Significance
Fibroblast growth factor 1 (FGF1) is released by white adipose tissue (WAT) in response to feeding or over-nutrition and plays a role in WAT remodeling and metabolic homeostasis. In addition, pharmacological administration of recombinant FGF1 improves glucose balance in diabetic mouse models by activating signaling in WAT. Autocrine effects on adipocyte function may thus play a role in the metabolic effects of FGF1. Here, we investigated the direct effects of FGF1 on adipocyte function. Our work establishes an FGF1-dependent autocrine pathway, independent of insulin, that controls glucose uptake in adipocytes.
Keywords: FGF1, glucose metabolism, adipocytes, insulin, fibroblast growth factors
Abstract
Fibroblast growth factor 1 (FGF1) is an autocrine growth factor released from adipose tissue during over-nutrition or fasting to feeding transition. While local actions underlie the majority of FGF1’s anti-diabetic functions, the molecular mechanisms downstream of adipose FGF receptor signaling are unclear. We investigated the effects of FGF1 on glucose uptake and its underlying mechanism in murine 3T3-L1 adipocytes and in ex vivo adipose explants from mice. FGF1 increased glucose uptake in 3T3-L1 adipocytes and epididymal WAT (eWAT) and inguinal WAT (iWAT). Conversely, glucose uptake was reduced in eWAT and iWAT of FGF1 knockout mice. We show that FGF1 acutely increased adipocyte glucose uptake via activation of the insulin-sensitive glucose transporter GLUT4, involving dynamic crosstalk between the MEK1/2 and Akt signaling proteins. Prolonged exposure to FGF1 stimulated adipocyte glucose uptake by MEK1/2-dependent transcription of the basal glucose transporter GLUT1. We have thus identified an alternative pathway to stimulate glucose uptake in adipocytes, independent from insulin, which could open new avenues for treating patients with type 2 diabetes.
Excessive nutrient intake and a sedentary lifestyle have resulted in a pandemic of obesity and related chronic metabolic diseases, including type 2 diabetes (T2D), nonalcoholic fatty liver disease (NAFLD), cardiovascular disease (CVD), and certain types of cancer (1). A critical player in the pathophysiology of these obesity-related diseases is the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ), a lipid sensor and master regulator of adipocyte differentiation and fat storage, and the molecular target of the thiazolidinedione (TZD) class of insulin-sensitizing drugs (2, 3).
We have previously identified fibroblast growth factor 1 (FGF1) as a downstream target of PPARγ and demonstrated that this growth factor is essential for white adipose tissue (WAT) expansion and glycemic control during energy excess (4). Activation of PPARγ by high-fat diet feeding or TZD treatment induces the local release of FGF1 in WAT (4), where it is thought to function as an autocrine or paracrine factor (5, 6). Mice deficient in FGF1 become severely insulin resistant upon high-fat diet feeding and display aberrant WAT architecture characterized by increased inflammation and fibrosis (4). In subsequent studies, we also demonstrated that pharmacological treatment of diabetic mouse models with recombinant FGF1 has potent glucose-lowering, insulin-sensitizing, and anti-steatotic effects. The effects of systemically administered FGF1 on insulin sensitivity and blood glucose levels are at least partly dependent on FGFR1 signaling in the WAT (7). However, besides adipose, FGF1 has also been shown to regulate glucose homeostasis via interactions with other organs, including the hypothalamus and pancreas (8–11).
In the current study, we investigated the direct effect of FGF1 on mature adipocyte function independent of interactions with other organs. To this end, we used ex vivo and in vitro adipose models. We show that FGF1 stimulates glucose uptake in eWAT and iWAT in an autocrine manner. Mechanistically, we show that FGF1 has distinct acute and prolonged effects on adipocyte glucose uptake. Acute FGF1-stimulated adipocyte glucose uptake is dependent on MEK1/2 and Akt crosstalk and activation of GLUT4. Chronic FGF1-stimulated adipocyte glucose uptake is mediated through MEK1/2-dependent transcriptional up-regulation of GLUT1. Together, we have identified an FGF1-dependent autocrine pathway that is distinct from insulin action to stimulate glucose uptake in adipocytes.
Results
FGF1 Stimulates Glucose Uptake in Adipose Tissue.
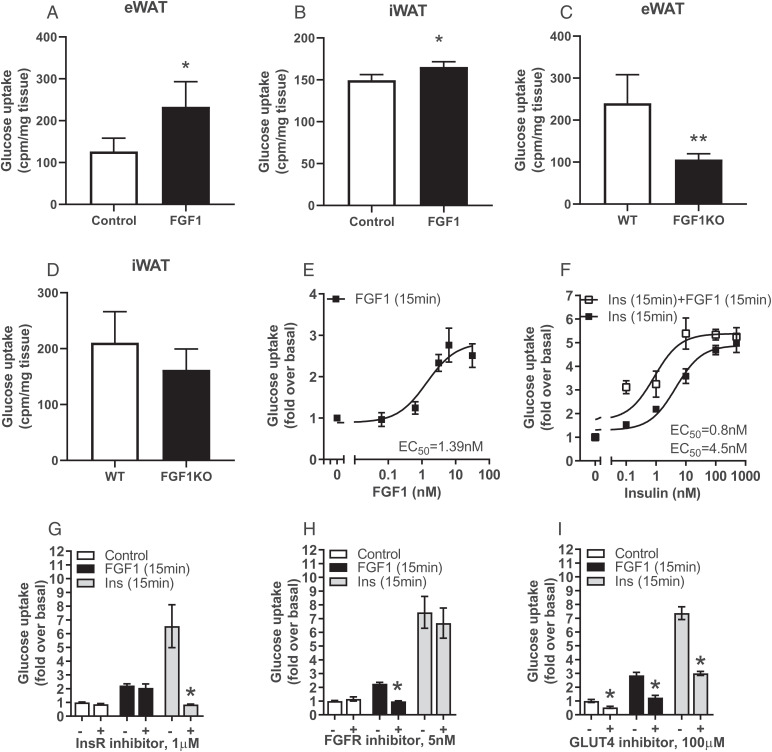
Glucose uptake in the adipose tissue contributes to systemic glucose homeostasis (12–14). We found that FGF1 stimulated ex vivo glucose uptake in both eWAT and iWAT (Fig. 1 A and B), with this effect being more pronounced in eWAT than in iWAT. Conversely, ex vivo glucose uptake was decreased in eWAT and, to a lesser extent, in iWAT isolated from FGF1 knockout mice (Fig. 1 C and D).
Fig. 1.
Acute stimulation of glucose uptake in adipocytes by FGF1. (A and B) Ex vivo FGF1-stimulated 3H-2-deoxy-d-glucose uptake in (A) epididymal WAT (eWAT) and (B) inguinal WAT (iWAT) 30 min after treatment. (C and D) Ex vivo 3H-2-deoxy-d-glucose uptake in (C) eWAT and (D) iWAT of wild-type (WT) and FGF1 knockout mice (FGF1KO) after 30 min incubation. (E) Dose–response curves of FGF1– and (F) FGF1+ insulin-stimulated 2-deoxy-d-[14C]-glucose uptake in 3T3-L1 adipocytes 15 min after treatment. (G) Effect of insulin receptor (InsR) inhibition by 1 μM Osi-906 (30 min pretreatment), (H) FGF receptor (FGFR) inhibition by 5 nM LY2874455 (30 min pretreatment), or (I) GLUT4 inhibition by 100 μM indinavir (cotreatment) on FGF1- and insulin-stimulated 2-deoxy-d-[14C]-glucose uptake in 3T3-L1 adipocytes 15 min after treatment. All data shown are mean ± SD (n = 4–6, *P < 0.05).
To study the underlying mechanism involved in FGF1-dependent adipocyte glucose uptake, we used differentiated 3T3-L1 adipocytes. The expression of FGF receptors (FGFRs) 1c and 2c in 3T3-L1 adipocytes is similar to normal adipocytes, making them a suitable model to assess FGF signaling (15). Stimulating 3T3-L1 cells with FGF1 increased glucose uptake after 15 min of treatment in a dose-dependent fashion with an EC50 of 1.4 nM (Fig. 1E). Glucose uptake could be further stimulated by FGF1 after cotreatment with insulin, suggesting that the effect of FGF1 is regulated via a pathway independent of insulin signaling (Fig. 1F).
FGF1-Stimulated Glucose Uptake Is Dependent on FGFR1-Signaling and GLUT4 Activity.
The FGFRs, insulin receptor (InsR), and insulin-like growth factor receptor (IGF-R) are structurally and functionally related. To determine the contribution of these receptors to the FGF1-stimulated glucose uptake in 3T3-L1 cells, we used the pan-FGFR inhibitor LY2874455 and the InsR/IGF-R inhibitor Osi-906 (16). As anticipated, insulin-stimulated glucose uptake was completely abrogated by Osi-906, whereas LY2874455 did not have any effect (Fig. 1G). Conversely, FGF1-stimulated glucose uptake was completely abrogated by LY2874455, but was not affected by Osi-906 (Fig. 1H). Thus, FGF1-stimulated glucose uptake is strictly dependent on FGFR signaling and we did not observe cross-activation of the InsR or IGF-R (Fig. 1 G and H).
FGF1 can exert its biological functions by activating four different receptor subtypes (FGFR1–FGFR4). 3T3-L1 cells primarily express high levels of Fgfr1. In contrast, Fgfr2 mRNA transcripts are present at much lower levels (10-fold lower than FGFR1), while Fgfr3 and Ffgr4 transcripts are not detected in 3T3-L1 cells (17). To address the relevance of FGFR1 in FGF1-stimulated glucose uptake, we generated Fgfr1-deficient 3T3-L1 cells by Cas9-mediated gene editing. We found that FGF1-stimulated glucose uptake was completely abolished in Fgfr1-deficient 3T3-L1 cells (SI Appendix, Fig. S1A). Small interfering RNA (siRNA)-mediated knockdown of Fgfr2 in wild-type or Fgfr1-deficient 3T3-L1 cells did not affect FGF1-stimulated glucose uptake (SI Appendix, Fig. S1A). In line, FGF1-driven ERK activation was largely abolished in Fgfr1-deficient 3T3-L1 adipocytes, whereas knockdown of Fgfr2 alone had minor effects on FGF1-driven ERK activation (SI Appendix, Fig. S1B).
The primary transporters involved in adipocyte glucose uptake are GLUT1 and GLUT4. While GLUT1 is responsible for basal glucose uptake, GLUT4 is primarily involved in insulin-stimulated glucose uptake, although there is substantial evidence that there is a functional overlap between them (18, 19). The GLUT4 inhibitor Indinavir (19–21) was used to study the relative importance of these glucose transporters in acute FGF1-stimulated glucose uptake. Inhibiting GLUT4 with Indinavir reduced acute FGF1-stimulated glucose uptake by ∼65% (±0.09%). Indinavir reduced insulin-stimulated glucose uptake by ∼62% (±0.02%), which is in line with previous studies regarding the contribution of GLUT4 to insulin-induced glucose uptake (18, 19) (Fig. 1I). In agreement with the consensus that GLUT1 and GLUT4 have overlapping functionalities, we found that GLUT4 inhibition reduced basal glucose uptake into adipocytes by ∼40% (±0.08%). Together, these observations indicate that the acute effects of FGF1 on glucose uptake are largely mediated by GLUT4.
FGF1-Stimulated Glucose Uptake Is Dependent on MEK-ERK Activation.
Next, we investigated the downstream signaling events upon FGF1 stimulation in 3T3-L1 adipocytes. To this end, we systematically mapped FGF1 and insulin signaling using the PathScan signaling array (SI Appendix, Fig. S2A), which allows the simultaneous detection of 16 phosphorylated proteins. Whereas a sustained (up to 60 min) activation of Akt was observed after stimulation with insulin, only transient (up to 5 min) phosphorylation of Akt was seen after stimulation with FGF1. In contrast, stimulation with FGF1 resulted in stable activation of ERK, while this was much less pronounced for insulin (SI Appendix, Fig. S2B). We did not observe interference of FGF1 with insulin-mediated phosphorylation of Akt at Ser473 (SI Appendix, Fig. S2C).
To determine the relative contribution of the PI3K-Akt and MEK-ERK pathways in FGF1- and insulin-stimulated glucose uptake, we used specific inhibitors targeting these signaling pathways and assessed their role in FGF1- and insulin-stimulated glucose uptake (Fig. 2 A–F and SI Appendix, Table S1). In line with previous reports (22, 23), insulin-stimulated glucose uptake could be entirely blocked by inhibition of PI3K or Akt using PIK-75 and MK-2206, respectively (Fig. 2 A and C), while no effect was seen of MEK1/2 inhibition using PD0325901 (Fig. 2E). In contrast, while inhibition of PI3K or Akt did not affect FGF1-stimulated glucose uptake (Fig. 2 B and D), this was reduced by inhibition of MEK1/2 (Fig. 2F).
Fig. 2.
FGF1-stimulated glucose uptake is dependent on MEK-ERK activation. (A and B) Dose–response curves of PI3K inhibition by PIK-75 (0–10 µM, 30 min pretreatment) on insulin- and FGF1-stimulated 2-deoxy-d-[14C]-glucose uptake in 3T3-L1 cells 15 min after treatment. (C and D) Dose–response curves of Akt inhibition by MK-2206 (0–10 µM, 30 min pretreatment) on insulin- and FGF1-stimulated 2-deoxy-d-[14C]-glucose uptake in 3T3-L1 cells 15 min after treatment. (E and F) Dose–response curves of MEK1/2 inhibition by PD0325901 (0–10 µM, 30 min pretreatment) on insulin- and FGF1-stimulated 2-deoxy-d-[14C]-glucose uptake in 3T3-L1 cells 15 min after treatment. All data shown are mean ± SD (n = 3).
Crosstalk between the MEK-ERK and PI3K-Akt Pathways Ensures Acute FGF1-Stimulated Glucose Uptake.
Although FGF1-stimulated glucose uptake was reduced upon MEK1/2 inhibition, it was not completely abolished. The observed residual glucose uptake led us to hypothesize that another pathway can compensate for MEK1/2 during acute FGF1-stimulated glucose transport. Upon inhibition of MEK1/2 with PD0325901, we observed an increase in the duration of Akt phosphorylation after FGF1 stimulation, indicating that MEK1/2 functions as a negative feedback loop that represses Akt activation in 3T3-L1 adipocytes (Fig. 3A). Similar effects on Akt activity were seen after 3T3-L1 cells were stimulated with EGF in the presence of PD0325901 (SI Appendix, Fig. S3A). In addition, FGF1-stimulated crosstalk between MEK1/2 and Akt could also be observed in HEK293 cells (SI Appendix, Fig. S3B), suggesting the existence of receptor- and cell type-independent wiring of MEK and Akt signaling proteins.
Fig. 3.
Crosstalk between MEK-ERK and PI3K-Akt in FGF1-stimulated glucose uptake. (A) Time course (0-15 min) of Akt (Thr308, Ser473) and ERK (Thr202/Tyr204) phosphorylation by FGF1 in the absence and presence of MEK1/2 inhibitor PD0325901 (1 µM, 30 min pretreatment) in 3T3-L1 adipocytes. (B) Effect of MEK1/2 (1 µM PD0325901, 30 min pretreatment) and Akt (1 µM MK2206, 30 min pretreatment) inhibition on acute (15 min) FGF1-stimulated 2-deoxy-d-[14C]-glucose uptake in 3T3-L1 cells. (C) Effect of MEK1/2 (1 µM PD0325901, 30 min pretreatment) and Akt (1 µM MK2206, 30 min pretreatment) inhibition on FGF1-stimulated ERK (Thr202/Tyr204) and Akt (Thr308, Ser473) phosphorylation in 3T3-L1 adipocytes. All data shown are mean ± SD (n = 6, *P < 0.05).
Because pharmacological MEK1/2 inhibitors are not suitable to determine the individual contribution of MEK1 and MEK2 in MEK/Akt crosstalk, we used Cas9-mediated gene editing to further dissect this conserved regulatory circuit. Given the previously identified role of MEK1 in EGF-dependent Akt regulation (24), we hypothesized a comparable role for this protein in FGF1 signaling. Similar to pharmacological MEK1/2 inhibition, we found that upon MEK1 ablation, FGF1 stimulation led to sustained Akt phosphorylation at Ser473 (SI Appendix, Fig. S3C). MEK1 ablation also increased FGF1-stimulated ERK phosphorylation (SI Appendix, Fig. S3C), which is explained by a role of MEK1 in downregulating MEK2-dependent ERK signaling (25). In addition, we determined whether crosstalk between MEK and Akt signaling occurs in vivo. Ob/ob mice were pretreated for 90 min with the MEK inhibitor PD0325901 (26, 27) followed by 15 min stimulation with FGF1. In all tissues examined (i.e., muscle, eWAT, liver, and heart), PD0325901 effectively inhibited ERK phosphorylation (SI Appendix, Fig. S4 A–D). When MEK was inhibited, FGF1 treatment led to sustained Akt phosphorylation, indicating that crosstalk between MEK and Akt signaling also occurs in vivo (SI Appendix, Fig. S4 A–D).
Finally, we questioned whether sustained Akt activation could rescue FGF1-stimulated glucose uptake under conditions of MEK inhibition. Indeed, simultaneous inhibition of both MEK and Akt signaling resulted in complete inhibition of FGF1-stimulated glucose uptake in 3T3-L1 adipocytes (Fig. 3 B and C). Together, these findings show that FGF1 stimulates adipocyte glucose uptake primarily through the MEK1/2 pathway. However, under conditions of decreased MEK1/2 activity, Akt can partially rescue FGF1-driven glucose uptake.
Chronic Stimulation of Glucose Uptake by FGF1 in 3T3-L1 Adipocytes.
The acute effect of FGF1 on glucose uptake that we describe here is different from the previously reported effect of FGF21, which requires at least 4 h of treatment and is dependent on the induction of GLUT1 expression (28). To test whether FGF1 also has chronic, FGF21-like effects, 3T3-L1 adipocytes were treated for 24 h with FGF1 and tested for glucose uptake. Dose-dependent stimulation of glucose uptake was also observed after chronic exposure to FGF1, but this effect was not as strong as the acute effects of FGF1 on adipocyte glucose uptake (Fig. 4A). This 24 h treatment with FGF1 had a stronger augmenting effect on the effects of insulin than the acute treatment, suggesting that the mechanisms underlying these effects are distinct (Fig. 4B).
Fig. 4.
Chronic stimulation of glucose uptake by FGF1 in 3T3-L1 adipocytes is mediated via MEK-ERK-dependent control of GLUT1. (A) Dose–response curve of FGF1-stimulated 2-deoxy-d-[14C]-glucose uptake 24 h after treatment. (B) Dose–response curve of acute (15 min) insulin-stimulated 2-deoxy-d-[14C]-glucose uptake and the effect of 24 h of FGF1 (100 ng/mL) treatment. (C) Time course analysis (2–8 h) of GLUT1 gene expression or (D) GLUT4 gene expression after treatment with FGF1. (E) Basal- and FGF1-induced GLUT1 gene expression upon 30 min pretreatment with 1 μM of the RNA synthesis Triptolide. (F) Effect of MEK1/2 inhibition by 1 μM PD0325901 (30 min pretreatment) on basal- and FGF1-stimulated GLUT1 gene expression (4 h), (G) GLUT1 protein levels, and (H) 2-deoxy-d-[14C]-glucose uptake (24 h) in 3T3-L1 adipocytes. (I) Basal- and FGF1-induced 2-deoxy-d-[14C]-glucose uptake after cotreatment with 100 μM GLUT4 blocker Indinavir in 3T3-L1 adipocytes. All data shown are mean ± SD (n = 4–8, *P < 0.05).
To investigate how chronic FGF1 exposure drives glucose uptake, we first determined its effect on the expression of GLUT1 and GLUT4, the primary glucose transporters in adipocytes (29). FGF1 strongly increased GLUT1 gene expression, which peaked at 4 h after treatment and remained significantly elevated up to 8 h (Fig. 4C). GLUT1 protein levels increased with some delay relative to GLUT1 mRNA expression (SI Appendix, Fig. S5A) and remained elevated for up to 24 h after FGF1 treatment, as shown by immunocytochemistry (SI Appendix, Fig. S5B). In contrast, GLUT4 mRNA expression decreased in response to FGF1 treatment (Fig. 4D). Inhibition of mRNA synthesis using Triptolide completely blocked FGF1-stimulated GLUT1 mRNA expression, indicating that GLUT1 expression is controlled by increased gene transcription rather than inhibition of mRNA degradation or increased mRNA stability (Fig. 4E). The MEK1/2 inhibitor PD0325901 could completely block the increased expression of GLUT1 by chronic FGF1 treatment, both at the mRNA and protein level (Fig. 4 F and G), and this was accompanied by loss of chronic FGF1-stimulated glucose uptake (Fig. 4H). Also, the chronic effects of FGF1 were repressed by only 9% (±0.08%) with Indinavir, providing additional evidence that GLUT1, and not GLUT4, is the major glucose transporter involved in this process (Fig. 4I).
Together, these findings indicate that the chronic effects of FGF1 on glucose uptake are mediated via MEK-ERK-dependent transcriptional control of GLUT1, which is similar to the effects previously described for FGF21 (28).
Discussion
In this study, we investigated the effect of FGF1 on glucose transport in adipocytes and the underlying signaling events. Our findings show that FGF1 stimulates glucose uptake and enhances insulin-stimulated glucose uptake, even at a maximally effective dose of insulin. We show that FGF1 has both immediate, signaling-dependent, and prolonged, transcription-dependent stimulatory effects on glucose transport. Whereas an FGFR-dependent engagement of GLUT4 mediates the acute response to FGF1, the prolonged response relies on transcriptional control of GLUT1. Insulin-induced glucose uptake is highly dependent on GLUT4 (30), whereas FGF21-induced glucose uptake is dependent on transcriptional regulation of GLUT1 (28). Thus, FGF1 has mixed insulin- and FGF21-like properties. However, in contrast to insulin, which relies on PI3K-Akt signaling to induce acute glucose transport (30), we show that FGF1 mediates its effect primarily through activation of the MEK1/2 pathway.
Intriguingly, when the MEK pathway was blocked, Akt was robustly activated and could rescue FGF1-driven glucose uptake, showing that there is considerable flexibility within the FGF1/FGFR signaling pathway with regards to adipocyte glucose uptake. This phenomenon was not observed for insulin-mediated adipocyte glucose uptake.
The FGF21-like actions of FGF1 are linked to its prolonged effects on adipocyte glucose uptake. Increased glucose uptake in response to FGF21 is regulated via two ERK-responsive transcription factors (Elk-1 and SRF) that increase GLUT1 expression by binding to its promoter (28, 31). Whether FGF1 also relies on these two transcription factors to increase GLUT1 is unknown. In addition, while the relative importance of adipocyte GLUT1 induction for the in vivo anti-diabetic activity of FGF21 and FGF1 remains unclear, it has been shown that the FGF21/FGF1 chimera FGF1ΔHBS-FGF21c-tail resolved impaired GLUT1 translocation in WAT of db/db mice (32).
To our knowledge, FGF1 is the first FGF family member that can acutely (within minutes) stimulate glucose uptake via MEK-ERK signaling and thus represents a novel pathway driving adipose glucose uptake. Two other members of the FGF family, FGF15/19 and FGF21, can also stimulate glucose uptake in a MEK-ERK dependent manner but can only do so via transcriptional up-regulation of GLUT1, which requires hours instead of minutes to manifest itself. FGF1-induced glucose uptake is thus fundamentally different from both insulin-, FGF15/19-, and FGF21-induced glucose uptake (17, 28). The intracellular signaling mechanisms underlying these differences are currently elusive. It has been shown in PC12 cells that the strength and duration of ERK activation determine the physiological outcome (33). A similar mechanism may underlie the differences between FGF1, FGF15/19, and FGF21 signaling. Since FGF1 does not recruit KLB, as opposed to FGF15/19 and FGF21 (34, 35), it is also conceivable that the recruitment of KLB limits the acute effects on GLUT4 and glucose transport.
Genetic models have demonstrated an important role for adipose tissue glucose uptake in systemic glucose homeostasis. However, under normal physiological circumstances, the absolute amount of glucose taken up by white adipose tissue is only minor compared to skeletal muscle (36–38). Therefore, adipose glucose uptake is not likely to play a significant role in the acute glucose-lowering effect of FGF1 in vivo. It has been shown that adipose glucose uptake channels glucose for lipid synthesis via a carbohydrate-responsive element binding protein (ChREBP) dependent pathway, and this effect strongly predicts systemic insulin sensitivity in humans (39).
In conclusion, we show that FGF1 has potent effects on adipocyte glucose uptake that may contribute to its endogenous and pharmacological functions in vivo. These effects are independent of insulin and could thus open new avenues for treating type 2 diabetes.
Materials and Methods
Details of animal experiments, ex vivo adipose glucose uptake assay, cell culture, inhibitors, Cas9-mediated gene editing, siRNA-mediated knockdown, 2-deoxy-d-glucose uptake, protein analysis, Akt signaling array, gene expression analysis, immunocytochemistry, and statistics are provided in SI Appendix.
Supplementary Material
Acknowledgments
This study was supported by grants from The Netherlands Organization for Scientific Research (VICI grant 016.176.640 to J.W.J.) and European Foundation for the Study of Diabetes (award supported by EFSD/Novo Nordisk), and the De Cock Stichting. This project was co-financed by the Ministry of Economic Affairs and Climate Policy by means of the PPP-allowance made available by Health∼Holland, Top Sector Life Sciences & Health to stimulate public-private partnerships. R.M.E. is a March of Dimes Chair in Molecular and Developmental Biology at the Salk Institute, and is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH (R01DK057978) and the Hillblom Foundation.
Footnotes
Reviewers: D.D.M., University of California Berkeley; and P.T., University of California Los Angeles.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122382119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Haslam D. W., James W. P. T., Obesity. Lancet 366, 1197–1209 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Tontonoz P., Spiegelman B. M., Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 77, 289–312 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Ahmadian M., et al. , PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 19, 557–566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonker J. W., et al. , A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature 485, 391–394 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mejhert N., et al. , Mapping of the fibroblast growth factors in human white adipose tissue. J. Clin. Endocrinol. Metab. 95, 2451–2457 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Beenken A., Mohammadi M., The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh J. M., et al. , Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513, 436–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J. M., Scarlett J. M., Matsen M. E., Nguyen H. T., The hypothalamic arcuate nucleus-median eminence is a target for sustained diabetes remission induced by fibroblast growth factor 1. Diabetes 68, 1054–1061 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarlett J. M., et al. , Peripheral mechanisms mediating the sustained antidiabetic action of FGF1 in the brain. Diabetes 68, 654–664 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarlett J. M., et al. , Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat. Med. 22, 800–806 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tennant K. G., Lindsley S. R., Kirigiti M. A., True C., Kievit P., Central and peripheral administration of fibroblast growth factor 1 improves pancreatic islet insulin secretion in diabetic mouse models. Diabetes 68, 1462–1472 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abel E. D., et al. , Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Gnudi L., Tozzo E., Shepherd P. R., Bliss J. L., Kahn B. B., High level overexpression of glucose transporter-4 driven by an adipose-specific promoter is maintained in transgenic mice on a high fat diet, but does not prevent impaired glucose tolerance. Endocrinology 136, 995–1002 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Carvalho E., Kotani K., Peroni O. D., Kahn B. B., Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am. J. Physiol. Endocrinol. Metab. 289, E551–E561 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M., et al. , betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 22, 1006–1014 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemmon M. A., Schlessinger J., Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams A. C., et al. , Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PLoS One 7, e38438 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao W., et al. , Lentiviral short hairpin ribonucleic acid-mediated knockdown of GLUT4 in 3T3-L1 adipocytes. Endocrinology 147, 2245–2252 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Rudich A., et al. , Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46, 649–658 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Murata H., Hruz P. W., Mueckler M., Indinavir inhibits the glucose transporter isoform Glut4 at physiologic concentrations. AIDS 16, 859–863 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Hresko R. C., Hruz P. W., HIV protease inhibitors act as competitive inhibitors of the cytoplasmic glucose binding site of GLUTs with differing affinities for GLUT1 and GLUT4. PLoS One 6, e25237 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hausdorff S. F., et al. , Identification of wortmannin-sensitive targets in 3T3-L1 adipocytes. DissociationoOf insulin-stimulated glucose uptake and glut4 translocation. J. Biol. Chem. 274, 24677–24684 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Knight Z. A., et al. , A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125, 733–747 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zmajkovicova K., et al. , MEK1 is required for PTEN membrane recruitment, AKT regulation, and the maintenance of peripheral tolerance. Mol. Cell 50, 43–55 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catalanotti F., et al. , A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat. Struct. Mol. Biol. 16, 294–303 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Haura E. B., et al. , A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin. Cancer Res. 16, 2450–2457 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Barrett S. D., et al. , The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg. Med. Chem. Lett. 18, 6501–6504 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Kharitonenkov A., et al. , FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia de Herreros A., Birnbaum M. J., The acquisition of increased insulin-responsive hexose transport in 3T3-L1 adipocytes correlates with expression of a novel transporter gene. J. Biol. Chem. 264, 19994–19999 (1989). [PubMed] [Google Scholar]
- 30.Taha C., Klip A., The insulin signaling pathway. J. Membr. Biol. 169, 1–12 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Ge X., et al. , Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J. Biol. Chem. 286, 34533–34541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L., et al. , Paracrine-endocrine FGF chimeras as potent therapeutics for metabolic diseases. EBioMedicine 48, 462–477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall C. J., Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995). [DOI] [PubMed] [Google Scholar]
- 34.Goetz R., et al. , Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell. Biol. 27, 3417–3428 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa Y., et al. , BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. U.S.A. 104, 7432–7437 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrannini E., et al. , Adipose tissue and skeletal muscle insulin-mediated glucose uptake in insulin resistance: Role of blood flow and diabetes. Am. J. Clin. Nutr. 108, 749–758 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Kolumam G., et al. , Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/βKlotho complex. EBioMedicine 2, 730–743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding X., et al. , βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 16, 387–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman M. A., et al. , A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 484, 333–338 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.