scholar]
- 27.Guo L, Zhang C, Guo Y. Changes and significance of serum LCN-2 and AOPP levels in patients with gestational diabetes mellitus complicated with preeclampsia. Shandong Med J (Chinese) 2019;59:12–15. [Google Scholar]
- 28.Kang Y. The value of adiponectin, resistin and lipocalin-2 tests in early diagnosis of gestational diabetes mellitus. Label Immun. Clin Med (Chinese) 2018;25:1192–1195. [Google Scholar]
- 29.Wang F, Ye L, Yang L et al. Changes of serum neutrophic gelatinase-associated lipocalin in gestational diabetes mellitus. Chin J Microcirculat (Chinese) 2013;23:19–21. [Google Scholar]
- 30.He X, Gu D, Chen S et al. Clinical significanle of NGAL in women with gestational diabetes mellitus. China Med Pharm (Chinese) 2018;8:35–38. [Google Scholar]
- 31.Plows J F, Stanley J L, Baker P N et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Liu S, Solomon C G et al. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29:2223–2230. doi: 10.2337/dc06-0266. [DOI] [PubMed] [Google Scholar]
- 33.Binder A M, LaRocca J, Lesseur C et al. Epigenome-wide and transcriptome-wide analyses reveal gestational diabetes is associated with alterations in the human leukocyte antigen complex. Clin Epigenet. 2015;7:79. doi: 10.1186/s13148-015-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De la Chesnaye E, Manuel-Apolinar L, Oviedo-de Anda N et al. [Gender differences in lipocalin 2 plasmatic levels are correlated with age and the triglyceride/high-density lipoprotein ratio in healthy individuals] Gac Med Mex. 2016;152:612–617. [PubMed] [Google Scholar]
- 35.Wu G, Li H, Zhou M et al. Mechanism and clinical evidence of lipocalin-2 and adipocyte fatty acid-binding protein linking obesity and atherosclerosis. Diabetes Metab Res Rev. 2014;30:447–456. doi: 10.1002/dmrr.2493. [DOI] [PubMed] [Google Scholar]
- 36.Abella V, Scotece M, Conde J et al. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers. 2015;20:565–571. doi: 10.3109/1354750X.2015.1123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Lam K S, Kraegen E W et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- 38.Yan Q W, Yang Q, Mody N et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 39.Sun W X, Lou K, Chen L J et al. Lipocalin-2: a role in hepatic gluconeogenesis via AMP-activated protein kinase (AMPK) J Endocrinol Invest. 2021;44:1753–1765. doi: 10.1007/s40618-020-01494-0. [DOI] [PubMed] [Google Scholar]
- 40.Law I K, Xu A, Lam K S et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan Y K, Sung H K, Jahng J W et al. Lipocalin-2 inhibits autophagy and induces insulin resistance in H9c2 cells. Mol Cell Endocrinol. 2016;430:68–76. doi: 10.1016/j.mce.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Chella Krishnan K, Sabir S, Shum M et al. Sex-specific metabolic functions of adipose lipocalin-2. Mol Metab. 2019;30:30–47. doi: 10.1016/j.molmet.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaberi S A, Cohen A, D’Souza C et al. Lipocalin-2: structure, function, distribution and role in metabolic disorders. Biomed Pharmacother. 2021;142:112002. doi: 10.1016/j.biopha.2021.112002. [DOI] [PubMed] [Google Scholar]
- 44.Kamble P G, Pereira M J, Sidibeh C O et al. Lipocalin 2 produces insulin resistance and can be upregulated by glucocorticoids in human adipose tissue. Mol Cell Endocrinol. 2016;427:124–132. doi: 10.1016/j.mce.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Auguet T, Quintero Y, Terra X et al. Upregulation of lipocalin 2 in adipose tissues of severely obese women: positive relationship with proinflammatory cytokines. Obesity (Silver Spring) 2011;19:2295–2300. doi: 10.1038/oby.2011.61. [DOI] [PubMed] [Google Scholar]
- 46.Catalán V, Gómez-Ambrosi J, Rodríguez A et al. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J Mol Med (Berl) 2009;87:803–813. doi: 10.1007/s00109-009-0486-8. [DOI] [PubMed] [Google Scholar]
- 47.Liu J T, Song E, Xu A et al. Lipocalin-2 deficiency prevents endothelial dysfunction associated with dietary obesity: role of cytochrome P450 2C inhibition. Br J Pharmacol. 2012;165:520–531. doi: 10.1111/j.1476-5381.2011.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, Yan Sun W, Fu B et al. Lipocalin-2 – the myth of its expression and function. Basic Clin Pharmacol Toxicol. 2020;127:142–151. doi: 10.1111/bcpt.13332. [DOI] [PubMed] [Google Scholar]
- 49.Clerico A, Galli C, Fortunato A et al. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50:1505–1517. doi: 10.1515/cclm-2011-0814. [DOI] [PubMed] [Google Scholar]
- 50.Valsamakis G, Kumar S, Creatsas G et al. The effects of adipose tissue and adipocytokines in human pregnancy. Ann N Y Acad Sci. 2010;1205:76–81. doi: 10.1111/j.1749-6632.2010.05667.x. [DOI] [PubMed] [Google Scholar]
- 51.Deis J A, Guo H, Wu Y et al. Adipose lipocalin 2 overexpression protects against age-related decline in thermogenic function of adipose tissue and metabolic deterioration. Mol Metab. 2019;24:18–29. doi: 10.1016/j.molmet.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan B K, Adya R, Shan X et al. Ex vivo and in vivo regulation of lipocalin-2, a novel adipokine, by insulin. Diabetes Care. 2009;32:129–131. doi: 10.2337/dc08-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno-Navarrete J M, Manco M, Ibáñez J et al. Metabolic endotoxemia and saturated fat contribute to circulating NGAL concentrations in subjects with insulin resistance. Int J Obes (Lond) 2010;34:240–249. doi: 10.1038/ijo.2009.242. [DOI] [PubMed] [Google Scholar]
- 54.Stroup D F, Berlin J A, Morton S C et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]


