Unstructured summary
Acute retinal vascular occlusions are common causes of visual impairment. While both retinal artery occlusions (RAOs) and retinal vein occlusions (RVOs) are associated with increased age and cardiovascular risk factors, their pathophysiology, systemic implications, and management differ significantly. Acute management of RAOs involves a multidisciplinary approach including neurologists with stroke expertise, while RVO treatment is provided by ophthalmologists; optimization of systemic risk factors by patients’ primary care providers is an important component of RAO and RVO management.
Introduction
Acute retinal vascular occlusions are common causes of visual loss. Retinal vein occlusions (RVOs) are much more common than retinal arterial occlusions (RAOs) and have a better prognosis.1–3 The pathophysiology and systemic implications of RVOs and RAOs differ greatly (Figure 1). While both occur more commonly in the older population and are associated with cardiovascular risk factors, RVOs do not usually require specific systemic work-up,3,4 whereas acute RAOs, including vascular transient monocular vision loss (TMVL), branch retinal arterial occlusion (BRAO), central retinal arterial occlusion (CRAO) and ophthalmic arterial occlusion (OAO), are associated with a higher risk of stroke and cardiac events that must be addressed acutely.5,6 Although acute management of RAOs is well codified and involves neurologists with stroke expertise, there is no proven ocular treatment for acute RAO.6 In contrast, evaluation and treatment of central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO) “belong” to ophthalmologists, with numerous studies addressing treatment of RVOs for which guidelines are available.7 Acute CRAO or BRAO with CRVO is rare and may indicate a systemic disease such as vasculitis, hypercoagulable state or malignancy.2 This article discusses acute RAOs and RVOs emphasizing the need for standardized multidisciplinary approach, and reviews recent advances regarding these two causes of visual loss.
Figure 1: Anatomy of the blood supply to the eye.
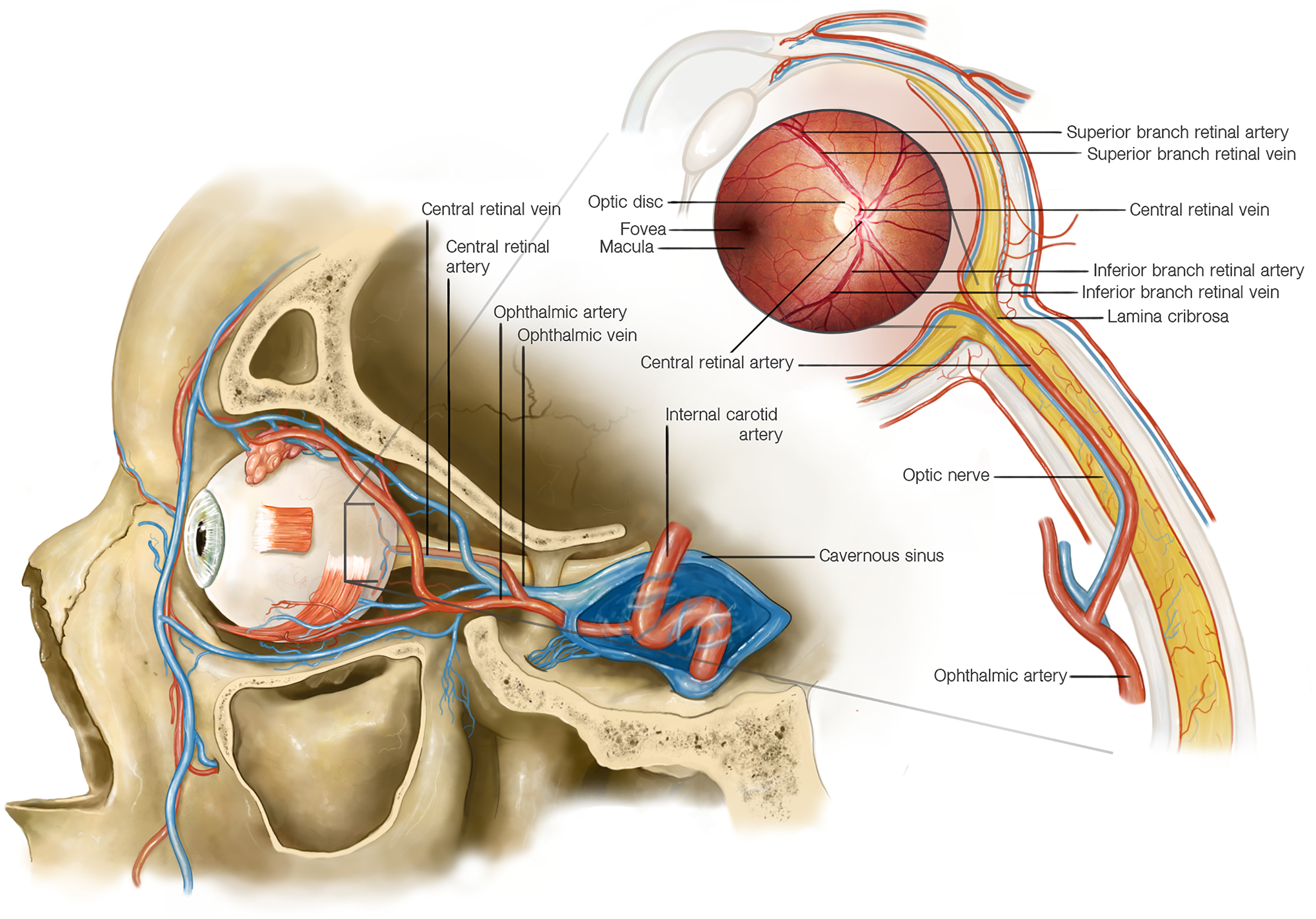
The arterial blood supply to the eye comes mostly from vascular networks originating from the ophthalmic artery, the most distal branch of the internal carotid artery. The central retinal artery is a branch of the ophthalmic artery that enters the optic nerve approximately 10–12 mm behind the eye and supplies the inner layers of the retina. At the level of the optic nerve head the central retinal artery divides into superior and inferior branches. The outer layers of the retina are supplied by the choroidal arteries, which originate from the posterior ciliary arteries (also branches of the ophthalmic artery). The retinal veins follow the retinal arteries, and the superior and inferior retinal veins join at the level of the optic disc where the central retinal vein enters the optic nerve, adjacent to the central retinal artery. The central retinal vein travels posteriorly in the optic nerve and exits the optic nerve in close proximity to the central retinal artery to join the superior and inferior ophthalmic veins which drain into the cavernous sinus. Both the central retinal artery and vein travel though the lamina cribrosa, a mesh-like connective tissue structure at the level of the scleral canal.
The optic nerve is vascularized by a different circulation derived from the ophthalmic artery: the posterior part of the optic nerve is supplied by a surrounding pial plexus originating from small branches off the ophthalmic artery posteriorly and from the posterior ciliary arteries anteriorly; the optic nerve head receives its arterial blood supply from an anastomotic arterial circle (the circle of Zinn–Haller), formed by anastomoses among side branches of the short posterior ciliary arteries, branches from the nearby pial arterial network, and branches from choroidal vessels.
Search strategy and selection criteria:
Data were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the terms “retinal vascular occlusion”, “central retinal artery occlusion”, “branch retinal artery occlusion”, “transient visual loss”, “stroke”, “central retinal vein occlusion”, “branch retinal vein occlusion”, and “hemiretinal vein occlusion”. Articles published in English between 1966 and 2020 were included.
Acute retinal arterial occlusions
Introduction
Acute retinal arterial ischemia, including vascular TMVL, BRAO, CRAO and OAO, requires immediate diagnosis and treatment.5 Indeed, TMVL of vascular origin is a retinal transient ischemic attack (TIA), whereas BRAO, CRAO and OAO result in retinal infarctions, with mechanisms and causes identical to those of acute cerebral infarctions in the internal carotid artery (ICA) territory. Transient ischemic attacks and strokes are on a spectrum of serious conditions involving cerebral and ocular ischemia, just as angina and acute myocardial infarction are on the continuum of acute coronary syndromes.8 It is, therefore, logical to combine vascular TMVL, BRAO, CRAO and OAO as “acute RAOs” and propose the same systematic management for these four entities.5 Although their respective visual outcomes are different, their overall significance and their systemic and neurologic implications are similar.
Epidemiology of RAOs
The estimated incidence of acute CRAO is 1–2 per 100,000, is higher in men than women,9,10 and increases with age with an incidence as high as 10 per 100,000 in older adults.11 A rising incidence of iatrogenic RAO has been reported in young women treated with cosmetic facial filler injections inadvertently injected into facial arteries.12,13 Ophthalmic artery occlusion is rare and BRAO is estimated to account for approximately one-third of acute RAOs.10 Because its diagnosis is challenging, the incidence of vascular TMVL is unknown.
Over the past decade, numerous studies emphasized the high risk of stroke and other cardiovascular events in patients with acute RAOs (Table 1).14–29 The incidence of cerebral small vessel disease is also increased in RAO patients,30,31 consistent with shared vascular risk factors. Most RAO patients are diagnosed with major vascular risk factors, including hypertension, hypertensive crisis, diabetes mellitus, dyslipidemia, and acute coronary syndrome at the time of RAO, prompting an immediate change in medication as a result of the RAO evaluation.5,21 Severe atheromatous ICA stenosis is found in up to 40% of acute RAO patients (Table 1)15–19 and should prompt urgent surgery per stroke guidelines.32–39 Recent studies emphasized the high rate of cardiac source of emboli, especially atrial fibrillation, in RAO patients.23,28,29,40–42 Studies also demonstrated a high rate of asymptomatic, often multiple, small cerebral infarctions on brain MRIs performed within 15 days of visual loss in patients with acute RAOs (in up to 53% of CRAO, 31% of BRAO, and 18% of TMVL patients) (Table 2).21,26,30,31,43–48 The findings of such infarctions in acute RAO patients who do not have neurologic symptoms indicates a higher risk of recurrent stroke and higher chance of identifying a major cause of stroke during the work-up, permitting early stroke risk stratification and intervention.5,8,39,49,50 Although studies provide heterogeneous numbers regarding the incidence of recurrent stroke and other cardiovascular events after acute RAO, numerous recent publications (Table 1)15–29 reported a high risk of complications within the first few days after onset of visual loss, emphasizing the need for prompt diagnosis and triage of patients with acute visual loss by an eye care provider to a stroke center. Indeed, stroke and myocardial infarction occurring during hospitalization for acute CRAO was reported in 15·3% and 7·7%, respectively, in the U.S. in 2014.25 Among 103 patients admitted to the hospital for acute CRAO, 79% were found to have other major acute cardiovascular problems that would have required hospitalization in the absence of CRAO.21 The combined risk of stroke, myocardial infarction and death at 2-year follow-up has been reported as high as 32% in the southern U.S. (stroke belt) and can be decreased by aggressive secondary prevention.21 A Korean study19 reported a 70-fold increase in ischemic stroke the first week after a CRAO, and a U.S. study22 using National Medicare datasets for 2013 found a 28-fold and 33-fold increased incidence of ischemic stroke in the first and second weeks following a CRAO. Additionally, a recent U.S. study29 showed that readmission for stroke after RAO was highest within the first 150 days after initial admission, nearly 1 out of 10 RAO patients were readmitted within 30 days and were twice as likely as patients with acute ischemic strokes to be readmitted for cardiac dysrhythmia or endocarditis. However, despite this evidence, appropriate care is often delayed and suboptimal.51–60
Table 1:
Recent large studies evaluating the risk of stroke, cardiac ischemia and vascular disease in patients with isolated acute retinal arterial occlusions
| Study | Patients Follow up | Clinical Presentation | Vascular Risk Factors and Cardiovascular Diseases in RAO patients | Risk of Stroke and of Acute Coronary Syndrome |
|---|---|---|---|---|
| Chang et al,15 Taiwan 2012 | N = 464 3 year f/u |
BRAO: 349 CRAO: 115 |
Hypertension (38%) Diabetes (22%) Dyslipidemia (10%) |
91/464 (20%) had a stroke/TIA at 3 years f/u (CRAO: 28% vs BRAO 17%) -25% within 1 month after RAO -60% within 6 months after RAO Risk of stroke higher if vascular risk factors and higher with age (≥70 yo) |
| Chang et al,16 Korea 2014 | N = 688 1 year f/u |
BRAO: 531 CRAO: 157 |
Hypertension (43%) Diabetes (24%) Dyslipidemia (12%) Atrial fibrillation (1%) |
37/688 (5%) had ACS at 1 year f/u (CRAO: 10% vs BRAO: 4%) Risk of ACS higher if vascular risk factors and higher with age (≥70 yo) |
| Park et al,17 Korea 2015 | N = 1585 1 year f/u |
CRAO: 1585 | Previous stroke or ACS within previous 6 months (4%) | 152/1585 (10%) had a stroke at 1 year f/u -34% within 1 month after CRAO (higher within first week after CRAO) -44% within 6 months after CRAO 15/1585 (9%) had MI at 6 months |
| Callizo et al,18 Germany 2015 | N = 77* 4 week f/u |
CRAO: 77 | Hypertension (73%) Diabetes (14%) Dyslipidemia (23%) Atrial fibrillation (20%) Ischemic heart disease (22%) Valvular heart disease (17%) ICA stenosis ≥70% (40%) |
11/77 (14%) had a stroke -5/11 (45%) had a stroke within 4 weeks after CRAO 1/77 (1.3%) had a TIA within 4 weeks after CRAO No patients had ACS within 4 weeks after CRAO |
| Rim et al,19 Korea 2016 | N = 401 10 year f/u |
BRAO: 32 CRAO: 119 |
Hypertension (77%) Diabetes (61%) Dyslipidemia (74%) Atrial fibrillation (9%) Ischemic heart disease (44%) |
60/401 (15%) had a stroke at 1 year f/u -59% within 2.5 years after RAO (higher immediately after RAO) Risk of stroke higher if vascular risk factors and higher with age (≥65 yo) |
| Hong et al,20 Korea 2017 | N = 151 1 year f/u |
BRAO: 32 CRAO: 119 |
Hypertension (58%) Diabetes (23%) Dyslipidemia (23%) Atrial fibrillation (6%) LA atherosclerosis (41%) Previous stroke (11%) |
13/151 (9%) had a stroke at 1 year f/u -57% had a stroke within 1 month after RAO -79% had a stroke within 3 months after RAO Risk of stroke higher if large artery atherosclerosis 1/151 (1%) had MI at 1 year |
| Lavin et al,21 USA 2018 | N = 103 2 year f/u |
CRAO: 103 | Hypertensive crisis (33%) Atrial fibrillation (14%) Cardiac disease (20%) ICA stenosis ≥70% (37%) |
24/75 (32%) had a stroke or MI or death at 2 year f/u 79% of CRAO patients found to have other severe medical problem requiring hospitalization |
| French et al,22 USA 2018 | N = 3338 National Medicare data set 2013 |
CRAO: 3338 | Hypertension (26%) Diabetes (8%) Atrial fibrillation (21%) Congestive heart failure (5%) Previous stroke (1%) |
141/3338 (4%) had a stroke at 6 months f/u −74/141 (52%) had a stroke within the first 2 weeks after CRAO −91/141 (64%) had a stroke within 30 days after CRAO |
| Christiansen et al,23 Denmark 2018 | N = 706 National Patient Registry |
RAO | Hypertension (73%) Diabetes (36%) Dyslipidemia (65%) Congestive heart failure (20%) Previous stroke (23%) |
Atrial fibrillation found in 74/706 (10%) RAO patients |
| Avery et al,24 Canada 2019 | N = 66 3 year f/u |
BRAO: 35 CRAO: 31 |
Hypertension (74%) Diabetes (24%) Dyslipidemia (47%) Atrial fibrillation (14%) ICA stenosis ≥70% (6%) Previous stroke (21%) |
1/21 (5%) with no previous stroke had a stroke at 3 year f/u 4/31 (13%) with no previous stroke had a stroke at 3 year f/u |
| Mir et al,25 USA 2019 | N = 17,117 inpatient admissions (NIS 2003–2014) | CRAO: 17,117 | Hypertension (72%) Diabetes (26%) Dyslipidemia (51%) Atrial fibrillation (16%) Ischemic heart disease (35%) Valvular heart disease (12%) ICA stenosis ≥70% (22%) Previous stroke (9%) |
Incidence of inpatient stroke: 13% Incidence of inpatient MI: 4% Combined risk of in-hospital stroke, transient ischemic attack, MI or death: 19% |
| Chodnicki et al,26 USA 2019 | N = 300 | CRAO: 300 | Previous stroke (2%) | 4/300 (1%) had a stroke simultaneously with CRAO 5/300 (2%) had a stroke within 15 days after CRAO |
| Kang et al,27 Taiwan 2019 | N = 3778 1 year f/u |
CRAO: 3778 | Hypertension (30%) Diabetes (15%) Dyslipidemia (7%) Atrial fibrillation (1%) |
151/3778 (4%) had a stroke at 1 year f/u -17/151 (11%) had a stroke within the first week after CRAO -33/151 (22%) had a stroke within the first 30 days after CRAO |
| Zarkali et al,28 UK 2019 | N = 400 | TMVL: 263 CRAO or BRAO: 137 |
Hypertension (51%) Diabetes (14%) Dyslipidemia (35) Atrial fibrillation (9%) ICA stenosis ≥70% (8%) Previous stroke (5%) |
42/400 (10%) had a stroke or TIA at 90 days f/u |
| Schorr et al,29 USA 2020 | N = 4871 (NRD 2013–2015) |
TMVL: 2066 CRAO: 2163 BRAO: 642 |
Hypertension (62%) Diabetes (24%) Dyslipidemia (60%) Atrial fibrillation (16%) Heart failure (9%) Valvular heart disease (13%) |
8.9% RAO readmitted to the hospital within 30 days 18.5% RAO readmitted to the hospital within 1 year -20.8% readmitted for stenosis/occlusion ICA -4% readmitted for stroke -1% readmitted for transient ischemic attack -5% readmitted for cardiac dysrhythmia |
N: number of patients;
indicates prospective study (all other studies were retrospective);
f/u: follow-up; TMVL: vascular transient monocular visual loss; BRAO: branch retinal artery occlusion; CRAO: central retinal artery occlusion; RAO: retinal artery occlusion; TIA: transient ischemic attack; LA: large artery; ACS: acute coronary syndrome; MI: myocardial infarction; ICA: internal carotid artery; NIS: Nationwide Inpatient Sample; NRD: Nationwide Readmissions Database; NA: information not available.
Table 2:
Recent studies evaluating brain magnetic resonance imaging (MRI) in patients with acute transient and permanent retinal arterial ischemia
| Study | Patients | Clinical Presentation | Timing of MRI and stroke workup | DWI-MRI Results | Correlation with Abnormal DWI-MRI |
|---|---|---|---|---|---|
| Hellenius et al,43 USA 2012 | N = 129 | Isolated TMVL: 66 Isolated BRAO/CRAO: 46 Neurologic sx + TMVL: 8 Neurologic sx + RAO: 9 |
≤7 days of visual loss | DWI+ in 31/129 (24%) (CRAO/BRAO: 33% vs TMVL: 18%) |
Neurologic sx+ Permanent VL > TMVL Identified cause Embolic cause |
| Lee et al,44 Korea 2014 | N = 33 | Isolated BRAO: 12 Isolated CRAO: 13 Neurlogic sx + BRAO: 3 Neurologic sx + CRAO: 5 |
≤7 days of visual loss | DWI+ in 8/33 (24%) (CRAO: 27% vs BRAO: 20%) |
Neurologic sx+ Identified cause Embolic cause |
| Tanaka et al,45 Japan, 2014 | N = 13 | Isolated TMVL: 12 Neurologic sx + TMVL: 1 |
≤7 days of visual loss | DWI+ in 4/13 TMVL (31%) | NA |
| Lauda et al,46 Germany 2015 | N = 213 | TMVL: 68 BRAO: 44 CRAO: 101 Neurologic sx + RAO: NA |
≤7 days of visual loss | DWI+ in 49/213 (23%) (CRAO: 53%; BRAO: 31% vs TMVL: 16%) |
Neurologic sx+ CRAO > BRAO > TMVL Identified cause Embolic cause |
| Cho et al,29 Korea, 2016 | N = 46 | Isolated BRAO: 46 | ≤14 days of visual loss | DWI+ in 6/46 BRAO (13%) | Identified cause Embolic cause |
| Golsari et al,31 Germany 2017 | N = 112* | Isolated TMVL: 35 Isolated BRAO: 8 Isolated CRAO: 69 |
≤1 day of visual loss | DWI+ in 17/112 (15%) (CRAO: 19%; BRAO: 12% vs TMVL: 12%) |
CRAO > BRAO > TMVL Identified cause Embolic cause |
| Tanaka et al,47 Japan, 2018 | N = 40* | Isolated TMVL: 35 Neurologic sx + TMVL: 5 |
≤7 days of visual loss | DWI+ in 7/40 (18%) | NA |
| Zhang et al,48 USA 2018 | N = 41 | Isolated TMVL: 23 Isolated BRAO: 12 Isolated CRAO: 5 Isolated OAO: 1 |
≤7 days of visual loss | DWI+ in 8/41 (19%) (OAO: 100%; CRAO: 25%; BRAO: 40% vs TMVL: 4.3%) |
NA |
| Lavin et al,21 USA, 2018 | N = 67 | Isolated CRAO: 67 | ≤7 days of visual loss | DWI+ in 25/67 (37%) | NA |
| Chodnicki et al,26 USA, 2019 | N = 94 | Isolated CRAO: 94 | ≤15 days of visual loss | DWI+ in 10/94 (11%) | Identified embolic cause |
N: number of patients;
indicates prospective study (all other studies were retrospective);
TMVL: vascular transient monocular visual loss; VL: visual loss; BRAO: branch retinal artery occlusion; CRAO: central retinal artery occlusion; RAO: retinal artery occlusion; OAO: ophthalmic artery occlusion; Neurologic sx+: indicates patients who had acute focal neurologic symptoms at the time of visual loss); MRI: magnetic resonance imaging; DWI: diffusion weighted imaging; DWI+: indicates patients with abnormal DWI indicating acute cerebral infarction on MRI. NA: information not available.
Pathogenesis of RAOs
Similar to cerebral infarctions, RAOs result from acute interruption of blood flow to the eye from diverse underlying conditions, the frequency of which varies depending on the populations studied (Figure 1, Table 1).1,5,14–29 Acute RAO commonly leads to permanent retinal ischemia and irreversible cell death within a few hours.
Emboli from the ICA, aortic arch or heart are the main causes of RAO (Table 1).1,5,14–28 Classic teaching suggested most acute RAOs are secondary to ICA disease;1,2,14 however, recent data demonstrated that cardiac emboli, especially related to atrial fibrillation, represent a major cause of acute RAO.23,28,29,40,43 Systematic prolonged cardiac rhythm monitoring after a stroke or RAO permits delayed diagnosis of atrial fibrillation in many patients previously considered as having a “negative work-up”.23,29,40–42,61,62 Non-embolic RAO may result from systemic vasculitis such as giant cell arteritis, as well as hematologic, immune-mediated and infectious disorders.1,2
Diagnosis and Investigation of RAOs
Acute RAO results in acute monocular visual loss, with severity based on the size of the occluded vessel (OAO, CRAO or BRAO) and duration of occlusion (transient vs. permanent). Transient occlusion results in TMVL and is better described as a “retinal TIA”, than “amaurosis fugax” which is a confusing term and should be avoided.5,8 The diagnosis of vascular TMVL is challenging and most often relies on the patient’s description of acute painless blackout of vision in one eye lasting a few minutes, with a normal ocular examination.5 Because many ocular disorders can result in transient blurry vision, it is essential that patients with TMVL be examined urgently by an eye care provider before receiving extensive testing for presumed vascular TMVL.5 Ophthalmic artery occlusion (usually by a large embolus or thrombus) is devastating, with vision reduced to hand motion or worse. The prognosis is poor and most affected eyes develop ischemic neovascularization with numerous ocular complications.2,63 Most patients with acute CRAO have profound visual loss at presentation with vision in the 20/200-counting fingers range and an ipsilateral relative afferent pupillary defect. Up to 25% of eyes have a cilioretinal artery which originates from the chorioretinal circulation and is therefore spared in acute CRAO and BRAO. Such patients may retain relatively good visual acuity despite very abnormal visual fields. Approximately 20% of patients with visual loss from acute CRAO have spontaneous visual improvement related to revascularization of the occluded artery.2,63 Smaller emboli often migrate in retinal arteries and result in BRAO which has a better visual prognosis.63 These patients complain of acute partial monocular visual loss, often described as a superior or inferior shade and visual acuity varies greatly depending on anatomic variations of retinal vascularization.2,63
The diagnosis of OAO, CRAO and BRAO is usually easy for an eye care provider who is able to visualize attenuation of the retinal arteries, segmentation of the retinal arterial blood columns with sometimes visible retinal arterial emboli, and acute retinal ischemia seen as retinal edema that appears as whitish retinal discoloration after a few hours (Figure 2). In acute CRAO, the fovea appears as a “cherry red spot” because the normal choroidal circulation is seen in contrast to the edematous inner retina surrounding the fovea where the inner retina is thinnest, whereas eyes with OAO have diffuse retinal edema typically associated with optic nerve ischemia and optic nerve edema and no cherry red spot. Reperfused CRAOs may not have arterial attenuation and are often difficult to diagnose acutely.
Figure 2: Acute left central retinal artery occlusion (CRAO) secondary to a left internal carotid artery stenosis.
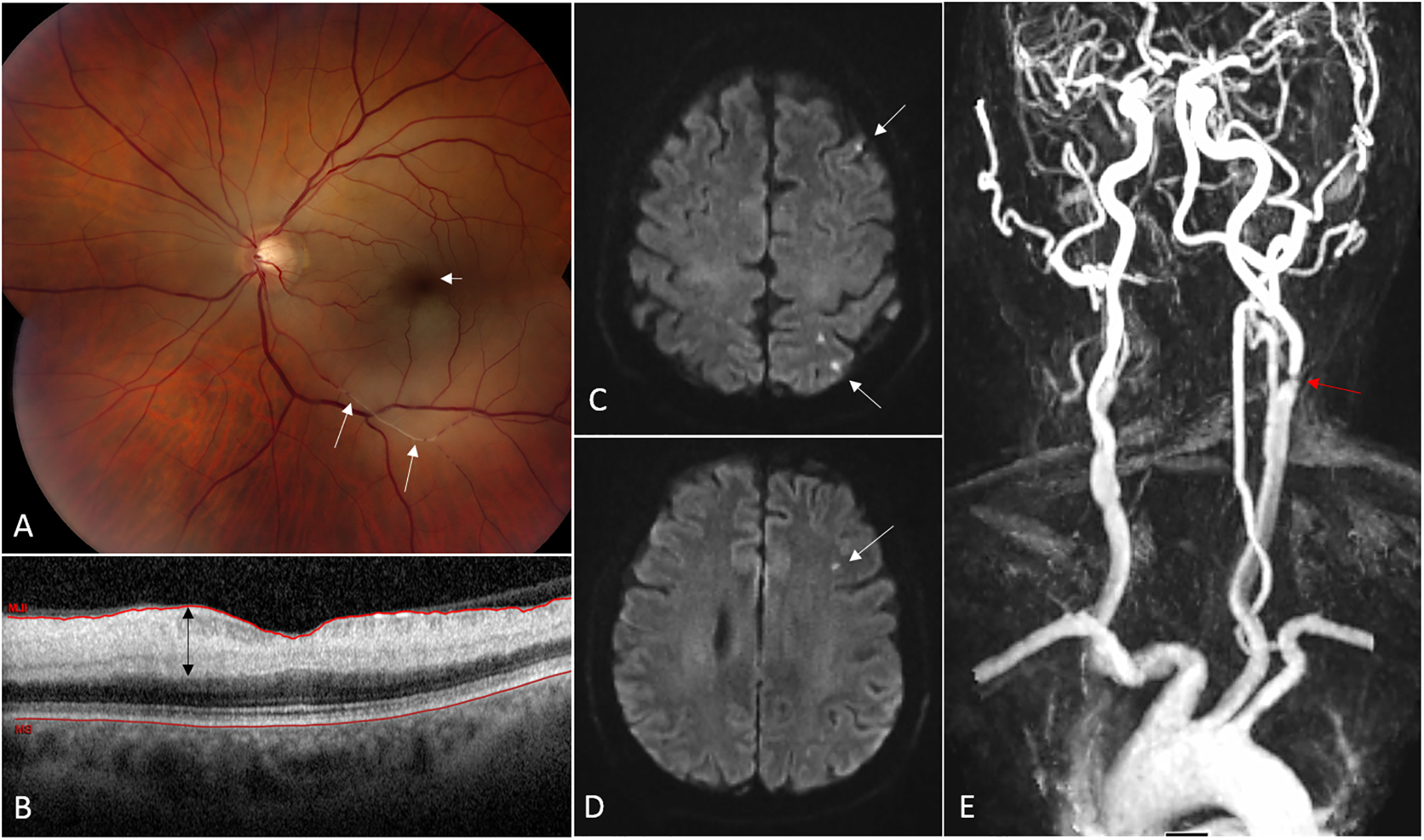
(A) Fundus photograph from a 68 year-old man with acute CRAO in the left eye. The ischemic retina appears whitish and the normally perfused fovea (from the choroidal circulation) is dark red in contrast, consistent with a so-called “cherry-red spot” (short arrow). Platelet-fibrin emboli are seen migrating in the inferior branches of the central retinal artery (long arrows). (B) Optical coherence tomography (OCT) of the macula showing a cut through the fovea. The ischemic inner retinal layers are thickened (black arrow) whereas the outer retinal layers are normal. (C, D) Brain MRI (axial cuts, diffusion-weighted images) performed 24 hours after onset of visual loss demonstrates multiple small acute areas of infarction as small hypersignals in the left hemisphere (arrows). (E) Magnetic resonance imaging (MRA) of the neck and great vessels shows a severe atheromatous stenosis at the origin of the left internal carotid artery.
An ocular examination is always necessary to rule out a nonvascular ocular problem in patients with acute visual loss, and confirm a diagnosis of vascular TMVL, BRAO, CRAO or OAO.5 Telephone diagnosis of visual loss is not possible and there should be a pathway for same-day appointment with an eye care provider for patients complaining of acute visual loss. Ideally, the examination should be performed in close proximity to an emergency department (ED) affiliated with a stroke center or in the ED itself. Diagnosis of acute retinal ischemia can be difficult within a few hours of visual loss, in which case optical coherence tomography (OCT) is useful by showing inner retinal edema acutely (Figure 2). Retinal fluorescein angiography (FA) may show arterial occlusions and delayed retinal arterial perfusion, but is time consuming and usually not necessary acutely. In clinical settings where no eye care provider is available, non-mydriatic digital fundus photography with remote interpretation may permit immediate diagnosis of BRAO, CRAO and OAO.64–66
Management of RAOs
Because giant cell arteritis is a classic cause of acute visual loss and ocular arterial ischemia, it must be ruled out in patients older than 50 with urgent blood tests (complete blood count, erythrocyte sedimentation rate and c-reactive protein) looking for an inflammatory biologic syndrome before further stroke work-up is initiated. Patients with retinal arterial ischemia complicating giant cell arteritis should receive emergent high-dose intravenous corticosteroids as soon as the diagnosis is suspected and before a temporal artery biopsy is obtained.6,67
Once the diagnosis of acute vascular TMVL, BRAO, CRAO or OAO is confirmed and giant cell arteritis is ruled out, guidelines recommend that patients with recent visual loss be immediately referred to the closest ED affiliated with a stroke center or a rapid-access TIA clinic.5,8,,37–39,59,66,68 Indeed, rapid access to specialized centers is the best way to expedite work-up and identify high-risk patients, facilitating early preventive treatments to reduce the risk of subsequent stroke and cardiovascular events.5,8,37–39 Many hospitals have ED-affiliated observation units that allow rapid outpatient work-up with a predefined accelerated diagnostic protocol. Hospitalization is usually only necessary if a dedicated outpatient center is unavailable or the initial work-up demonstrates a cause that requires urgent treatment, such as associated acute cerebral ischemia, ICA stenosis or a cardiac source of emboli. Studies have shown that such pathways for acute stroke patients result in improved outcomes and dramatic decrease in stroke recurrence.5,8,38,39,69,70 For these reasons, widespread access to stroke centers is a public health priority in most countries.
Most recommendations are aimed at preventing further vascular events, similar to what is done for patients with acute cerebral TIA or infarction,5,8,37–39,69,70 but there is currently no specific treatment proven to reverse acute RAO and improve visual outcome beyond its natural history.6,66,68 Numerous treatments aimed at reperfusing acutely occluded retinal arteries or reversing retinal cell death have been suggested (Table 3).6,51 However, most proposed interventions are based on anecdotal reports of improvement in retrospective small case series and few have been evaluated in randomized clinical trials.6 Theoretically, the sooner the retina is reperfused following an acute RAO, the better the chance of improving visual function. The ideal therapeutic window remains unknown, but animal studies suggest treatment would need to be administered within 3 hours of visual loss to prevent permanent retinal ischemia.2,6,63 The highly variable clinical presentations and causes of CRAO, in addition to its relative low incidence, make clinical trials challenging.68 Not only do presenting visual acuity and visual field defects vary tremendously, but the size and type of emboli influence visual outcome. Large emboli occluding the artery at the level of the lamina cribrosa (Figure 1) result in profound visual loss and are difficult to dislodge, especially when made of calcium or large fragments of atheromatous plaques. Identification of such calcified emboli may be possible with transorbital ultrasonography, and usually predicts poor visual outcome with limited effect of thrombolysis.71 Platelet-fibrin emboli are often smaller and multiple, reaching smaller retinal arteries and often migrating spontaneously, with improved vision; such emboli are expected to dissolve quickly after administration of thrombolysis. Iatrogenic embolization such as from cosmetic fillers has a poor visual prognosis since arterial occlusion is usually extensive and permanent.12,13
Table 3:
Treatments proposed for the treatment of acute central retinal artery occlusion
| Mechanism | Proposed effect | Comments |
|---|---|---|
| Dislodging emboli | Reperfuse retina | |
| -Ocular massage | Decrease intraocular pressure and induce retinal arteriolar dilation | No effect shown |
| -Nd:Yag laser embolectomy | Removes embolus | Controversial 50% vitreous hemorrhage |
| Increasing retinal artery perfusion pressure | Reperfuse retina | |
| -Lower intraocular pressure with medications | Decrease intraocular pressure and induce retinal arteriolar dilation | No effect shown |
| -Anterior chamber paracentesis | Rapid decrease in intraocular pressure leading to dilation of retinal arteries with subsequent distal migration of embolus | Anecdotal effect |
| Vasodilation | Stimulates distal migration of embolus | |
| -Hyperventilation or inhalation of carbogen | Increase blood CO2 with subsequent vascular dilation | No effect shown |
| -Induce vasodilation (sublingual isosorbide dinitrate) | Induce retinal arteriolar dilation with subsequent distal migration of embolus | No effect shown |
| -Increase erythrocyte flexibility (pentoxifylline) | Increase red blood cell flexibility, reduce blood viscosity, increase tissue perfusion | Shown to increase retinal artery blood flow |
| Increase blood oxygen tension | Increase amount of oxygen delivered to ischemic retina | |
| -Hyperbaric oxygen | Supportive measure until spontaneous reperfusion of retina occurs | Anecdotal effect |
| Thrombolysis | Dissolution of fibrin-based clots | |
| -Intravenous tPA | Reperfusion of retina | Retrospective studies with variable results |
| -Intra-arterial tPA in ophthalmic artery | Selective reperfusion of retina | EAGLE trial negative Retrospective studies with variable results |
The most promising treatment for acute CRAO is thrombolysis, which, when administered shortly after onset of visual loss, may induce rapid recanalization of the occluded arteries and reperfusion of the ischemic retina prior to retinal cell death. Despite the lack of clinical trials proving their safety and efficacy, thrombolytics have been administered either intravenously or directly into the ophthalmic artery for decades, usually following established protocols for cerebral infarction.6,66,68 The ideal therapeutic window is unknown, but it is assumed that the earlier, the better. Recent reports have suggested a window of 4.5 hours,66,68,72–75 the window currently used in a European randomized clinical trial using intravenous tissue plasminogen activator (tPA) for acute CRAO (ClinicalTrials.gov Identifier: NCT03197194). Two previous randomized clinical trials evaluating intravenous tPA in 16 patients within 24 hours of visual loss76 and intra-arterial tPA in 84 patients within 20 hours of visual loss77 did not show a benefit of thrombolysis and reported a significant number of complications. However, the negative results could be explained by the long treatment windows. Other studies suggested some improvement with intravenous66,68,73,74,75,78 and intra-arterial tPA.66,79,80 In a recent survey, 53% of U.S. centers reported offering thrombolysis for acute CRAO, more often intravenous than intra-arterial, emphasizing the need for clinical trials.51
Patients with CRAO and OAO must be followed every few weeks by eye care providers to monitor for ocular neovascularization that may lead to further ocular complications such as vitreous hemorrhage and neovascular glaucoma.1,2,63
Future research
The combination of clinical features and urgent brain MRI and vascular imaging can identify TMVL, BRAO, CRAO and OAO patients at highest risk for recurrent stroke, providing the opportunity to start early preventive treatments to reduce the risk of subsequent stroke and cardiovascular events.5,8 Because stroke risk is highest within the first few days after onset of visual loss, prompt diagnosis and triage by an eye care provider are mandatory. Integration of diagnostic tools such as non-mydriatic fundus photography in EDs should allow efficient remote diagnosis of acute RAOs when there is no immediate access to an eye care provider.64–66 Automated interpretation of fundus photographs with artificial intelligence should also facilitate the diagnosis of acute RAOs.81,82 Existing telestroke networks can used to facilitate remote acute care of RAO patients.39,66 Increased public awareness of stroke and ocular emergencies,83–85 education of health care providers, and development of local networks enabling collaborations among optometrists, ophthalmologists and neurologists with stroke expertise is a priority as it should expedite such multidisciplinary evaluations and facilitate clinical trials evaluating potential treatments for acute CRAO.5
Retinal vein occlusions
Introduction
There are two types of RVOs, central RVO (CRVO) and branch RVO (BRVO). Thrombosis within the central retinal vein (the major outflow vessel of the eye) causes CRVO,86 while thrombosis within a branch retinal vein causes BRVO.87 Hemiretinal vein occlusion, considered by some a subgroup of CRVO and by others a subgroup of BRVO, is an occlusion resulting in involvement of about half of the retina. While CRVO and BRVO share many features and are therefore often lumped together, they have important differences in pathogenesis, natural history, and response to treatment.
Epidemiology of RVOs
The prevalence of RVOs in predominantly white populations is 0·6–1·2% (BRVO) and 0·1–0·4% (CRVO), with an incidence per year of 0·12% (BRVO) and 0·03% (CRVO).88–91 Disease burden is similar among diverse populations and pooled data from population studies estimated the 2010 worldwide prevalence of CRVO and BRVO at 2·5 million and 13·9 million, respectively.92
The risk of BRVO and CRVO increases with age.88–91,93,94 Hypertension and arteriolar narrowing or nicking are particularly important risk factors for BRVO, but also increase CRVO risk.4,88–91,94,95 Diabetes, glaucoma and increased cup-to-disc ratio are strong risk factors for CRVO, but may also increase BRVO risk.88,89,93,96,97 Case-control studies demonstrated a significantly higher proportion of RVO patients versus age-matched controls have hypertension, diabetes, and cardiovascular disease.4,98,99 Mortality rate is significantly increased in RVO patients, but not different from controls when adjusted for these comorbidities.
Pathogenesis of RVOs
CRVO
The central retinal vein exits the eye and enters the optic nerve at the optic disc, traveling within it through the lamina cribrosa, a connective tissue structure providing support to it and axons as they pass through the sclera (Figure 1). Postmortem eyes with CRVO showed fresh or recanalized thrombus in the central retinal vein near the lamina cribrosa,86 where its lumen normally narrows100 and blood flow increases.101 Vessel narrowing or irregularity promotes turbulent flow and endothelial cell stress. Glaucoma or increased intraocular pressure causes displacement of the lamina cribrosa102,103 which may alter the central retinal vein’s shape and course, increasing turbulence and endothelial stress; this may explain the increased CRVO risk in eyes with glaucoma. Systemic vascular comorbidities may compromise endothelial cells in the central retinal vein,104 and a prothrombotic state (e.g., elevated homocysteine or anticardiolipin antibodies) also increases CRVO risk.105
Hayreh classified eyes with CRVO as ischemic or nonischemic.106 Eyes with ischemic CRVOs presented with poor vision, a relative afferent pupillary defect, and large areas of retinal nonperfusion (RNP) on FA, and frequently developed iris neovascularization often complicated by neovascular glaucoma.107 Eyes with nonischemic CRVOs presented with better vision, little or no RNP, and leakage from perifoveal capillaries resulting in macular edema (ME). Some clinicians suggested eyes with nonischemic CRVO had partial rather than complete central vein obstruction.108 Others described acute hemorrhagic retinopathy with prominent optic disc swelling in young patients that usually resolved spontaneously, referring to it as papillophlebitis,109,110 benign retinal vasculitis,111 or optic disc vasculitis.112 These likely represent CRVO in young patients, and while the visual prognosis is generally good, about 20% suffer substantial vision loss.113
Why does RNP vary so much in CRVO eyes at presentation? Insight is provided by experimental retinal vascular occlusion in primates.114 Central vein occlusion in the orbit caused venous engorgement, perivenous hemorrhages, and optic disc edema/hyperemia that normalized after 10 days; FA showed no RNP and the vasculature was normal after 2 weeks (analogous to CRVO in young patients). However, combined central retinal vein and central retinal artery clamping for 6–7·5 hours caused a typical picture of severe CRVO with extensive hemorrhages, disc edema and hyperemia, ME, and RNP that worsened over time (analogous to older patients with atherosclerosis and compromised retinal arterial perfusion). “Nonperfused” or “ischemic” CRVO is not a separate entity from “perfused” CRVO; it is CRVO occurring in an eye with pre-existing retinal arterial disease and marginal perfusion prior to venous occlusion. The suboptimal, but well-compensated, perfusion is decreased below a critical threshold by the sudden increase in resistance caused by venous occlusion, resulting in closure of retinal capillaries throughout a large area of retina.
Why does RNP often worsen over time? The answer was provided by clinical trials investigating the effect of ranibizumab, an antibody fragment directed against vascular endothelial growth factor-A (VEGF-A).115–120 Those trials demonstrated that VEGF-A is a major stimulus for ME, retinal hemorrhages, and RNP, because sustained suppression of VEGF-A markedly reduced ME, accelerated resolution of retinal hemorrhages, prevented progression of RNP and, in some eyes, was associated with RNP improvement.120–122 The observation that VEGF suppression is associated with improvement in RNP in patients with CRVO was confirmed in large multicenter trials investigating the effect of aflibercept.123 The mechanism of worsening RNP is VEGF-A-induced leukostasis and vessel closure from leukocytic plugging; VEGF-A suppression decreases leukostasis and allows previously plugged vessels to reperfuse.124
BRVO
BRVOs occur at arteriovenous crossing sites, predominantly those in which the artery passes over the vein (Figure 1).125,126 Postmortem eyes with BRVO showed fresh or recanalized thrombus within a retinal vein compressed by a retinal artery with a thickened wall crossing over the vein.87 As in CRVO, a narrowed and/or irregular lumen results in turbulent flow which perturbs endothelial cells. In addition to this indirect effect from altered vessel morphology, hypertension and atherosclerosis directly compromise the vascular endothelium. The luminal surface of compromised endothelial cells is less able to prevent thrombosis after minor injury from turbulent blood flow.
Similar to CRVO, variable amounts of RNP occur acutely depending upon the health of the arterial circulation, but even in eyes with substantial arterial disease the total amount of RNP is less than in most CRVO eyes because less retina is distal to the occlusion. The association of increased VEGF-A levels with RNP progression and the mechanism by which it occurs are the same as for CRVO.
Clinical Features of RVOs
CRVO
Figure 3 illustrates the variability in CRVO presentation. Some eyes show mild retinal venous dilation/tortuosity, small retinal hemorrhages, blockage from hemorrhages and good filling of retinal capillaries on FA, and moderate ME (Figure 3A–C). Other eyes show marked venous dilation/tortuosity with large retinal hemorrhages, cotton wool patches caused by infarcts in the nerve fiber layer, blockage from hemorrhages and large areas of RNP on FA, and severe ME (Figure 3D–F).
Figure 3: Variability at presentation in patients with central retinal vein occlusion (CRVO).
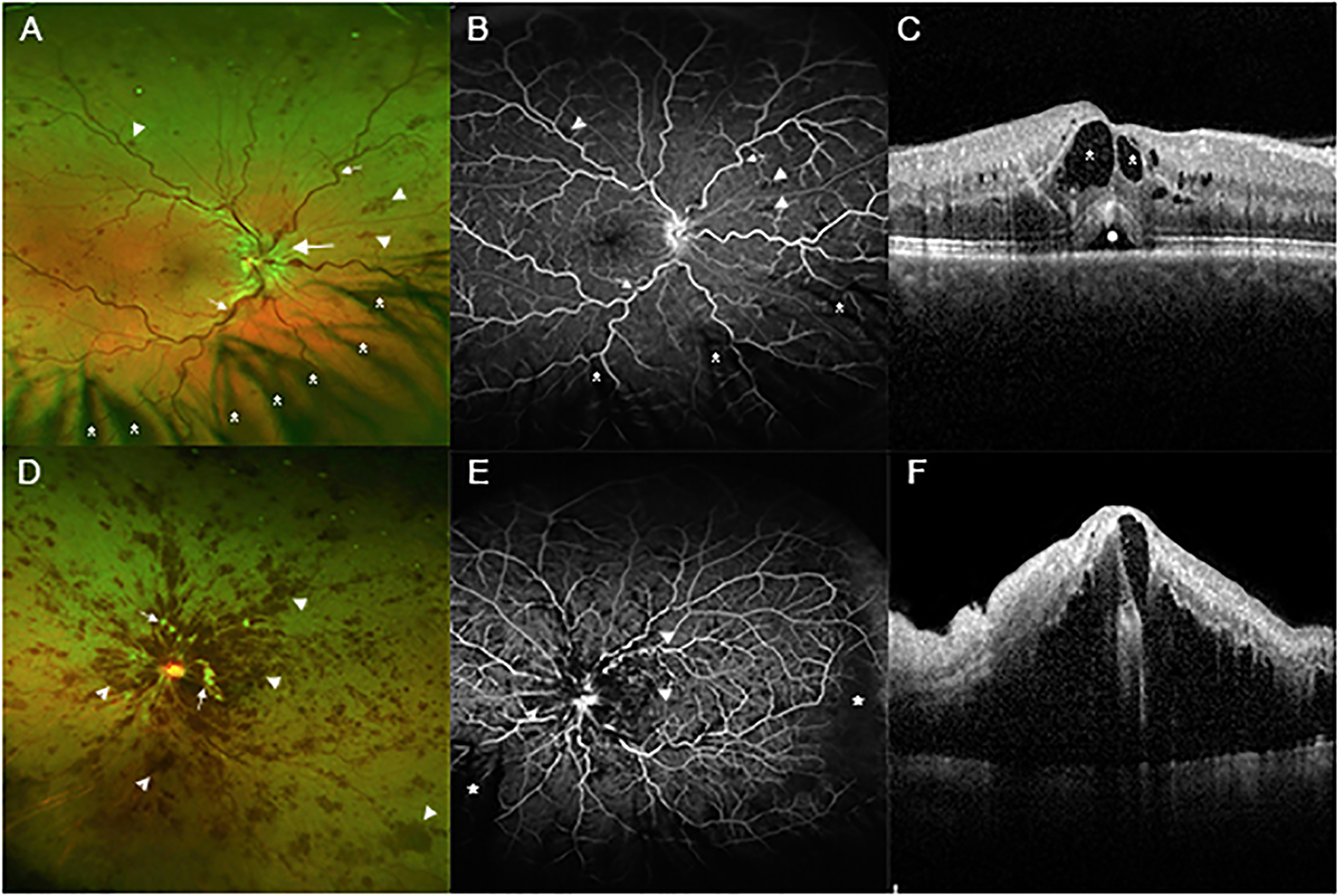
(A) A wide angle fundus photograph from a patient who presented with a mild CRVO shows dilated and tortuous retinal veins (small arrows), edema of the optic disc and surrounding retina (large arrow), and small hemorrhages scattered in all 4 quadrants of the retina (arrowheads). There is eye lash artifact inferiorly due to shadows cast on the retina from the eye lashes (asterisks). (B) Fluorescein angiography of same patient shows tortuous veins (small arrows) and blocked fluorescence from the small hemorrhages (arrowheads). The retinal capillaries are well-perfused throughout the posterior retina. Most of the dark areas inferiorly are due to shadowing from eye lashes (asterisks). (C) A spectral domain optical coherence tomography (SD-OCT) scan through the fovea shows intraretinal fluid (dark spaces within the retina, asterisks) and a small collection of fluid under the retina (circle). (D) A wide angle fundus photograph from a patient who presented with a moderately severe CRVO shows retinal hemorrhages (arrowheads) which are confluent in the posterior pole and scattered throughout the peripheral retina. There are several white cotton wool patches caused by infarcts in the nerve fiber layer (small arrows). (E) Fluorescein angiography shows blocked fluorescence from the retinal hemorrhages (arrowheads) and retinal nonperfusion (RNP) temporally and inferonasally (asterisks). (F) SD-OCT through the fovea shows massive edema.
BRVO
Some BRVO eyes show retinal hemorrhages, RNP, and ME that are mild (Figure 4A–C). Other eyes show marked venous dilation/tortuosity distal to the occlusion, extensive retinal hemorrhages and cotton wool patches, widespread RNP, and severe ME (Figure 4D–F).
Figure 4: Variability at presentation in patients with branch retinal vein occlusion (BRVO).
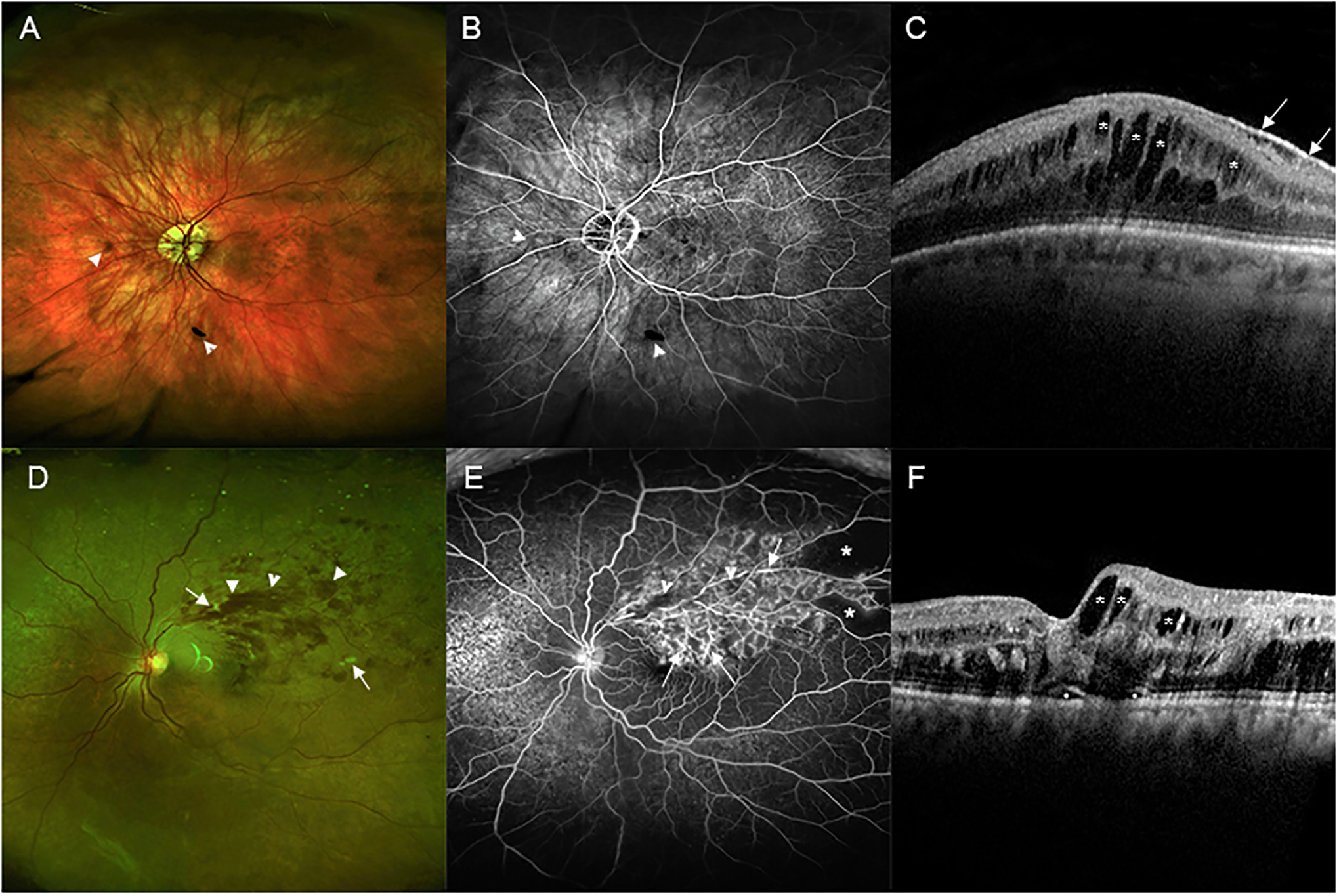
(A) A wide angle fundus photograph from a patient who presented with a mild BRVO shows only a few small hemorrhages (arrowheads). (B) Fluorescein angiography shows blocked fluorescence from hemorrhages (arrowheads) and good retinal perfusion. The dark area superiorly is sometimes seen at the edge of an image due to inadequate pupillary dilation and is not RNP. (C) A spectral domain optical coherence tomography (SD-OCT) scan through the fovea shows many dark cystoid spaces (asterisks). The white line along the surface is an epiretinal membrane that is incidental to the BRVO and is not visually significant (small arrows). (D) A wide angle fundus photograph from a patient who presented with a moderately severe BRVO shows retinal hemorrhages extending from the site of occlusion out to the periphery of the superotemporal retina (arrowheads). There are a few cotton wool patches (small arrows). (E) Fluorescein angiography shows mild blocked fluorescence from retinal hemorrhages (arrowheads) and staining of vessel walls (arrows) throughout the region drained by the occluded vein with RNP in the periphery (asterisks). (F) SD-OCT shows small areas (circles) of fluid under the fovea and cystoid spaces of intraretinal fluid (asterisks) that are larger temporal to the fovea than nasal to the fovea.
Natural History of RVOs
CRVO
The Central Vein Occlusion Study was designed to define the natural history of CRVO and evaluate scatter laser for ischemic CRVO and grid laser for ME.3,127–129 Eyes with ≥10 optic disc areas of RNP measured in 7 central photographic fields of FAs were defined as ischemic. Many initially perfused eyes, particularly those with best-corrected visual acuity (BCVA) <20/200, CRVO duration <1 month, and/or 5–9 optic disc areas of RNP, developed ≥10 optic disc areas of RNP and/or iris neovascularization within 4 months and there was progressive RNP throughout the 3-year trial in many eyes,127 which was also observed over 2 years in the control group of the Standard Care vs COrticosteroid for REtinal Vein Occlusion (SCORE)-CRVO trial.130 The SCORE-CRVO trial demonstrated spontaneous improvement in BCVA ≥15 letters in 6·8% of eyes with CRVO over 12 months, while 43·8% showed a reduction of ≥15 letters.130
Thus, eyes with CRVO present along a spectrum ranging from no RNP and good vision, which usually retain good vision, to severe RNP and poor vision, which are at high risk for iris neovascularization, neovascular glaucoma, and blindness. Eyes presenting with mild or moderate RNP are less predictable, but many show progressive RNP, loss of vision, and neovascular complications.
BRVO
Since half or less of the retina is affected by BRVO, neovascular glaucoma and severe vision loss are much less common than in CRVO. Retinal rather than iris neovascularization is more likely and its major vision-threatening complication is vitreous hemorrhage and occasionally traction retinal detachment. Macular edema is the most common cause of vision loss. The Branch Vein Occlusion Study was designed to determine whether panretinal photocoagulation could prevent retinal neovascularization and vitreous hemorrhage, and whether macular grid laser could benefit eyes with ME.131,132 At the 3-year endpoint, 37% of untreated eyes in the ME control group had improved ≥10 letters, 17% had decreased ≥10 letters, 34% had BCVA ≥20/40, and mean BCVA was 20/70. Thus, while some eyes improve spontaneously (roughly 2-fold higher percentage than in CRVO) and catastrophic vision loss is uncommon, only about one-third of eyes have visual outcome sufficient for reading and driving.
Diagnosis and Investigation of RVOs
CRVO and BRVO usually present as acute painless loss of vision in one eye. Some patients are unaware of the unilateral vision loss and, hence, delay seeking medical attention. Many patients become aware of the problem because of reduction in depth perception, which requires good vision in both eyes. Other patients become aware when, during activities of daily life, the unaffected eye is inadvertently obstructed. Occasionally patients fail to detect the problem for months and first become aware when a secondary complication occurs, such as pain in the affected eye from neovascular glaucoma (more likely in eyes with CRVO than those with BRVO).
Retina specialists/ophthalmologists who make a diagnosis of RVO should communicate with the patient’s primary care provider. Medical history/physical is important to evaluate for comorbidities, make sure they are optimally controlled, and screen for symptoms and signs that could indicate systemic illness associated with a hypercoagulable state (Table 4A). Laboratory testing is guided by history and physical findings, and if there are nonspecific findings, serum protein electrophoresis is indicated to rule out multiple myeloma. Even in the absence of an abnormal history or physical, there are some situations, such as bilateral RVO or RVO in a young patient (Table 4B), that warrant additional testing to screen for thrombophilia or other systemic conditions associated with a hypercoagulable state (Table 4A).
Table 4.
Systemic Diseases and Retinal Vein Occlusion (RVO)
| A. Systemic conditions associated with increased risk of RVO |
| Hypertension |
| Marked elevation of red or white blood cells, or platelets |
| Leukemia |
| Lymphoma |
| Polycythemia vera |
| Thrombocythemia |
| Marked elevation of plasma proteins |
| Multiple myeloma |
| Waldenström’s macroglobulinemia |
| Rheumatologic diseases |
| Rheumatoid arthritis |
| Systemic lupus erythematosus |
| Systemic sclerosis |
| Vasculitis |
| Sarcoidosis |
| Syphilis |
| Hypoxic states |
| Sleep apnea |
| Thrombophilias |
| Antiphospholipid antibodies |
| Cryoglobulinemia |
| Factor V Leiden |
| Hyperhomocysteinemia |
| Protein C deficiency |
| Protein S deficiency |
| Drugs |
| Oral or transdermal contraceptives |
| Diuretics |
| B. Factors that increase suspicion of systemic disease |
| Bilateral RVO |
| Lack of common risk factors |
| Hypertension, diabetes, glaucoma, dyslipidemia |
| Age <50 years |
| Symptom or sign of systemic disease |
Management of RVOs
VEGF-A plays a critical role in RVO pathogenesis,115–120 which is also the case for two other highly prevalent retinal diseases, neovascular age-related macular degeneration (AMD) and diabetic retinopathy, and the development of VEGF-A antagonists that can be injected into the eye has revolutionized the management of these conditions.133 Monthly injections for 6 months of ranibizumab or aflibercept, a recombinant protein containing the VEGF binding domains of VEGFR1 and VEGFR2 fused to an Fc domain,134 was associated with mean BCVA improvement >15 letters in eyes with BRVO or CRVO.116,117,135–137 This dramatic vision improvement occurred because ME was eliminated in most eyes. Bevacizumab is a full-length antibody directed against VEGF-A approved for use in oncology138 that was used off-label in RVO due to perceived efficacy and cost savings. The Study of COmparative Treatments for REtinal Vein Occlusion 2 (SCORE2) trial demonstrated non-inferiority of monthly injections of bevacizumab versus aflibercept with mean BCVA improvement >18 letters for each at the 6-month endpoint.139 In the Lucentis, Eylea, Avastin in Vein Occlusion (LEAVO) Study, aflibercept was non-inferior to ranibizumab at 100 weeks and bevacizumab was not non-inferior to ranibizumab.140 Thus, aflibercept and ranibizumab may be slightly better than bevacizumab for the long-term treatment of some patients with CRVO, but in general the three available anti-VEGF agents have excellent efficacy in RVO, and since bevacizumab is much less expensive, it is widely used at the outset of treatment. If outcomes are suboptimal with bevacizumab, a switch to aflibercept or ranibizumab is considered.
Contrary to expectations, approximately half of BRVO patients and 56–75% of CRVO patients still require anti-VEGF injections to control ME ≥5 years after starting treatment.141,142 Evaluation of patients over time demonstrated large improvements in BCVA during initial periods of frequent treatment, that declined when injection frequency was reduced.142 An alternative dosing approach, treat-and-extend, seeks to identify the longest period between injections without recurrent edema, and short-term outcomes with this strategy are good.143,144 SCORE2 investigators compared treat-and-extend with monthly injections and could not conclude treat-and-extend was non-inferior with regard to visual outcomes due to wide confidence intervals on the differences between groups,144 and long-term outcomes are unknown.
In some patients with CRVO or BRVO, monthly anti-VEGF injections for 3–6 months does not eliminate ME. A possible explanation is that multiple pro-permeability factors are upregulated in hypoxic retina, and while VEGF suppression is sufficient to eliminate edema in most patients, other factors may contribute enough in some patients to cause persistent edema despite VEGF suppression.145 Steroids reduce production of several pro-permeability factors, providing rationale for steroid-based treatments. In the SCORE trial, approximately 25% of eyes with CRVO or BRVO that received intravitreous injections of 1 or 4mg of triamcinolone acetonide every 4 months for 12 months improved ≥15 letters.130,146 This demonstrates benefit of steroid monotherapy in RVO, although it should be noted that in BRVO the benefit was similar to grid laser,146 and in both BRVO and CRVO, the benefit was less than the 45–70% of RVO eyes that improve ≥15 letters after monthly anti-VEGF injections.116,135–137,139 The dexamethasone intraocular implant, a sustained delivery formulation of dexamethasone, also showed benefit in RVO eyes.147 The dexamethasone implant was approved for injection every 6 months and with that regimen, outcomes have been inferior to prn ranibizumab.148–150 In clinical practice, is the dexamethasone implant is often given as frequently as every 3 months. Intraocular steroids promote cataract and may increase intraocular pressure, which has relegated them tosecond-line treatment, but they can be useful in RVO eyes that respond suboptimally to frequent anti-VEGF injections, particularly in patients who have already had cataract surgery and are not prone to steroid-induced glaucoma. There is no indication for thrombolytics in RVO and administration of anticoagulants is only recommended in patients with a known underlying thrombophilia.
Future research
In most patients with RVO, frequent intravitreous injections are needed for many years and it is difficult to maintain the visit and injection frequency needed for optimal outcomes.142 The situation is similar in patients with neovascular AMD and diabetic ME and, therefore, there is high motivation to develop more durable treatments. One approach is a surgically implanted refillable reservoir that slowly releases ranibizumab into the eye. In patients with neovascular AMD in which the reservoir was filled with 100 mg/ml ranibizumab, the median time to first refill was 15 months and visual outcomes were similar to monthly ranibizumab injections.151 A second approach also being tested in neovascular AMD is incorporation of a VEGF receptor antagonist into polymeric microparticles that provide sustained delivery after intravitreous injection (ClinicalTrials.gov Identifier: NCT03249740). A third approach is gene transfer to provide sustained expression of a VEGF-neutralizing protein in the eye. Proof-of-concept for this approach has been obtained with intravitreous injection of an AAV2 vector expressing modified soluble VEGFR1 which resulted in detectable expression of the therapeutic protein in the highest dose cohort and evidence of benefit in some patients.152 Compared with intravitreous injection, subretinal injection of AAV vectors provides much higher expression, and subretinal injection of an AAV8 vector expressing an anti-VEGF protein resulted in strong efficacy in models of VEGF-induced vascular leakage or neovascularization.153
Conclusion
Acute retinal vascular occlusions represent an important cause of visual loss. Although current diagnosis requires a detailed ocular examination, teleophthalmology and new technology such as artificial intelligence may allow immediate diagnosis when no eye care provider is available. Increased awareness from patients and healthcare providers will allow for prompt multidisciplinary management, which will hopefully translate into better visual outcomes. Thrombolysis in CRAO and various drug delivery approaches in RVOs are being evaluated with the goal of improving patients’ outcomes.
Acknowledgements
The authors thank Calla Head for preparing Figure 1 and Grace Tsai, MD for her assistance in preparation of Figures 3 and 4.
Declaration of interest
IUS has received research support from NIH/NEI grants 1U10EY023533-01 and 2UG1EY023533-06, and has served as a consultant for Allergan and on Data Safety and Monitoring Committees for Novartis and Thrombogenics.
PAC receives research support from NIH/NEI (R01 EB 016121), an unrestricted grant to the Wilmer Eye Institute from Research to Prevent Blindness (New York, NY), Aerpio Pharmaceuticals, Asclepix Therapeutics, Genentech/Roche Inc., Sanofi Genzyme, Graybug Vision, Novartis Pharmaceuticals Corporation, Oxford Biomedica, Perfuse, Regeneron Pharmaceuticals, Inc., and Regenexbio, Inc.
PAC serves as a consultant for Aerpio Pharmaceuticals, Allegro, Asclepix Therapeutics, Exonate, Ltd., Genentech/Roche, Inc. Graybug Vision, Merck &Co., Inc., Novartis Pharmacueticals Corporation, Perfuse, and Wave Life Sciences.
VB has received research support from NIH/NEI core grant P30-EY006360 (Department of Ophthalmology) and from NIH/PHS (UL1-RR025008). VB is a consultant for GenSight Biologics.
NJN has received research support from NIH/NEI core grant P30-EY006360 (Department of Ophthalmology) and from NIH/PHS (UL1-RR025008). NJN is a consultant for GenSight Biologics, Santhera and Stealth Pharmaceuticals. NJN is on the Data Monitoring and Safety Committee for Quark Pharmaceuticals
None of the authors have stocks, equity, contract of employment or named position on company boards.
References
- 1.Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology 2009; 116: 1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res 2011; 30: 359–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol 1997; 115: 486–91. [DOI] [PubMed] [Google Scholar]
- 4.Ponto KA, Scharrer I, Binder H, et al. Hypertension and multiple cardiovascular risk factors increase the risk for retinal vein occlusions: results from the Gutenberg Retina lVein Occlusion Study J Hypertension 2019; 37: 1372–83. [DOI] [PubMed] [Google Scholar]
- 5.Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia. Follow the guidelines! Ophthalmology 2018; 125: 1597–607. [DOI] [PubMed] [Google Scholar]
- 6.Sharma RA, Dattilo M, Newman NJ, Biousse V. Treatment of nonarteritic acute central retinal artery occlusion. Asia Pac J Ophthalmol (Phila) 2018; 7: 235–41. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, Midena E, Sivaprasad S, Tadayoni R, Wolf S, Loewenstein A. Guidelines for the management of retinal vein occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2019; 242: 123–62. [DOI] [PubMed] [Google Scholar]
- 8.Amarenco P Transient Ischemic Attack. N Engl J Med 2020; 382: 1933–41. [DOI] [PubMed] [Google Scholar]
- 9.Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol 1999; 128: 733–8. [DOI] [PubMed] [Google Scholar]
- 10.Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol 2011; 152: 820–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SJ, Choi NK, Seo KH, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology 2014; 121: 1933–8. [DOI] [PubMed] [Google Scholar]
- 12.Park SW, Woo SJ, Park KH, Huh JW, Jung C, Kwon OK. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol 2012; 154: 653–62. [DOI] [PubMed] [Google Scholar]
- 13.Chatrath V, Banerjee PS, Goodman GJ, Rahman E. Soft-tissue filler-associated blindness: A systematic review of case reports and case series. Plast Reconstr Surg Glob Open 2019; 7: e2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt D, Hetzel A, Geibel-Zehender A, Schulte-Monting J. Systemic diseases in non-inflammatory branch and central retinal artery occlusion--an overview of 416 patients. Eur J Med Res 2007; 12: 595–603. [PubMed] [Google Scholar]
- 15.Chang YS, Jan RL, Weng SF, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol 2012; 154: 645–652. [DOI] [PubMed] [Google Scholar]
- 16.Chang YS, Chu CC, Weng SF, Chang C, Wang JJ, Jan RL. The risk of acute coronary syndrome after retinal artery occlusion: a population-based cohort study. Br J Ophthalmol 2015; 99: 227–231. [DOI] [PubMed] [Google Scholar]
- 17.Park SJ, Choi NK, Yang BR, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology 2015; 122: 2336–43. [DOI] [PubMed] [Google Scholar]
- 18.Callizo J, Feltgen N, Pantenburg S, et al. ; European Assessment Group for Lysis in the Eye. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination. Ophthalmology 2015; 122: 1881–8. [DOI] [PubMed] [Google Scholar]
- 19.Rim TH, Han J, Choi YS, et al. Retinal artery occlusion and the risk of stroke development: Twelve-year nationwide cohort study. Stroke 2016; 47: 376–82. [DOI] [PubMed] [Google Scholar]
- 20.Hong JH, Sohn SI, Kwak J, et al. Retinal artery occlusion and associated recurrent vascular risk with underlying etiologies. PLoS One 2017; 12: e0177663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavin P, Patrylo M, Hollar M, Espaillat KB, Kirshner H, Schrag M. Stroke risk and risk factors in patients with central retinal artery occlusion. Am J Ophthalmol 2019; 200: 271–2 [DOI] [PubMed] [Google Scholar]
- 22.French DD, Margo CE, Greenberg PB. Ischemic stroke risk in Medicare beneficiaries with central retinal artery occlusion: A retrospective cohort study. Ophthalmol Ther 2018; 7: 125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christiansen CB, Torp-Pedersen C, Olesen JB, et al. Risk of incident atrial fibrillation in patients presenting with retinal artery or vein occlusion: a nationwide cohort study. BMC Cardiovasc Disord 2018; 18: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avery MB, Magal I, Kherani A, Mitha AP. Risk of stroke in patients with ocular arterial occlusive disorders: A retrospective canadian study. J Am Heart Assoc 2019; 8: e010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mir TA, Arham AZ, Fang W, et al. Acute vascular ischemic events in patients with central retinal artery occlusion in the United States: A Nationwide Study 2003–2014. Am J Ophthalmol 2019; 200: 179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chodnicki KD, Pulido JS, Hodge DO, Klaas JP, Chen JJ. Stroke risk before and after central retinal artery occlusion in a US cohort. Mayo Clin Proc 2019; 94: 236–41. [DOI] [PubMed] [Google Scholar]
- 27.Kang EY, Lin YH, Wang NK, et al. Aspirin use in central retinal arterial occlusion to prevent ischaemic stroke: a retrospective cohort study in Taiwan. BMJ Open 2019; 9: e025455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarkali A, Cheng SF, Dados A, Simister R, Chandratheva A. Atrial fibrillation: An underestimated cause of ischemic monocular visual loss? J Stroke Cerebrovasc 2019; 28: 1495–9. [DOI] [PubMed] [Google Scholar]
- 29.Schorr EM, Rossi KC, Stein LK, Park BL, Tuhrim S, Dhamoon MS. Characteristics and outcomes of retinal artery occlusion: Nationally representative data. Stroke 2020; 51: 800–7. [DOI] [PubMed] [Google Scholar]
- 30.Cho KH, Kim CK, Woo SJ, Park KH, Park SJ. Cerebral small vessel disease in branch retinal artery occlusion. Invest Ophthalmol Vis Sci 2016; 57: 5818–24. [DOI] [PubMed] [Google Scholar]
- 31.Golsari A, Bittersohl D, Cheng B, et al. Silent brain infarctions and leukoaraiosis in patients with retinal ischemia: A prospective single-center observational study. Stroke 2017; 48: 1392–6. [DOI] [PubMed] [Google Scholar]
- 32.Jetty P, Husereau D, Kubelik D et al. Wait times among patients with symptomatic carotid artery stenosis requiring carotid endarterectomy for stroke prevention. J Vasc Surg 2012; 56: 661–7. [DOI] [PubMed] [Google Scholar]
- 33.Brott TG, Halperin JL, Abbara S, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Stroke Association; American Association of Neuroscience Nurses; American Association of Neurological Surgeons; American College of Radiology; American Society of Neuroradiology; Congress of Neurological Surgeons; Society of Atherosclerosis Imaging and Prevention; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of NeuroInterventional Surgery; Society for Vascular Medicine; Society for Vascular Surgery. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/ SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Vasc Med 2011; 16: 35–77 [DOI] [PubMed] [Google Scholar]
- 34.Johansson E, Cuadrado-Godia E, Hayden D, et al. Recurrent stroke in symptomatic carotid stenosis awaiting revascularization: A pooled analysis. Neurology 2016; 86: 498–504. [DOI] [PubMed] [Google Scholar]
- 35.Gocan S, Bourgoin A, Blacquiere D, Shamloul R, Dowlatshahi D, Stotts G. Fast-track systems improve timely carotid endarterectomy in stroke prevention outpatients. Can J Neurol Sci 2016; 43: 648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng SF, Zarkali A, Richards T, Simister R, Chandratheva A. Carotid artery stenosis, an underestimated cause of stroke recurrence in patients with ischaemic monocular visual loss. Ann R Coll Surg Engl 2019. Jun 3:1–5. 10.1308/rcsann.2019.0071. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston SC, Albers GW, Gorelick PB, et al. National Stroke Association recommendations for systems of care for transient ischemic attack. Ann Neurol. 2011; 69: 872–7. [DOI] [PubMed] [Google Scholar]
- 38.Furie KL, Kasner SE, Adams RJ, et al. ; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 227–76. [DOI] [PubMed] [Google Scholar]
- 39.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 40.Yen JC, Lin HL, Hsu CA, Li YC, Hsu MH. Atrial fibrillation and coronary artery disease as risk factors of retinal artery occlusion: A nationwide population-based study. Biomed Res Int 2015; 2015: 374616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callizo J, Feltgen N, Ammermann A, et al. Atrial fibrillation in retinal vascular occlusion disease and non-arteritic anterior ischemic optic neuropathy. PLoS One 2017; 12: e0181766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kewcharoen J, Tom ES, Wiboonchutikula C, et al. Prevalence of atrial fibrillation in patients with retinal vessel occlusion and its association: A systematic review and meta-analysis. Curr Eye Res 2019. Jul 29:1–8. doi: 10.1080/02713683.2019.1641826. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Helenius J, Arsava EM, Goldstein JN, et al. Concurrent acute brain infarcts in patients with monocular visual loss Ann Neurol. 2012; 72: 286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J, Kim SW, Lee SC, Kwon OW, Kim YD, Byeon SH. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion weighted magnetic resonance imaging study. Am J Ophthalmol 2014; 157: 1231–8. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka K, Uehara T, Kimura K, et al. ; Japan TIA Research Group 2009–2011. Features of patients with transient monocular blindness: a multicenter retrospective study in Japan. J Stroke Cerebrovasc Dis 2014; 23: e151–5. [DOI] [PubMed] [Google Scholar]
- 46.Lauda F, Neugebauer H, Reiber L, Jüttler E. Acute silent brain infarction in monocular visual loss of ischemic origin. Cerebrovasc Dis 2015; 40: 151–6. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka K, Uehara T, Kimura K, et al. ; PROMISE-TIA study Investigators. Comparison of clinical characteristics among subtypes of visual symptoms in patients with transient ischemic attack: analysis of the PROspective Multicenter registry to identify subsequent cardiovascular events after TIA (PROMISE-TIA) registry. J Stroke Cerebrovasc Dis 2018; 27: 1711–6 [DOI] [PubMed] [Google Scholar]
- 48.Zhang LY, Zhang J, Kim RK, et al. Risk of acute ischemic stroke in patients with monocular vision loss of vascular etiology. J Neuroophthalmol 2018; 38: 328–33. [DOI] [PubMed] [Google Scholar]
- 49.Valls J, Peiro-Chamarro M, Cambray S, Molina-Seguin J, Benabdelhak I, Purroy F. A current estimation of the early risk of stroke after transient ischemic attack: A systematic review and meta-analysis of recent studies. Cerebrovasc Dis 2017; 43: 90–8. [DOI] [PubMed] [Google Scholar]
- 50.Kelly PJ, Albers GW, Chatzikonstantinou A, et al. Validation and comparison of imaging-based scores for prediction of early stroke risk after transient ischaemic attack: a pooled analysis of individual-patient data from cohort studies. Lancet Neurol 2016; 15: 1238–47. [DOI] [PubMed] [Google Scholar]
- 51.Youn TS, Lavin P, Patrylo M, Schindler J, Kirshner H, Greer DM, Schrag M. Current treatment for central retinal artery occlusion: a national survey. J Neurol 2018; 265: 330–5. [DOI] [PubMed] [Google Scholar]
- 52.Zarkali A, Cheng SF, Dados A, Simister R, Chandratheva A. Undertreatment of vascular risk factors in patients with monocular ischaemic visual loss. Cerebrovasc Dis 2018; 45: 228–35. [DOI] [PubMed] [Google Scholar]
- 53.Chandratheva A, Lasserson DS, Geraghty OC, Rothwell PM; Oxford Vascular Study. Population-based study of behavior immediately after transient ischemic attack and minor stroke in 1000 consecutive patients: lessons for public education. Stroke 2010; 41: 1108–14. [DOI] [PubMed] [Google Scholar]
- 54.Sprigg N, Machili C, Otter ME, Wilson A, Robinson TG. A systematic review of delays in seeking medical attention after transient ischaemic attack. J Neurol Neurosurg Psychiatry 2009; 80: 871–5. [DOI] [PubMed] [Google Scholar]
- 55.Hurst K, Lee R, Sideso E, Giles M, Handa A. Delays in the presentation to stroke services of patients with transient ischaemic attack and minor stroke. Br J Surg 2016; 103: 1462–6. [DOI] [PubMed] [Google Scholar]
- 56.Naylor AR, Robinson TG, Eveson D, Burns J. An audit of management practices in patients with suspected temporary monocular blindness. Br J Ophthalmol 2014; 98: 730–3. [DOI] [PubMed] [Google Scholar]
- 57.Kvickström P, Lindblom B, Bergström G, Zetterberg M. Amaurosis fugax – delay between symptoms and surgery by specialty. Clin Ophthalmol 2016; 10: 2291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kvickström P, Lindblom B, Bergström G, Zetterberg M. Amaurosis fugax: risk factors and prevalence of significant carotid stenosis. Clin Ophthalmol 2016; 10: 2165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subramanian PS. How urgent is the treatment of transient visual loss? Br J Ophthalmol 2014; 98: 719–20. [DOI] [PubMed] [Google Scholar]
- 60.Biousse V Acute retinal arterial ischemia: an emergency often ignored. Am J Ophthalmol 2014; 157: 1119–21. [DOI] [PubMed] [Google Scholar]
- 61.Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015;14: 377–87. [DOI] [PubMed] [Google Scholar]
- 62.Brachmann J, Morillo CA, Sanna T, et al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: Three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol 2016; 9: e003333. [DOI] [PubMed] [Google Scholar]
- 63.Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res 2014; 41: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biousse V, Bruce BB, Newman NJ. Ophthalmoscopy in the 21st century: The 2017 H. Houston Merritt Lecture. Neurology 2018; 90: 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vasseneix C, Bruce BB, Bidot S, Newman NJ, Biousse V. Nonmydriatic fundus photography in patients with acute vision loss. Telemed J E Health 2018. Dec 20. doi: 10.1089/tmj.2018.0209. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dumitrascu OM, Newman NJ, Biousse V. Thrombolysis for Central Retinal Artery Occlusion in 2020: Time is Vision! J Neuro-ophthalmol 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hellmich B, Agueda A, Monti S, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2019. Jul 3. pii: annrheumdis-2019–215672. doi: 10.1136/annrheumdis-2019-215672.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 68.Mac Grory B, Lavin P, Kirshner H, Schrag M. Thrombolytic therapy for acute central retinal artery occlusion. Stroke. 2020; 51: 687–95. [DOI] [PubMed] [Google Scholar]
- 69.Rothwell PM, Giles MF, Chandratheva A, et al. ; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370: 1432–42. [DOI] [PubMed] [Google Scholar]
- 70.Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic withround-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol 2007; 6: 953–60. [DOI] [PubMed] [Google Scholar]
- 71.Nedelmann M, Graef M, Weinand F, et al. Retrobulbar spot sign predicts thrombolytic treatment effects and etiology in central retinal artery occlusion. Stroke 2015; 46: 2322–4. [DOI] [PubMed] [Google Scholar]
- 72.Schrag M, Youn T, Schindler J, Kirshner H, Greer D. Intravenous fibrinolytic therapy in central retinal artery occlusion: A patient-level meta-analysis. JAMA Neurol 2015; 72: 1148–54. [DOI] [PubMed] [Google Scholar]
- 73.Dumitrascu OM, Shen JF, Kurli M, et al. Is intravenous thrombolysis safe and effective in central retinal artery occlusion? A critically appraised topic. Neurologist 2017; 22: 153–6. [DOI] [PubMed] [Google Scholar]
- 74.Préterre C, Godeneche G, Vandamme X, et al. Management of acute central retinal artery occlusion: Intravenous thrombolysis is feasible and safe. Int J Stroke 2017; 12: 720–3. [DOI] [PubMed] [Google Scholar]
- 75.Schultheiss M, Härtig F, Spitzer MS, et al. Intravenous thrombolysis in acute central retinal artery occlusion - A prospective interventional case series. PLoS One 2018; 13: e0198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen CS, Lee AW, Campbell B, Lee T, Paine M, Fraser C, Grigg J, Markus R. Efficacy of intravenous tissue-type plasminogen activator in central retinal artery occlusion: report from a randomized, controlled trial. Stroke 2011; 42: 2229–34. [DOI] [PubMed] [Google Scholar]
- 77.Schumacher M, Schmidt D, Jurklies B, et al. ; EAGLE-Study Group. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology 2010; 117: 1367–75. [DOI] [PubMed] [Google Scholar]
- 78.Hattenbach LO, Kuhli-Hattenbach C, Scharrer I, Baatz H. Intravenous thrombolysis with low-dose recombinant tissue plasminogen activator in central retinal artery occlusion. Am J Ophthalmol 2008; 146: 700–6. [DOI] [PubMed] [Google Scholar]
- 79.Page PS, Khattar NK, White AC, Cambon AC, Brock GN, Rai SN, James RF. Intra-arterial thrombolysis for acute central retinal artery occlusion: A systematic review and meta-analysis. Front Neurol 2018; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aldrich EM, Lee AW, Chen CS, et al. Local intraarterial fibrinolysis administered in aliquots for the treatment of central retinal artery occlusion: the Johns Hopkins Hospital experience. Stroke 2008; 39: 1746–50. [DOI] [PubMed] [Google Scholar]
- 81.Ting DSW, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, Tan GSW, Schmetterer L, Keane PA, Wong TY. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol 2019; 103: 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Korot E, Wood E, Weiner A, Sim DA, Trese M. A renaissance of teleophthalmology through artificial intelligence. Eye (Lond) 2019; 33: 861–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawlor M, Perry R, Plant GT. Is the ‘Act FAST’ stroke campaign lobbyist? The implications of including symptoms of occipital lobe and eye stroke in public education campaigns. J Neurol Neurosurg Psychiatry 2015; 86: 818–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uhr JH, Mishra K, Wei C, Wu AY. Awareness and Knowledge of Emergent Ophthalmic Disease Among Patients in an Internal Medicine Clinic. JAMA Ophthalmol 2016; 134: 424–3. [DOI] [PubMed] [Google Scholar]
- 85.Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. Stroke 2017; 48: 479–81. [DOI] [PubMed] [Google Scholar]
- 86.Green WR, Chan CC, Hutchins GM, Terry JM. Central vein occlusion: a prospective histological study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc 1981; 79: 371–421. [PMC free article] [PubMed] [Google Scholar]
- 87.Frangieh GT, Green WR, Barraquer-Somers E, Finkelstein D. Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol 1982; 100: 1132–40. [DOI] [PubMed] [Google Scholar]
- 88.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc 2000; 98: 133–41. [PMC free article] [PubMed] [Google Scholar]
- 89.Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol 2008; 126: 513–8. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. Arch Ophthalmol 1996; 114: 1243–7. [DOI] [PubMed] [Google Scholar]
- 91.Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol 2006; 124: 726–32. [DOI] [PubMed] [Google Scholar]
- 92.Rogers S, McIntosh RL, Journ BGD, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010; 117: 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barnett EM, Fantin A, Wilson BS, Kass MA, Gordon MO, Group ftOHTS. The incidence of retinal vein occlusion in the ocular hypertension treatment study. Ophthalmology 2010; 117: 484–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arakawa S, Yasuda M, Nagata M, et al. Nine-year incidence and risk factors for retinal vein occlusion in a general Japanes population: the Hisayama Study. Invest Ophthalmol Vis Sci 2011; 52: 5905–9. [DOI] [PubMed] [Google Scholar]
- 95.Koh V, Cheung CY, Li X, et al. Retinal vein occlusion in a multi-ethnic asian population: the Singapore Epidemiology of Eye Disease Study. Ophthalmic Epidemiol 2016; 23: 6–13. [DOI] [PubMed] [Google Scholar]
- 96.Klein BE, Meuer SM, Knudtson MD, Klein R. The relationship of optic disk cupping to retinal vein occlusion: The Beaver Dam Eye Study. Am J Ophthalmol 2006; 141: 859–62. [DOI] [PubMed] [Google Scholar]
- 97.Keel S, Xie J, Forman J, van Wijngaarden P, Taylor HR, Dirani M. Prevalence of retinal vein occlusion in the Australian National Eye Health Survey. Clin & Exp Ophthalmol 2018; 46: 260–5. [DOI] [PubMed] [Google Scholar]
- 98.Eye Disease Case-Control Study Group. Risk factors for branch retinal vein occlusion. Am J Ophthalmol 1993; 116: 286–96. [PubMed] [Google Scholar]
- 99.Bertelsen M, Linneberg A, Christoffersen N, Vorum H, Gade E, Larsen M. Mortality in patients with central retinal vein occlusion. Ophthalmology 2014; 121: 637–42. [DOI] [PubMed] [Google Scholar]
- 100.Taylor AW, Sehu W, Williamson TH, Lee WR. Morphometric assessment of the central retinal artery and vein in the optic nerve head. Can J Ophthalmol 1993; 28: 320–4. [PubMed] [Google Scholar]
- 101.Williamson TH. A “throttle” mechanism in the central retinal vein in the region of the lamina cribrosa. Br J Ophthalmol 2007; 91: 1190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takusagawa HL, Hoguet A, Junk AK, Nouri-Hahdavi K, Radhakrishnan S, Chen TC. Swept-source OCT for evaluating the lamina cribrosa. Ophthalmology 2019; 126: 1315–23. [DOI] [PubMed] [Google Scholar]
- 103.Tan NYQ, Tham Y-C, Thakku SG, et al. Changes in the anterior lamina cribrosa morphology with glaucoma severity. Sci Reports 2019; 9: 6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang MH, Galaratnasingam C, Yu PK, et al. Alterations to vascular endothelium in the optic nerve head in patients with vascular comorbidites. Exp Eye Res 2013; 111: 50–60. [DOI] [PubMed] [Google Scholar]
- 105.Janssen MC, den Heijer M, Cruysberg JR, Wollersheim H, Bredie SJ. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost 2005; 93: 1021–6. [DOI] [PubMed] [Google Scholar]
- 106.Hayreh SS. Classification of central reinal vein occlusion. Ophthalmology 1983; 90: 458–74. [DOI] [PubMed] [Google Scholar]
- 107.Hayreh SS, Rojas P, Podhajsky P, Montague P, Wollson RF. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology 1983; 90: 488–506. [DOI] [PubMed] [Google Scholar]
- 108.Zegarra H, Gutman F, Zakov N, Carim M. Partial occlusion of the central retinal vein. Am J Ophthalmol 1983; 96: 330–7. [DOI] [PubMed] [Google Scholar]
- 109.Lonn LI, Hoyt WF. papillophlebitis: a cause of protracted yet benign optic disc edema. Eye Ear Nose Throat Mon 1966; 45: 62. [PubMed] [Google Scholar]
- 110.Vandervliet SK. Papillophlebitis associated with systemic thrombophlebitis: case report. Ann Ophthalmol 1977; 6: 171–4. [PubMed] [Google Scholar]
- 111.Hart CD, Sanders MD, Miller SJH. Benign retinal vasculitis. Br J Ophthalmol 1971; 55: 721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Appen RE, DeVenecia G, Ferwerda J. Optic disc vasculitis. Am J Ophthalmol 1980; 90: 352–9. [DOI] [PubMed] [Google Scholar]
- 113.Fong ACO, Schatz H. Central retinal vein occlusion in young adults. Surv Ophthalmol 1993; 37: 393–417. [DOI] [PubMed] [Google Scholar]
- 114.Hayreh SS, van Heuven WAJ, Hayreh MS. Experimental retinal vascular occlusion. I. Pathogenesis of central retinal vein occlusion. Arch Ophthalmol 1978; 96: 311–23. [DOI] [PubMed] [Google Scholar]
- 115.Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions; implication of VEGF as a critical stimulator. Molec Ther 2008; 16: 791–9. [DOI] [PubMed] [Google Scholar]
- 116.Brown DM, Campochiaro PA, Singh RP, et al. Efficacy and safety of ranibizumab in the treatment of macular edema secondary to central retinal vein occlusion:6-month results of the phase III CRUISE study. Ophthalmology 2010; 117: 1124–33. [DOI] [PubMed] [Google Scholar]
- 117.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: 6-month primary endpoint results of a phase III study. Ophthalmology 2010; 117: 1102–12. [DOI] [PubMed] [Google Scholar]
- 118.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011; 118: 2041–9. [DOI] [PubMed] [Google Scholar]
- 119.Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011; 118: 1594–602. [DOI] [PubMed] [Google Scholar]
- 120.Campochiaro PA, Bhistikul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 2013; 120: 795–802. [DOI] [PubMed] [Google Scholar]
- 121.Sophie R, Hafiz G, Scott A, et al. Long term outcomes in ranibizumab-treated patients with retinal vein occlusion; the role of progression of retinal nonperfusion. Am J Ophthalmol 2013; 156: 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mir TA, Kherani S, Hafiz G, et al. Changes in retinal nonperfusion associated with suppression of vascular endothelial growth factor in retinal vein occlusion. Ophthalmology 2016; 123: 625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feltgen N, Ogura Y, Boscia F, et al. Impact of baseline retinal nonperfusion and macular retinal capillary nonperfusion on outcomes in the COPERNICUS and GALILEO studies. Ophthalmol Retina 2019; 3: 553–60. [DOI] [PubMed] [Google Scholar]
- 124.Liu Y, Shen J, Fortmann SD, Wang J, Vestweber D, Campochiaro PA. Reversible retinal vessel closure from VEGF-inudce leukocyte plugging. JCI Insight 2017; 2: pii: 95530. doi: 10.1172/jci.insight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.The Seitz R. ‘crossing phenomenon’. In: The Retinal Vessels. St. Louis: Mosby, 1964: 20–74. [Google Scholar]
- 126.Zhao J, Sastry SM, Sperduto RD, Chew EY, Remaley NA. Arteriovenous crossing patterns in branch retinal vein occlusion: The Eye Disease Case-Control Study Group. Ophthalmology 1993; 100: 423–8. [DOI] [PubMed] [Google Scholar]
- 127.Central Vein Occlusion Study Group. Baseline and early natural history report. The Central Vein Occlusion Study. Arch Ophthalmol 1993; 111: 1087–95. [DOI] [PubMed] [Google Scholar]
- 128.Central Vein Occlusion Study Group. Photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology 1995; 102: 1434–44. [PubMed] [Google Scholar]
- 129.Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The central vein occlusion study group M report. Ophthalmology 1995; 102: 1425–33. [DOI] [PubMed] [Google Scholar]
- 130.Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion. The Standard care vs COrticosteroid for REtinal vein occlusion (SCORE) study report 5. Arch Ophthalmol 2009; 127: 1101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol 1984; 98: 271–82. [DOI] [PubMed] [Google Scholar]
- 132.Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Arch Ophthalmol 1986; 104: 34–41. [DOI] [PubMed] [Google Scholar]
- 133.Campochiaro PA, Aiello LP, Rosenfeld PJ. Anti-vascular endothelial growth factor agents in the treatment of retinal disease. From bench to bedside. Ophthalmology 2016; 123: S78–S88. [DOI] [PubMed] [Google Scholar]
- 134.Holash J, Davis S, Papadoupoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 2002; 99: 11393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor trap-eye for macular edema secondary to central retinal vein occlusion. Six-month results of the phase 3 COPERNICUS study. Ophthalmolgy 2012; 119: 1024–32. [DOI] [PubMed] [Google Scholar]
- 136.Holz FG, Roider J, Ogura Y, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the pahse III GALILEO study. Br J Ophthalmol 2013; 97: 278–84. [DOI] [PubMed] [Google Scholar]
- 137.Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch reitnal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology 2015; 122: 538–44. [DOI] [PubMed] [Google Scholar]
- 138.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391–400. [DOI] [PubMed] [Google Scholar]
- 139.Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA 2017; 317: 2072–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hykin P, Prevost T, Vasconcelos JC, et al. Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion. A randomized clinical trial. JAMA Ophthalmol 2019; 137: 1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: The RETAIN Study. Ophthalmology 2014; 121: 209–19. [DOI] [PubMed] [Google Scholar]
- 142.Iftikhar M, M TA, Hafiz G, et al. Loss of peak vision in retina vein occlusion patients treated for macular edema. Amer J Ophthalmol 2019; 205:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rahimy E, Rayess N, Brady CJ, Regillo CD. Treat-and-extend regimane for macular edema secondary to central retinal vein occlusion: 12 month results. Ophthalmol Retina 2017; 1: 118–23. [DOI] [PubMed] [Google Scholar]
- 144.Scott IU, VanVeldhuisen PC, Ip MS, et al. Comparison of monthly vs treat-and-extend regimens for individuals with macular edema who respond well to anti-vascular endothelial growth factor medications: secondary outcomes from the SCORE2 randomized clinical trial. JAMA Ophthalmol 2018; 136: 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Campochiaro PA, Hafiz G, Mir TA, et al. Pro-permeability factors after dexamethasone implant in retinal vein occlusion; the Ozurdex for retinal vein occlusion (ORVO) study. Am J Ophthalmol 2015; 160: 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion. The Standard Care vs COrticosteroid for REtinal vein occlusion (SCORE) study report 6. Arch Ophthalmol 2009; 127: 1115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haller JA, Bandello F, Belfort RJ, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-months study results. Ophthalmology 2011; 118: 2453–60. [DOI] [PubMed] [Google Scholar]
- 148.Hattenbach L-O, Feltgen N, Bertelmann T, et al. Head-to-head comparison of ranibizumab PRN versus single-dose dexamethasone for branch retinal vein occlusion (COMRADE-B). Acta Ophthalmol (Copenh) 2018; 96: e10–8. [DOI] [PubMed] [Google Scholar]
- 149.Hoerauf H, Feltgen N, Weiss C, et al. Clinical Efficacy and Safety of Ranibizumab Versus Dexamethasone for Central Retinal Vein Occlusion (COMRADE C): A European Label Study. Am J Ophthalmol 2016; 169: 258–67. [DOI] [PubMed] [Google Scholar]
- 150.Bandello F, Augustin A, Tufail A, Leaback R. A 12-month, multicenter, parallel group comparison of dexamethasone intravitreal implant versus ranibizumab in branch retinal vein occlusion. Eur J Ophthalmol 2018; 28: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 Ladder clinical trial. Ophthalmology 2019; In press. [DOI] [PubMed] [Google Scholar]
- 152.Heier JS, Kherani S, Desai S, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. The Lancet 2017; 389: 50–61. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y, Fortmann SD, Shen J, et al. AAV8-antiVEGFfab ocular gene transfer for neovascular age-related macular degeneration. Mol Ther 2017; 26: 542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


