Abstract
Objective
This investigation was conducted to analyze and evaluate the impact of Chinese herbal medicine on glucolipid metabolism in women with polycystic ovary syndrome (PCOS).
Methods
We used manual and computer-aided search methods, and the search scopes included Chinese databases (China National Knowledge Infrastructure, Wanfang, the China Science and Technology Journal Database, and the Chinese Biomedical Literature Database) and English databases (PubMed, Embase, Web of Science, and the Cochrane Library). We searched these eight databases for randomized controlled trials investigating the effects of Chinese herbal medicine on glucolipid metabolism in women with PCOS, with the retrieval deadline being June 2021. Two reviewers screened, selected, and extracted data and verified the results independently. The NoteExpress software was used to manage and screen the literature, the risk of bias assessment tool was used to evaluate the methodological quality of the included studies, and the RevMan 5.4 software was used for meta-analysis.
Results
A total of 13 trials were included, including 825 patients with PCOS. Because the drugs used in the control group were different, we divided the results into two parts, with four trials using placebo and nine trials using metformin as the control. The results of the meta-analysis showed that fasting insulin (MD = −2.45, 95% CI = [−4.74, −0.17], P = 0.04), 2 h fasting plasma glucose (MD = −0.33, 95% CI = [−0.64, −0.02], P = 0.04), serum total cholesterol (MD = −0.38, 95% CI = [−0.58, −0.18], P = 0.0002), triglycerides (MD = −0.36, 95% CI = [−0.58, −0.14], P = 0.001), and low-density lipoprotein cholesterol (MD = −0.58, 95% CI = [−0.75, −0.41], P < 0.00001) were significantly improved in the Chinese herbal medicine group compared with the placebo group. In addition, compared with metformin, body mass index (MD = −1.04, 95% CI = [−1.55, −0.53], P < 0.0001), serum total cholesterol (MD = −0.27, 95% CI = [−0.46, −0.07] P = 0.007), and low-density lipoprotein cholesterol were significantly reduced (MD = −0.12, 95% CI = [−0.22, −0.02], P = 0.02) and high-density lipoprotein cholesterol (MD = 0.09, 95% CI = [0.02, 0.17], P = 0.01) was significantly improved after treatment with Chinese herbal medicine.
Conclusion
Compared with the placebo group, Chinese herbal medicine had positive effects on glucolipid metabolism in women with PCOS. Chinese herbal medicine had a positive effect on lipid metabolism when the control group was metformin, but no effect on glucose metabolism. These findings need to be verified in high-quality, large-sample, randomized controlled trials in the future.
1. Introduction
Polycystic ovary syndrome (PCOS) is a complex and heterogeneous endocrine and metabolic disorder in reproductive-age women [1]. PCOS commonly manifests as ovulatory dysfunction, elevated androgen levels, polycystic ovaries, insulin resistance (IR), and obesity. Studies have shown that hyperandrogenism and IR are the core etiology and main endocrine features of PCOS [2]. IR not only affects the reproductive function of PCOS patients, but also significantly increases the risk of chronic metabolic diseases—such as hyperlipidemia, hyperglycemia, cardiovascular disease, and type2 diabetes [3]—and metabolic disorders are considered to be the most important long-term concerns related to PCOS [4, 5]. In addition, the healthcare system bears a huge burden for treating the direct and indirect diseases related to IR [6]. Thus, IR has become the main focus of treatment for patients with PCOS.
There are no effective curative treatments for PCOS due to its requirement for long-term treatment. At present, syndrome differentiation dictates the main clinical treatments. Lifestyle interventions such as strength training, diet, and changing poor habits (for example, giving up smoking and drinking) have positive effects in patients with PCOS and are currently the first-line treatments [7]. However, lifestyle interventions face various challenges as the first-line management, for instance, suboptimal response and lack of adherence. Therefore, patients require additional pharmacological interventions. Metformin, the most extensively studied insulin-sensitizing agent for the treatment of women with PCOS, reduces serum insulin and androgen levels and improves ovulatory function [8]. In addition, metformin can reduce low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels to lower the risk of complications such as cardiovascular disease [9]. However, oral metformin has a high incidence of adverse reactions such as gastrointestinal distress, nausea, vomiting, anorexia, etc., and can even lead to death [10, 11]. It is difficult for some patients to accept these possible risks, and thus metformin is not an ideal choice for long-termfirst-line medication for patients. Therefore, the use of complementary treatments has increased in recent years [10, 11].
Traditional Chinese medicine (TCM), as a form of complementary medicine, has the clinical advantages of obvious curative effects, minimal side effects, low cost, and fewer complications in the treatment of IR in PCOS patients and has gradually become a new choice for people [12]. The treatment of PCOS should be sustainable and dynamic and should be adapted to the changing circumstances and expectations of the individual patient. In addition, patients with PCOS have different clinical symptoms, and TCM is amenable to individualized treatments. Doctors can analyze the physical condition of patients with PCOS and then formulate corresponding treatment plans. Owing to the lack of efficacy and the debilitating side effects of pharmaceuticals, complementary and alternative drugs are becoming more and more popular in the treatment of PCOS [13]. Clinical studies have further shown that Chinese herbal medicine has a definite effect in regulating glucolipid metabolism disorders [14, 15], and numerous experimental studies have found that Chinese herbal medicine also has the effect of improving glucolipid metabolism in animal models of PCOS and IR [16–18]. Overall, the available evidence suggests that Chinese herbal medicine has higher efficacy and safety for patients with PCOS compared to pharmaceutical treatments.
However, the effects of Chinese herbal medicine on glucolipid metabolism in PCOS patients have not been comprehensively analyzed and studied. Therefore, on the basis of the existing evidence, we conducted a comprehensive search of domestic and foreign literature to objectively evaluate the clinical efficacy and safety of Chinese herbal medicine on glucolipid metabolism in PCOS patients, aiming to provide the latest basis for clinical medication.
2. Methods and Materials
2.1. PRISMAP
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMAP) was followed for the systematic review and meta-analysis.
2.2. Search Strategy
Two reviewers conducted a systematic literature search in English databases (PubMed, Embase, Web of Science, and the Cochrane Library) and Chinese databases (China National Knowledge Infrastructure (CNKI), Wanfang, the China Science and Technology Journal Database (VIP), and the Chinese Biomedical Literature Database (CBM)). Search terms were related to Chinese herbal medicine (e.g., “Oriental medicine,” “East Asian medicine,” and “Chinese herbal drugs”), PCOS (e.g., “polycystic ovary syndrome”), glucolipid metabolism (e.g., “insulin sensitivity,” “glucose tolerance tests,” “lipid profile,” “HbA1c,” “triglycerides,” “total cholesterol,” “high-density lipoprotein cholesterol,” and “low-density lipoprotein cholesterol”), and randomized controlled trials (e.g., “clinical trial,” “RCT,” “random,” “randomize,” and “randomization”). Both text and MeSH terms were used. We searched all the above databases until June 22, 2021, and two reviewers screened, selected, and extracted data and cross-verified the results of the data extraction independently.
2.3. Eligibility Criteria
The PICOS (population, intervention, comparison, results, and study design) framework was used to establish the selection criteria.
2.3.1. Types of Studies
We only included randomized controlled trials (RCTs) in Chinese or English that used Chinese herbal medicine to treat PCOS patients. The status or date of the study had no effect on the systematic review.
2.3.2. Participants
Participants in all included RCTs were adult patients with PCOS, and all participants were diagnosed using the 2003 Rotterdam criteria [19]. This definition proposes that PCOS can be diagnosed in any woman presenting with at least two of the three following criteria: hyperandrogenism (either clinical or hyperandrogenemia), ovulation dysfunction, and polycystic ovaries on ultrasound plus the exclusion of other diagnoses that could result in hyperandrogenism or ovulatory dysfunction. The participants were not excluded by their race, background, or body size, but participants with other serious diseases (such as cancer, liver disease, or kidney disease) were excluded from the RCTs.
2.3.3. Intervention Groups
The included RCTs used various forms of Chinese herbal medicine treatment, including Chinese herbal decoctions or proprietary Chinese medicines derived from botanicals, minerals, animals, or chemicals. The dosage forms of Chinese medicine included decoctions, tablets, powders, pills, granules, capsules, ointments, oral liquids, plasters, and injections. Nonherbal interventions (such as massage, acupuncture, cupping, and other TCM treatments), herbal injections, or combined interventions using two or more different types of herbal medicines were excluded from the included RCTs. There were no restrictions on the herbal composition, dosage, frequency of intake, or duration of treatment in the included RCTs.
2.3.4. Comparison Groups
Patients in the control groups received Western medicine (including insulin sensitizers such as metformin), placebo, Western medicine combined with placebo, or lifestyle management, including weight loss through diet and exercise. There were no restrictions on the dosage form, quantity, or duration of the medicine or placebo.
2.3.5. Outcome Measures
The primary outcome was homeostatic model assessment of insulin resistance (HOMA-IR), and the secondary outcomes were fasting blood glucose (FPG), fasting plasma insulin (FINS), 2-hour fasting blood glucose (2hFPG), 2-hour fasting insulin (2hFINS), serum total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), LDL-C, body mass index (BMI), and waist-to-hip ratio (WHR). The safety indicator was any adverse event.
2.4. Literature Screening
The identified articles were initially imported into NoteExpress. After reading the title and abstract, initial screening was performed according to the inclusion criteria. The full texts of the qualifying trials were then read to check whether the papers met the aforementioned inclusion criteria. All duplicate trials were excluded. If there was any disagreement between the two researchers, a third researcher was consulted.
2.5. Data Extraction and Management
The following data were collected from each study: (a) Year of publication; (b) Name of first author; (c) Country; (d) Basic characteristics of the included patients; (e) Sample size; (f) Intervention measures; (g) Outcome measures; (h) Adverse reactions; (i) Random allocation methods; and (j) Other relevant information. Microsoft Excel was used for data extraction. If there was any disagreement between the two researchers, a third researcher was consulted.
2.6. Bias Risk Assessment of the Included Studies
Two researchers independently assessed the quality of the literature by using the Cochrane collaborative bias risk assessment tool. The evaluation was conducted according to the standards proposed in the Cochrane Intervention System Evaluation Manual, and the risk of bias was divided into three levels of low, high, and unclear. The bias risk assessment included the following seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. If there was any disagreement between the two researchers, a third researcher was consulted.
2.7. Data Synthesis
Data analysis was performed with the RevMan 5.4 software. When the included studies used the same measurement scale, the mean difference (MD) and 95% confidence interval (CI) were used to describe continuous variables. Because the prescriptions used in TCM are different and vary from person to person, there is inevitably heterogeneity in clinical indicators. Therefore, in this meta-analysis, the random-effects model was adopted uniformly.
3. Results
3.1. Literature Search and Screening Flowchart
The selection of the studies is shown in Figure 1. A total of 1277 trials were retrieved in the initial search. After deleting duplicates and filtering through the titles and abstracts, we obtained 25 full texts. Finally, 13 studies were included in the systematic review and meta-analysis [20–32].
Figure 1.
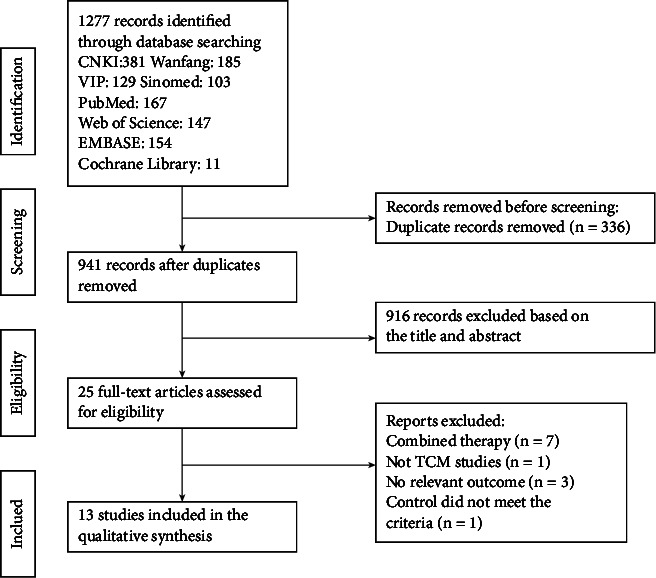
Literature search and screening flowchart.
3.2. Features of the Included Trials
A total of 825 patients were included in the 13 RCTs. All participants were diagnosed with PCOS according to the Rotterdam criteria, and they only received Chinese herbal medicine treatment or treatment with placebo or metformin. The specific characteristics of these studies are summarized in Table 1, including author, year, country, sample size, age, intervention measures, duration of treatment, and outcome indicators, and the main ingredients and usages of Chinese herbal medicine are summarized in Table 2.
Table 1.
Features of the included studies.
| Study ID | Sample size | Age | Treatment versus control | Duration of treatment (months) | Outcomes |
|---|---|---|---|---|---|
| Chen [23] China | T: 47 | T: 28.43 ± 3.83 | T: ban-xia-xie-xin-tang, decocted. | 3 | BMI, WHR, HOMA-IR, FPG, FINS, TC, TG, LDL-c, HDL-c |
| C: 15 | C: 27.27 ± 5.75 | C: metformin, 500 mg, po, tid | |||
|
| |||||
| Fang et al. [25] China | T: 45 | T:25.41 ± 3.48 | T: wen-shen-tiao-jing-tang, decocted. | 2 | BMI, HOMA-IR, FPG, 2hFPG, FINS, 2hINS, TC, TG, HDL-c, LDL-c |
| C: 45 | C:25.32 ± 3.37 | C: metformin, 500 mg, po, bid. | |||
|
| |||||
| Hong et al. [22] China | T: 23 | T: 24.3 ± 5.8 | T: jian-pi-qu-tan-tong-luo-tang, decocted. | 3 | BMI, WHR, HOMA-IR, FPG, FINS, TC, TG, HDL-c, LDL-c |
| C: 22 | C: 25.1 ± 6.2 | C: metformin, 500 mg, po, tid. | |||
|
| |||||
| Li [24] China | T: 45 | T: 25.33 ± 3.97 | T: wu-ji-san, powdered medicine. | 3 | BMI, WHR, FPG, 2hFBG, FINS, 2hFINS, TC, TG, HDL-C |
| C: 45 | C: 25.33 ± 4.32 | C: metformin, 500 mg, po, tid. | |||
|
| |||||
| Liang et al. [31] China | T: 48 | 24.69 ± 3.55 | T: HYKT (Heyan Kuntai capsule) | 6 | BMI, WHR, HOMA-IR, FPG, 2hFBG, FINS, 2hFINS, TC, TG, HDL-C, LDL-c |
| C: 47 | C: placebo tid, 4 capsules each time. | ||||
|
| |||||
| Liang [28] China | T: 25 | T: 23.20 ± 3.73 | T: zi-shen-qing-re-li-shi-hua-yu-fang (granules). | 3 | HOMA-IR, FPG, FINS, 2hFPG,2hINS, TC, TG, HDL-C, LDL-C |
| C: 25 | C: 24.20 ± 3.08 | C: placebo granules bid, 1 bag each time. | |||
|
| |||||
| Luo [30] China | T: 19 | T: 23.1 ± 5.7 | T: bu-shen-hua-tan-qu-yu-fang, decocted. | 6 | BMI, FPG, FINS, T C, TG, HDL-C, LDL-C |
| C: 16 | C: 24.1 ± 4.3 | C: metformin, 500 mg, po, qd. | |||
|
| |||||
| Mahdie [29] Iran | T: 29 | T: 28.62 ± 5.74 | T: cinnamon powder capsules | 3 | BMI, HOMA-IR, FPG, FINS, 2hFPG, TC, TG, LDL-C, HDL-C |
| C: 30 | C: 26.53 ± 6.35 | C: placebo 500 mg, po, tid. | |||
|
| |||||
| Mehri [32] Iran | T: 24 | T: 28.6 ± 4.7 | T: curcumin | 3 | BMI, HOMA-IR, FPG, FINS, TC, TG, LDL-C, HDL-C |
| C: 36 | C: 27.2 ± 3.4 | C: placebo 500 mg/day | |||
|
| |||||
| Wang et al. [20] China | T: 40 | 29.43 ± 4.35 | T: cang-fu-dao-tan-tang modified, decocted. | 3 | BMI, HOMA-IR, FPG, FINS, TC, TG, HDL-C, LDL-C |
| C: 35 | C: metformin, 500 mg, po, tid. | ||||
|
| |||||
| Zhao et al. [27] China | T: 36 | 18~35 | T: yang-yin-yi-qi-huo-xue-fang, decocted. | 3 | BMI, HOMA-IR, FPG, FINS, TC, HDL-C, LDL- C |
| C: 26 | C: metformin, 500 mg, po, tid. | ||||
|
| |||||
| Zhao et al. [26] China | T: 30 | T: 23.2 ± 3.1 | T: yang-yin-yi-qi-huo-xue-fang, decocted. | 6 | BMI, HOMA-IR, FPG, FINS, TC, TG, HDL-C, LDL-C |
| C: 22 | C: 24.1 ± 2.9 | C: metformin, 500 mg, po, tid. | |||
|
| |||||
| Zheng et al. [21] China | T: 26 | T: 27.02 ± 4.98 | T: duo-nang-yin, decoction. | 6 | BMI, WHR, FPG, FINS, TC, TG, HDL-C, LDL-C |
| C: 24 | C: 27.45 ± 4.67 | C: metformin, 500 mg, po, tid. | |||
T: treatment; C: control; po: peros; qd: quaque die; bid: bis in die; tid: ter in die; BMI: body mass index; WHR: waist-to-hip ratio; HOMA-IR: homeostasis model assessment of insulin resistance; FPG: fasting plasma glucose; 2hFPG: 2 h fasting plasma glucose; FINS: fasting insulin; 2hFINS: 2 h fasting insulin; TC: serum total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
Table 2.
Ingredients and usages of Chinese herbal medicine.
| Study ID | Chinese medicine decoction | Main ingredients and usage |
|---|---|---|
| Chen [23] China | Ban-xia-xie-xin-tang | Banxia 9 g, huangqin 30 g, huanglian 15 g, ganjiang 15 g, dangshen 12 g, shengyimi 30 g, gouqi 30 g. Add or subtract Chinese herbals according to symptoms. Decoction, 150 ml per dose. One dose each time, two times a day, once in the morning and once in the evening. |
|
| ||
| Fang et al. [25] China | Wen-shen-tiao-jing-tang | Zishiying 15 g, xianlingpi 15 g, chuanduan 10 g, tusizi 15 g, baishao 10 g, heshouwu 15 g, xiangfu 10 g, danggui 15 g, chuanniuxi 10 g, chuanjiao 10 g. Decoction in water, 1 dose a day, 250 ml per dose, orally two times, once in the morning and once in the evening. |
|
| ||
| Hong et al. [22] China | Jian-pi-qu-tan-tong-Luo-tang | Baizhu 15 g, dangshen 15 g, fuling 15 g, cangzhu 15 g, xiangfu 15 g, xianglingpi 15 g, guizhi 10 g, chaihu 10 g, danggui 15 g, taoren 10 g, shudihuang 15 g, jixueteng 15 g, chenpi 10 g. Decoction, 200 ml, 1 dose/time, 2 times/day. |
|
| ||
| Li [24] China | Wu-ji-san, powdered medicine. | Baizhi 9 g, chuanxiong 9 g, gancao 9 g, fuling 9 g, danggui 9 g, rougui 9 g, baishao 9 g, jiangbanxia 9 g, shengmahuang 9 g, chenpi 18 g, zhiqiao 18 g, cangzhu 30 g, ganjiang 10 g, jiegeng 12 g, houpo 12 g. Dissolve the medicine powder with 300 ml warm boiled water and take it two times a day, once in the morning and once in the afternoon. Do not stop taking the medicine during menstruation. |
|
| ||
| Liang et al. [31] China | HYKT (Heyan Kuntai capsule) | Dihuang, huanglian, baishao, huangqin, ejiao, fuling. 3 times a day, 4 capsules each time. |
|
| ||
| Liang [28] China | Zi-shen-qing-re-li-shi-hua-yu-fang (granules). | Zhimu 10 g, shanzhuyu 10 g, danshen 10 g, taoren 10 g, yiyiren 15 g, baijiezi 10 g, huangbai 10 g, xuanshen 10 g, gancao 6 g. Boil 1 sachet in water two times a day, take in the morning and evening, drink while still warm. |
|
| ||
| Luo [30] China | Bu-shen-hua-tan-qu-yu-fang | Tusizi 15 g, xianlingpi 15 g, roucongrong 15 g, shengdi 15 g, danggui 12 g, chuanxiong 6 g, zelan 15 g, fuling 15 g, fabanxia 10 g, cubiejia 12 g, zaojiaoci 15 g, gancao 6 g. Take one dose a day, decoct to 300 ml, and take it twice per day, once in the morning and once in the evening. Stop taking during menstruation. |
|
| ||
| Wanget al. [20] China | Cang-fu-dao-tan-tang modified | Cangzhu 12 g, xiangfu 12 g, chenpi 15 g, fabanxia 15 g, zaojiaoci 15 g, danggui 12 g, chuanxiong 12 g, shichangpu 12 g, fuling 20 g, danshen 30 g, heye 15 g, shanyao 15 g, huangqi 30 g, xianlingpi 15 g, lujiaoshuang 12 g, zishiying 30 g, sharen 6 g, chuanniuxi 15 g. Take 1 dose a day. Decoct in water two times, drink while warm, once in the morning and once in the evening. Start taking it from the 10th day of menstruation and stop taking it during menstruation, and continue for 3 consecutive months. |
|
| ||
| Zhao et al. [27] China | Yang-yin-yi-qi-huo-xue-fang | Gouqizi 10 g, ejiaozhu 10 g, zhihuangjing 10 g, hanliancao 10 g, buguzhi 10 g, nvzhenzi 10 g, digupi 10 g, maidong 10 g, nanshashen 10 g, beishashen 10 g, ziheche 10 g, shengdi 10 g, shengbaishao 10 g, huangqi 30 g, gancao 6 g. Add or subtract Chinese herbs according to menstrual symptoms. |
|
| ||
| Zhao et al. [26] China | Yang-yin-yi-qi-huo-xue-fang | Ejiaozhu 10 g, huangqin 10 g, huangbo 10 g, hanliancao 10 g, buguzhi 10 g, nvzhenzi 10 g, digupi 10 g, maidong 10 g, nanshashen 10 g, beishashen 10 g, shengbaishao 10 g, huangqi 30 g, gancao 6 g, ziheche 3 g. Add or subtract Chinese herbs according to menstrual symptoms. |
|
| ||
| Zheng [21] China | Duo-nang-yin | Tusizi 15 g, bajitian 10 g, chaihu 10 g, yinyanghuo 10 g, baishao 10 g, longdancao 6 g. Add or subtract Chinese herbs according to symptoms. Soak the herbs in 300 ml of water for 30 minutes, then boil with high heat and then simmer for 20 minutes. Take 200 ml of the first decoction, then add 200 ml of water and continue to decoct for 15 minutes. Take 150 ml of the second decoction, mix with the first 200 ml, and divide it into two doses. Take each dose in the morning and evening. |
3.3. Evaluation of the Quality of the Trials
Cochrane collaborative bias risk assessment was used to evaluate the quality of the literature. Based on standards outlined in the Cochrane Intervention System Evaluation Manual, two researchers independently assessed the literature. The risk of bias of these 13 trials was divided into three levels of low, high, and unclear. All 13 trials mentioned the word “random” or “randomization,” and six studies described the specific randomization methods, of which only three studies described both allocation concealment and randomization methods. For participant and personnel blinding, three trials had a low risk of bias [28, 29, 32], one study did not have enough information to judge the risk level [31], and the remaining studies were at high risk of bias due to the lack of blinding during implementation. Measurements were generally made by third parties other than the researchers, so the blinding of outcome assessment was defined as low risk. In addition, all studies described the missing data and reported both the glucose metabolism and lipid profile indicators. Detailed information of the quality evaluation is shown in Figure 2. If there was any discrepancy between the two researchers, a third researcher was consulted.
Figure 2.
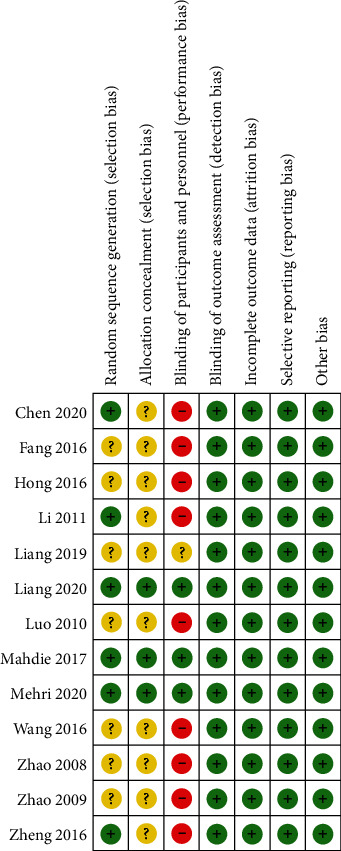
Evaluation of the risk biases of the included studies.
3.4. Results of the Meta-Analysis
3.4.1. Compared with the Placebo Group
(1) General Indicators.
Meta-analysis showed no statistically significant differences for BMI (P = 0.84) or WHR (P = 0.06) between the Chinese herbal medicine groups and the placebo groups. The results of the meta-analysis are shown in Figure 3 (3.1.1–3.1.2).
Figure 3.
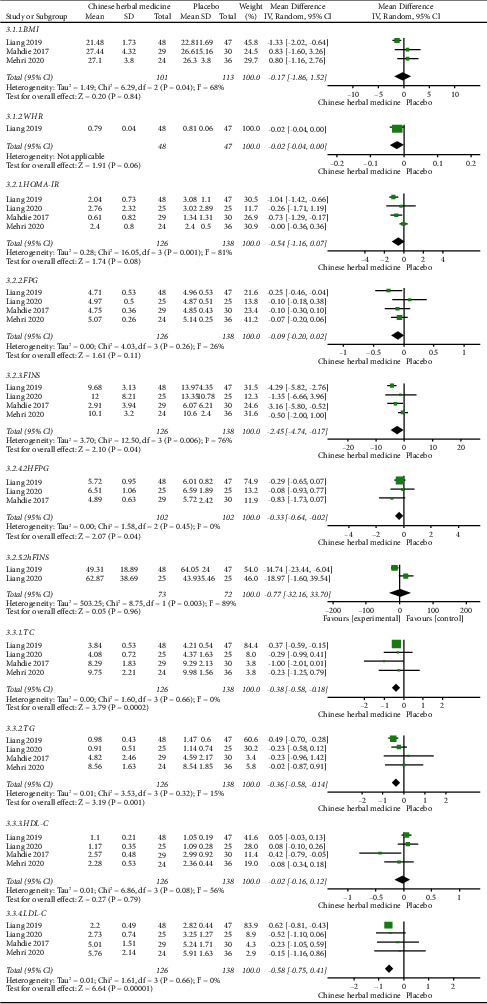
Comparisons of glucolipid metabolism between the Chinese herbal medicine group and placebo group in the treatment of PCOS.
(2) Glucose Metabolism Indicators.
The analysis showed that compared with the placebo group, FINS (MD = −2.45, 95% CI = [−4.74, −0.17], P = 0.04) and 2hFPG (MD = −0.33, 95% CI = [−0.64, −0.02], P = 0.04) were significantly improved in the Chinese herbal medicine group. However, no significant difference was seen in the HOMA-IR (P = 0.08), FPG (P = 0.11), or 2hFINS (P = 0.96) between the two groups. The results of the meta-analysis are shown in Figure 3 (3.2.1–3.2.5).
(3) Lipid Profile Indicators.
The analysis showed that the indicators of TC (MD = −0.38, 95% CI = [−0.58, −0.18], P = 0.0002), TG (MD = −0.36, 95% CI = [−0.58, −0.14], P = 0.001), and LDL-C (MD =−0.58, 95% CI = [−0.75, −0.41], P < 0.00001) were statistically different when compared with the placebo group, but there was no statistical difference in HDL-C (P = 0.79) when the control group was placebo. The results of the meta-analysis of blood lipid metabolism are shown in Figure 3 (3.3.1–3.3.4).
3.4.2. Compared with the Metformin Group
(1) General Indicators.
The analysis of BMI and WHR showed that compared with the metformin group, the BMI of the Chinese herbal medicine group was significantly improved (MD = −1.04, 95% CI = [−1.55, −0.53], P < 0.0001), while there was no statistical difference in WHR (P = 0.47). The results of the meta-analysis are shown in Figure 4 (4.1.1–4.1.2).
Figure 4.
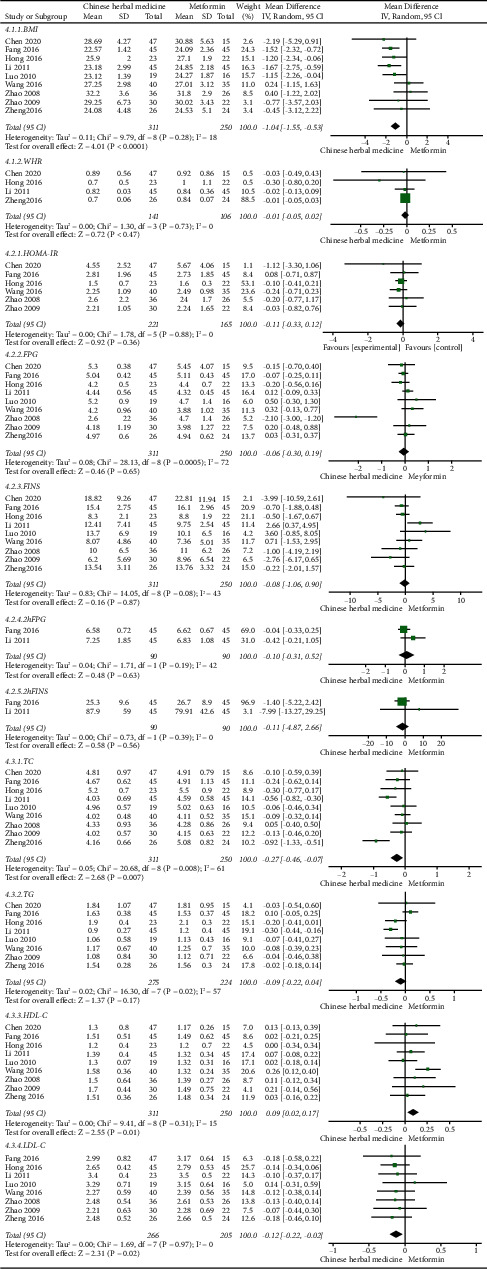
Comparisons of glucolipid metabolism between the Chinese herbal medicine and metformin group in the treatment of PCOS.
(2) Glucose Metabolism Indicators.
Compared with the metformin group, the analysis showed that no significant difference was seen in the HOMA-IR (P = 0.36), FPG (P = 0.65), FINS (P = 0.87), 2hFPG (P = 0.63), or 2hFINS (P = 0.56). The results of the meta-analysis are shown in Figure 4 (4.2.1–4.2.5).
(3) Lipid Profile Indicators.
The analysis showed that in the Chinese herbal medicine group, TC (MD = −0.27, 95% CI = [−0.46, −0.07], P = 0.007) and LDL-C (MD = −0.12, 95% CI = [−0.22, −0.02], P = 0.02) were significantly reduced and HDL-C (MD = 0.09, 95% CI = [0.02, 0.17], P = 0.01) was significantly improved. However, no significant difference was seen in the TG (P = 0.17). The results of the meta-analysis of blood lipid metabolism are shown in Figure 4 (4.3.1–4.3.4).
3.4.3. Reporting Bias Assessment
When more than 10 RCTs were included in the meta-analysis, a funnel plot was used to assess bias. We tried to test the indicators that included 9 RCTs of metformin as the control group in this study. The results showed that there was no obvious asymmetry in FINS and HDL-C, but the funnel plots of BMI, FPG, and TC were not symmetrical enough, suggesting that there may be publication bias. The reasons for this might be unpublished negative results, low methodological quality, or small sample sizes. They are shown in Figure 5.
Figure 5.
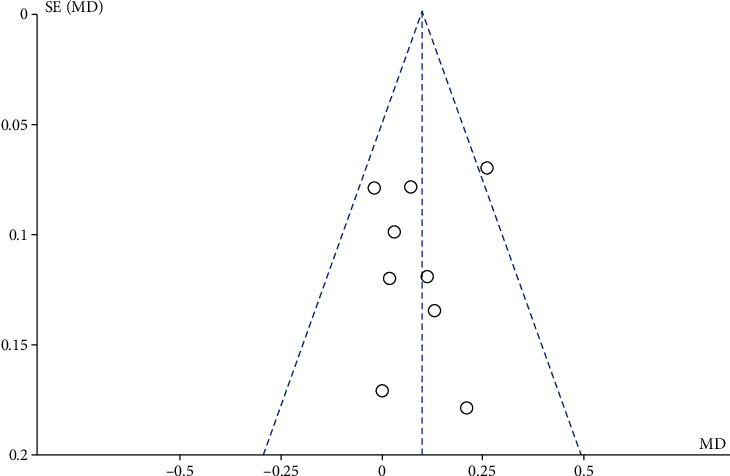
Funnel plot of comparison: HDL-C.
3.4.4. Adverse Events
Five studies reported the existence of adverse events. Three studies reported gastrointestinal side effects such as nausea, vomiting, diarrhea, or weakness in the metformin group (control group) and the patients chose to discontinue treatment [24, 26, 27]. Two of the studies reported that there were patients with abdominal distension and anorexia in the Chinese herbal medicine group, but the patients could tolerate these effects and they resolved spontaneously [26, 31]. In another study, a patient in the Chinese herbal medicine group developed skin rash and itching after using cinnamon capsules for 5 days, but the adverse effect disappeared after discontinuation of the treatment without any intervention [32].
4. Discussion
In this study, a meta-analysis was performed to systematically evaluate the effect of Chinese herbal medicine on glucolipid metabolism in women with PCOS. The results showed that compared with the placebo group, Chinese herbal medicine has a relatively positive effect on glucolipid metabolism in women with PCOS. Chinese herbal medicine has relatively positive effects on lipid metabolism when the control group was metformin, but had no significant effect on glucose metabolism. Overall, the effects of Chinese herbal medicine on glucose metabolism were not as significant as metformin, but were more effective than placebo. Although the results of this meta-analysis showed that insulin resistance was not significantly improved, there have still been many related reports of TCM improving insulin resistance [31, 33]. There is also basic research suggesting that Chinese herbal medicine has the potential to modulate the gut microbiota in order to control inflammation and improve insulin resistance in PCOS patients [34]. Chinese herbal medicine rarely caused adverse events, and the reported adverse events, such as abdominal distension, did not have a significant impact, indicating that Chinese herbal medicine is relatively safe and reliable for treating PCOS. Therefore, we conclude that, compared with standard treatments, Chinese herbal medicine is more effective and safer in ameliorating lipid metabolism in patients with PCOS.
However, current meta-analyses of the Chinese herbal medicine treatment of PCOS have mainly focused on infertility and obesity [35, 36], thus there is a lack of research on using Chinese herbal medicine to improve glucolipid metabolism in PCOS patients. At present, the treatments for PCOS are mainly symptomatic treatments, but Chinese herbal medicine treatment focuses on the overall health of the patient, not just their specific symptoms. In the included trials of this meta-analysis, a large percent of the prescriptions were adjusted by physicians based on clinical experience. There are also many classical prescriptions commonly used in clinical practice that were selected, such as Banxia Xiexin decoction, Cangfu Daotan decoction, and Wuji San. The compositions of the prescription are different, but most of them are mainly based on resolving phlegm and removing blood stasis. PCOS patients are often troubled by obesity, and TCM believes that the blockage of “phlegm turbidity” or “static blood” is the main pathological factor behind obesity, and thus the constitution of phlegm dampness and kidney deficiency are likely to be the important pathological basis of obesity and infertility in patients with PCOS.
Modern pharmacology has found that the active polysaccharide in Angelica sinensis has a variety of pharmacological activities, including hematopoietic activity, immune promotion, anti-inflammation, antioxidation, and liver protection. [37]. Citrinin, the active ingredient in tangerine peels, has the effect of inhibiting obesity and can also improve insulin resistance by regulating the inflammatory response caused by obesity in adipose tissue [38]. In addition, some studies have shown that after screening and network pharmacology prediction, a variety of active ingredient monomers in Cangfu Daotan Tang were found to be useful in the treatment of PCOS [39]. Among them, quercetin plays a role in regulating metabolic disorders in the treatment of PCOS [40], and kaempferol can improve insulin resistance by inhibiting the inflammatory response [41].
The results of this study also show that Chinese herbal medicine has a better effect than control interventions. In addition to herbal medicine, there are other TCM treatments such as acupuncture, cupping, and massage. Among these, acupuncture greatly improves glucolipid metabolism in patients with PCOS [42]. This meta-analysis demonstrates sufficient evidence for the effectiveness of Chinese herbal medicine in the treatment of PCOS, suggesting that more PCOS patients might choose Chinese medicine as an alternative adjuvant treatment. However, the evidence for the influence of Chinese herbal medicine on glucolipid metabolism in PCOS patients needs more high-quality, large-sample, randomized controlled trials to further confirm these conclusions.
The meta-analysis presented here improves our understanding of the therapeutic effect of Chinese herbal medicine on glucolipid metabolism in women with PCOS. However, this study also has certain limitations. First, the qualities of the included RCTs varied. Only 6 of the 13 RCTs described the specific randomization methods, of which only three studies described both randomization methods and allocation concealment, while the rest only mentioned the use of randomization methods to allocate patients, thus the accuracy and objectivity of the results are reduced. Second, the sample size of the included RCTs was small, and the clinical and physiological characteristics of PCOS were heterogeneous. Third, the choice of intervention in this meta-analysis was Chinese herbal medicine treatment, but the dosage forms, types, quantities, and courses of treatment with Chinese herbal medicine were not the same, and the treatment doses of metformin or placebo in the control groups were also different, resulting in diverse interventions in the included RCTs. Thus, our results can provide a reference for clinical practice, but further accurately designed clinical trials are needed to obtain more rigorous treatment results to overcome these shortcomings.
5. Conclusion
The RCTs analyzed here have shown that, compared with the placebo group, Chinese herbal medicine has relatively positive effects on glucolipid metabolism in women with PCOS. Chinese herbal medicine has a relatively positive effect on lipid metabolism when the control group is metformin, but no positive effect on glucose metabolism. Further large-scale, long-term RCTs that meet strict methodological standards are needed to prove this conclusion.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 81804137), the Open laboratory project for college students of Guangzhou Medical University, (Grant 20205016 (Dr. J. Li)), and the Scientific Research Project of Guangdong Provincial Administration of Traditional Chinese Medicine (Grant 20212131 (Dr. H. Tan)).
Contributor Information
Mei Han, Email: hanmeizoujin@163.com.
Juan Li, Email: lijua@163.com.
Data Availability
This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Disclosure
Jie Li and Ruqun Zheng are the co-first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Juan Li and Mei Han contributed equally. Juan Li conceptualized and designed the study. Jie Li and Ruqun Zheng collected and analyzed the data. Jie Li drafted the manuscript. Juan Li and Mei Han reviewed the protocol for important content and revised the manuscript. Juan Li and Huiyan Tan sought funding. All authors contributed to the further editing and final publication of the trial.
References
- 1.McCartney C. R., Marshall J. C. Polycystic ovary syndrome. New England Journal of Medicine . 2016;375(1):54–64. doi: 10.1056/nejmcp1514916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J., Wu D., Guo H., Li M. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sciences . 2019;236:p. 116940. doi: 10.1016/j.lfs.2019.116940. [DOI] [PubMed] [Google Scholar]
- 3.Palomba S., Santagni S., Falbo A., La Sala G. B. Complications and challenges associated with polycystic ovary syndrome: current perspectives. International Journal of Women’s Health . 2015;7:745–763. doi: 10.2147/ijwh.s70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conway G., Dewailly D., Diamanti-Kandarakis E., et al. European survey of diagnosis and management of the polycystic ovary syndrome: results of the ESE PCOS Special Interest Group’s Questionnaire. European Journal of Endocrinology . 2014;171(4):489–498. doi: 10.1530/eje-14-0252. [DOI] [PubMed] [Google Scholar]
- 5.Anagnostis P., Tarlatzis B. C., Kauffman R. P. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism . 2018;86:33–43. doi: 10.1016/j.metabol.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Teede H., Deeks A., Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Medicine . 2010;8(1):p. 41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin P., Xie Y. Treatment strategies for women with polycystic ovary syndrome. Gynecological Endocrinology . 2018;34(4):272–277. doi: 10.1080/09513590.2017.1395841. [DOI] [PubMed] [Google Scholar]
- 8.Seli E., Duleba A. J. Treatment of PCOS with metformin and other insulin-sensitizing agents. Current Diabetes Reports . 2004;4(1):69–75. doi: 10.1007/s11892-004-0014-8. [DOI] [PubMed] [Google Scholar]
- 9.Rosilio C., Ben-Sahra I., Bost F., Peyron J. F. Metformin: a metabolic disruptor and anti-diabetic drug to target human leukemia. Cancer Letters . 2014;346(2):188–196. doi: 10.1016/j.canlet.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Hostalek U., Gwilt M., Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs . 2015;75(10):1071–1094. doi: 10.1007/s40265-015-0416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harborne L., Fleming R., Lyall H., Norman J., Sattar N. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. The Lancet . 2003;361(9372):1894–1901. doi: 10.1016/s0140-6736(03)13493-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X. Y., Zhou L., Sun Z. Y. [Research progress in mechanism of traditional Chinese medicine treatment of polycystic ovary syndrome] Zhongguo Zhongyao Zazhi . 2016;41(20):3715–3720. doi: 10.4268/cjcmm20162003. [DOI] [PubMed] [Google Scholar]
- 13.Ong M., Peng J., Jin X., Qu X. Chinese herbal medicine for the optimal management of polycystic ovary syndrome. The American Journal of Chinese Medicine . 2017;45(3):405–422. doi: 10.1142/s0192415x17500252. [DOI] [PubMed] [Google Scholar]
- 14.Tian J., Lian F., Yang L., Tong X. Evaluation of the Chinese herbal medicine Jinlida in type 2 diabetes patients based on stratification: results of subgroup analysis from a 12-week trial. Journal of Diabetes . 2018;10(2):112–120. doi: 10.1111/1753-0407.12559. [DOI] [PubMed] [Google Scholar]
- 15.Li J., Bai L., Wei F., et al. Therapeutic mechanisms of herbal medicines against insulin resistance: a review. Frontiers in Pharmacology . 2019;10:p. 661. doi: 10.3389/fphar.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W., Tang M., Wang J., Wang L. Clinical effects of Shou-WuJiang-Qi decoction combined acupuncture on the treatment of polycystic ovarian syndrome with kidney deficiency, phlegm and blood stasisness: study protocol clinical trial (SPIRIT Compliant) Medicine . 2020;99(12):p. e19045. doi: 10.1097/md.0000000000019045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H., Zhou D., Chen Y., Liu D., Chu S., Zhang S. Beneficial effects of Heqi san on rat model of polycystic ovary syndrome through the PI3K/AKT pathway. Daru Journal of Pharmaceutical Sciences . 2017;25(1):p. 21. doi: 10.1186/s40199-017-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang D., Li Q., Lu F. [Effects of bushen tongmai recipe on expression of phosphatidylinositol-3-kinase in PCOS rats accompanying with insulin resistance] Zhongguo Zhongyao Zazhi . 2010;35(13):1735–1739. doi: 10.4268/cjcmm20101318. [DOI] [PubMed] [Google Scholar]
- 19.Escobar-Morreale H. F. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nature Reviews Endocrinology . 2018;14(5):270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 20.Wang C., Sun X., Ding C., Shen Y. Effect of modified cangfudaotan decoction and acupuncture on glucose and lipid metabolism and ovulation rate in obese patients with polycystic ovary syndrome. Modern Journal of Integrated Traditional Chinese and Western Medicine . 2016;25(36):4056–4058. [Google Scholar]
- 21.Zheng Y., Sun M., Zheng J. Effects on endocrine, lipid metabolism and polycystic ovary morphology in patients with deficiency of kidney, liver depression and polycystic ovary syndrome treated by traditional Chinese medicine polycystic drink. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care . 2016;23:609–612. [Google Scholar]
- 22.Hong Y., Sun X., Zhou Y. Treatment of 23 cases of obese polycystic ovary syndrome with jianpi qutan tongluo decoction. Jiangxi Journal of Traditional Chinese Medicine . 2016;47(10):48–50. [Google Scholar]
- 23.Chen R. Beijing, China: Beijing University of Chinese Medicine; 2020. Evaluation of the curative effect of banxia xiexin decoction on pcos hyperinsulinemia of stomach heat and spleen deficiency. Master thesis. [Google Scholar]
- 24.Li S. Nanjing, China: Nanjing University of Chinese Medicine; 2011. Clinical and empirical study of reproductive hormones and glucose and lipid metabolism with wujisan in the treatment of polycystic ovary syndrome with type of stagnation of phlegm-damp. Ph.D. thesis. [Google Scholar]
- 25.Fang R., Liang W., Zhang J. Influence of wenshen tiaojing decoction on endocrine and lipid metabolism of polycystic ovary syndrome patients. Chinese Journal of Modern Applied Pharmacy . 2016;33(8):1073–1077. [Google Scholar]
- 26.Zhao H., Bao W. F., Zhang T. Advantages of Chinese medicine for treatment of blood sugar and lipid metabolic disorders in patients with polycystic ovarian syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Za Zhi . 2009;29(7):595–598. [PubMed] [Google Scholar]
- 27.Zhao H., Wang X. E. Hangzhou, “the effect of metabolic status of blood sugar and lipid and reproductive endocrine of Chinese traditional medicine”. Journal of Zhejiang University of Traditional Chinese Medicine . 2008;4:458–460. [Google Scholar]
- 28.Liang R., Sun X., Li P. Effect of zishen qingre lishi huayu recipe on spontaneous ovulation and menstruation in polycystic ova- ry syndrome patients with shen yin deficiency syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi . 2021;41(2):189–193. [Google Scholar]
- 29.Jamilian M., Foroozanfard F., Kavossian E., et al. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition ESPEN . 2020;36:128–133. doi: 10.1016/j.clnesp.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Luo J. Hubei, China: Hubei University of Traditional Chinese Medicine; 2010. The clinical research of bushen huatan quyu treatment on insulin resistance and dyslipidaemia in patients with polycystic ovary syndrome. Master thesis. [Google Scholar]
- 31.Liang R., Liu Z., Li P., et al. Kuntai capsules improve glucolipid metabolism in patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Medicine (Baltimore) . 2019;98(39) doi: 10.1097/md.0000000000016788.e16788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajimonfarednejad M., Nimrouzi M., Heydari M., Zarshenas M. M., Raee M. J., Jahromi B. N. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: a randomized double-blind placebo controlled clinical trial. Phytotherapy Research . 2018;32(2):276–283. doi: 10.1002/ptr.5970. [DOI] [PubMed] [Google Scholar]
- 33.Deng Y., Xue W., Wang Y. F., et al. Insulin resistance in polycystic ovary syndrome improved by Chinese medicine dingkun pill: a randomized controlled clinical trial. Chinese Journal of Integrative Medicine . 2019;25(4):246–251. doi: 10.1007/s11655-018-2947-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y., Li Y., Liu M., Hu X., Zhu H. Guizhi fuling wan, Chinese herbal medicine, ameliorates insulin sensitivity in PCOS model rats with insulin resistance via remodeling intestinal homeostasis. Frontiers in Endocrinology . 2020;11:p. 575. doi: 10.3389/fendo.2020.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ried K. Chinese herbal medicine for female infertility: an updated meta-analysis. Complementary Therapies in Medicine . 2015;23(1):116–128. doi: 10.1016/j.ctim.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Ding N., Yue R., Wang L., Yang H. Chinese herbal medicine on treating obese women with polycystic ovary syndrome: a systematic review and meta-analysis protocol. Medicine (Baltimore) . 2020;99(49) doi: 10.1097/md.0000000000022982.e22982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nai J., Zhang C., Shao H., et al. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. International Journal of Biological Macromolecules . 2021;183:2337–2353. doi: 10.1016/j.ijbiomac.2021.05.213. [DOI] [PubMed] [Google Scholar]
- 38.Ya X., Yide F., Yapeng H., Yang D. Research progress on pharmacological effects of citrin. Chinese Patent Medicine . 2018;40(09):2030–2033. [Google Scholar]
- 39.Xu W., Tang M., Wang J., Wang L. Identification of the active constituents and significant pathways of Cangfu daotan decoction for the treatment of PCOS based on network pharmacology. Evidence-Based Complementary and Alternative Medicine . 2020;2020:15. doi: 10.1155/2020/4086864.4086864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabrizi F. P. F., Hajizadeh-Sharafabad F., Vaezi M., Jafari-Vayghan H., Alizadeh M., Maleki V. Quercetin and polycystic ovary syndrome, current evidence and future directions: a systematic review. Journal of Ovarian Research . 2020;13(1):p. 11. doi: 10.1186/s13048-020-0616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo C., Yang H., Tang C., et al. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. International Immunopharmacology . 2015;28(1):744–750. doi: 10.1016/j.intimp.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Zheng R., Qing P., Han M., et al. The effect of acupuncture on glucose metabolism and lipid profiles in patients with PCOS: a systematic review and meta-analysis of randomized controlled trials. Evidence-Based Complementary and Alternative Medicine . 2021;2021:11. doi: 10.1155/2021/5555028.5555028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


