Even though inflammation has long been associated with neurodegenerative diseases, reducing microglial activation and cytokine induction has not been a common therapeutic strategy compared with efforts to reduce amyloid deposition. The limited success of amyloid-specific antibodies in recent clinical trials has created interest in alternative strategies including modulating inflammation in Alzheimer’s disease (AD) (1). In this issue of PNAS, a treatment with an agonist of tumor necrosis factor receptor 2 (TNFR2, TNFRSF1B) was shown to reduce amyloid beta deposition, levels of beta-secretase 1 (BACE-1) and increase cognition in an animal model of AD (2). The paper is significant for focusing on the role of inflammation in AD, demonstrating the marked differences in the roles of the two receptors for tumor necrosis factor alpha (TNFα) and revealing the possible importance of the immune system in AD therapy.
Even though both TNF receptors are stimulated by TNFα, their cellular distribution, mode of activation, and the biological functions they activate vary significantly. TNFR1 is expressed in virtually all cells, whereas TNFR2 expression is more limited, being predominant on CD4+ FoxP3+, and CD8+ regulatory T cells (Tregs), monocytes, granulocytes, and in the central nervous system (CNS), microglia, astrocytes, and myelin-producing oligodendrocytes (3). The different biological outcomes of the receptors are thought to explain the puzzling fact that anti-TNFα therapy, which inhibits signaling in both receptors, reduces inflammation in rheumatoid arthritis, Crohn’s disease, and psoriasis but exacerbates the symptoms of multiple sclerosis (4, 5). Theoretically, the systemic diseases benefited from blocking TNFR1 signaling by reducing cytokine levels, whereas in the CNS blocking TNFR2 increased demyelination and reduced Treg activity, and its inhibition led to enhancement of the symptoms. The hypothesis that selective TNFR2 activation will regulate neuroinflammation could not be tested effectively until a selective TNFR2 agonist was developed.
The development of the agonist was not trivial because even though both TNF receptors are activated by trimers of TNFα, the form of the activating ligand differs. TNFR1 is activated predominantly by soluble, proteolytically cleaved trimers of TNF, while the majority of TNFR2 activation is induced by cell–cell contact, binding the membrane form of TNFα (6).
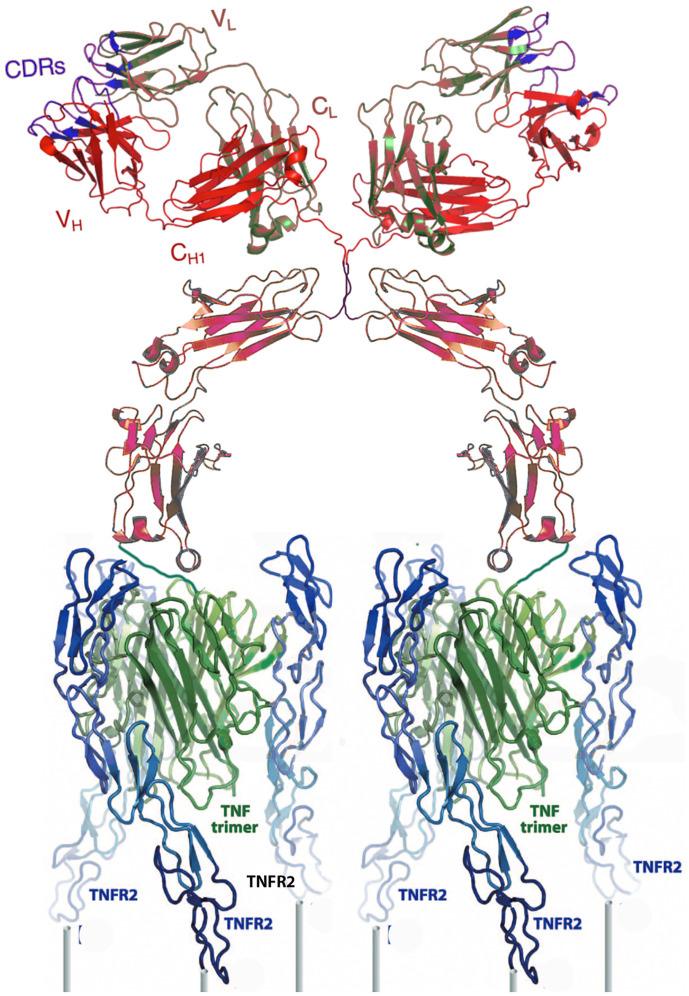
Engineering an effective, TNFR2-selective agonist capable of binding receptor trimers and cross-linking at least two aggregated triplets of receptors with a sufficiently long biological half-life required several iterations, which delayed the investigation of this promising pathway. The agonist in this study accomplished selective binding by using linkers to connect three copies of a mutated TNFα sequence previously shown to be specific for TNFR2 (7). Expressing the linked trimers fused to the carboxyl terminus of either the heavy or light chain of an immunoglobulin G1 (IgG1) was sufficient to bind, cross-link, and fully activate TNFR2 in transfected cells (Fig. 1 and ref. 8).
Fig. 1.
A cartoon ribbon diagram depicting the TNFR2 selective agonist cross-linking two sets of three TNFR2s (blue) on a cell surface (posts). The agonist is composed of an IgG1 (red/brown) covalently linked through its carboxyl termini to triplets of a mutated TNFα (green) selective for TNFR2. The cartoon was constructed using data in refs. 12 and 13.
Administration of the selective agonist, either centrally via implantation of osmotic pumps or systemically, by intraperitoneal injections to transgenic Aβ-overexpressing mouse model of AD (J20) reduced amyloid levels, limited expression of the enzyme central to amyloid formation, and rescued AD-related cognitive impairments. In addition, microglial and astrocytes were activated in both routes of administration, indicating either the drug effectively crossed the blood–brain barrier or the TNFR2 agonist activated cells in the periphery and they, or their secreted products, were responsible for the therapeutic benefit. The experimental results focus on the importance of reducing inflammation and underappreciated role of the immune system in not only AD but the full spectrum of neurodegenerative diseases. The results also question whether amyloid fibrils are the basis of inflammation, or whether amyloid fibrils result from inflammation. This is not simply a rhetorical question but one central to the development of effective therapeutics.
This work proves the importance of selective TNFR2 signaling and represents a foundation for the development of more sophisticated and effective biologics and small molecules capable of stimulating TNFR2 with optimal pharmacodynamics and pharmacokinetics.
The paper also leads to the question of whether neuroinflammation is fundamentally different from systemic inflammation and which of the manifold drugs that limit specific cytokines will be most effective. Clearly, TNFR2 stimulation has unique characteristics of stimulating both myelination and expansion of Tregs, which might be essential in the CNS and less important systemically. However, other drugs inhibiting related signaling pathways also might be effective, either alone or when coadministered. The latter question is reminiscent of the state of rheumatology research prior to the demonstration of value of anti-TNFα and interleukin 6 receptor blockade. For example, Jak inhibitors have been shown to effective therapeutics in animal model of Parkinson’s disease (9). Drugs specific for the nucleotide-binding oligomerization domain, leucine-rich repeat-containing protein 3 (NLRP3)/caspase-1 axis (10), TREM2 expressed on myeloid cells, and cGAS-STING (11) should be tested for therapeutic activity in AD animal models and in other degenerative diseases.
If systemic autoimmune diseases are a guide, there will be clinical value in many of these approaches and combinations might be optimal treatment for a complex polygenic neuroinflammatory diseases such as AD, Parkinson’s, multiple sclerosis, and amyotrophic lateral sclerosis.
Footnotes
The author declares no competing interest.
See companion article, “A TNF Receptor 2 agonist ameliorates neuropathology and improves cognition in an Alzheimer’s disease mouse model,” 10.1073/pnas.2201137119.
References
- 1.Mullard A., Anti-amyloid failures stack up as Alzheimer antibody flops. Nat. Rev. Drug Discov. 18, 327 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Ortí-Casañ N., et al. , A TNF Receptor 2 agonist ameliorates neuropathology and improves cognition in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. U.S.A. 119, e2201137119, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajant H., Beilhack A., Targeting regulatory T cells by addressing tumor necrosis factor and its receptors in allogeneic hematopoietic cell transplantation and cancer. Front. Immunol. 10, 2040–2054 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregory A. P., et al. , TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 488, 508–511 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group, TNF neutralization in MS: Results of a randomized, placebo-controlled multi-center study. Neurology 53, 457–465 (1999). [PubMed] [Google Scholar]
- 6.Grell M., et al. , The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83, 793–802 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Loetscher H., Stueber D., Banner D., Mackay F., Lesslauer W., Human tumor necrosis factor alpha (TNF alpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J. Biol. Chem. 268, 26350–26357 (1993). [PubMed] [Google Scholar]
- 8.Vargas J. G., et al. , A, TNFR2-specific TNF fusion protein with improved in vivo activity. Front. Immunol. 13, 888274–888287 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin H., et al. , Inhibition of the JAK/STAT pathway protects against α-synuclein-induced neuroinflammation and dopaminergic neurodegeneration. J. Neurosci. 36, 5144–5159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson F. L., et al. , NLRP3 inflammasome in neurodegenerative disease. Transl. Res., 10.1016/j.trsl.2022.08.006 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiser C., Kim B., Vincent J., Ascano M., Small molecule inhibition of human cGAS reduces total cGAMP output and cytokine expression in cells. Sci. Rep. 10, 7604–7615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris L. J., Skaletsky E., McPherson A., Crystallographic structure of an intact IgG1 monoclonal antibody. J. Mol. Biol. 275, 861–872 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Mukai Y., et al. , Solution of the structure of the TNF-TNFR2 complex. Sci. Signal. 3, ra83 (2010). [DOI] [PubMed] [Google Scholar]