Abstract
Arterial hypertension is the most prevalent global modifiable risk factor for cardiovascular morbidity and mortality. Despite the availability of numerous pharmacologic treatments, many patients do not achieve guideline-recommended blood pressure targets. Therefore, renal sympathetic denervation (RDN), a process in which catheter-directed techniques are used to ablate portions of the renal artery to reduce sympathetic activity, has been extensively investigated as a complementary and nonpharmacologic approach for the treatment of arterial hypertension. This review seeks to discuss the pathophysiological rationale of this strategy, to survey its history and development, and to highlight the current clinical evidence and possible future directions of its employment. In sum, RDN has demonstrated itself to be a safe and well-tolerated endovascular intervention that can reliably contribute to improved blood pressure control and, perhaps ultimately, significant cardiovascular prognosis.
Keywords: Denervation, Endovascular, Renal, Radiofrequency, Ultrasound, Hypertension
With an estimated global incidence of over 25% and 10 million deaths annually, arterial hypertension is the most prevalent modifiable risk factor for cardiovascular morbidity and mortality worldwide.1 In the United States alone, the adjusted annual incremental cost for the hypertensive adult population compared with the nonhypertensive population is $131 billion per year.2,3 Despite the availability of lifestyle interventions and numerous medications to treat high blood pressure, rates of control are poor.4 Especially in light of the ongoing challenge of an aging and more sedentary population, alternative, nonpharmacologic interventions for the management of hypertension may be attractive adjuncts to conventional therapies. Among these interventions, RDN has been widely studied over the past decade. This review aims to summarize the history, underlying physiology, and current evidence surrounding RDN in the management of arterial hypertension.
Physiology of Renal Sympathetic Regulation of Blood Pressure
Despite its multifactorial pathogenesis, the correlation between sympathetic activation and arterial hypertension is well documented. This relationship is mediated through both renal efferent and afferent sympathetic signaling mechanisms. Increased activity of sympathetic renal efferent nerves results in renal arteriolar vasoconstriction, reduced glomerular filtration, and increased renin secretion with subsequent angiotensin and aldosterone stimulation. These changes ultimately lead to downstream sodium and water retention.5 Activation of renal afferent fibers can be mediated by renal ischemia, hypoxia, or oxidative stress, which facilitate increased hypothalamic stimulation and central sympathetic outflow to the juxtaglomerular apparatus, further increasing the aforementioned mediators of vascular resistance.6 Increased sympathetic outflow can be quantified using norepinephrine spillover, which measures renal-specific concentrations of endogenous norepinephrine. In 10 patients with resistant hypertension treated with radiofrequency RDN, there was a norepinephrine spillover reduction of 48% from the left kidney and 75% from the right kidney, with a marked “whole-body” norepinephrine spillover reduction of 42%.7
Splanchnicectomy and the Historical Efficacy of RDN
In the 1940s and 1950s, an era with limited oral medications for the treatment of hypertension, subdiaphragmatic lumbodorsal splanchnicetomies were explored as a possible treatment for malignant hypertension. Benefit was most successfully demonstrated in 1953 in a surgical series of 1266 patients treated with splanchnicetomy, which showed a 5 year mortality of 19% versus 54% among 467 medically-treated controls.8 Nevertheless, high perioperative morbidity and mortality coupled with severe adverse events including orthostatic hypotension, syncope, hyperhidrosis, impotence, incontinence, and depression limited its use. As the advent of new oral medications were developed, this modality was largely abandoned.9
Catheter-Directed Ablation
As the understanding of the close anatomical proximity between renal nerves and renal arteries developed, the novel idea of utilizing intraarterial catheter-based ablation systems to interrupt nerve signaling was explored. The ultimate goal of ablation is to disturb the afferent and efferent sympathetic nerves within the adventitia of the renal arteries with minimal disruption to the artery’s external elastic lamina or proximal endoluminal surface. The proof of principle of percutaneous intervention for this goal was originally done by Krum et al.,10 who, utilizing high focused radiofrequency ablative energy, demonstrated a sustained reduction in blood pressure in patients with resistant hypertension without serious adverse events, in addition to sustained postprocedure reduction in measured renal norepinephrine spillover. This initial procedure has been refined with the advent of more dedicated radiofrequency catheters that conform to the artery. In addition, investigation of other ablative modalities have been developed over time, including ultrasound and ethanol ablation. As one surveys the scientific and technological innovations that enabled the development of these new modalities, its historical progression can be discussed in 3 discrete therapeutic phases.
Phase 1: The Advent of Catheter-Directed Radiofrequency Ablation Techniques
The first open-label and randomized trial using patients with severe and drug-resistant hypertension was the SYMPLICITY HTN-1 trial, which enrolled 153 patients with at least a systolic blood pressure (SBP) of 160 mmHg, were on at least 3 antihypertensive medications at optimal doses, and utilized the Medtronic Symplicity Flex monoelectrode radiofrequency denervation device for artery ablation. The study did not have a control group, nor did it utilize 24-hour ambulatory blood pressure measurements, which is a preferred method of blood pressure assessment in modern clinical trials.11 At 6 months there was a reduction in SBP of 22 mmHg (95% confidence interval [CI], −32 to −12 mmHg) and a reduction in SBP of 32 mmHg at 36 months (95% CI, −35.7 to −28.2 mmHg). Furthermore, more than 93% of patients at 36 months had a greater than 10 mmHg fall in SBP.12
The SYMPLICITY HTN-2 study advanced the gains of its namesake, utilizing the same device, and randomized 106 patients 1:1 to radiofrequency RDN plus medical therapy, or medical therapy alone. Unlike the previous investigation, this study utilized a control group in addition to 24-hour ambulatory pressure monitoring. However, there was no blinding to study group allocation, and the control group did not undergo a sham procedure. Patients with hemodynamically significant renal artery stenosis, previous renal artery interventions or unsuitable anatomy, estimated glomerular filtration rates less than 45 ml/min per 1.73 m2 and type 1 diabetes mellitus were excluded. The change in mean office blood pressure at 6 months in the patients in the radiofrequency RDN group was −32 mmHg systolic and −12 mmHg diastolic, with no significant change in the control group. The between-group difference in 24-hour ambulatory monitoring was also significant, with a reduction of 31 mmHg systolic and 11 mmHg diastolic.13
In both the SYMPLICITY HTN-1 and SYMPLICITY HTN-2 trials there were no reported procedure-related or device-related adverse events. That being said, potential complications of catheter-directed radiofrequency ablation include secondary arterial stenosis, thrombosis, perforation or arterial dissection.14
Phase 1A: Sham-Controlled Catheter-Directed Radiofrequency Ablation - SYMPLICITY HTN-3
On the basis of the SYMPLICITY HTN-2 trial, there was great excitement for the clinical use of RDN, but the United States Food and Drug Administration required an additional trial for device approval. Therefore, an additional, multicenter, single-blind, randomized controlled trial incorporating a sham group was conducted. 535 patients with an office SBP 160 mmHg despite the prescription of at least 3 antihypertensive medications including a diuretic were randomized in a 2:1 fashion into RDN or sham groups. This trial required 24-hour SBP of at least 135 mmHg for inclusion in the study; exclusion criteria were similar to the previous study protocol. Patients were blinded to the type of intervention using sedation, sensory isolation and lack of familiarity with the procedure, and clinical assessors were additionally blinded to treatment assignment. After 6 months, blood pressure dropped in both arms, but the mean change in office SBP in the RDN group was −14 ± 24 mmHg compared with −12 ± 26 mmHg in the sham procedure group. The between-group differences were not significant, which was a failure of the trial’s primary outcome measure. There was also no significant between-group difference in mean change in 24-hour ambulatory SBP.15 These results were maintained at 12 months, showing no further reduction in office or ambulatory blood pressures.16
Extensive post hoc analysis of SYMPLICITY HTN-3 has been conducted to reconcile the stark contrast between these study findings and the observations from the prior SYMPLICITY trials. For example, the effect of adjunctive medications used to treat blood pressure has been carefully examined. As the study required only 2 weeks of antihypertensive therapy prior to subject enrollment, it is possible that patients were on fluctuating regimens at the time of randomization, in part discerned by the profound drop in blood pressure observed within the sham group. Notably, 39% of patients within the study underwent medication changes, with at least one-third of patients changing 2 or more pharmacological agents.17 This suggests that variable medical adherence may have influenced the study results. The study may have also been confounded by limited operator experience and device capability. Subgroup analysis demonstrated that approximately 35 study operators performed the procedure only once.18 Moreover, only 6% of the subjects in the RDN group had a complete, circumferential ablation pattern. The first-generation Symplicity Flex system utilizes a catheter tip electrode which requires operator maneuvering and rotation to achieve appropriate ablation positions. It has been shown that this procedural necessity only ablates to a depth of approximately 4 mm, theoretically missing approximately 30% of renal artery nerves, which can be as deep as 10 mm.19 Finally, subsequent studies have demonstrated that radiofrequency ablation is more effective at interrupting renal nerves when energy was directed at the distal segment of the renal artery as well as branch arteries, locations that were not targeted in this study.20
As a result of the identified challenges uncovered in the SYMPLICITY HTN-3 study, several academic-industry-regulatory partnership working groups were convened to better standardize trial methodology as well as the devices and protocols within future clinical trials. For example, focus was made on technologies that would enable more consistent and circumferential ablation patterns, stricter criteria for study subject inclusion, for run-in phases, and for methods that would ensure appropriate medication standardization and adherence.21 These efforts laid the groundwork for newer trials to investigate the efficacy of catheter-directed RDN.
Phase 2: New Ablation Techniques, New Patient Populations
The first set of studies to address the challenges identified in the SYMPLICITY HTN-3 study and to incorporate the newly described guidelines was made possible by the development of the second-generation Symplicity Spyral catheter, a multielectrode and helical radiofrequency device with the ability to reach distal renal artery segments and branch arteries as small as 3 mm.22 This new device was utilized in the SPRYAL HTN Global Clinical Trial Program, a parallel feasibility study fashioned in 2 parts. The first was SPYRAL HTN-OFF MED, a multicenter, randomized, sham-controlled RDN study in 331 patients with untreated hypertension and office SBP greater than 150 mmHg and less than 180 mmHg (Table 1). If patients were on antihypertensive medications, they were required to discontinue them, and urine and plasma screens were monitored for their absence. The study’s primary efficacy endpoint was based on 24-hour ambulatory blood pressure monitoring. All operators had previous RDN experience, and a standardized approach was used to successfully target accessible renal vessels. There were approximately 9 main renal artery ablations and 13 branch artery ablations per kidney in each trial. At 3 months the RDN group had a greater reduction in both office blood presssure (−10/−5.3 mmHg) and 24-hour ambulatory blood pressure (−5.5/−4.4 mmHg) compared to the sham-procedure group, which showed no significant changes. This was a marked difference from the results in SYMPLICITY HTN-3.23 Interestingly, post hoc analysis demonstrated that higher heart rates at baseline were predictors of greater blood pressure reductions after the procedure, potentially identifying patient parameters that would yield greater utility of the treatment.17 There were no reported safety concerns using the new technology.24
Table 1.
Landmark trials and primary outcomes
| Study (Date) | Design | Sample size | RDN (N) | Control (N) | Catheter | Ablation method | Primary outcome | Longest follow-up |
|---|---|---|---|---|---|---|---|---|
| SYMPLICITY HTN -1 (2009)11 | Open-label | 153 | 153 | N/A | Symplicity flex | Monoelectrode radiofrequency | Change in office SBP at 6 months: SBP −22 mmHg (95% CI −32 to −12 mmHg), P < 0.001 |
36 mos |
| SYMPLICITY HTN-2 (2010)13 | Open-label, parallel, RCT | 190 | 52 | 54 | Symplicity flex | Monoelectrode radiofrequency | Change in mean office SBP at 6 months: RDN −32 ± 23 mmHg vs. control 1 ± 21 mmHg, P < 0.0001 | 6 mos |
| SYMPLICITY HTN-3 (2014)16 | RCT, double-blinded, multicenter | 1441 | 364 | 171 | Symplicity flex | Monoelectrode radiofrequency | Change in office SBP at 6 months: RDN −14.1 ± 23.9 mmHg vs. sham −11.7 ± 25.9 mmHg, P = 0.26 | 12 mos |
| SPYRAL HTN-OFF MED (2017)23 | RCT, sham-controlled | 1519 | 166 | 165 | Symplicity spyral | Multielectrode radiofrequency | Change in 24-h ambulatory SBP at 3 months: RDN −5.5 mmHg (95% CI −9.1 to −2.0 mmHg) vs. sham −0.5 mmHg (95% CI −3.9 to 2.9 mmHg), P = 0.0414 | 12 mos |
| SPYRAL HTN-ON MED (2018)25 | RCT, double-blinded, sham-controlled | 467 | 38 | 42 | Symplicity spyral | Multielectrode radiofrequency | Change in 24-h ambulatory SBP at 6 months: RDN −9.0 mmHg (95% CI −12.7 to −5.3 mmHg) vs. sham −1.6 mmHg (95% CI −5.2 to 2.0 mmHg), P = 0.0051 | 36 mos |
| RADIANCE-HTN SOLO (2018)29 | RCT, single-blinded, sham-controlled | 803 | 73 | 73 | PARADISE | Ultrasound | Change in daytime SBP at 2 months baseline-adjusted SBP difference vs. sham −6.3 mmHg [95% CI −9.4 to −3 mmHg], P < 0.001 | 12 mos |
| RADIOSOUND-HTN (2019)37 | 3-armed RCT, single-blinded | 1884 | 39 (Main) 39 (Branch) 42 |
-- | Symplicity spyral Symplicity spyral PARADISE |
Multielectrode radiofrequency Multielectrode radiofrequency Ultrasound |
Change in daytime SBP at 3 months: radiofrequency-based RDN of main renal artery −6.5 ± 10.3 mmHg vs. radiofrequency-based RDN of main renal artery and branches −8.3 ± 11.7 mmHg vs. ultrasound-based RDN −13.2 ± 13.7 mmHg; overall change −9.5 ± 12.3 mmHg (P < 0.001) | 3 mos |
| RADIANCE-HTN TRIO (2021)33 | RCT, single-blinded sham-controlled | 989 | 69 | 67 | PARADISE | Ultrasound | Change in daytime ambulatory SBP at 2 months significantly greater in RDN than sham median between-group difference −4.5 mmHg ([95% CI −8.5 to 0.3], P = 0.022) | 6 mos |
| REQUIRE (2021)36 | RCT, single-blinded sham-controlled | 411 | 72 | 71 | PARADISE | Ultrasound | Change in 24-h ambulatory SBP at 3 months was not significantly different between 2 groups (between-group difference at 3 mos: −0.1, 95% CI −5.5, 5.3; P = 0.971) | 3 mos |
CI, confidence interval; N/A; not available; RCT, randomized controlled trial; RDN, renal sympathetic denervation; SBP, systolic blood pressure.
The second study, SPYRAL HTN-ON MED (pilot), was a multicenter, sham-controlled single-blind trial in 80 patients with uncontrolled hypertension on 1 to 3 medications and randomized to RDN or sham. Like its predecessor, urine and serum assays before and after RDN or sham treatment were utilized, however now to confirm medication adherence rather than absence. At 6 months, the change in 24-hour ambulatory SBP was −9.0 mmHg (95% CI −12.7 to −5.3 mmHg) in the RDN group compared to −1.6 mmHg (95% CI −5.2 to +2.0 mmHg) in the sham group.25 Furthermore, at 3 year follow-up, those 35 patients who underwent denervation had a 10 mmHg greater reduction in 24-hour ambulatory SBP, despite similar use of antihypertensive agents. There was also a 5.9 mmHg larger fall in mean ambulatory diastolic blood pressure, and a 37.9% difference in the proportion of patients controlled to below 140 mmHg in the denervation arm.26 Notably, in the pilot study, only 60% of patients in either group were fully adherent to the prescribed antihypertensive regimens, but these rates were consistent between both groups. This discovery identified the inherent challenge of consistent antihypertensive adherence in all patients. Nevertheless, the success of these 2 studies was encouraging, fully supporting the idea that with novel technologies and rigorous protocols RDN therapy can significantly reduce blood pressure in patients with arterial hypertension (Figure 1).
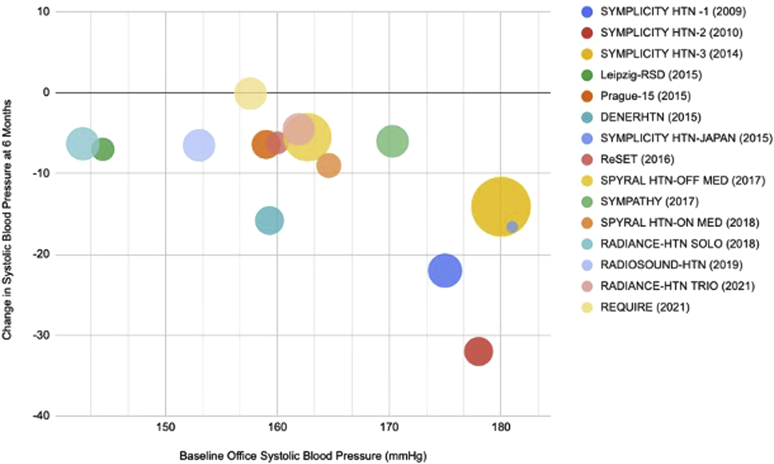
Figure 1.
Change in systolic blood pressure with renal sympathetic denervation. Association between baseline office systolic blood pressure and systolic pressure reductions at 6 months after renal denervation in major clinical trials. Trial sizes are reflected by the sizes of the bubbles.
Phase 2a: Endovascular Ultrasound-Based Renal Denervation Therapy
Ultrasound-directed therapies which use frictional thermal energy through circumferentially-distributed ultrasonic waves emitted from a piezoelectric crystal have also demonstrated efficacy in lowering blood pressure. The most studied ultrasound-based system is the PARADISE system, a 6 French balloon catheter with a cylindrical transducer that emits ultrasonic energy to a depth of 1 mm to 6 mm. The PARADISE system effects circumferential nerve injury, because the ultrasound balloon is centered in the renal artery by the inflation of a water-cooled balloon which also protects the endothelial wall from frictional heat. The safety and efficacy of the PARADISE system was first demonstrated in 11 patients with resistant hypertension who received an average of 5 ultrasound emissions for a total denervation duration of less than 4 minutes. Results showed comparable 3-month efficacy of this modality, with mean office and home blood pressure-lowering of −36/−17 mmHg and −22/−12 mmHg, respectively.27 These results were expanded upon in the ACHIEVE study, which is a prospective, multicenter, nonrandomized, and postmarket study evaluating the safety and efficacy of the PARADISE system in 96 patients with resistant hypertension. At 12 months the average 24-hour ambulatory blood pressure change was −7.5/−3.8 ± 18.3/10.6 mmHg (P = 0.0024) with an average office blood pressure change of −15.0/−7.0 ± 27.0/12.3 mmHg (P < 0.0001).28 There were no safety concerns reported.
Three recent randomized and sham-controlled clinical trials aimed to evaluate the safety and efficacy of the PARADISE ultrasound ablation system have been completed. The RADIANCE-HTN trials were designed to evaluate 2 specific patient populations, namely the RADIANCE-HTN SOLO study, which included patients with mild-to-moderate hypertension after 4 weeks of antihypertensive medication discontinuation; and the RADIANCE-HTN TRIO study, which included patients with resistant hypertension despite the use of 3 antihypertensive medications. The REQUIRE study, completed in Japan and South Korea, was also designed to evaluate the PARADISE system among Asian patients with resistant hypertension who were on standard-of-care antihypertensive treatment.29
In the RADIANCE-HTN SOLO trial, 146 patients were randomized 1:1 to RDN with ultrasound or sham according to the aforementioned study design. After 2 months of antihypertensive medication discontinuation, “stepped-up” pharmacotherapy was reinitiated if monthly home blood pressure readings exceeded 135/85 mmHg, regardless if patients were in the sham or RDN groups, and with study blinding maintained. After 2 months, reduction in daytime ambulatory systolic pressure was greater in the ultrasound-mediated denervation group than the sham group (baseline-adjusted SBP difference of −6.3 mmHg [95% CI −9.4 to −3 mmHg], P = 0.0001).30 At 6 months, 65% of patients in the ultrasound denervation group and 85% of patients in the sham group required reinitiation of pharmacological treatment, however the blood pressure-lowering effects of the treatment continued to be maintained (SBP difference −4.3 mmHg [95% CI −7.9 to −0.6 mmHg], P = 0.024).31 At 12 months when the patient, investigator and other treating physicians were unblinded to the original treatment assignments and allowed to adjust antihypertensive regimens, the decrease in daytime ambulatory SBP from baseline in the RDN group remained stable (−16.5 mmHg [95% CI −3.6 to −29.4 mmHg]) and the adjusted between-group differences in change in office SBP and home SBP favored the RDN group (−6.3 mmHg [95% CI: −11.1 to −1.5 mmHg; P < 0.010] and −3.4 mm Hg [95% CI: −6.9 to 0.1 mmHg; P = 0.062], respectively). The overall proportion of patients receiving any antihypertensive medications and those on 2 or more antihypertensive medications remained lower in the RDN group than in the sham group.32 Further, among 33 patients initially randomized to sham who underwent crossover RDN for persistently elevated blood pressure, mean changes in this group’s daytime systolic ambulatory blood pressure from precrossover to 2-months and 6-months post-RDN was lowered (−11.2 mmHg [95% CI: −24.9 to 2.5 mmHg], n = 33; P < 0.001) and −10.8 [95% CI: −28.5 to 6.5 mmHg], n = 27; P < 0.001), respectively. Among these patients, 18 (54.5%), had their daytime ambulatory blood pressure controlled at 2 months, and 44.4% were controlled at 6 months post-RDN.33 Finally, this trial highlighted a potential theoretical treatment advantage of RDN in demonstrating significant blood pressure-lowering effects throughout the 24-hour circadian cycle when compared to the sham group. This “always on” effect may differentiate its mechanism of action from conventional time-dependent pharmacological interventions, highlighting itself as an adjunctive strategy that works outside the limitations of standard dosing regimens and unpredictable patient adherence. There were no major adverse events reported in any treatment group of any of the aforementioned trials.
The RADIANCE-HTN TRIO study was a multicenter, single-blind, and sham-controlled trial with 136 patients with resistant hypertension (office blood pressure of >140/90 mmHg despite 3 or more antihypertensive medications including a diuretic) and randomized 1:1 to ultrasound RDN using the PARADISE system, or to sham. In order to reduce pill burden and to optimize medication adherence, prior to randomization, patients were switched from their baseline medication regimens to 4 weeks of a fixed-dosed, single pill combination of amlodipine 10 mg (or 5 mg if they had leg edema), valsartan 160 mg (or olmesartan 40 mg given availability) and hydrochlorothiazide 25 mg if blood pressure remained elevated. Adherence to medications was evaluated using urine samples, and at 2 months was found to be similar between the 2 treatment groups (82% in the renal denervation arm and 82% in the sham arm). At 2 months ultrasound-mediated RDN reduced daytime ambulatory SBP significantly more than sham (8 mmHg [95% CI −16.4 to 0] vs. −3.0 mmHg [95% CI 10.3 to −1.8 mmHg]; median between-group difference −4.5 mmHg ([95% CI −8.5 to 0.3], P = 0.022).34 Importantly, the SBP-lowering effect of ultrasound RDN was demonstrated to be consistent over the entire 24-hour circadian cycle, similar to the RADIANCE-HTN SOLO and the SPYRAL trials. This deserves particular emphasis, because night-time blood pressure elevations have been associated with cardiovascular events.35 The increment in antihypertensive medications was also more common in the sham group than in the RDN group at the 2-month, and 6-month follow-ups, stemming from less use of spironolactone.36 Medication adherence remained high between both groups at 6 months, and there were no reported differences in safety outcomes.
With similar goals to the RADIANCE-HTN SOLO trial, the recently published REQUIRE study sought to evaluate the efficacy of the PARADISE ultrasound system exclusively in an Asian population with resistant hypertension, a cohort which had not yet been robustly investigated. A total of 143 patients were randomized 1:1 to ultrasound RDN or to sham with the primary endpoint of change in baseline 24-hour ambulatory SBP at 3 months. Surprisingly, this measure was not significantly different between the renal denervation (−6.6 mmHg) and the sham control (−6.5 mmHg) groups (mean difference: −0.1, [95% CI −5.5 to 5.3 mmHg]; P = 0.971).37 Nevertheless, the absolute change of the reduction from baseline in 24-hour ambulatory SBP in the renal denervation group was of a similar magnitude to decreases in the 2 previous prospectively-powered sham-controlled ultrasound-based RDN studies.30,34 Similar to the SYMPLICITY HTN-3 trial, the key difference between the REQUIRE trial and these trials was the sizable reduction from baseline in 24-hour ambulatory SBP in the sham group, approximately double that of its predecessors. A possible explanation to this finding is that, unlike RADIANCE-HTN TRIO, this study did not standardize patients on a fixed medication regimen prior to initiation, and did not monitor medication adherence or witness intake of medications. Study assessors were also not blinded within the trial. Similar to the lessons learned in the SYMPLICITY HTN-3 trial, its subsequent reappraisal and noteworthy future trial design changes, this study appears to have also been affected by methodological concerns that its investigators aim to address in an upcoming trial.
Finally, the RADIOSOUND-HTN trial was the first of its kind to compare the different treatment modalities and anatomical targets of catheter-based RDN in a tripartite design as follows: (i) radiofrequency RDN (using the Symplicity Spyral system) of the main renal artery, (ii) radiofrequency RDN (using the Symplicity Spyral system) of both the main renal artery and side branches, and (iii) endovascular ultrasound-based RDN (using the PARADISE system) of the main renal artery. The study randomized 120 patients with resistant hypertension (office SBP >160 mmHg or > 90 mmHg diastolic despite treatment with more than 3 different classes of antihypertensives including a diuretic) in a 1:1:1 manner. At 3 months, systolic daytime ambulatory blood pressure decreased by a total of −9.5±12.3 mmHg in the whole cohort (P < 0.001). Nevertheless, blood pressure was significantly more reduced in the ultrasound-based ablation group than in the radiofrequency group when specifically applied to the main renal artery (−13.2 ± 13.7 vs. −6.5 ± 10.3 mmHg; mean difference −6.7 mmHg P = 0.038). No difference was found between the ultrasound and radiofrequency side branch ablation groups (mean difference −1.8 mmHg, P > 0.99). In sum, this study demonstrated the technological superiorities of main renal artery ultrasound ablation and main artery plus side-branch radiofrequency ablation in comparison to radiofrequency ablation solely to the main artery.38 The study results additionally infer that the ablation patterns created by ultrasound ablation obviates the need for deeper branch treatment using this modality.
Phase 2B: Endovascular Pharmacological Ablation Therapy
As the development of radiofrequency and ultrasonic ablative technologies were evolving, a third technology was introduced: pharmacological ablation therapy. The Peregrine system is a catheter-guided system which use 3 microneedles to locally inject 0.6 ml of alcohol into renal artery adventitial and periadventitial tissue with the goal of causing circumferential renal nerve ablation while sparing the vessel intima or media (Figure 2). This therapy was first validated in 2016 in a preclinical histopathological study of adult swine which demonstrated a linear dose-response between injected alcohol volume and norepinephrine reduction (up to 88%).39 Following this animal proof of concept was a European open-label trial to evaluate the safety and efficacy of the paradigm, where 45 patients with drug-resistant hypertension on at least 3 agents underwent alcohol-mediated renal denervation using the Peregrine system. At 6 months, mean 24-hour ambulatory blood pressure reduction versus baseline was −11 mmHg (95% CI −15 to −7 mmHg) for SBP and −7 mmHg (95% CI: −9 to −4 mmHg) for diastolic blood pressure (P < 0.001 for both). Office systolic BP was reduced by −18/−10 mmHg (95% CI: −25 to −12/−13 to −6 mmHg), and antihypertensive medications were reduced in 23% of patients, with adherence remaining stable over time. The primary safety endpoint was met in 96% of patients, suggesting that this system indeed safely reduces blood pressure.40 Nevertheless, this investigation was open-label and not sham-controlled. Therefore, 2 large-scale, multinational, randomized, blinded, and sham-controlled trials to investigate alcohol-mediated RDN, namely the TARGET BP trials, are ongoing in patients with (TARGET BP-I) and without (TARGET BP OFF-MED), concomitant antihypertensive medications.41
Figure 2.
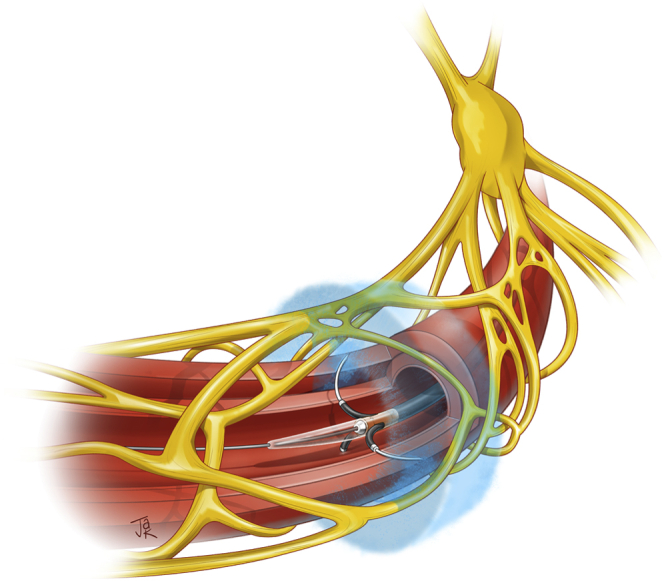
Alcohol-mediated renal denervation using the Peregrine system infusion catheter. Reprinted with permission from Medical Illustration by Justin A. Klein, CMI © 2022
Long-Term Safety of RDN
There have been some concerns regarding renal artery reinnervation after RDN. This has been experimentally observed in sheep, where electrical stimulation of the whole renal nerve after radiofrequency ablation procedures resulted in normalization of afferent and efferent response, in contrast to initial muted post-RDN responses.42 There are also observations of neural sprouting as early as 5 months after kidney transplant in humans, suggestive of possible reinnervation mechanisms, although without elucidation of healthy nerve functioning.43 Nevertheless, other hypertensive and chronic kidney disease sheep models have demonstrated that SBP, renin levels, and sodium excretion responses are curtailed 2 months to 5 months after RDN procedures, and evidence from rat models shows that norepinephrine content within the same timeline does not fully recover to predenervation levels.44,45
The elucidation of the long-term efficacy and safety of RDN has been drawn from registry data, the largest being the GSR (Global proSpective registrY for syMPathetic renaL denervatIon in seleCted IndicatIons Through 3 Years Registry), which is a single-group and open-label international registry utilizing data from 196 active sites worldwide in patients treated with the Symplicity radiofrequency ablation system. Adverse events and blood pressure reduction data after 3 years have been published about 2652 patients with uncontrolled hypertension, with pooled reductions in 24-hour SBP of −8.9 ± 20.1 mmHg in the overall cohort.46 Similar reductions were found in subgroup analyses, including patients older than 65 years (−8.7 ± 17.4 mmHg), patients with diabetes (−10.2 ± 17.9 mmHg), isolated systolic hypertension (−8.6 ± 18.7 mmHg), chronic kidney disease (−10.1 ± 20.3 mmHg), and atrial fibrillation (AF) (−10.0 ± 19.1 mmHg) (P < 0.0001 compared with baseline for all). The pooled safety data of the entire registry demonstrated low rates of adverse events as follows: 1.9% cardiovascular death, 4.4% hospitalization for hypertensive crisis, 1.9% new-onset end-stage renal disease, and 0.2% new renal artery stenosis. Intriguingly, comparing the blood pressure changes within the registry after 6 months, 1 year, 2 years, and 3 years from RDN did not show diminution of SBP reduction, giving credence to possible long-term durability of treatment.
Cardiovascular Outcomes Following RDN
Long-term epidemiological studies as well as randomized clinical trials have found a strong association between the pharmacological reduction of hypertension and decreased cardiovascular events across all patient populations. In 3 meta-analyses, a linear relationship was found between pharmacological reduction of in-office SBP and the incidence of major cardiovascular events, concluding that a decrease of 5 to 10 mmHg of in-office SBP conferred a decrease in major cardiovascular events by 10% to 20% and in stroke by 13% to 26%.47,48 Such data reiterate the rationale why regulatory bodies have consistently used blood pressure as a surrogate endpoint for stroke and cardiovascular events. Nonetheless, up until the present, there has not been a randomized clinical trial available that has assessed the direct effect of RDN on the incidence of cardiovascular or cerebrovascular events. In its absence, regression analyses from registry data using mean decreases in in-office SBP, assuming these levels would be maintained, have computed relative risk reductions of 26% for major cardiovascular events and 34% for strokes, respectively.49 In a retrospective analysis of the SYMPLICITY HTN-2 trial investigating the cost-effectiveness and long-term clinical benefits of RDN versus standard-of-care on 10-year probabilities of stroke, myocardial infarction, all coronary disease, heart failure, end-stage renal disease and median survival, investigators determined a median survival of 18.4 years for patients in the RDN group compared to 17.1 years in standard-of-care group. The 95% credible interval for incremental cost-effectiveness was computed as $31,460 per quality-adjusted life-year.50 Finally, using data from SYMPLICITY HTN-3, an Australian analysis predicted that RDN would be cost effective if it was offered to patients with a 10-year cardiovascular risk of at least 13.2%.51
Future Directions for RDN
As the field of endovascular RDN continues to develop, several open questions regarding novel technologies to aid in optimal vessel targeting, reliable patient predictors of blood pressure response, and RDN’s effect on other cardiovascular comorbities are paramount. As previously discussed, because blood pressure response to RDN depends on the overall responses of a variety of nerve fibers within the renal artery, there is thought that selective denervation guided by renal nerve stimulation may provide an opportunity to target treatment to the most anatomically sensitive sites. Preclinical studies have compared the effects of selective RDN using renal nerve stimulation in dogs, demonstrating that “strong-response sites” conferred a greater reduction in total body norepinephrine than weak sites.52 The first human feasibility study of 14 patients using renal nerve stimulation demonstrated clear blood pressure responses in vivo, and renal nerve stimulation-induced SBP elevation before RDN at sites of maximal response were significantly correlated with changes in ambulatory SBP-monitoring at 4.5 month follow-up.53 These results motivated a multicenter study designed to assess the feasibility of renal nerve mapping in 20 hypertensive patients using the ConfidenHT System. This device delivers an electrical stimulation to the renal nerves using a catheter with simultaneous intra-arterial BP monitoring, recording, and analysis, and displays the subsequent responses to stimulation. In the 20 cases described, there were no periprocedural adverse events, and electrical stimulation determined clear location-dependent responses along the renal artery with increases in SBP ranging from 0 mmHg to 30 mmHg.54 Therefore, investigators concluded that the possible benefits of this new technology would be in aiding the optimal targeting locations of RDN in hypertensive patients. That being said, large randomized trials have yet to confirm its benefit.
The variability of blood pressure responses to RDN remains considerable and widely demonstrated in the aforementioned randomized clinical trials.23, 24, 25,31 Therefore, predicting and selecting patients with more efficacious responses to therapy is of paramount importance. Other than Wilder’s principle, where pretreatment BP value portends post-treatment response, direct preprocedure patient parameters correlated to treatment effect have yet to be rigorously validated.
Beyond arterial hypertension, RDN is being studied in other cardiovascular conditions with etiologies related to sympathetic overactivation, notably AF. A small pilot study using implantable cardiac monitors in 20 patients with hypertension suggested that RDN might improve AF burden over a 6-month to 12-month time frame.55 This motivated the ERADICATE-AF trial, a multicenter and single-blind randomized clinical trial in Russia, Poland, and Germany, which randomized 302 patients with hypertensive (on greater than 1 agent) and paroxysmal AF 1:1 to pulmonary vein isolation with radiofrequency RDN compared to pulmonary vein isolation alone. After 12 months, the RDN group had a greater proportion of patients free from AF, atrial flutter, or tachycardia (72.4% vs. 56.5%; hazard ratio, 0.57 [95% CI, 0.38 to 0.85], P = 0.006).56 There was also a significant reduction in mean SBP between the 2 groups (between-group difference −13 mmHg [95% CI, −15 to −11 mmHg], P < .001). Both groups had the same rates of procedural complications. Results of the SYMPLICITY AF trial, an open-label multicenter study that aims to randomize 265 patients with persistent or paroxysmal AF and hypertension (on 1 or more medications) to radiofrequency RDN using the Spyral system and pulmonary vein isolation compared to pulmonary vein isolation alone are eagerly awaited.
As the use of beta blockade to downregulate pathologic sympathetic overactivation in heart failure has repeatedly been shown to reduce cardiovascular morbidity and mortality, there remains the intriguing possibility that effects of RDN may help attenuate the progression of heart failure.57 The REACH-Pilot Study, which treated 7 patients with an average ejection fraction of 43% on maximally-tolerated heart failure therapy with bilateral radiofrequency RDN demonstrated improved heart failure symptoms and exercise capacity as measured by a 6-minute walk test at 6 months. There were no safety concerns within this study. Nevertheless, there is limited data to suggest the generalizability of this study and no randomized and placebo-controlled blinded trials have been completed to robustly determine the efficacy of RDN in the diminution of heart failure symptoms, or in the improvement of its clinical trajectory.
Conclusion
Over the last decade the development of RDN for the treatment of arterial hypertension has undergone a dramatic evolution from rapid early interest generated by open-label and nonrandomized trials, through setbacks with unanticipated challenges from the SYMPLICITY HTN-3 trial, to gradual reconsideration with technological improvements, operator know-how, more informed study designs, and larger randomized trials. Through this progress, RDN has demonstrated itself to be a safe and well-tolerated endovascular intervention that can reliably enhance blood pressure control through potential novel mechanisms of action. This may ultimately lead to improved cardiovascular prognosis. Nevertheless, larger randomized controlled trials with longer study follow-ups and a better understanding of patient predictors of blood pressure response are necessary to better define the role of this procedure in the long-term control of arterial hypertension.
DISCLOSURE
AJK reports institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, and SoniVie. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for consulting and/or speaking engagements in which AJK controlled the content. AJK also reports personal consulting from IMDS; Travel Expenses/Meals from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron.
The other author declared no conflicts of interest.
References
- 1.Kearney P.M., Whelton M., Reynolds K., et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Kirkland E.B., Heincelman M., Bishu K.G., et al. Trends in healthcare expenditures among US adults with hypertension: national estimates, 2003-2014. J Am Heart Assoc. 2018;7:e008731. doi: 10.1161/JAHA.118.008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muntner P., Carey R.M., Gidding S., et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol. 2018;71:109–118. doi: 10.1016/j.jacc.2017.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou B., Danaei G., Stevens G.A. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet. 2019;394:639–651. doi: 10.1016/S0140-6736(19)31145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannan A., Medina R.I., Nagajothi N., Balamuthusamy S. Renal sympathetic nervous system and the effects of denervation on renal arteries. World J Cardiol. 2014;6:814–823. doi: 10.4330/wjc.v6.i8.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp U.C. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–R95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlaich M.P., Sobotka P.A., Krum H., et al. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 8.Smithwick R.H., Thompson J.E. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 9.Peet M.M. Hypertension and its surgical treatment by bilateral supradiaphragmatic splanchnicectomy. Am J Surg. 1948;75:48–68. doi: 10.1016/0002-9610(48)90284-0. [DOI] [PubMed] [Google Scholar]
- 10.Krum H., Schlaich M., Whitbourn R., et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 11.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 12.Krum H., Schlaich M.P., Sobotka P.A., et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 13.Esler M.D., Böhm M., Sievert H., et al. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the Symplicity HTN-2 randomized clinical trial. Eur Heart J. 2014;35:1752–1759. doi: 10.1093/eurheartj/ehu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rippy M.K., Zarins D., Barman N.C., et al. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol. 2011;100:1095–1101. doi: 10.1007/s00392-011-0346-8. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt D.L., Kandzari D.E., O’Neill W.W., et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 16.Bakris G.L., Townsend R.R., Flack J.M., et al. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: the Symplicity HTN-3 trial. J Am Coll Cardiol. 2015;65:1314–1321. doi: 10.1016/j.jacc.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Kandzari D.E., Bhatt D.L., Brar S., et al. Predictors of blood pressure response in the Symplicity HTN-3 trial. Eur Heart J. 2015;36:219–227. doi: 10.1093/eurheartj/ehu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandzari D.E., Bhatt D.L., Sobotka P.A., et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 Trial. Clin Cardiol. 2012;35:528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakakura K., Ladich E., Cheng Q., et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–643. doi: 10.1016/j.jacc.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 20.Mahfoud F., Tunev S., Ewen S., et al. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J Am Coll Cardiol. 2015;66:1766–1775. doi: 10.1016/j.jacc.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Mahfoud F., Schmieder R.E., Azizi M., et al. Proceedings from the 2nd European Clinical Consensus Conference for device-based therapies for hypertension: state of the art and considerations for the future. Eur Heart J. 2017;38:3272–3281. doi: 10.1093/eurheartj/ehx215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborn J.W., Banek C.T. Catheter-based renal nerve ablation as a novel hypertension therapy: lost, and then Found, in Translation. Hypertension. 2018;71:383–388. doi: 10.1161/HYPERTENSIONAHA.117.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend R.R., Mahfoud F., Kandzari D.E., et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 24.Böhm M., Mahfoud F., Townsend R.R., et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019;40:743–751. doi: 10.1093/eurheartj/ehy871. [DOI] [PubMed] [Google Scholar]
- 25.Kandzari D.E., Böhm M., Mahfoud F., et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 26.Mahfoud F., Kandzari D.E., Kario K., et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399:1401–1410. doi: 10.1016/S0140-6736(22)00455-X. [DOI] [PubMed] [Google Scholar]
- 27.Mabin T., Sapoval M., Cabane V., et al. First experience with endovascular ultrasound renal denervation for the treatment of resistant hypertension. EuroIntervention. 2012;8:57–61. doi: 10.4244/EIJV8I1A10. [DOI] [PubMed] [Google Scholar]
- 28.Daemen J., Mahfoud F., Kuck K.H., et al. Safety and efficacy of endovascular ultrasound renal denervation in resistant hypertension: 12-month results from the ACHIEVE study. J Hypertens. 2019;37:1906–1912. doi: 10.1097/HJH.0000000000002120. [DOI] [PubMed] [Google Scholar]
- 29.Mauri L., Kario K., Basile J., et al. A multinational clinical approach to assessing the effectiveness of catheter-based ultrasound renal denervation: the RADIANCE-HTN and REQUIRE clinical study designs. Am Heart J. 2018;195:115–129. doi: 10.1016/j.ahj.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Azizi M., Schmieder R.E., Mahfoud F., et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 31.Azizi M., Schmieder R.E., Mahfoud F., et al. Six-month results of treatment-blinded medication titration for hypertension control after randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO trial. Circulation. 2019;139:2542–2553. doi: 10.1161/CIRCULATIONAHA.119.040451. [DOI] [PubMed] [Google Scholar]
- 32.Azizi M., Daemen J., Lobo M.D., et al. 12-month results from the unblinded phase of the RADIANCE-HTN SOLO trial of ultrasound renal denervation. JACC Cardiovasc Interv. 2020;13:2922–2933. doi: 10.1016/j.jcin.2020.09.054. [DOI] [PubMed] [Google Scholar]
- 33.Mahfoud F., Bloch M.J., Azizi M., et al. Changes in blood pressure after crossover to ultrasound renal denervation in patients initially treated with sham in the RADIANCE-HTN SOLO trial. EuroIntervention. 2021;17:e1024–e1032. doi: 10.4244/EIJ-D-21-00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azizi M., Sanghvi K., Saxena M., et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN Trio): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–2486. doi: 10.1016/S0140-6736(21)00788-1. [DOI] [PubMed] [Google Scholar]
- 35.Kario K., Hoshide S., Mizuno H., et al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020;142:1810–1820. doi: 10.1161/CIRCULATIONAHA.120.049730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirtane A. Six-month outcomes of a randomized trial of renal denervation versus a sham procedure for resistant hypertension-impact of treatment blinded medication titration. tctMD. 2021. Accessed November 4, 2021. https://www.tctmd.com/slide/radiance-htn-six-month-outcomes-randomized-trial-renal-denervation-versus-sham-procedure
- 37.Kario K., Yokoi Y., Okamura K., et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2021;15 doi: 10.1038/s41440-021-00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fengler K., Rommel K.P., Blazek S., et al. A three-arm randomized trial of different renal denervation devices and techniques in patients with resistant hypertension (RADIOSOUND-HTN) Circulation. 2019;139:590–600. doi: 10.1161/CIRCULATIONAHA.118.037654. [DOI] [PubMed] [Google Scholar]
- 39.Fischell T.A., Ebner A., Gallo S., et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv. 2016;9:589–598. doi: 10.1016/j.jcin.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 40.Mahfoud F., Renkin J., Sievert H., et al. Alcohol-mediated renal denervation using the peregrine system infusion catheter for treatment of hypertension. JACC Cardiovasc Interv. 2020;13:471–484. doi: 10.1016/j.jcin.2019.10.048. [DOI] [PubMed] [Google Scholar]
- 41.Mahfoud F., Weber M., Schmieder R.E., et al. Catheter-based alcohol-mediated renal denervation for the treatment of uncontrolled hypertension: design of two sham-controlled, randomized, blinded trials in the absence (TARGET BP OFF-MED) and presence (TARGET BP I) of antihypertensive medications. Am Heart J. 2021;239:90–99. doi: 10.1016/j.ahj.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Booth L.C., Nishi E.E., Yao S.T., et al. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension. 1979;65:393–400. doi: 10.1161/HYPERTENSIONAHA.114.04176. [DOI] [PubMed] [Google Scholar]
- 43.Mauriello A., Rovella V., Borri F., et al. Hypertension in kidney transplantation is associated with an early renal nerve sprouting. Nephrol Dial Transplant. 2017;32:1053–1060. doi: 10.1093/ndt/gfx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh R.R., McArdle Z.M., Iudica M., et al. Sustained decrease in blood pressure and reduced anatomical and functional reinnervation of renal nerves in hypertensive sheep 30 months after catheter-based renal denervation. Hypertension. 1979;73:718–727. doi: 10.1161/HYPERTENSIONAHA.118.12250. [DOI] [PubMed] [Google Scholar]
- 45.Mulder J., Hökfelt T., Knuepfer M.M., Kopp U.C. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R675–R682. doi: 10.1152/ajpregu.00599.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahfoud F., Böhm M., Schmieder R., et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global Symplicity Registry. Eur Heart J. 2019;40:3474–3482. doi: 10.1093/eurheartj/ehz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomopoulos C., Parati G., Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–2295. doi: 10.1097/HJH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 48.Ettehad D., Emdin C.A., Kiran A., et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 49.Pietzsch J., Mahfoud F., Williams B., et al. Clinical event reductions in high-risk hypertension patients treated with renal denervation: a model-based estimate based on 36-month data from the global symplicity registry. J Am Coll Cardiol. 2021;77:1644. doi: 10.1016/S0735-1097(21)03000-X. [DOI] [Google Scholar]
- 50.Geisler B.P., Egan B.M., Cohen J.T., et al. Cost-effectiveness and clinical effectiveness of catheter-based renal denervation for resistant hypertension. J Am Coll Cardiol. 2012;60:1271–1277. doi: 10.1016/j.jacc.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Chowdhury E.K., Reid C.M., Zomer E., et al. Cost-effectiveness of renal denervation therapy for treatment-resistant hypertension: a best case scenario. Am J Hypertens. 2018;31:1156–1163. doi: 10.1093/ajh/hpy108. [DOI] [PubMed] [Google Scholar]
- 52.Liu H., Chen W., Lai Y., et al. Selective renal denervation guided by renal nerve stimulation in canine. Hypertension. 1979;74:536–545. doi: 10.1161/HYPERTENSIONAHA.119.12680. [DOI] [PubMed] [Google Scholar]
- 53.de Jong M.R., Hoogerwaard A.F., Gal P., et al. Persistent increase in blood pressure after renal nerve stimulation in accessory renal arteries after sympathetic renal denervation. Hypertension. 1979;67:1211–1217. doi: 10.1161/HYPERTENSIONAHA.115.06604. [DOI] [PubMed] [Google Scholar]
- 54.Tsioufis K., Mahfoud F., Feyz L., et al. TCT-32 ConfidenHTTM system - diagnostic electrical mapping of renal nerves for optimizing renal denervation procedures. J Am Coll Cardiol. 2018;72:B14–B15. doi: 10.1016/j.jacc.2018.08.1113. [DOI] [Google Scholar]
- 55.Feyz L., Theuns D., Bhagwandien R., et al. Atrial fibrillation reduction by renal sympathetic denervation: 12 months’ results of the AFFORD study. Clin Res Cardiol. 2019;108:1–9. doi: 10.1007/s00392-018-1391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinberg J.S., Shabanov V., Ponomarev D., et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA. 2020;323:248–255. doi: 10.1001/jama.2019.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heidenreich P.A., Lee T.T., Massie B.M. Effect of beta-blockade on mortality in patients with heart failure: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 1997;30:27–34. doi: 10.1016/s0735-1097(97)00104-6. [DOI] [PubMed] [Google Scholar]