Significance
The astounding ubiquity of ants has prompted many naturalists to contemplate their exact number on Earth, but systematic and empirically derived estimates are lacking. Integrating data from all continents and major biomes, we conservatively estimate 20 × 1015 (20 quadrillion) ants on Earth, with a total biomass of 12 megatons of dry carbon. This exceeds the combined biomass of wild birds and mammals and equals 20% of human biomass. Ant abundance is distributed unevenly on Earth, peaking in the tropics and varying sixfold among habitats. Our global map of ant abundance expands our understanding of the geography of ant diversity and provides a baseline for predicting ants’ responses to worrying environmental changes that currently impact insect biomass.
Keywords: Formicidae, density, diversity, insect, worldwide
Abstract
Knowledge on the distribution and abundance of organisms is fundamental to understanding their roles within ecosystems and their ecological importance for other taxa. Such knowledge is currently lacking for insects, which have long been regarded as the “little things that run the world”. Even for ubiquitous insects, such as ants, which are of tremendous ecological significance, there is currently neither a reliable estimate of their total number on Earth nor of their abundance in particular biomes or habitats. We compile data on ground-dwelling and arboreal ants to obtain an empirical estimate of global ant abundance. Our analysis is based on 489 studies, spanning all continents, major biomes, and habitats. We conservatively estimate total abundance of ground-dwelling ants at over 3 × 1015 and estimate the number of all ants on Earth to be almost 20 × 1015 individuals. The latter corresponds to a biomass of ∼12 megatons of dry carbon. This exceeds the combined biomass of wild birds and mammals and is equivalent to ∼20% of human biomass. Abundances of ground-dwelling ants are strongly concentrated in tropical and subtropical regions but vary substantially across habitats. The density of leaf-litter ants is highest in forests, while the numbers of actively ground-foraging ants are highest in arid regions. This study highlights the central role ants play in terrestrial ecosystems but also major ecological and geographic gaps in our current knowledge. Our results provide a crucial baseline for exploring environmental drivers of ant-abundance patterns and for tracking the responses of insects to environmental change.
“Ants make up two-thirds of the biomass of all the insects. There are millions of species of organisms and we know almost nothing about them.”
- Edward O. Wilson
“The little things that run the world” is how eminent biologist Edward O. Wilson encapsulated the ecological importance of insects and other invertebrates (1). More than 80% of described eukaryotic species on Earth are invertebrates (2), with the majority being insects (3). They affect many ecosystem functions and services (4) and provide extensive benefits to human societies (5, 6). Knowledge on the taxonomic diversity and functional roles of insects remains nonetheless very limited, with estimates suggesting that around 80% of species remain to be described (3).
Biodiversity is increasingly threatened globally (7–10), with a burgeoning number of local-scale studies now documenting alarming declines in terrestrial insect abundance and biomass (11–13) (but see (14)). Threats contributing to these declines include habitat destruction and fragmentation, land-use change, invasive species, and climate change (4, 15–17). Information on the abundances of invertebrates and their spatial patterns is paramount for predicting how community-level changes may influence ecosystem functioning and terrestrial food webs. While recent studies have documented global patterns in the abundances of nematodes and earthworms (18, 19), efforts to compile a global view of insect abundance patterns are still lacking.
Due to their ubiquity, ants are a useful model system for studying biodiversity across different dimensions and scales, with several global databases developed to this end (20–25). Ants are highly diverse, comprising more than 15,700 named species and subspecies (26) and possibly as many undescribed ones (27). Ants are integral components of terrestrial ecosystems, owing to their manifold interactions with other organisms. They serve as seed dispersers for plants (myrmecochory) (28), mutualists with sap-sucking insects (trophobionts) (29), and hosts for a wide range of associate organisms (myrmecophiles) (30) and act as both predator and prey. While ant diversity is undoubtedly crucial for the functioning and maintenance of many ecosystems (31, 32), it is the sheer number of ants—their abundance and by extension their biomass—and their activity that determines the scale of their impact (33). The effects of ants on nutrient decomposition, soil turnover, and perturbation can be enormous: they are estimated to excavate up to 13 tons of soil per hectare annually and increase local nutrient availability by an order of magnitude (34). By facilitating the creation and maintenance of sustainable microhabitats for a plethora of other organisms (35–37), ants are key ecosystem engineers in multiple biomes.
The sizes of ant populations have occasionally been estimated at the local scale and found to exhibit unexpectedly high densities (e.g., Lévieux (38) reports 20 million ants per hectare in an Ivory Coast forest), yet such estimates are often based on subjective methodologies, such as extrapolations from nest counts or localized observations. Additionally, extreme values are probably reported more often and may not be representative. On the whole, our understanding of regional and global patterns of ant abundance is surprisingly limited, with recent efforts focusing on local scales and relying on community assessments (22). As with other insects, it remains unclear whether latitudinal gradients observed in ant species richness and phylogenetic diversity (21,25) are mirrored in their patterns of abundance.
As yet, there is no detailed evaluation of total global ant abundance or biomass based on empirical data, but more general estimates have been published (further details can be found in SI Appendix). Renowned ecologists Bert Hölldobler and Edward O. Wilson ventured a rough calculation to suggest that there were ∼1016 ants globally (39), assuming that ants comprised ∼1% of an estimated global insect population of 1018 individuals (40). In a second estimate, the same authors revised their first estimate downward to a range of 1015 to 1016 ants (41). Their assessments of corresponding ant biomass ranged from 2.5 to 25 Mt C (megatons of dry carbon). The underlying value of global insect abundance was, however, based on a simple extrapolation of insect densities from southeastern England to the entire terrestrial landmass, a “questionable occupation” in the words of its author (40). Similarly, a more-recent estimate of ant biomass (∼70 Mt C) published by Tuma et al. (42) used an estimation of global terrestrial arthropod biomass (43) in combination with only two ant studies from lowland rainforests of Southeast Asia (Borneo and Seram islands), which are particularly rich in ants (44). Global ant biomass has occasionally been proclaimed to equal human biomass (39, 42), but there is limited empirical evidence to support this claim.
Nonetheless, a wealth of local population surveys of epigaeic (i.e., ground surface-dwelling) arthropods using standard methods has been published in the scientific literature for several decades. In addition to measures of biodiversity, local abundances of insects—particularly of omnipresent ants—are commonly reported. Some of these abundance data have been collated in a database of ant diversity measures (The Global Ants Database [GLAD]) (22). However, data are still lacking from many regions on Earth, especially areas where studies are reported in languages other than English. Here, we compile a global dataset of published data on ant abundances, significantly expanding on previous efforts by increasing geographic coverage and including non-English literature (Fig. 1). All the compiled data are derived from standardized samples, thereby allowing a global quantification of ant abundance patterns.
Fig. 1.
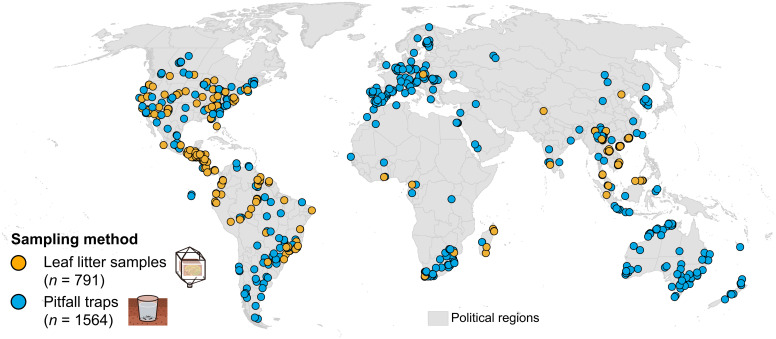
Global map of sampling locations (n = 1,306) in the dataset, which comprises unique samples (n = 2,355) obtained from leaf-litter extractions (orange circles) or pitfall traps (blue circles) and which reported ant abundances. Some locations contain multiple samples (e.g., from different habitats). The global land surface area is divided into national or regional administrative entities.
Data were sourced from studies reporting epigaeic ant abundances from leaf-litter samples and pitfall traps (i.e., the two most widely used standardized methods for sampling ants (45)) and which included relevant additional information (Materials and Methods and SI Appendix include details). Leaf-litter samples provide a “snapshot” of ant abundance in a defined area (i.e., density); they extract all ants foraging in the leaf litter as well as small nests in fallen branches and other cavities. Pitfall traps measure both abundance and activity over defined time periods by capturing foraging ants that fall into them. In this regard, data from pitfall traps reflect activity densities rather than worker densities and may best be understood as a reflection of the local encounter rate. Nonetheless, several parameters, such as body size and the physical structure of the environment, may influence pitfall capture rates (46, 47). Data from the two methods—leaf-litter samples and pitfall traps—should be considered separately, as they sample the active epigaeic ant fauna in different ways. Both are standardized and can be used for regional comparisons, but only leaf-litter samples allow for extrapolations, as they provide absolute values of abundances within defined surface areas (i.e., densities). Apart from the ground surface, ants are much less commonly sampled in a standardized manner from other vegetation layers. Therefore, we compiled the available published (but much more limited) abundance data on arboreal ants from standardized insecticidal fogging samples (SI Appendix, Table S4) to complement our epigaeic ant dataset and provide a more complete overview of global ant abundances.
Using this dataset, we aim to (1) estimate the total global ant abundance and dry biomass, (2) provide a global overview of epigaeic ant density and activity density patterns, and (3) compare epigaeic ant densities and activity densities geographically and among habitats.
Results
Global Ant Dataset.
To assess the global abundance of epigaeic ants, we identified 465 suitable studies (Dataset S5) encompassing 1,306 sampling locations, covering all continents and major biomes where ants occur (Fig. 1). The dataset includes 2,355 unique entries, of which 791 (34%) represent leaf-litter samples and 1,564 (66%) pitfall traps (Dataset S1). In total, we compiled data from 2.68 × 104 standard leaf-litter units (1 m2) and 2.17 × 106 standard pitfall units (24 h trap). Altogether, these are distributed across 176 (out of 477) geographic entities, expanding the geographic coverage of comparable data on ant abundance (i.e., meeting the same stringent selection criteria for standardization) by 160% (GLAD) (22). Our dataset therefore provides an improved baseline for global estimations, as the large number of sampling locations worldwide can account for more regional variation and reduce the uncertainty of calculations. The sampling locations in our dataset remain, however, unevenly distributed, reflecting both geographic disparities in sampling effort over the past decades as well as regional idiosyncrasies in preferred sampling methods (48). Regions such as the Americas and East Asia are well represented by both sampling methods, while others, such as Australia and Europe, contribute primarily pitfall trap data. Much of Africa and Northern Asia is poorly represented; these regions either lack available data, have been sampled with other methodologies (e.g., hand collections in Japan), or report data from sampling protocols that do not meet our stringent selection criteria (criteria for sampling completeness are detailed in SI Appendix). Our large dataset on epigaeic ant abundances is complemented by a second, smaller dataset of 24 published studies on arboreal ant abundances (SI Appendix, Table S4).
Global Ant Abundance and Biomass.
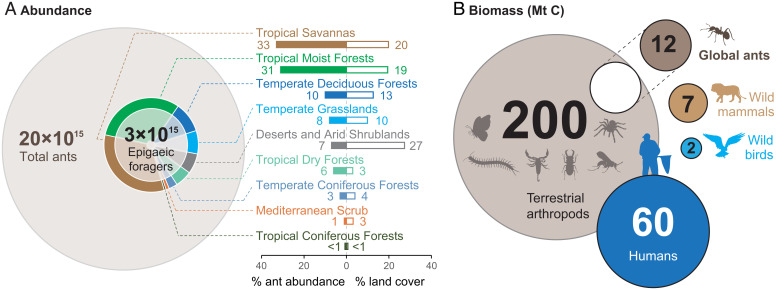
Using only data compiled from leaf-litter samples (as pitfall samples provide activity densities instead of densities), we first calculate biome-specific epigaeic ant densities, extrapolate these to the total areas of biomes, and aggregate the abundances of all biomes. Thereby, we estimate the global epigaeic ant abundance at 3 × 1015 (±0.7 × 1015) (mean ± SEM), i.e., ∼3 quadrillion, individuals (Fig. 2A and SI Appendix, Fig. S1). This corresponds to a dry biomass of 1.9 (±0.5) Mt C or approximately the dry biomass of all wild birds, ∼25% of the dry biomass of all wild mammals, and ∼3% of total human dry biomass (43). Nonetheless, epigaeic ants in the leaf litter comprise only a fraction of the global ant fauna. Adding up our estimates of epigaeic, arboreal, and nonforaging ants (see Materials and Methods), we estimate the number of all ants on Earth at any given time to be 19.8 × 1015 (±5 × 1015), i.e., ∼20 quadrillion, individuals with a total dry biomass of 12.3 (±3.1) Mt C (Fig. 2B). This exceeds the combined dry biomass of all wild birds and mammals and represents ∼20% of total human dry biomass (43). Our estimates remain conservative, as particular regions, biomes, and habitats lacking data (e.g., boreal forests, mangroves, and all subterranean habitats) could not be included (SI Appendix for alternative calculations of global ant abundance and biomass, based on altered parameters).
Fig. 2.
Estimates of global ant abundance and biomass. (A) The pie chart at center shows the global abundance of epigaeic foraging ants in the leaf litter and the contribution of each biome to the epigaeic ant fauna. The bars on the right show the relative contribution of each biome to global epigaeic ant abundance and land cover. Note that not all biomes are included. The area of the larger gray circle in the background corresponds to the total global abundance of all ants, including arboreal ants and nonforaging individuals. (B) The biomass of the total global ant population in comparison to other selected taxa (data for other taxa from Bar-On et al. (43)). Note that the uncertainty of the terrestrial arthropod biomass estimate is ∼15-fold and that of wild bird biomass is ∼twofold). Biomass values are in megatons of dry carbon (Mt C).
Patterns of Ant Density.
In absolute numbers, it is remarkable that almost two-thirds (61%) of global epigaeic ant abundance occurs in only two biomes: tropical moist forests and tropical savannas (Fig. 2A). As these two biomes represent about 38% of ant-inhabited terrestrial surfaces, their contributions are disproportionately high and underscore the importance of tropical regions in maintaining the abundance and biomass of ants globally.
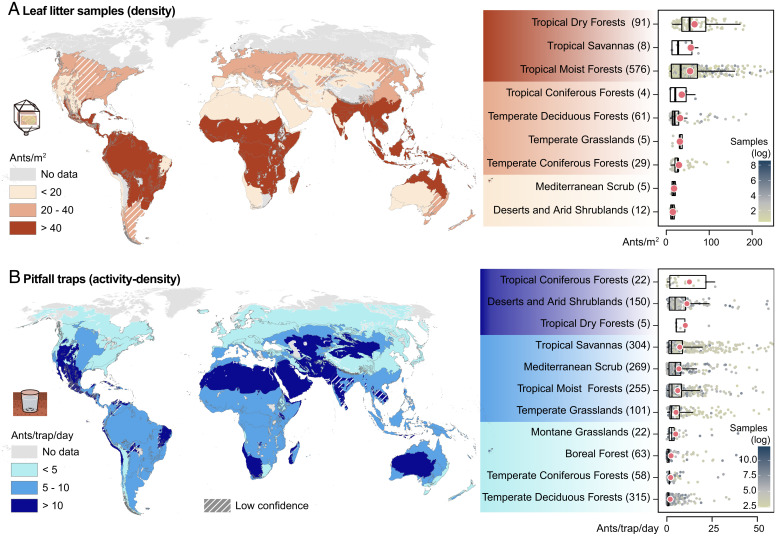
To achieve a geographic overview of the distribution of ants on Earth, and to compare individual biomes, we calculate average ant abundances per sampling unit and project those onto global maps. The two sampling methods in our dataset are analyzed separately. Leaf-litter ant densities are highest in tropical and subtropical biomes (Fig. 3A and SI Appendix, Fig. S3), mirroring previously observed patterns in the species richness of ants and other taxa, such as birds, fish, and flowering plants (21, 49). All (sub)tropical forests and grasslands rank twice as high as their temperate counterparts, while the lowest leaf-litter abundances are observed in Mediterranean scrub and arid shrublands (Fig. 3A). Pitfall activity densities are consistently two to three times higher in (sub)tropical forests and grasslands than in their temperate counterparts (Fig. 3B). Arid and drought-prone biomes, such as arid shrublands, dry forests, and Mediterranean scrub, rank among the highest in terms of activity density. The broad classification of biomes may, however, conceal extensive environmental heterogeneity across different habitats.
Fig. 3.
Biome-level maps and plots showing mean values of (A) ant density from leaf-litter samples and (B) ant activity density from pitfall trap samples. Mean values were binned into three categories to generate a color gradient, where light and dark shades indicate low and high values, respectively. Hatched patterns mark regions with low confidence for mean value calculations (fewer than six entries). Boxplots show the sample size-weighted mean (red dot), median, upper, and lower quartile. The number of data entries is denoted after each biome name. Data points show values per entry, colored by sample size (natural log scale). Axes are truncated for increased readability. Numerical values of weighted means and SEM are provided in SI Appendix, Table S3.
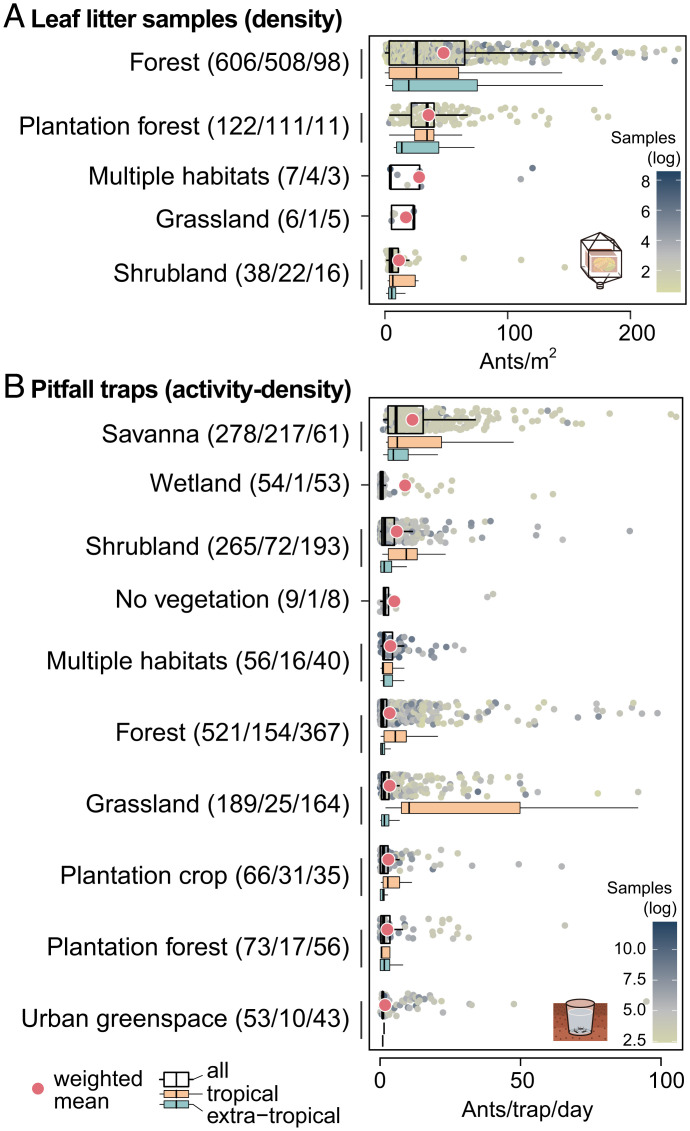
Based on the descriptions of habitats provided in the publications and a distinction between tropical and extratropical samples (SI Appendix), we find that the contribution of tropical regions to the global ant abundance is disproportionally high (Fig. 4). Again keeping the two sampling methods separate for analyses, we observe that densities of leaf-litter ants are four times higher in forests—including both native and plantation forests—than in shrubland (Fig. 4A). However, it is also evident that the vast majority of leaf-litter data have been collected in a small number of habitats with sufficient leaf litter, while several other habitats are poorly represented. Pitfall samples, on the other hand, cover a wider range of habitats, as this method can be applied more generally (45). Pitfall activity densities are highest in savannas, followed by wetlands and shrubland (Fig. 4B). Unsurprisingly, the values are lowest (six times lower than savannas) in urban green spaces.
Fig. 4.
Habitat-level plots showing mean values of (A) ant density from leaf-litter samples and (B) ant activity density from pitfall trap samples. The wide boxplots show the sample size-weighted mean (red dot), median, upper, and lower quartile per habitat; the narrow subboxplots show the data separated into two latitudinal zones: tropical (latitude −23.5° to 23.5°, orange boxplots) and extratropical (latitude <−23.5° and >23.5°, blue boxplots). The numbers in brackets after each habitat denote the number of data entries (overall/tropical/extratropical). Datapoints show values per entry, colored by sample size (natural log scale). Axes are truncated for increased readability, and habitats with fewer than six entries are excluded. Numerical values of weighted means and SEM are provided in SI Appendix, Table S3.
Discussion
Global Ant Abundance and Biomass.
Our estimated global ant abundance of ∼20 × 1015 (±5 × 1015) individuals, though conservative, is 2–20 times higher than previous estimates of 1015–1016 individuals (39, 41). These previous estimates employed a “top-down” approach by assuming that ants comprise ∼1% of the world’s estimated insect population of 1018 individuals. In contrast, our approach can be considered a “bottom-up” estimate based on empirical evidence from an extensive dataset of globally distributed ant samples. We argue that this provides a more reliable estimate of ant abundance, as it relies on fewer assumptions and instead directly extrapolates abundance values from observations. Nevertheless, our estimate remains conservative because we cautiously applied the lowest estimate of the nonforaging ant population in our calculations and did not include several data-deficient segments of the global ant fauna, such as those from boreal forests, subterranean habitats, or any brood or reproductive castes. As these presumably constitute a substantial portion of ant abundance, the true abundance of ants globally is likely to be considerably higher.
Although our estimate of global ant abundance surpasses previous estimates, our estimate of global ant biomass is considerably lower than several previous estimates (39, 41, 42). Regularly cited by popular science and news outlets, these estimates have suggested that global ant dry biomass may be—rather satisfyingly—equivalent to global human dry biomass, which is estimated at ∼60 Mt C (43). However, these claims are supported by limited empirical evidence, and while Tuma et al.’s (42) ant biomass estimate (∼70Mt C) is at the magnitude of human biomass, the estimates of Hölldobler and Wilson (39, 41) are in fact consistently lower (∼2.5–25 Mt C, SI Appendix). We calculate global ant dry biomass at ∼12 Mt C or ∼20% of estimated global human biomass. Still, these results should be interpreted with some caution, as not all biome-specific mean values of ant abundance are derived from similar amounts of data. In particular, there is sparse data on ant abundances in extratropical regions and in savannas or other open environments (50). Additional efforts to document the ant faunas of such under-sampled areas are needed to improve our understanding of ant macroecology. As with our abundance estimate, we exclude some substantial but data-deficient segments of the global ant fauna, such as subterranean ants. In addition, while our estimates of the proportion of nonforaging ants and the average body mass of an individual ant are based on published values from the literature, it remains uncertain how representative these are. We present alternative calculations in SI Appendix that highlight how our estimates may change as more data are published in the future and assumptions are modified. In any case, it is clear that, in proportion to the total biomass of all organisms on Earth (550 Gt C) (43), the biomass of ants is exceedingly small—as is that of humans. Yet, like humans (51), the impacts of ants on the world’s ecosystems are enormous (34, 39).
Patterns of Ant Density.
This study provides an overview and comparison of ant densities at the global scale. We show that, for leaf-litter ants, the highest densities are found in tropical biomes (i.e., savannas and rainforests, Fig. 3A) and habitats (Fig. 4A). This general geographic trend is intriguing, as it broadly aligns with patterns of nest abundance (52) and species richness in ants (21, 53), the biomass distribution of termites (54), as well as patterns in species richness of other terrestrial animals (55). Environmental parameters, such as temperature (56) or net primary productivity (57), may regulate ant densities at the global scale. It should be noted, however, that, owing to the limited availability of data from certain biomes (e.g., savannas) or areas (e.g., extratropical regions), confidence of the calculated mean values varies (SI Appendix, Table S3). Our results strongly contrast with patterns observed in soil invertebrates, which peak in density in the subarctic (soil nematodes) (19) and temperate regions (earthworms) (18) (but see (58)). Although climatic variables do play a role in shaping soil nematode density patterns, at the global scale, this pattern is overwhelmingly driven by characteristics of the soil (19). While data on subterranean ants are largely deficient for high-latitude regions, similar patterns are unlikely to be found in ants (59). Apart from nematodes, earthworms, and now ants, our knowledge of large-scale patterns of invertebrate density remains extremely limited. As such, our understanding of what regulates invertebrate density globally, and potentially some vertebrates and plants that depend on invertebrates, remains incomplete.
Our analyses also provide a global overview of ant activity densities, i.e., the number of ants captured in a standardized pitfall trap over a 24-h period (Figs. 3B and 4B). Despite variation in data availability between different biomes and habitats, we find that ant activity densities are generally higher in tropical biomes and habitats (again broadly aligning with the global pattern of species richness described above) but are actually peaking in arid and drought-prone environments (Figs. 3B and 4B). The physical structure of such open environments may lead to increased ant activity levels and their rates of encounter with pitfall traps (47).
The ubiquity and extremely high abundance of ants has led to specialized ecological interactions with other organisms (60). Recent evidence suggests that a range of evolutionary shifts in vertebrate clades, such as the miniaturization of certain dinosaurs (61) or the loss of dentition in some amphibian lineages (62), are a consequence of diet specializations toward ants and other abundant invertebrates. Further evidencing the ecological importance of high ant abundance and biomass, the global patterns in ant density (Fig. 3A) broadly align with the diversity patterns of other organisms that depend on ants. For instance, the convergent evolution of specializations for eating ants among several mammals (63) in the tropics of the Americas (i.e., anteater), Africa (i.e., aardvark), Asia (i.e., pangolin), and Australasia (i.e., echidna), may be a result of the increased availability of ants in these regions (64). Similarly, the prevalence of ant-specialized diets in lizards is associated with the relative abundance of ant prey (65, 66). The number of myrmecomorphic arthropod species, which mimic ants morphologically or behaviorally to escape predation or hunt ants, also increases toward the tropics (67), where the model organisms (ants) are more abundant, allowing mimicry to remain effective (68, 69). Even for myrmecochorous plants, which are most commonly found and most diverse in low-nutrient, fire-prone environments (70), our results may suggest that the surprisingly high activity density of ants in such biomes (arid shrublands, savannas, and Mediterranean scrub, Fig. 3B) could have favored the emergence of such ant–plant interactions to ensure the quick removal and dispersal of seeds; on the other hand, such myrmecochoric strategies also appear to be prevalent and diverse among tropical epiphytic plants (71) but remain largely understudied.
Beyond the gainful inclusion of abundance data for comprehending patterns of coevolution and diversification within particular biomes or taxonomic groups, a precise picture of current density patterns is paramount for evaluating the ecosystem functions of specific taxa. Population density is known to affect ecosystem function (72), and our findings underscore the fact that ants form a particularly abundant group of invertebrates: with only 1.2% of all terrestrial arthropod species (3), they comprise at least 6% of their biomass (Fig. 2B). Recent studies on the density of earthworms (73) and spiders (74) have highlighted the considerable global impacts of invertebrates on plant-litter decomposition, soil-carbon regulation, and predation levels. Our efforts in compiling a global overview of ant densities have brought similar quantifications of impacts by ants within reach. Moreover, we can now begin to evaluate environmental drivers of insect abundance and biomass, providing a baseline for monitoring responses to environmental change. With our extensive dataset, we plan to perform a formal analysis of the environmental drivers of ant density patterns in the future.
Despite the global coverage of the compiled samples, our dataset highlights considerable shortcomings and gaps in our global picture of ant abundances. Numerous studies could not be included, due to a lack of standardization in procuring or reporting of ant fauna samples. While standardization may not be seen as essential for certain study aims (e.g., regional species checklists can be compiled from a wide range of collection methods), adherence to published and widely available standard sampling methods (e.g., (45)) ensures that findings are comparable between studies and can add immense value to scientific data. Our global ant abundance samples are also geographically clustered, leaving several regions from which barely any data were available (Fig. 1). Such notable areas include central Africa, which is also known to be a center of ant diversity (75), as well as most of Central Asia. Furthermore, data on ant abundances from important sampling methods (e.g., leaf-litter extractions) are lacking in places such as Australia, Europe, or the southern parts of South America. Until recently, hardly any leaf-litter samples had been collected in China and most of Southeast Asia (north of Malaysia), but recent efforts by members of our research group have made important contributions (see Fig. 1). As such, a small number of individuals can make a substantial difference through strategic and targeted sampling in areas where data are needed most. In contrast to diversity estimates, which require specialist taxonomic knowledge, assessments of local ant abundances are straightforward, as they require simple counts of individuals in standardized samples. They are eminently suited for concerted global sampling programs, including citizen science and science education projects (76, 77).
Finally, our study further calls attention to the fact that the vast majority of samples have been collected from the ground layer, leaving a dearth of information on the arboreal and subterranean strata and thus only providing an incomplete picture of true ant abundance. While there are established protocols for the standardized sampling of these strata—the insecticide fogging technique for tree canopies (78) and modified pitfall traps or soil extraction methods for subterranean assemblages (59)—such work can be labor intensive and logistically demanding and is still infrequently performed. Nevertheless, it has become clear that vertical stratification can be substantial in the tropics, with distinct insect communities inhabiting the different vegetative and ground-surface strata (79–81). Although direct comparisons of ant abundances between strata remain scarce, ants are known to be particularly abundant in the tropical forest canopy (44, 82), but not in temperate forests (83), as can be seen in SI Appendix, Table S4. Subterranean ant diversity and local abundance appear to be high in the tropics too (81, 84, 85), but this “final frontier” of ant diversity remains largely unexplored (59, 86, 87). Thus, the global pattern we have identified in this study, where ant density and biomass increase toward tropical regions, will likely be even more pronounced if all strata are considered. It is of utmost importance that we fill these remaining gaps to achieve a comprehensive picture of insect diversity and a truly global understanding of global biodiversity patterns, their drivers, and ramifications. As a global community, we should focus our efforts on the regions, habitats, and biomes we know the least about while we still can. To achieve this goal, we should fervently respond to the call of the late Edward O. Wilson for “more boots on the ground” (88).
Materials and Methods
Literature Search.
We performed a literature search for relevant published studies (including scientific articles, technical reports, and books) in two steps. First, studies published before 2014 were identified from the Global Ant Biodiversity Informatics (GABI) database (see details in Guénard et al. (24)) and from a global study on ant community assembly (89), which together include details of over 9,300 publications. Further studies from before 2014 were sourced from the GLAD, which encompasses 369 studies (22). Second, studies published in any language between 1 January 2014 and 28 February 2020 were identified through searches in Scopus and Google Scholar. In addition, we specifically searched for Chinese literature in the China National Knowledge Infrastructure and Airiti Library. A small number of unpublished datasets from our own research group and from collaborators was also included (SI Appendix includes more details on methods).
We only considered studies that sampled ants using standardized methods, i.e., leaf-litter extractions and pitfall traps. Both techniques provide reliable abundance measures of the epigaeic ant fauna. Leaf-litter extractions yield 90–98% of individuals in the leaf-litter ant community (90, 91), but this approach is restricted to habitats with leaf litter. The commonly used pitfall trap is effective at capturing ground-active, foraging ants; while capture rates can be species specific (46), this effect decreases strongly with sample size (92), and overall, this method is reliable across a wide range of habitats (45,50,93,94). A study was deemed relevant for our purposes if it used leaf-litter extractions and/or pitfall traps and reported both sampling effort and the number of ants collected. Sizeable portions of the GABI database and GLAD did not meet our criteria and could not be included.
Data Acquisition.
The dataset on epigaeic ant abundances was organized in separate entries—each corresponding to a number (count) of individual ants observed at the highest possible spatial resolution. If a study reported separate values of ant abundance for different locations, habitats, or methods, these were included as separate entries. For each entry, we recorded the number and details of sampling units. When leaf-litter sampling was performed, each litter sample was one unit; we recorded its area (m2). When pitfall traps were used, each trap was considered a unit; we recorded the diameter of the trap container (in cm) and the duration of exposure (in hours). Abundance values were standardized by transformation to individuals per 1 m2 for leaf-litter data and 24 h trap duration for pitfall trap data. If reported, we also recorded details of the sampling habitat (SI Appendix includes further details). Entries were only retained in our database if (1) the reported abundances could be assigned to a single method (leaf litter extractions or pitfall traps), (2) sufficient detail was provided on sampling effort and location, and (3) the data had not yet been entered into our dataset as part of another study.
Global Abundance and Biomass Estimation.
To estimate global epigaeic ant abundance, we only used abundances derived from leaf-litter extractions, as these can be related to surface area. Entries were assigned to biomes based on their geographic location, using ArcGIS version 10.2 (95) and the biome definitions of Dinerstein et al. (96) (SI Appendix, Table S1). The use of biomes as categories for extrapolation accounted for some of the geographic bias in our dataset, where most of the leaf-litter samples were sourced from tropical areas (Fig. 1). For each biome, we calculated the mean abundance per m2 (density), extrapolated this to determine the overall abundance of epigaeic ants in the total area occupied by that biome globally, and finally summed the abundance values of all biomes to derive a global estimate of epigaeic ant abundance. As our separate data entries differed substantially in sample size, we weighted each entry accordingly (weighted mean) and calculated weighted SEM as a measure of confidence. The upper and lower limits were estimated by summing biome means +SEM or –SEM, respectively.
Significant ant populations also inhabit and forage in arboreal and subterranean spaces and are not captured by litter sampling (44, 59, 82). Arboreal insects, including ants, have been collected with the arboreal fogging technique, where a nebulized insecticide is blown into the tree canopy and all falling insects are collected in sheets or funnels (78). We identified 24 studies that published values of arboreal ant densities using this method, mostly from tropical rainforests (SI Appendix, Table S4). We calculated the weighted mean ant abundance per m2 separately for tropical and temperate forests and extrapolated to global biome area (only for forest biomes) as above. With the limited data available on arboreal ant densities, these calculations were not intended to provide reliable numerical values but merely a general indication of the size of the global arboreal ant population. We could not perform similar calculations for subterranean ants, as studies are scarce and subterranean trapping devices are often baited (59).
All the ants collected by leaf litter samples or tree canopy fogging merely constitute a subset of the total global ant fauna. They consist—to a very large degree—of foragers. Additional ants occur on vegetation or do not leave the nest to forage and are not captured by leaf-litter samples (some nonforaging ants may of course be present in leaf-litter samples where entire small colonies are collected; their proportion is presumably small and they are not further considered here). We conservatively estimate the proportion of foragers to comprise (on average) only ∼22% of individuals in an ant colony (SI Appendix, Table S5), of which only a portion are actively foraging at any given time. Thus, to account for nonforaging ants, we calculate total global ant abundance as
| [1] |
We consider this to be a minimum estimate, as it is highly unlikely that all foraging ants leave the nest to collect food at the same time.
For estimating ant biomass, an average worker ant was considered to have a dry weight of 1.24 mg (Dataset S4), a value obtained from the body mass measurements of 534 species ranging from 0.004 to 57 mg. Considering a carbon content of 50% dry weight for arthropods (43), a single ant has an average dry carbon (C) weight of 0.62 mg, and the dry carbon biomass of an ant population in megatons (Mt) is then calculated as
| [2] |
We estimated upper and lower biomass ranges by using our upper and lower abundance estimates (mean +SEM or –SEM) for calculations. Alternative calculations based on different assumptions of forager proportion and ant dry weight can be found in SI Appendix.
Regional Patterns.
To investigate geographic patterns of ant distribution on Earth, we assigned data entries to biomes as described above. We used the complete epigaeic dataset encompassing leaf-litter samples and pitfall samples, while keeping the two methods entirely separate for analysis. For each biome, the weighted mean ant abundance per sampling unit (leaf-litter samples: density; pitfall samples: activity density) was calculated and projected onto maps. Because the number of data entries in each biome varied substantially, we calculated the weighted SEM as a measure of confidence. Biome values with low confidence (fewer than six data entries) have been highlighted as such in Fig. 3 (hatched pattern).
To investigate regional patterns in terms of the local environment, we assigned data entries to habitats where possible, while again carrying out separate analyses for data from the two methods. The descriptions of sampled habitats across all studies did not follow any standardized nomenclature, and these were therefore reclassified into general categories, such as “forest”, “grassland”, or “shrubland” (SI Appendix, Table S2). As habitat categories do not convey any information on geographic location, we further separated the data within each habitat category into the two subcategories “tropical” and “extratropical” based on latitude (SI Appendix) for comparative purposes. This subcategorization also accounted for some of the geographic bias in our dataset, where most leaf-litter samples were from tropical regions and most pitfall samples from temperate regions (Fig. 1). For each habitat category, we calculated mean values weighted according to each data entry’s sample size in standard units and the weighted SEM as a measure of confidence. Habitat values with low confidence (i.e., fewer than six data entries) were excluded from Fig. 4.
Supplementary Material
Acknowledgments
This work was supported by a Division of Ecology and Biodiversity Postdoctoral Fellow Research Award from the University of Hong Kong and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—project No. 499479766 to P.S. Further support came from the Early Career Scheme of the Research Grant Council of Hong Kong (No. ECS-27106417), and fieldwork in SE Asia was supported by a National Geographic Grant to B.G. S.S.N. received funding by the DFG—project No. 445715161. M.K.L.W. is supported by a postdoctoral fellowship from the Forrest Research Foundation. The authors are grateful to Crisanto Gómez and Xavier Espadaler for providing data on ant body mass and to Mostafa Sharaf for providing abundance data.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. V.N. is a guest editor invited by the Editorial Board.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201550119/-/DCSupplemental.
Data, Materials, and Software Availability
All data are included in the manuscript and/or supporting information. Some study data are available (All data used in our analyses are provided in the supporting information to this article. The geographic locations of data entries are not part of our current analyses and have been removed, as we are currently performing further analyses in this regard. This information will be made available upon reasonable request.).
References
- 1.Wilson E. O., The little things that run the world (the importance and conservation of invertebrates). Conserv. Biol. 1, 344–346 (1987). [Google Scholar]
- 2.Brusca R. C., Moore W., Shuster S. M., Invertebrates (Sinauer Associates, Sunderland, ed. 3, 2016). [Google Scholar]
- 3.Stork N. E., How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 63, 31–45 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Prather C. M., et al. , Invertebrates, ecosystem services and climate change. Biol. Rev. Camb. Philos. Soc. 88, 327–348 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Losey J. E., Vaughan M., The economic value of ecological services provided by insects. Bioscience 56, 311–323 (2006). [Google Scholar]
- 6.Potts S. G., et al. , Safeguarding pollinators and their values to human well-being. Nature 540, 220–229 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Blowes S. A., et al. , The geography of biodiversity change in marine and terrestrial assemblages. Science 366, 339–345 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Dirzo R., et al. , Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Pimm S. L., et al. , The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Soroye P., Newbold T., Kerr J., Climate change contributes to widespread declines among bumble bees across continents. Science 367, 685–688 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer N., Bonn A., A Guerra C., Recognizing the quiet extinction of invertebrates. Nat. Commun. 10, 50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallmann C. A., et al. , More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12, e0185809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Klink R., et al. , Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368, 417–420 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Crossley M. S., et al. , No net insect abundance and diversity declines across US Long Term Ecological Research sites. Nat. Ecol. Evol. 4, 1368–1376 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Hafernik J. E., “Threats to invertebrate biodiversity: Implications for conservation strategies” in Conservation Biology, Fiedler P. L., Jain S. K., Eds. (Springer, Boston, 1992), pp. 171–195. [Google Scholar]
- 16.Seibold S., et al. , Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574, 671–674 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Wagner D. L., Insect declines in the Anthropocene. Annu. Rev. Entomol. 65, 457–480 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Phillips H. R. P., et al. , Global distribution of earthworm diversity. Science 366, 480–485 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Hoogen J., et al. , Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Dunn R. R., et al. , Global ant (Hymenoptera: Formicidae) biodiversity and biogeography - A new database and its possibilities. Myrmecol. News 10, 77–83 (2007). [Google Scholar]
- 21.Economo E. P., Narula N., Friedman N. R., Weiser M. D., Guénard B., Macroecology and macroevolution of the latitudinal diversity gradient in ants. Nat. Commun. 9, 1778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibb H., et al. , A global database of ant species abundances. Ecology 98, 883–884 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Guénard B., Weiser M. D., Dunn R. R., Global models of ant diversity suggest regions where new discoveries are most likely are under disproportionate deforestation threat. Proc. Natl. Acad. Sci. U.S.A. 109, 7368–7373 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guénard B., Weiser M. D., Gómez K., Narula N., Economo E. P., The Global Ant Biodiversity Informatics (GABI) database: Synthesizing data on the geographic distribution of ant species (Hymenoptera: Formicidae). Myrmecol. News 24, 83–89 (2017). [Google Scholar]
- 25.Kass J. M., et al. , The global distribution of known and undiscovered ant biodiversity. Sci. Adv. 8, eabp9908 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolton B., An online catalog of the ants of the world. https://antcat.org. Accessed May 28, 2021
- 27.Ward P. S., The phylogeny and evolution of ants. Annu. Rev. Ecol. Evol. Syst. 45, 23–43 (2014). [Google Scholar]
- 28.Beattie A. J., Hughes L., “Ant-plant interactions” in Plant-animal Interactions: An Evolutionary Approach, Herrera C. M., Pellmyr O., Eds. (Blackwell Science, Oxford, 2002), pp. 334. [Google Scholar]
- 29.Way M. J., Mutualism between ants and honeydew-producing Homoptera. Annu. Rev. Entomol. 8, 307–344 (1963). [Google Scholar]
- 30.Kronauer D. J., Pierce N. E., Myrmecophiles. Curr. Biol. 21, R208–R209 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Del Toro I., Ribbons R. R., Pelini S. L., The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 17, 133–146 (2012). [Google Scholar]
- 32.Folgarait P. J., Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 7, 1221–1244 (1998). [Google Scholar]
- 33.Brian M. V., Production Ecology of Ants and Termites, International Biological Programme (Cambridge University Press, Cambridge, 1978), pp. 409. [Google Scholar]
- 34.Frouz J., Jilková V., The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecol. News 11, 191–199 (2008). [Google Scholar]
- 35.Meyer S. T., et al. , Leaf-cutting ants as ecosystem engineers: Topsoil and litter perturbations around Atta cephalotes nests reduce nutrient availability. Ecol. Entomol. 38, 497–504 (2013). [Google Scholar]
- 36.Sanders D., van Veen F. J. F., Ecosystem engineering and predation: The multi-trophic impact of two ant species. J. Anim. Ecol. 80, 569–576 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Swanson A. C., et al. , Welcome to the Atta world: A framework for understanding the effects of leaf‐cutter ants on ecosystem functions. Funct. Ecol. 33, 1386–1399 (2019). [Google Scholar]
- 38.Levieux J., “A comparison of the ground dwelling ant populations between a Guinea savanna and an evergreen rain forest of the Ivory Coast” in The Biology of Social Insects, Breed M. D., Michener C. D., Evans H. E., Eds. (Westview Press, Boulder, Colorado, 1982), pp. 48–53. [Google Scholar]
- 39.Hölldobler B., Wilson E. O., Journey to the Ants (Harvard University Press, Cambridge, MA, 1994). [Google Scholar]
- 40.Williams C. B., The range and pattern of insect abundance. Am. Nat. 94, 137–151 (1960). [Google Scholar]
- 41.Hölldobler B., Wilson E. O., The Superorganism (W.W. Norton & Co., New York, 2009). [Google Scholar]
- 42.Tuma J., Eggleton P., Fayle T. M., Ant-termite interactions: An important but under-explored ecological linkage. Biol. Rev. Camb. Philos. Soc. 95, 555–572 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Bar-On Y. M., Phillips R., Milo R., The biomass distribution on Earth. Proc. Natl. Acad. Sci. U.S.A. 115, 6506–6511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson D. W., Cook S. C., Snelling R. R., Chua T. H., Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Bestelmeyer B. T., et al. , “Field techniques for the study of ground-dwelling ants” inAnts: Standard Methods for Measuring and Monitoring Biodiversity, Agosti D., Majer J. D., Alonso L. E., Schultz T. R., Eds. (Smithsonian Institution Press, Washington, London, 2000), chap. 9, pp. 122–144. [Google Scholar]
- 46.Gotelli N. J., Ellison A. M., Dunn R. R., Sanders N. J., Counting ants (Hymenoptera: Formicidae): Biodiversity sampling and statistical analysis for myrmecologists. Myrmecol. News 15, 13019 (2011). [Google Scholar]
- 47.Majer J. D., The use of pitfall traps for sampling ants - A critique. Mem. Mus. Vic. 56, 323–329 (1997). [Google Scholar]
- 48.Hughes A. C., et al. , Sampling biases shape our view of the natural world. Ecography 44, 1259–1269 (2021). [Google Scholar]
- 49.Barlow J., et al. , The future of hyperdiverse tropical ecosystems. Nature 559, 517–526 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Parr C. L., Chown S. L., Inventory and bioindicator sampling: Testing pitfall and Winkler methods with ants in a South African savanna. J. Insect Conserv. 5, 27–36 (2001). [Google Scholar]
- 51.Elhacham E., Ben-Uri L., Grozovski J., Bar-On Y. M., Milo R., Global human-made mass exceeds all living biomass. Nature 588, 442–444 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Kaspari M., Alonso L., O’Donnell S., Three energy variables predict ant abundance at a geographical scale. Proc. Biol. Sci. 267, 485–489 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn R. R., et al. , Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol. Lett. 12, 324–333 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Sanderson M. G., Biomass of termites and their emissions of methane and carbon dioxide: A global database. Global Biogeochem. Cycles 10, 543–557 (1996). [Google Scholar]
- 55.Currie D. J., Energy and large-scale patterns of animal- and plant-species richness. Am. Nat. 137, 27–49 (1991). [Google Scholar]
- 56.Allen A. P., Brown J. H., Gillooly J. F., Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297, 1545–1548 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Kaspari M., Global energy gradients and size in colonial organisms: Worker mass and worker number in ant colonies. Proc. Natl. Acad. Sci. U.S.A. 102, 5079–5083 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James S. W., et al. , Comment on “Global distribution of earthworm diversity”. Science 371, eabe4629 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Wong M. K. L., Guénard B., Subterranean ants: Summary and perspectives on field sampling methods, with notes on diversity and ecology (Hymenoptera: Formicidae). Myrmecol. News 25, 1–16 (2017). [Google Scholar]
- 60.Lach L., Parr C. L., Abbott K. L., Ant Ecology (Oxford University Press, Oxford, 2010). [Google Scholar]
- 61.Qin Z., et al. , Growth and miniaturization among alvarezsauroid dinosaurs. Curr. Biol. 31, 3687–3693.e5 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Paluh D. J., et al. , Rampant tooth loss across 200 million years of frog evolution. eLife 10, e66926 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo Z. X., Transformation and diversification in early mammal evolution. Nature 450, 1011–1019 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Redford K. H., “Ants and termites as food: Patterns of mammalian myrmecophagy” inCurrent Mammalogy, Genoways H. H., Ed. (Springer, New York, 1987), chap. 9, pp. 349–399. [Google Scholar]
- 65.Abensperg-Traun M., The influence of climate on patterns of termite eating in Australian mammals and lizards. Aust. J. Ecol. 19, 65–71 (1994). [Google Scholar]
- 66.Abensperg-Traun M., Steven D., Ant- and termite-eating in Australian mammals and lizards: A comparison. Aust. J. Ecol. 22, 9–17 (1997). [Google Scholar]
- 67.McIver J. D., Stonedahl G., Myrmecomorphy: Morphological and behavioral mimicry of ants. Annu. Rev. Entomol. 38, 351–379 (1993). [Google Scholar]
- 68.Finkbeiner S. D., et al. , Frequency dependence shapes the adaptive landscape of imperfect Batesian mimicry. Proc. Biol. Sci. 285, 20172786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richardson J. M. L., Anholt B. R., “Defensive morphology” in Encyclopedia of Animal Behavior, Breed M. D., Moore J., Eds. (Academic Press, 2010), pp. 493–499. [Google Scholar]
- 70.Lengyel S., Gove A. D., Latimer A. M., Majer J. D., Dunn R. R., Ants sow the seeds of global diversification in flowering plants. PLoS One 4, e5480 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Youngsteadt E., Nojima S., Häberlein C., Schulz S., Schal C., Seed odor mediates an obligate ant-plant mutualism in Amazonian rainforests. Proc. Natl. Acad. Sci. U.S.A. 105, 4571–4575 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spaak J. W., et al. , Shifts of community composition and population density substantially affect ecosystem function despite invariant richness. Ecol. Lett. 20, 1315–1324 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Huang W., González G., Zou X., Earthworm abundance and functional group diversity regulate plant litter decay and soil organic carbon level: A global meta-analysis. Appl. Soil Ecol. 150, 103473 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nyffeler M., Birkhofer K., An estimated 400-800 million tons of prey are annually killed by the global spider community. Naturwissenschaften 104, 30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janicki J., Narula N., Ziegler M., Guénard B., Economo E. P., Visualizing and interacting with large-volume biodiversity data using client–server web-mapping applications: The design and implementation of antmaps.org. Ecol. Inform. 32, 185–193 (2016). [Google Scholar]
- 76.Chandler M., et al. , Contribution of citizen science towards international biodiversity monitoring. Biol. Conserv. 213, 280–294 (2017). [Google Scholar]
- 77.Mitchell N., et al. , Benefits and challenges of incorporating citizen science into university education. PLoS One 12, e0186285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erwin T. L., Canopy arthropod diversity: A chronology of sampling techniques and results. Rev Per Ent 32, 71–77 (1990). [Google Scholar]
- 79.Basset Y., Hammond P. M., Barrios H., Holloway J. D., Miller S. E., “Vertical stratification of arthropod assemblages” in Arthropods of Tropical Forests, Basset Y., Novotny V., Miller S. E., Kitching R. L., Eds. (Cambridge University Press, Cambridge, 2003), chap. 3, pp. 17–27. [Google Scholar]
- 80.Brühl C. A., Gunsalam G., Linsenmair K. E., Stratification of ants (Hymenoptera, Formicidae) in a primary rain forest in Sabah, Borneo. J. Trop. Ecol. 14, 285–297 (1998). [Google Scholar]
- 81.Ryder Wilkie K. T., Mertl A. L., Traniello J. F., Species diversity and distribution patterns of the ants of Amazonian Ecuador. PLoS One 5, e13146 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stork N. E., Insect diversity: Facts, fiction and speculation. Biol. J. Linn. Soc. Lond. 35, 321–337 (1988). [Google Scholar]
- 83.Southwood T. R. E., Moran V. C., Kennedy C. E. J., The richness, abundance and biomass of the arthropod communities on trees. J. Anim. Ecol. 51, 635–649 (1982). [Google Scholar]
- 84.Andersen A. N., Brault A., Exploring a new biodiversity frontier: Subterranean ants in northern Australia. Biodivers. Conserv. 19, 2741–2750 (2010). [Google Scholar]
- 85.Longino J., Colwell R. K., Biodiversity assessment using structured inventory: Capuring the ant fauna of a tropical rain forest. Ecol. Appl. 7, 1263–1277 (1997). [Google Scholar]
- 86.Castro D., et al. , A preliminary checklist of soil ants (Hymenoptera: Formicidae) of Colombian Amazon. Biodivers. Data J. 6, e29278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Houadria M., Menzel F., . Digging Deeper into the Ecology of Subterranean Ants: Diversity and Niche Partitioning across Two Continents. Diversity 13, 53 (2021). [Google Scholar]
- 88.Wilson E. O., Biodiversity research requires more boots on the ground. Nat. Ecol. Evol. 1, 1590–1591 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Guénard B., Perrichot V., Economo E. P., Integration of global fossil and modern biodiversity data reveals dynamism and stasis in ant macroecological patterns. J. Biogeogr. 42, 2302–2312 (2015). [Google Scholar]
- 90.Guénard B., Lucky A., Shuffling leaf litter samples produces more accurate and precise snapshots of terrestrial arthropod community composition. Environ. Entomol. 40, 1523–1529 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Ivanov K., Milligan J., Keiper J., Efficiency of the Winkler method for extracting ants (Hymenoptera: Formicidae) from temperate-forest litter. Myrmecol. News 13, 73–79 (2010). [Google Scholar]
- 92.Engel J., et al. , Pitfall trap sampling bias depends on body mass, temperature, and trap number: Insights from an individual-based model. Ecosphere 8, e01790 (2017). [Google Scholar]
- 93.Lopes C. T., Vasconcelos H. L., . Evaluation of three methods for sampling ground-dwelling ants in the Brazilian Cerrado. Neotrop. Entomol. 37, 399–405 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Menke S. B., Vachter N., A comparison of the effectiveness of pitfall traps and Winkler litter samples for characterization of terrestrial ant (Formicidae) communities in temperate savannas. Great Lakes Entomol. 47, 149–165 (2014). [Google Scholar]
- 95.ESRI, ArcGIS: Release 10.2 (Environmental Systems Research Institute, Redlands, CA, 2020). [Google Scholar]
- 96.Dinerstein E., et al. , An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67, 534–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and/or supporting information. Some study data are available (All data used in our analyses are provided in the supporting information to this article. The geographic locations of data entries are not part of our current analyses and have been removed, as we are currently performing further analyses in this regard. This information will be made available upon reasonable request.).