Abstract
Vision loss is primarily caused by age-related macular degeneration (AMD) due to oxidative retinal pigment epithelial (RPE) cell injury. Carotenoid utilization is deemed a possible strategy for treating AMD. Cordyceps militaris has advantages like immunomodulatory, anti-inflammatory, and antioxidative characteristics. This paper assessed the possible protective influence of carotenoids obtained by isolating and purifying the Cordyceps militaris (CMCT) into human RPE cells (ARPE-19) damaged by hydrogen peroxide (H2O2). The findings demonstrated that CMCT safeguarded the ARPE-19 cells against the damage and apoptosis caused by H2O2 and oxidative stress via Bcl-2 protein upregulation, as well as the expression of Bax and cleaved caspase-3 protein. In addition, CMCT treatment increased cell survival and restricted the generation of H2O2-induced reactive oxygen species (ROS) and the protein expression of NADPH oxidase-1 (NOX1). Additionally, the CMCT treatment of H2O2-induced ARPE-19 cells ameliorated high malondialdehyde (MDA) levels in oxidative stress-induced cells. The catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH) returned to standard levels, which were governed by the higher expression of nuclear Nrf2 protein in the ARPE-19 cells. Moreover, this study showed that CMCT safeguarded the ARPE-19 cells against the damage caused by oxidative stress via its antioxidant activity and antiapoptotic functionality, suggesting the potential therapeutic role of CMCT in AMD prevention and mitigation.
1. Introduction
Age-related macular degeneration (AMD) is affected by daily living habits and the environment [1, 2]. It is estimated that up until 2020, AMD has affected 196 million people globally, a number anticipated to rise to 288 million by 2040 [3]. Furthermore, the pathogenesis of AMD is complicated, with no specific therapeutic strategy available. Increasing research demonstrates that injured retinal pigment epithelial (RPE) cells are vulnerable to oxidative stress. Additionally, reactive oxygen species (ROS)-induced RPE, in particular, is considered an initial pathological trigger of AMD occurrence [4–6]. RPE represents a pigment cell layer between the neural retina and the choriocapillaris and is responsible for external retinal protection [7]. These cells display the most active cellular metabolism in ocular tissues and are essential for regenerating and repairing photoreceptor cells, and restricting the retinal entry of toxic plasma and molecules [7].
In addition, oxidative stress produces ROS in cells, causing cell damage and apoptosis [8]. Various clinical studies suggest that damage to RPE cells from prolonged exposure to oxidative stress eventually leads to AMD [9–11]. The RPE cell density decline over time increases the lipofuscin granules, impeding the protective effect against oxidative stress. Consequently, increasing interventions against oxidative stress in RPE cells may provide a therapeutic approach to slow down early AMD progression.
Extensive research data have demonstrated that natural antioxidants derived from plants prevent and ameliorate AMD by protecting RPE cells from oxidative stress injury [12, 13]. Modulating retinal oxidation via daily dietary strategies is vital in slowing or attenuating AMD development. Recent research has indicated that the natural antioxidants found in legumes, plants, vegetables, fruit, and medicinal herbs, including flavonols, penicillin, carotenoids, vitamin E, vitamin C, resveratrol, polyphenols, and curcumin, are effective in reducing functional retinal damage and essential for retinal tissue microcirculation and protecting against oxidative stress changes [14–16]. This suggests that moderately consuming antioxidants via diet daily can help alleviate or delay the occurrence of AMD [17]. Some studies have indicated that specific carotenoids constitute macular pigments and protect the macula from oxidative damage. Chrysanthemum contains various natural bioactive components and pharmacological characteristics like antitumor, antioxidative, immunomodulatory, antiaging, and antibacterial activity [18], and is widely used in many Asian countries for medicinal and health purposes. Furthermore, Cordyceps militaris primarily consists of carotenoids, ergosterol, adenosine, cordycepin, proteins, ascorbic acid, phenols, and polysaccharides, providing it with unique medicinal properties [19]. In particular, carotenoids exhibit natural immunomodulatory, antioxidative, antitumor, antiaging, and antibacterial properties, as evidenced by several related studies [20]. However, the antioxidative effect of Cordyceps militaris carotenoids on RPE cells damaged by hydrogen peroxide remains known. Therefore, this study investigates the protective effect of Cordyceps militaris carotenoids on undifferentiated human retinal epithelial cells (ARPE-19) cells to explore their activity against oxidative damage while discussing the possible mechanisms behind this effect.
2. Methods and Materials
2.1. Test Kits and Reagents
The Cordyceps militaris was acquired from the Nanjiang Hongxing Biological Company in Bazhong City (Sichuan Province, China) (Figure 1), while dimethyl sulfoxide (DMSO), Dulbecco's Modified Eagle Medium (DMEM), acetonitrile, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), were supplied by Sigma-Aldrich, Inc. in St. Louis (MO, USA). Mitsubishi Chemical Holdings (Japan) provided the HP Macroporous Adsorption Resin, while the Jiancheng Institute of Bioengineering (Nanjing, China) supplied the total capacity test kit, catalase (CAT), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH). Biotechnology Hyclone Inc. (Los Angeles, USA) supplied trypsin (0.25%), streptomycin, penicillin, and fetal bovine serum (SV3087), while ALPCO (Salem, NH, USA) provided the annexin V-FITC kit for apoptosis detection. Furthermore, Santa Cruz Biotechnology in Santa Cruz (CA, USA) provided the anti-NADPHoxidase-1 (NOX1), anti-Nrf2, β-actin, caspase-3, Bcl-2, and Bax principal antibodies. All additional reagents were provided by Sigma-Aldrich (St. Louis, MO, USA) unless stated to the contrary.
Figure 1.

Cordyceps militaris samples.
2.2. Isolating and Purifying the Cordyceps militaris Carotenoids
Fresh Cordyceps militaris was subjected to air-drying at 60°C until a steady weight was reached, it was then crushed and sieved. Next, a 2 g sample was mixed with acetone-60% ethanol (2 : 1, v/v) in 20 ml, followed by the addition of 0.5% compound enzymes at pH 4 for 45 min at 50°C. The sample was sonicated for 1.5 h, after which the supernatant was diluted 100 times via centrifugation at 4500 rpm for 10 min to obtain the subsequent Cordyceps militaris crude carotenoid extract (CMC).
The CMC samples were purified further via adsorption using an HP-20 macroporous resin column (particle sizes: 0.3–1.2 mm) ≥ 90%, using a 60% ethanol eluent. The eluate was concentrated to a paste at lower pressure (60 rpm, 50°C/min) using a rotary evaporator. The concentrated, dried CMCT was collected and stored at an ultra-low temperature for later use.
2.3. Analysis of the Carotenoids and Pigments
The freeze-dried CMCT was dissolved in methanol in an ultrasonic bath, after which a 0.22 μm membrane was used to filter the sample solution while protected from light for carotenoid identification and subjected to ultra-performance liquid chromatography (UPLC). The analysis occurred according to a modified technique delineated before [21]. This process used a Waters C18 reversed-phase column (dimensions: 2.1 × 50 mm and 1.7 μm) at a steady 30°C temperature, 2 μl injection volume, and a flow rate of 0.25 ml/min. The elution process included a pure methanol solution for A, while B comprised a 0.1% formic acid solution. Furthermore, the program consisted of 0 min, 100% A; 4 min, 70% A; 16 min, 45% A; 25∼28 min, 10% A; 32∼40 min, 45% A, at a detection wavelength of 445 nm. High-resolution mass spectrometry conditions: electrospray ion source, positive ion mode, perimeter scan range m/z: 50–1000, drying temperature: 450°C, capillary voltage: 5500 V, drying gas flow rate: 40 L/min, and DAD detection wavelength range: 380 nm∼600 nm. Fourier transform infrared (FTIR) spectrometry: the CMCT-potassium bromide mixture (2 : 1, v/v) was ground into a powder and pressed into tablets, after which the infrared absorption was detected in a wavelength range of 500–4000 cm−1 using the transparent potassium bromide tablets.
2.4. Culturing the ARPE-19 Cells
The ARPE-19 cells, provided by the American Type Culture Collection (ATCC), were cultivated in DMEM/F12 supplemented with streptomycin at 100 μg/ml (SV3010 Los Angeles, CA, USA), penicillin at 100 U/ml (Los Angeles, CA, USA), and 10% fetal bovine serum inactivated by heat (Los Angeles, CA, USA) via incubation in 5% CO2 at 37°C. After reaching 90% fusion, the cells were passaged every 3–4 d using trypsin at 0.25% (Biotechnology Hyclone Inc., LA, USA) and inoculated into 96-well culture plates for subsequent experiments.
2.5. Oxidative Stress Induction Using H2O2
The ARPE-19 cells were placed on 96-well plates at 1 × 105 cells/well. They were left overnight and allowed to replicate and fuse. DMEM/F12 containing H2O2 (0–500 μM) was used to culture the cells for 12 h, while cells without H2O2 served as a control.
2.6. MTT Assays
The cell viability was ascertained via the MTT technique. To determine the performance of CMCT against the toxicity caused by H2O2, 96-well plates were inoculated with the ARPE-19 cells at 1 × 105 cell/well, treated with different CMCT (1–10 μg/ml) and H2O2 concentrations for 12 h. Next, 20 μL of the MTT (5 mg/ml) was added to each well for a 4 h incubation period at 37°C. The aspiration of the culture solution from each well was followed by the addition of DMSO at 150 μL of DMSO and electric shaking for 10 min. The reaction was terminated via β-crystal dissolution. Using a microplate reader, the absorbance of the samples was immediately measured at 490 nm, while three replicates were obtained for each experiment. The results were illustrated as the absorbance value percentage vs. the control, i.e., the absorbance value of the plate wells, divided by the control percentage.
2.7. The Analysis of Cell Apoptosis via Annexin and Flow Cytometry
The apoptosis level was analyzed with an annexin V-FITC/PI apoptosis kit as per the instructions of the manufacturer. The ARPE-19 cells were inoculated into the culture plate at 1 × 105 cells/ml and subjected to treatment with and without CMCT, as well as H2O2 for 12 h. The collected cells were rinsed two times using cold PBS, after which they were resuspended in a new medium and stained at room temperature for 15 min away from light with annexin V-FITC/PI. Then, they were assessed via flow cytometry (BD Bioscience, USA), while a Cell Quest analysis tool (BD Biosciences, Franklin Lakes, NJ, USA) was used to calculate the degree of apoptosis. The results were as follows: normal cells: annexin-V-negative-PI-negative; early apoptotic cells: annexin-V-positive-PI-negative; late apoptotic or necrotic cells: annexin-V-positive-PI-positive. All experiments were repeated three times.
2.8. Measurement of the ROS in the Cells
A ROS assay kit was used following the protocols of the manufacturer to detect the ROS levels in the ARPE-19 cells. The DCF-DA fluorescent probe reacted with the ROS produced by cells to form DCF. The collected cells were subjected to a 30 min incubation period using 10 μM of DCFH-DA reagent in darkness at 37°C, after which ice-cold PBS (1 × 105 cells/ml) was used to rinse them twice, followed by resuspension in phosphate buffer. The fluorescence intensity was determined via flow cytometry at respective emission and excitation wavelengths of 525 nm and 488 nm.
2.9. Determination of the MDA, GSH, CAT, and SOD Levels
The CMCT impact on the oxidative stress in the ARPE-19 cells was determined after the different treatments, using the SOD, MDA, CAT, and GSH oxidative biomarkers. The ARPE-19 cells were subjected to a 24 h incubation period in 6-well plates (1 × 106 cells/well), followed by applying different CMCT concentrations for 12 h and H2O2 (400 μg/ml) for 12 h. Next, the collected cells were rinsed two times using ice-cold PBS. The oxidative biomarkers were then measured using the appropriate analytical kits using the protocols of the manufacturers.
2.10. Western Blot Analysis
This analysis occurred according to a previously delineated method. Ice-cold PBS was used to wash the ARPE-19 cells twice. The cell lysates were extracted from the collected cells with RIPA lysis buffer, centrifuged at 4°C and 12,000 rpm for 15 min to obtain the supernatant, and measured via a BCA Protein Assay Kit to determine the protein level. Equal quantities of 30 μg of protein were relocated to polyvinyl fluoride (PVDF) membranes along with 12% sodium dodecyl sulfate-polyacrylamide for electrophoretic separation. The samples were exposed for 1 h to 5% skim milk and subjected to overnight incubation at 4°C with the β-actin (1 : 1000) Bc1-2 (1 : 200), Bax (1 : 500), caspase-3 (1 : 500), anti-Nrf2 (1 : 300), and NOX1 (1 : 500) primary antibodies. This was followed by room temperature incubation for 2 h using the secondary, horseradish peroxidase-conjugated antimouse antibody (1 : 2000). After repeatedly rinsing with PBST, the individual protein expression was observed via an ECL western blotting assay test kit as per the prescribed procedure.
2.11. Statistical Evaluation
All experiments were performed at least three times, and the data after experiments were expressed as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism software. Data were analyzed by one-way ANOVA. P < 0.05 was considered significant.
3. Results
3.1. CMCT Preparation and Characterization
The CMCTs were produced using a technique previously described with some modifications [22]. The CMCTs were obtained via crushing, acetone, and ethanol mixed solution extraction and complex enzyme precipitation at an extraction rate of 3888.34 μg/g. Chrysin carotenoids are intracellular pigments. The cell walls were disrupted and extracted with cellulase and pectinase (extraction ratio of 2 : 1), after which the CMC extract was dissolved using acetone and a 60% ethanol solution. ELISA was employed for absorbance determination at 445 nm (Figure 2(a)). The standard curve was configured as Y = 0.1093x + 0.0801 and R2 = 0.9992 (Y denoted the absorbance, while X signified the pigment level). The macroporous adsorbent resin was used to further separate the CMC, increasing the carotenoid purity for subsequent cellular experiments to 66.24% (Figure 2(b)). Then, the CMCTs were exposed to concentrated sulfuric acid to obtain a blue-green color. Furthermore, exposure to an antimony trichloride chloroform solution yielded a green color, while the color values were consistent with those of olefin carotenoids. Spectroscopic and mass spectrometric analyses confirmed that the pigment purification exhibited general carotenoid characteristics. Five main pigment components were isolated, as shown in (Figure 2(c)). The exact mass numbers were determined from 1 to5 in positive ion [M + H]+ mode, yielding values m/z 629.1862, m/z 525.4265, m/z 495.3362, m/z 523.2778, and m/z 537.1624. The absorption peaks of the five carotenoids displayed identical absorption spectra and conjugation systems, which were consistent with the essential carotenoid characteristics. Therefore, they are compatible with the basic attributes of carotenoids.
Figure 2.
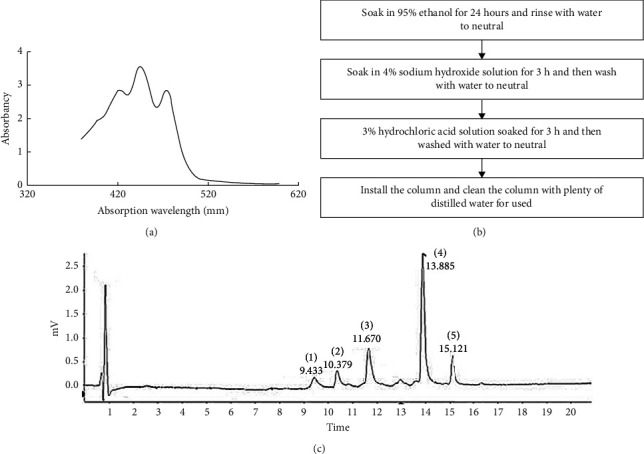
Isolating and purifying Cordyceps militaris. (a) A spectrum image of the Cordyceps militaris carotenoid extraction solution with three characteristic absorption peaks evident at 420 nm, 445 nm, and 475 nm, the most prominent of which is apparent at 445 nm. (b) Macroporous resin activation. (c) The UPLC chromatogram of the carotenoids identified in Cordyceps militaris.
3.2. The Influence of CMCT Treatment on the ARPE-19 Cells
The CMCT influence on cell survival was investigated via an MTT assay and presented as the survival rate of the untreated control samples. A slight increase in cell viability increase was evident in the cells subjected to different CMCT treatments at concentrations in a range from 1 μg/ml to 2.5 μg/ml (Figure 3(a)). However, the cell viability declined significantly after exposure to 5 μg/ml CMCT, while a 10 μg/ml CMCT concentration decreased the cell survival rate to 79% in comparison with the untreated samples (P > 0.05). Consequently, 1 μg/ml and 2.5 μg/ml CMCT concentrations were utilized for the subsequent experiments. To determine the correct H2O2 concentration for assessing the impact of CMCT on the cytotoxicity of H2O2, the ARPE-19 cell viability was examined after 12 h of exposure to H2O2 (0–500 μM). The cell viability decreased dose-dependently from 100 ± 3.6% in the untreated group to 91.1 ± 1.94% at 50 μM, 78.76 ± 3.3% at 100 μM, 69.1 ± 2.9% at 200 μM, 63.5 ± 2.48% at 250 μM, 41.6 ± 1.85% at 400 μM, and 35.2 ± 1.3% at 500 μM, respectively (Figure 3(b)). Therefore, an H2O2 concentration of 400 μM was used during the experiments.
Figure 3.
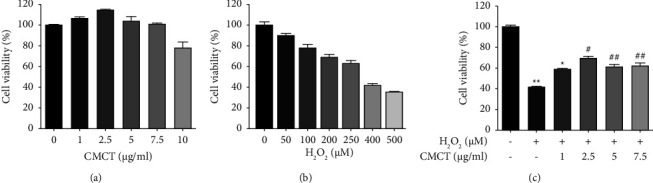
The CMCT treatment impact on the ARPE-19 cells. (a) Treatment with different CMCT concentrations (0–10 μg/ml) for 12 h (b) Treatment with different H2O2 concentrations (0–500 μM) for 12 h (c) Pretreatment with different CMCT concentrations (0–100 μg/ml) and H2O2 (400 μM) for 12 h, respectively. The information is shown as mean ± SD for three separate replicates. Compared to the control samples: ∗P < 0.05 and ∗∗P < 0.01. Compared to the H2O2 control samples: #P < 0.05 and ##P < 0.01 Compared to the H2O2 group: ##P < 0.01.
This study investigated if the target CMCT safeguarded ARPE-19 cells from destruction by H2O2. The cells were precultured for 12 h and 24 h in a medium containing CMCT (1 μg/ml, 2.5 μg/ml), which was changed to a new medium containing H2O2 (400 μM), followed by culturing for 12 h. The results showed significant protection against H2O2-induced AEPE-19 cell death after 12 h CMCT treatment (1 μg/ml, 2.5 μg/ml) (P < 0.05). However, although 24 h CMCT treatment (2.5 μg/ml, 1 μg/ml) displayed only a slight protective effect, no substantial differences were apparent from the control group (P > 0.05). Some toxicity was evident in the cells after prolonged pretreatment. Therefore, a 12 h incubation period was selected to assess CMCT protection against H2O2 damage in the ARPE-19 cells. As illustrated in (Figure 3(c)), pretreatment with 2.5 μg/ml and 1 μg/ml CMCT significantly increased cell viability to 75.6 ± 4.7% and 69.4 ± 6.5%, respectively, displaying substantial variation from the control samples (P < 0.05).
3.3. CMCT Protection against the RPE Cell Death Caused by H2O2
RPE cell damage is usually due to oxidative stress. The ability of CMCT to protect against the ARPE-19 cell death caused by H2O2 was examined by incubating the cells at 1 μg/ml and 2.5 μg/ml CMCT concentrations for 12 h, followed by H2O2 for 12 h, after which they were observed via phase-contrast microscopy, as shown in (Figure 4(a)). The population dispersion of the stained annexin V-positive cells is demonstrated in (Figure 4(b)). H2O2 (400 μM) treatment for 12 h significantly increased the apoptotic cell proportion to 63.87 ± 1.7% compared with the control group (5.16 ± 0.34%). However, CMCT (1 μg/ml and 2.5 μg/ml) pretreatment and H2O2 exposure for 12 h significantly decreased the apoptosis induced by H2O2 (27.21 ± 1.78% and 38.84 ± 1.58%, P < 0.05), when compared to the control cells (3.10 ± 0.67%).
Figure 4.
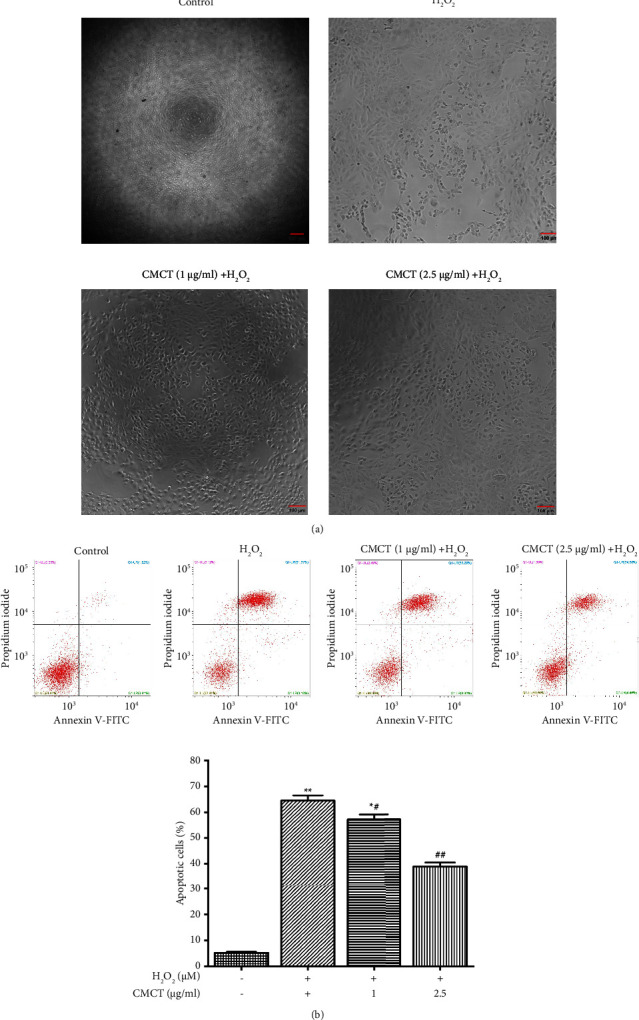
The CMCT protective impact against the RPE cell death caused by H2O2. (a) Images of the ARPE-19 cell morphology after CMCT treatment. (b) The influence of CMCT treatment on the apoptosis in the ARPE-19 cells caused by H2O2 was assessed via flow cytometry and annexin V-FITC/PI. The information is presented as mean ± SD for three separate replicates. Compared to the control group: ∗P < 0.05 and ∗∗P < 0.01. Compared to the H2O2 control samples: #P < 0.05 and ##P < 0.01.
3.4. The Impact of CMCT Treatment on the Production of Intracellular ROS and Expression of NOX1 Proteins in the ARPE-19 Cells
Research has shown that the production of ROS exceeds the in vivo clearance limit due to oxidative stress. The subsequent imbalance in the oxidative and antioxidant systems leads to functional and morphological damage in the ganglion, endothelial, and RPE cells [23]. Therefore, this study investigated whether CMCT affected the ROS levels in the ARPE-19 cells after stress. The cells were exposed to different CMCT concentrations (1 μg/ml, 2.5 μg/ml), as well as H2O2 (400 μM) for 12 h, respectively, after which the ROS levels were measured directly via DCFH-DA staining. H2O2 significantly elevated the production of intracellular ROS compared to the untreated samples (Figure 5(a)). However, CMCT (2.5 μg/ml, 1 μg/ml) treatment for 12 h significantly reduced the upregulated ROS activity induced by H2O2 (400 μM). According to (Figure 5(b)), pretreatment with different CMCT concentrations (2.5 μg/ml, 1 μg/ml) attenuated the NOX1 protein expression induced by H2O2 oxidative stress. Furthermore, some of the differences from the H2O2 control were statistically significant (P < 0.01 or P < 0.05).
Figure 5.
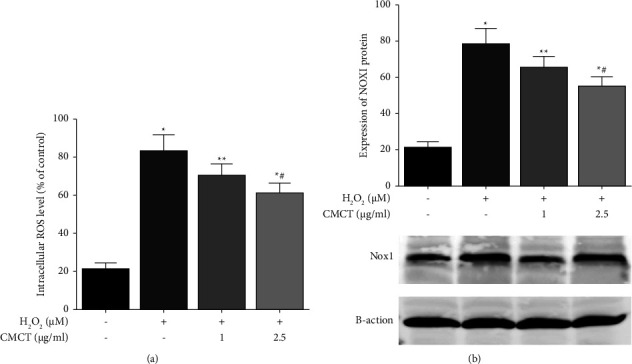
The influence of CMCT treatment on ROS generation and the protein expression of NOX1 in the ARPE-19 cells. (a) The influence of CMCT treatment on the production of ROS caused by H2O2. (b) The impact of CMCT (1 μg/ml and 2.5 μg/ml) on the protein expression of NOX1 in the ARPE-19 cells after H2O2 treatment was assayed via flow cytometry. The information is presented as mean ± SD for three separate replicates. Compared with the control group: ∗P < 0.05 and ∗∗P < 0.01. Compared with the H2O2 control samples: #P < 0.05 and ##P < 0.01.
3.5. The Influence of CMCT on Regulating the Antioxidant Content of the ARPE-19 Cells Exposed to H2O2
Since oxidative stress is crucial in the senescence state of normal ARPE-19 cells, this study examined the activity of MDA, an indicator of lipid oxidation, and GSH, CAT, and SOD, biomarkers of oxidative in the ARPE-19 cells subjected to various treatments. Compared with the untreated cells, H2O2 pretreatment alone for 12 h yielded higher MDA levels but decreased the GSH, CAT, and SOD activity (Figures 6(a)–6(d)). CMCT treatment alone did not affect the MDA level and three oxidative stress biomarkers. However, after incubation with CMCT (2.5 μg/ml, 1 μg/ml) for 12 h, the MDA level decreased according to the concentration, while the SOD, CAT, and GSH activity wer restored to normal levels. The cellular response to oxidative stress is regulated by Nrf2 to maintain redox homeostasis. It can mitigate the ROS and electrophile damage in cells by inducing and controlling antioxidant protein expression to stabilize the cells and facilitate the dynamic redox balance of the organism. This study assessed the impact of CMCT on the nuclear translocation expression of Nrf2 proteins in the ARPE-19 cells, while observing the upregulation of Nrf2 protein epitope expression after treatment with different CMCT concentrations (1 μg/ml, 2.5 μg/ml) (Figure 6(e)).
Figure 6.
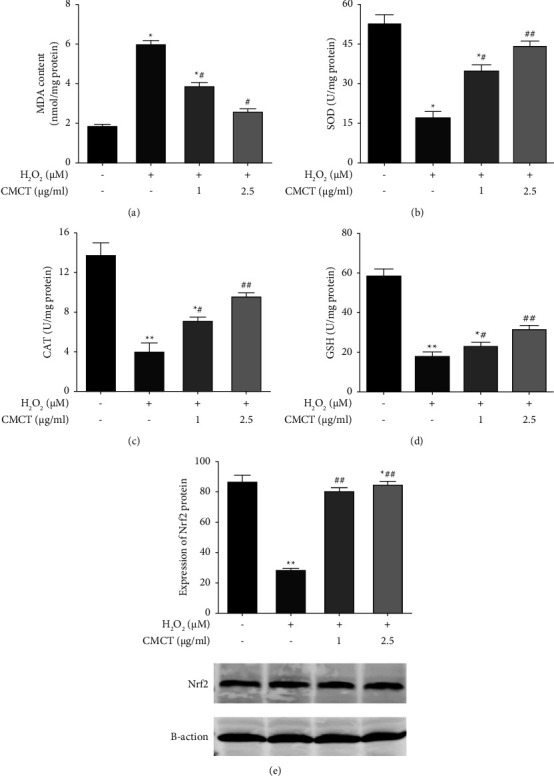
The antioxidant activity in the ARPE-19 cells exposed to H2O2 is down-modulated by CMCT. (a) The CMCT (1 μg/ml, 2.5 μg/ml) impact on the MDA degradation by lipid peroxide in the ARPE-19 cells after H2O2 treatment. (b) The CMCT (1 μg/ml and 2.5 μg/ml) impact on the SOD behavior in the ARPE-19 cells after H2O2 treatment. (c) The CMCT (1 μg/ml, 2.5 μg/ml) impact on the CAT levels in ARPE-19 cells after H2O2 exposure. (d) The CMCT (1 μg/ml, 2.5 μg/ml) impact on the GSH content in the ARPE-19 cells after H2O2 exposure. (e) CMCT encourages H2O2 to down-modulate the degree of intracellular antioxidant levels in ARPE-19 cells. The data are shown as mean ± SD for three separate replicates. Compared with the control group: ∗P < 0.05 and ∗∗P < 0.01. Compared with the H2O2 control samples: #P < 0.05 and ##P < 0.01.
3.6. The Influence of CMCT on the Protein Expression Associated with ARPE-19 Cell Apoptosis after H2O2 Exposure
Apoptosis and cell damage are often the result of oxidative stress. Therefore, this study explored whether CMCT effectively protected ARPE-19 cells against apoptosis and assessed the impact of CMCT on the expression of proteins associated with apoptosis. The results of the protein blot analysis are presented in (Figure 7). The ARPE-19 cells subjected to H2O2 (400 μM) treatment for 12 h showed significantly higher downregulation in the protein expression of Bcl-2, as well as higher cleaved caspase-3 and Bax expression levels than the control group, which was consistent with the flow cytometry results. Moreover, CMCT (1 μg/ml, 2.5 μg/ml) pretreatment for 12 h dose-dependently reversed this, increasing the protein expression of Bcl-2 and significantly decreasing these levels in cleaved caspase-3 and Bax.
Figure 7.
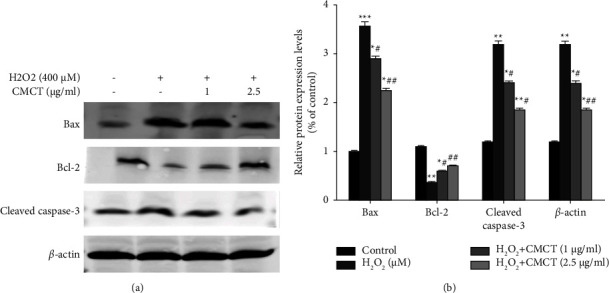
The impact of CMCT treatment on the expression of proteins associated with ARPE-19 cell apoptosis after H2O2 exposure. (a) The cells received a 12 h CMCT pretreatment at different concentrations (0–10 μg/ml), and H2O2 (400 μM) treatment for 12 h to examine the changes in the intracellular ROS levels. (b) Flow cytometry was used to analyze the expression of NOX1 proteins in the ARPE-19 cells following exposure to H2O2 and CMCT (2.5 μg/ml, 1 μg/ml). The data are shown as mean ± SD for three separate replicates. Compared to the control group: ∗P < 0.05 and ∗∗P < 0.01. Compared to the H2O2 control samples: #P < 0.05 and ##P < 0.01.
4. Discussion
Oxidative stress causes apoptosis in retinal endothelial cells. Excess ROS usually generates oxidative stress, leading to intracellular mitochondrial dysfunction and antioxidant system damage, ultimately causing degenerative diseases of the human retinal epithelium, such as AMD [24, 25]. Oxidative stress is primarily caused by the discrepancy between biological scavenging ability and active free radical generation. Fat accumulation, nucleic acid molecule cleavage mutations, protein inactivation and degradation, and photosensitive cell regeneration and repair decline in conjunction with environmental changes and increased age [26]. Consequently, reducing the cellular oxidative stress is critical for slowing AMD progression and facilitating potential treatment options for vision loss.
Clinical trials and research data have shown that the consumption of lutein, vitamins E and C, flavonoids, anthocyanins, zeaxanthin, and carotenoids can enhance cells and strengthen the antioxidant system to maintain good retinal function [27–31]. The minimal level of toxicity and powerful antioxidant capacity of the carotenoids found in natural plants have attracted considerable attention from researchers, rendering them effective as potential therapeutic agents. Singlet oxygen is quenched by carotenoids, scavenging free radicals to restrict lipid peroxidation reactions in vivo and blue light filtering, modulating light stress recovery time and neural processing speed [32]. Functions, Cordyceps militaris, as reported to contain many essential components required by the body, especially CMCT, has high antioxidant efficiency. As far as is known, this study is the first involving the antioxidant impact of CMCT on RPE cells. However, the mechanism underlying its anti-H2O2-induced oxidative stress effect remains unclear. Therefore, this research investigated the possible effect of CMCT in protecting against the death of retinal endothelial cells caused by H2O2.
Several studies have shown that H2O2 exposure induces ROS production while accelerating cell damage and apoptosis [33]. Since RPE cells are located behind photoreceptor cells and are mainly responsible for scavenging the oxidants generated by photoreceptor conversion, ARPE-19 cellular oxidative stress is used as a typical in vitro model to examine the functionality of human RPE cells. This study also revealed the pathogenesis of AMD by inducing oxidative stress and cellular damage [34]. The survival rate of the ARPE-19 cell exposed to 400 μM H2O2 decreased, while CMCT pretreatment (2.5 μg/ml, 1 μg/ml) substantially restricted the cytotoxicity caused by H2O2 and increased cell survival while reducing oxidative stress-induced apoptosis. Reports have indicated that protein restricts Bcl-2 and Bax. The increased protein expression of Bax and Bcl-2 alters the permeability of cellular mitochondrial membranes, subsequently inhibiting mitochondrial disruption and prompting the release of cytosolic c and cystathionine activation [35]. Moreover, caspase-3 selectively and efficiently cleaves proteins in various signal transduction pathways and is essential for controlling programmed cell death. Bcl-2 overexpression reportedly increases the survival of cells exposed to H2O2 [36]. The present study indicated that ARPE-19 cell exposure to H2O2 upregulated the caspase-3 and Bax protein expression. However, Bcl-2 protein expression was downregulated compared to the untreated control groups. H2O2 exposure elevated the expression of caspase-3 and Bax protein receptors and reduced that of Bcl-2. However, 12 h of CMCT pretreatment (1 μg/ml, 2.5 μg/ml) prior to H2O2 exposure successfully reversed this, decreasing the protein expression of caspase-3 and Bax, while enhancing that of Bcl-2. This suggests that the ROS generation in the ARPE-19 cells is associated with H2O2-induced apoptosis. CMCT protects against the ARPE-19 cell damage caused by H2O2 by inhibiting apoptosis.
ROS generation is associated with the co-interaction between nanoparticles and redox proteins such as mitochondria and NOX1, and cell surface receptors [37]. NOX1 is reportedly a vital enzymatic regulator of redox reactions in vivo and an identified system for ROS production. As one of the primary sources of ROS, NOX1 is crucial for the formation of RPE cell lesions in humans [38]. In addition, the GSH, CAT, and SOD activity, MDA levels, and NOX1 protein expression were examined. This study showed that exposing ARPE-19 cells to H2O2 significantly decreased the SOD, CAT, and GSH levels, while substantially increasing ROS and MDA production and NOX1 protein expression. However, CMCT treatment reversed these effects. Previous research indicated that the retinal cell apoptosis caused by oxidative stress was responsible for AMD pathogenesis, while the nuclear transcription factor, Nrf2, is crucial for oxidative stress regulation [39]. Nrf2 can upregulate antioxidant enzyme expression, scavenge free radicals, reduce intracellular oxidative stress levels, and decrease cell degeneration. It can also protect cells from oxidative damage by inducing ROS detoxification enzymes [40]. Moreover, Nrf2 is considered one of the most critical intracellular antioxidative stress mechanisms and plays a vital role in AMD treatment [41]. Therefore, the impact of CMCT on the translocation of Nrf2 in the ARPE-19 cells was examined via Western blotting, indicating that the Nrf2 expression was upregulated after CMCT treatment. Furthermore, it highlighted the potential of CMCT to safeguard RPE cells against the oxidative stress resulting from H2O2 by controlling NOX1 protein expression to reduce the ROS and MDA levels. Contrarily, the nuclear activation of Nrf2 protein increased the antioxidant enzyme activity of SOD, CAT, and GSH. Studies have shown that oxidative stress can reduce cell apoptosis, increase the total amount of apoptosis and ROS production, and prevent cells from oxidative stress-induced damage [42].
Therefore, this study shows that CMCT treatment successfully safeguards ARPE-19 cells against the oxidative damage resulting from exposure to H2O2 by inhibiting apoptosis, regulating ROS production and MDA formation, and increasing the activity of antioxidant enzymes. It also provides new insight into the ability of CMCT to help forestall and mitigate ocular diseases like AMD. Future research should explore the in vivo preventative and therapeutic effect of CMCT in AMD disease models to determine its complete mechanisms.
Acknowledgments
The authors did not receive specific funding.
Data Availability
The data supporting the findings of this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Lin Lan and Yufeng Li conceptualized and designed the study. Lin Lan, Shengyu Wang, Shuhua Duan, and Xiangyu Zhou collected and analyzed the data and experimental methods. Yufeng Li participated in drafting the manuscript and reviewing the final manuscript. All authors have read and approved the manuscript.
References
- 1.Chen Y., Bedell M., Zhang K. Age-related macular degeneration: genetic and environmental factors of disease. Molecular Interventions . 2010;10(5):271–281. doi: 10.1124/mi.10.5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seddon J. M., Cote J., Page W. F., Aggen S. H., Neale M. C. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Archives of Ophthalmology . 2005;123(3):321–327. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Wong W. L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health . 2014;2(2):p106–e116. doi: 10.1016/s2214-109x(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.Ding X., Patel M., Chan C. C. Molecular pathology of age-related macular degeneration. Progress in Retinal and Eye Research . 2009;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf S. Current status of anti-vascular endothelial growth factor therapy in Europe. Japanese Journal of Ophthalmology . 2008;52(6):433–439. doi: 10.1007/s10384-008-0580-4. [DOI] [PubMed] [Google Scholar]
- 6.Nowak J. Z. J. P. R. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacological Reports: PR . 2006;58:353–363. [PubMed] [Google Scholar]
- 7.Spraul C. W., Lang G. E., Grossniklaus H. E. Morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Investigative Ophthalmology & Visual Science . 1996;37(13):2724–2735. [PubMed] [Google Scholar]
- 8.Panieri E., Santoro M. M. ROS homeostasis and metabolism: a dangerous liaison in cancer cells. Cell Death & Disease . 2016;7(6) doi: 10.1038/cddis.2016.105.p2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson R. E., Rapp L. M., Wiegand R. D. J. C. E. R. Lipid peroxidation and retinal degeneration. Current Eye Research . 1984;3(1):223–227. doi: 10.3109/02713688408997203. [DOI] [PubMed] [Google Scholar]
- 10.Liang F.-Q., Godley B. F. J. E. E. R. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Experimental Eye Research . 2003;76(4):397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 11.Heesterbeek T. J., Lechanteur Y. T. E., Lores-Motta L., et al. Complement activation levels are related to disease stage in AMD. Investigative Opthalmology & Visual Science . 2020;61(3):p. 18. doi: 10.1167/iovs.61.3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao H., Chen Y., Liu J., et al. Iron-generated hydroxyl radicals kill retinal cells in vivo: effect of ferulic acid. Human & Experimental Toxicology . 2008;27(4):327–339. doi: 10.1177/0960327108092294. [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Solís I., Bosch-Morell F., Villagrasa V., et al. Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration. Neural Regeneration Research . 2020;15(12):p. 2207. doi: 10.4103/1673-5374.284978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong J. M., Chia L. S., Goh N. K., Chia T. F., Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry . 2003;64(5):923–933. doi: 10.1016/s0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 15.Evans J. R. Antioxidant vitamin and mineral supplements for slowing the progression of age‐related macular degeneration. Cochrane Database of Systematic Reviews . 2006;2 doi: 10.1002/14651858.cd000254.pub2.CD000254 [DOI] [PubMed] [Google Scholar]
- 16.Różanowska M., Bakker L., Boulton M. E., Rozanowski B. Concentration dependence of vitamin C in combinations with vitamin E and zeaxanthin on light-induced toxicity to retinal pigment epithelial cells. Photochemistry and Photobiology . 2012;88(6):1408–1417. doi: 10.1111/j.1751-1097.2012.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souied E. H., Delcourt C., Querques G., et al. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration. Ophthalmology . 2013;120(8):1619–1631. doi: 10.1016/j.ophtha.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Yu H. M., Wang B. S., Huang S. C., Duh P. D. Comparison of protective effects between cultured Cordyceps militaris and natural cordyceps sinensis against oxidative damage. Journal of Agricultural and Food Chemistry . 2006;54(8):3132–3138. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- 19.He B. L., Zheng Q. W., Guo L. Q., et al. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. International Journal of Biological Macromolecules . 2020;145:11–20. doi: 10.1016/j.ijbiomac.2019.12.115. [DOI] [PubMed] [Google Scholar]
- 20.Gong X., Draper C. S., Allison G. S., Marisiddaiah R., Rubin L. Effects of the macular carotenoid lutein in human retinal pigment epithelial cells. Antioxidants . 2017;6(4):p. 100. doi: 10.3390/antiox6040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijttebier S., D’Hondt E., Noten B., Hermans N., Apers S., Voorspoels S. Ultra high performance liquid chromatography versus high performance liquid chromatography: stationary phase selectivity for generic carotenoid screening. Journal of Chromatography A . 2014;1332:46–56. doi: 10.1016/j.chroma.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Q., Wei T., Lin Y., et al. Developing a novel two-stage process for carotenoid production by Cordyceps militaris (ascomycetes) International Journal of Medicinal Mushrooms . 2019;21(1):47–57. doi: 10.1615/intjmedmushrooms.2018029002. [DOI] [PubMed] [Google Scholar]
- 23.Simpson D. S. A., Oliver P. L. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants . 2020;9(8):p. 743. doi: 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bora N. S., Matta B., Lyzogubov V. V., Bora P. S. Relationship between the complement system, risk factors and prediction models in age-related macular degeneration. Molecular Immunology . 2015;63(2):176–183. doi: 10.1016/j.molimm.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Lim L. S., Mitchell P., Seddon J. M., Holz F. G., Wong T. Y. Age-related macular degeneration. The Lancet . 2012;379(9827):1728–1738. doi: 10.1016/s0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 26.Mitter S. K., Song C., Qi X., et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy . 2014;10(11):1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang J. W., Kim E. K., Lee S. J., et al. Antioxidant activity and protective effect of anthocyanin oligomers on H2O2-triggered G2/M arrest in retinal cells. Journal of Agricultural and Food Chemistry . 2012;60(17):4282–4288. doi: 10.1021/jf205321j. [DOI] [PubMed] [Google Scholar]
- 28.Kalariya N. M., Ramana K. V., Srivastava S. K., van Kuijk F. J. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Experimental Eye Research . 2008;86(1):70–80. doi: 10.1016/j.exer.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung H. H., Galano J. M., Crauste C., Durand T., Lee J. C. Y. Combination of lutein and zeaxanthin, and DHA regulated polyunsaturated fatty acid oxidation in H2O2-stressed retinal cells. Neurochemical Research . 2020;45(5):1007–1019. doi: 10.1007/s11064-020-02994-4. [DOI] [PubMed] [Google Scholar]
- 30.Muangnoi C., Sharif U., Ratnatilaka Na Bhuket P., Rojsitthisak P., Paraoan L. Protective effects of curcumin ester prodrug, curcumin diethyl disuccinate against H2O2-induced oxidative stress in human retinal pigment epithelial cells: potential therapeutic avenues for age-related macular degeneration. International Journal of Molecular Sciences . 2019;20(13):p. 3367. doi: 10.3390/ijms20133367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yiğit M., Güneş A., Uğuz C., et al. Effects of astaxanthin on antioxidant parameters in ARPE-19 cells on oxidative stress model. International Journal of Ophthalmology . 2019;12(6):930–935. doi: 10.18240/ijo.2019.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi S., Huang W., Chen S., et al. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. International Journal of Biological Macromolecules . 2020;150:261–280. doi: 10.1016/j.ijbiomac.2020.02.050. [DOI] [PubMed] [Google Scholar]
- 33.Plafker S. M., O’Mealey G. B., Szweda L. I. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. International Review of Cell and Molecular Biology . 2012;298:135–177. doi: 10.1016/B978-0-12-394309-5.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ke Y., Fan X., Rui H., et al. Exosomes derived from RPE cells under oxidative stress mediate inflammation and apoptosis of normal RPE cells through Apaf1/caspase‐9 axis. Journal of Cellular Biochemistry . 2020;121(12):4849–4861. doi: 10.1002/jcb.29713. [DOI] [PubMed] [Google Scholar]
- 35.Brentnall M., Rodriguez-Menocal L., De Guevara R. L., Cepero E., Boise L. H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biology . 2013;14(1):32–39. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang B., Ye D., Wang Y. Caspase-3 as a therapeutic target for heart failure. Expert Opinion on Therapeutic Targets . 2013;17(3):255–263. doi: 10.1517/14728222.2013.745513. [DOI] [PubMed] [Google Scholar]
- 37.Wang L., Azad N., Kongkaneramit L., et al. The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. The Journal of Immunology . 2008;180(5):3072–3080. doi: 10.4049/jimmunol.180.5.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bedard K., Krause K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews . 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 39.Pendyala S., Natarajan V. Redox regulation of Nox proteins. Respiratory Physiology & Neurobiology . 2010;174(3):265–271. doi: 10.1016/j.resp.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue D., Zhou X., Qiu J. Emerging role of NRF2 in ROS-mediated tumor chemoresistance. Biomedicine & Pharmacotherapy . 2020;131 doi: 10.1016/j.biopha.2020.110676.110676 [DOI] [PubMed] [Google Scholar]
- 41.Ferino A., Rapozzi V., Xodo L. E. The ROS-KRAS-Nrf2 axis in the control of the redox homeostasis and the intersection with survival-apoptosis pathways: implications for photodynamic therapy. Journal of Photochemistry and Photobiology B: Biology . 2020;202 doi: 10.1016/j.jphotobiol.2019.111672.111672 [DOI] [PubMed] [Google Scholar]
- 42.Liu H., Liu W., Zhou X., et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Molecular Medicine Reports . 2017;16(2):2069–2074. doi: 10.3892/mmr.2017.6838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available on reasonable request from the corresponding author.


