Abstract
Introduction
Complement 3 glomerulopathy (C3G) is a rare kidney disease characterized by dysregulation of the alternative pathway (AP) of the complement system. About 50% of patients with C3G progress to kidney failure within 10 years of diagnosis. Currently, there are no approved therapeutic agents for C3G. Iptacopan is an oral, first-in-class, potent, and selective inhibitor of factor B, a key component of the AP. In a Phase II study, treatment with iptacopan was associated with a reduction in proteinuria and C3 deposit scores in C3G patients with native and transplanted kidneys, respectively.
Methods
APPEAR-C3G (NCT04817618) is a randomized, double-blind, and placebo-controlled Phase III study to evaluate the efficacy and safety of iptacopan in C3G patients, enrolling 68 adults with biopsy-confirmed C3G, reduced C3 (<77 mg/dl), proteinuria ≥1.0 g/g, and estimated glomerular filtration rate (eGFR) ≥30 ml/min per 1.73 m2. All patients will receive maximally tolerated angiotensin-converting enzyme inhibitor/angiotensin receptor blocker and vaccination against encapsulated bacteria. Patients with any organ transplantation, progressive crescentic glomerulonephritis (GN), monoclonal gammopathy of undetermined significance, or kidney biopsy with >50% interstitial fibrosis/tubular atrophy, will be excluded. Patients will be randomized 1:1 to receive either iptacopan 200 mg twice daily or placebo for 6 months, followed by open-label treatment with iptacopan 200 mg twice daily for all patients for 6 months. The primary objective is to evaluate the efficacy of iptacopan versus placebo on proteinuria reduction urine protein:creatinine ratio (UPCR) (24 h urine). Key secondary endpoints will assess kidney function measured by eGFR, histological disease total activity score, and fatigue.
Conclusion
This study aims to demonstrate the clinical benefits of AP inhibition with iptacopan in C3G.
Keywords: alternative pathway, C3G, clinical trials, complement system, factor B, iptacopan, LNP023
Graphical abstract
INTRODUCTION
C3G is a chronic and rare kidney disease caused by dysregulation of the complement AP in the plasma and glomerular microenvironment and is characterized by isolated or dominant complement C3 deposition in kidney biopsies.1 Two subtypes of C3G are distinguished by electron microscopic examination of kidney biopsy based on the location and density of deposits, namely dense deposit disease and C3GN.2, 3, 4, 5 Previously, C3G was classified as a subtype of membranoproliferative GN (MPGN); however, a better understanding of pathogenesis has led to the reclassification of MPGN immunoglobulin-associated (immune complex-MPGN), with glomerular IgG and C3 deposits, and C3G, with predominant C3 deposits.6 Both entities share overlapping pathophysiological features, with AP dysregulation found in both immune complex-MPGN and C3G.6, 7, 8
The onset of C3G is typically seen in children and young adults3,9,10 with approximately 50% of patients progressing to kidney failure within 10 years of diagnosis, although cases of more rapid progression are not unusual.3,9 Patients require maintenance dialysis or kidney transplantation following the development of terminal kidney failure;11 however, there is a substantial risk of disease recurrence post-transplant, with allograft loss reported in about 50% of patients at 5 years post-transplantation.12 Though C3G carries one of the highest risks for kidney failure of all primary glomerular diseases, there are currently no approved therapies targeting the underlying cause of the disease.
The complement system, triggered by 1 of 3 initiating pathways (the classical, lectin, and APs), plays an integral role in innate immunity and serves as a first line of defense against foreign pathogens.13 Unlike the other 2 pathways, the AP is constitutively active at a low level, making it particularly susceptible to dysregulation.14 The AP also plays a pivotal role in amplifying the complement response and ultimately accounts for more than 80% of terminal pathway activation regardless of the activating pathway.15
Dysregulation of the AP in the fluid phase underlies the pathogenesis of C3G.16 The consequence is C3 glomerular deposition within the glomeruli, which triggers inflammation and glomerular injury.2 Genetic factors such as mutations in complement proteins or their regulators, and/or autoantibodies against complement components or nephritic factors that stabilize the convertases, drive this complement dysregulation. Evidence from mouse models has provided important insights into the role of AP overactivation in C3G pathogenesis. Mice deficient in factor H (Cfh−/−), a negative regulator of C3 convertase, develop a C3G-like phenotype whereas mice lacking both Cfh and Cfb, which encodes complement factor B (FB), a key regulator of the AP, do not.17 In addition, though C5 deficiency in Cfh-/- mice reduces renal inflammation and tissue damage, it does not prevent C3 deposition, an observation consistent with the role C5 plays in the terminal but not the initiating pathway of the complement cascade.18 These observations demonstrate that targeting AP activation, by inhibiting or preventing the formation of C3 convertase, is an attractive therapeutic strategy to ameliorate C3G disease progression.14
Iptacopan is an oral, first-in-class, and highly potent selective inhibitor of FB that efficiently blocks the AP.19 FB includes a serine protease domain (Bb), which is the proteolytically active component of the AP C3 (C3bBb) and C5 (C3bBb3b) convertases (see Figure 1 and Supplementary Video for further information). As a consequence, iptacopan-mediated inhibition of FB suppresses C3 convertase activity, blocking the cleavage of C3 and activation of the amplification loop. In turn, this blockade prevents downstream generation of the C5 convertase complex and triggering of the terminal complement cascade and its effector consequences, which include opsonization, formation of the potent C5a anaphylatoxin and generation of membrane attack complex.19 Preclinical studies have shown that inhibition of FB prevents complement activation in sera from C3G patients.19 In a Phase II study, treatment with iptacopan was associated with a mean increase in eGFR of 3.1 ml/min per 1.73 m2 from baseline to 12 weeks in patients with native C3G, corresponding to a mean predicted eGFR preservation of 6.4 ml/min per 1.73 m2 over 12 weeks as a result of iptacopan administration. A significant 45% reduction in proteinuria was reported in patients with naïve C3G, and a significant reduction in the histologic C3 deposit score in follow-up kidney biopsy in patients with recurrent C3G post-transplant at week 12.21 Data collected about 7 patients who entered the extension study confirmed ongoing eGFR stability until 25 weeks. Treatment with iptacopan in patients with native or recurrent C3G was well tolerated and led to sustained normalization of plasma C3 levels.22 These results support the rationale for further evaluating the benefits of iptacopan in C3G.
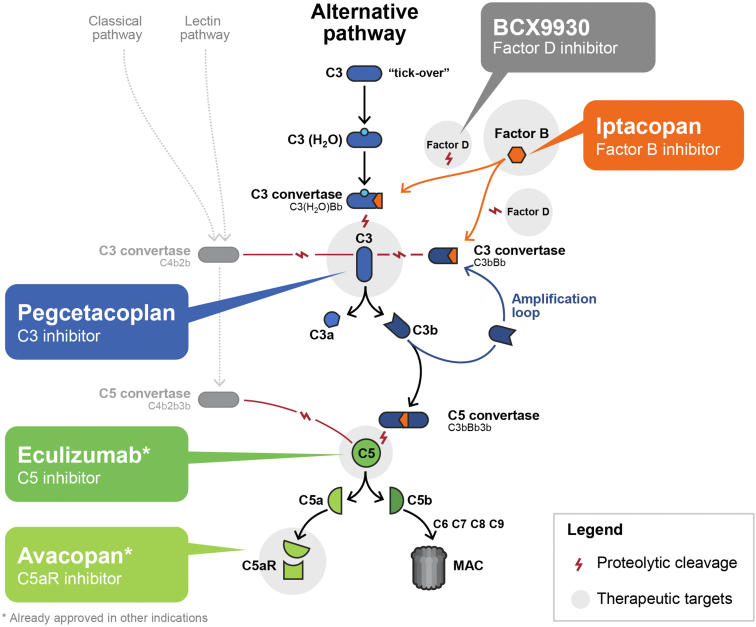
Figure 1.
Targeting complement in C3G. Eculizumab is not being evaluated in C3G but is to be considered in certain circumstances for off-label use.20 The complement system can be activated via 3 pathways: the classical pathway, triggered by antigen-antibody/immune complexes or by direct binding of complement component C1q to the pathogen surface; the lectin pathway, triggered by mannose-binding lectin; and the alternative pathway, which is activated by spontaneous hydrolysis of complement protein 3. Pegcetacoplan is a C3 inhibitor that targets C3 and activation of fragment C3b. Avacopan selectively blocks the effects of C5a through the C5a receptor. Eculizumab, an anti-C5 monoclonal antibody, targets complement protein 5. Iptacopan, an inhibitor of FB, blocks the alternative pathway. C3G, complement 3 glomerulopathy; FB, factor B; MAC, membrane attack complex.
Here, we describe the rationale and design of the APPEAR-C3G Phase III trial, which aims to evaluate the clinical efficacy and safety of iptacopan as compared to placebo in patients with C3G.
Methods
Study Population
Approximately 68 participants will be randomized 1:1 to either the iptacopan or placebo treatment arm. All patients are to provide written consent and fulfill all the criteria for inclusion and meet no exclusion criteria (Table 1).
Table 1.
Key inclusion and exclusion criteria
| Inclusion | Exclusion |
|---|---|
| Aged ≥18 yr and ≤60 yr | Organ transplant, including kidney |
| Biopsy-confirmed diagnosis of C3G in the past 12-mo | Rapidly progressive crescentic GN renal biopsy with interstitial fibrosis/tubular atrophy >50% |
On maximally recommended or tolerated dose of ACEi or ARB for ≥90 d
|
The use of inhibitors of complement factors (e.g., factor B, factor D, C3 inhibitors, anti-C5 antibodies, C5a receptor antagonists) within 6-months prior to the Screening visit The use of immunosuppressants (except mycophenolic acids), cyclophosphamide or systemic prednisone at a dose >7.5 mg/day (or equivalent) within 90 days of study drug administration |
| Reduced C3 (< 77 mg/dl) | Monoclonal gammopathy of undetermined significance |
| UPCR ≥1.0 g/g | SBP < 80 mmHg or >160 mmHg, or DBP < 50 mmHg or >100 mmHg, or pulse rate < 45 bpm or >100 bpm |
| eGFRa or measured GFR ≥30 ml/min/1.73 m2 | BMI > 38 kg/m2 |
|
Liver disease, infection, or injury |
| Evidence of urinary obstruction or difficulty in voiding | |
| Active systemic bacterial, viral, or fungal infection within 14 days or the presence of fever ≥38 °C (100.4 °F) within 7 days prior to study treatment administration | |
| History of recurrent invasive infections caused by encapsulated organisms | |
| Severe concurrent comorbidities or medical condition deemed likely to interfere with study participation |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; C3G, complement 3 glomerulopathy; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GN, glomerulonephritis; IMP, investigational medicinal products; LLN, lower limit of normal; SBP, systolic blood pressure; SGLT2, sodium-glucose cotransporter-2; UPCR, urine protein:creatinine ratio.
Using the Chronic Kidney Disease Epidemiology (CKD-EPI) formula for ≥18 years.
Study Design
APPEAR-C3G (ClinicalTrials.gov Identifier NCT04817618) is a multicenter, randomized, double-blind, parallel group, placebo-controlled, and pivotal Phase III study to evaluate the efficacy and safety of iptacopan in patients with native C3G. It is currently recruiting adult patients in 38 centers across 18 countries (Figure 2).
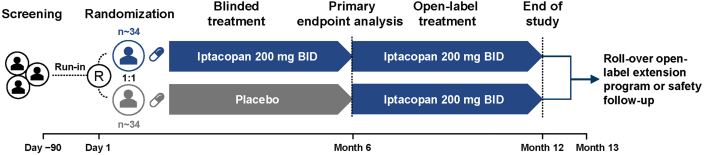
Figure 2.
Study design C3G Phase III—APPEAR- C3G. A multicenter, randomized, double-blind, parallel group, placebo-controlled study to evaluate the efficacy and safety of iptacopan in patients with C3G (NCT04817618). At study completion, patients have the option to roll over into the extension study. bid, twice daily; C3G, complement 3 glomerulopathy; n, number of patients; R, randomization.
The study comprises 3 periods (screening, 360-day treatment, 30-day safety follow-up) of 8 visits (on treatment) over 12-months with an initial screening or run-in period of up to 90 days, followed by 6 months of randomized double-blinded treatment and then 6 months of open-label treatment (Figure 2). Patients will be randomized 1:1 to one of the treatment arms and will receive either iptacopan 200 mg twice daily or matching placebo. Randomization will be stratified according to whether or not patients are receiving corticosteroid and/or mycophenolic acid at randomization. Upon completion of study treatment at 12-months, participants have the option to either discontinue iptacopan treatment and enter a 30-day safety follow-up period or continue open-label iptacopan treatment by transitioning to the C3G extension study (NCT03955445).
A summary of key study assessments is provided in Table 2. Kidney function will be assessed by, proteinuria as measured by UPCR from a 24-hour urine collection, a proteinuria-eGFR composite endpoint and eGFR. Historical eGFR and proteinuria data will be collected for 2 years prior to the screening visit, as available. Duplicate 24-hour urine tests will be performed at baseline and at 6 months to minimize risks associated with collection error for the primary end point. To examine the status of C3G disease progression, renal histopathology will be evaluated in mandatory renal biopsies obtained at day 45 and day 180 (6 months), with an additional optional biopsy at day 360 (12 months). Glomerular deposits and characteristics will also be evaluated by electron microscopy. C3 deposition will be scored independently by 3 blinded pathologists based on immunofluorescence microscopy. These data will allow evaluation of histopathologic improvements in glomerular inflammation, fibrosis, and C3 deposition and their correlation with the functional benefits of iptacopan. The effect of iptacopan on serum C3 and other complement pathway biomarkers (Bb and sC5b-9) will also be assessed as exploratory outcomes.
Table 2.
Summary of study assessments
| Assessment category | Assessment |
|---|---|
| Efficacy | Proteinuria (UPCR) |
| eGFR | |
| Composite renal endpoint | |
| Disease total activity score (scored using C3G-HI) | |
| Patient reported outcomes–FACIT-Fatigue | |
| Key safety | Adverse event monitoring |
| Laboratory evaluations in blood and urine | |
| Electrocardiogram | |
| Other | C3 deposit score and total chronicity score |
| Complement pathway and renal injury biomarkers | |
| Patient reported outcomes–SF-36, EQ-5D-5L and PGIS | |
| Iptacopan levels at trough (PK assessment) |
C3G-HI, C3G Histologic Index; eGFR, estimated glomerular filtration rate; EQ-5D-5L, EuroQol-5 dimensions-5 levels; FACIT-Fatigue, functional assessment of chronic illness therapy-fatigue; PGIS, patient global impression of severity; PK, pharmacokinetic; SF-36, short form 36; UPCR, urine protein: creatinine ratio.
The primary patient reported outcome for this study is the Functional Assessment of Chronic Illness Therapy Fatigue Scale (FACIT-Fatigue). The purpose of FACIT-Fatigue in this study is to assess the experience and effect of fatigue on patients with C3G. To further understand the participants’ symptoms, functioning, and overall well-being, as well as their changes during the study, questionnaires for Short Form 36, EuroQol-5 dimensions-5 levels, and patient global impression of severity will be completed. In addition, a patient interview will be conducted within 7 days after the 6 month visit to validate these patient reported outcomes in the C3G patient population. This interview, which allows patients to give feedback on their experience of meaningful changes in their condition as well as the patient reported outcome measures, is optional.
Study Objectives
The primary objective is to demonstrate the superiority of iptacopan versus placebo on proteinuria (reduction as measured by UPCR [24h urine collection]) at 6 months. The key objectives and endpoints are reported for the double-blind and open-label treatment period in Table 3 and Table 4, respectively.
Table 3.
Objectives and related endpoints for double-blind treatment period
| Objectives | Endpoints | |
|---|---|---|
| Primary objective | To demonstrate the superiority of iptacopan vs. placebo on proteinuria (UPCR) reduction at 6-mo | Log-transformed ratio to baseline in UPCR (sampled from a 24h urine collection) at 6-mo |
| Secondary objectives | To demonstrate the superiority of iptacopan vs. placebo on improvement from baseline in eGFR at 6-mo | Change from baseline in eGFR at 6-mo |
| To demonstrate the superiority of iptacopan vs. placebo in the proportion of patients who achieve a composite renal endpoint at 6-mo | A participant meets the requirements of the composite renal endpoint if they satisfy the following criteria at the 6-mo time point: (i) a stable or improved eGFR compared to the baseline visit (≤15% reduction in eGFR) and (ii) a ≥50% reduction in UPCR compared to the baseline visit | |
| To assess the effect of iptacopan vs. placebo on reduction of glomerular inflammation in the kidney at 6-mo | Change from baseline in disease total activity score in a renal biopsy at 6-mo | |
| To assess the effect of iptacopan vs. placebo on patient reported fatigue at 6-mo | Change from baseline to 6-mo in FACIT-Fatigue score | |
| To evaluate the safety and tolerability of iptacopan vs. placebo during the 6-mo double-blind period | Vital signs, ECGs, safety laboratory measurements, AEs, AESIs |
AEs, adverse events; AESIs, adverse events of special interest; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FACIT-Fatigue, functional assessment of chronic illness therapy-fatigue; UPCR, urine protein:creatinine ratio.
Table 4.
Objectives and related endpoints for open-label treatment period
| Objectives | Endpoints | |
|---|---|---|
| Primary objective | To evaluate the effect of iptacopan on proteinuria at 12-mo | • Change from baseline in log-transformed UPCR at the 12-mo visit (both study treatment arms) |
| • Change in log-transformed UPCR from the 6-mo visit to the 12-mo visit in the placebo arm | ||
| Secondary objectives | To evaluate the effect at 12-mo of iptacopan on a composite renal endpoint, in reducing glomerular inflammation in the kidney and in improvement of patient reported fatigue | • A participant is defined as meeting the requirements of the composite renal endpoint if they satisfy the eGFR and UPCR criteria at the 12-mo time point (both treatment arms) |
| • Proportion of participants who achieved the composite renal endpoint at 12-mo | ||
| • Change from baseline in total activity score in a renal biopsy at 12-mo (both treatment arms) | ||
| • Change in total activity score in a renal biopsy from the 6-mo visit to the 12-mo visit in the placebo arm | ||
| • Change from baseline in the FACIT-Fatigue score at 12-mo (both study treatment arms) | ||
| • Change in the FACIT-Fatigue score from the 6-mo visit to the 12-mo visit of the placebo arm | ||
| To evaluate the safety and tolerability of iptacopan during the 6-mo open-label treatment period as well as the entire 12-mo treatment period | Vital signs, ECGs, safety laboratory measurements, AEs, AESIs |
AEs, adverse events; AESIs, adverse events of special interest; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FACIT-Fatigue, functional assessment of chronic illness therapy-fatigue; UPCR, urine protein:creatinine ratio.
Primary Treatment Effect and Study Design Rationale
The precise definition of the primary treatment effect is the reduction in proteinuria at 6 months for iptacopan versus placebo in patients with biopsy-confirmed C3G without confounding for initiation or intensification of antiproteinuric (including any complement pathway modifying agent, corticosteroid, or immunosuppressant for a kidney indication) or renal replacement therapies administered after randomization. Patients discontinuing randomized medication will continue to be followed up and will contribute to the treatment effect, according to the intent to treat principle.
Statistical Considerations
Primary Efficacy Analysis
The primary comparison between iptacopan and placebo will be a 1-sided hypothesis test at a significance level of 0.025.
24-hour UPCR measurements collected after antiproteinuric or renal replacement therapies will be excluded from the analysis and multiple imputation approaches will be applied, which accounts for the use of such therapies as indicative of a worsening of condition. Statistical analysis will be carried out in each imputed dataset using a mixed model for repeated measures for the log ratio to baseline in UPCR and the results will be combined using Rubin’s rules to obtain the estimate of the percent reduction from baseline in UPCR (iptacopan vs. placebo).
Secondary Efficacy Analyses
For the composite renal endpoint, the initiation or intensification of antiproteinuric therapies or renal replacement therapy will be considered as treatment failures and therefore become part of a composite endpoint definition. The change from baseline to month 6 in the histology total activity score will be analyzed using an analysis of covariance model. The other secondary endpoints (change from baseline to month 6 in eGFR and FACIT-Fatigue total score) will be evaluated using similar methodology as the primary analysis.
Safety Analyses
Safety data (adverse events, deaths, vital signs, and laboratory data) will be collected throughout the study from all enrolled patients. Treatment emergent adverse events, death, serious adverse events, and other significant adverse events, including those leading to treatment or study discontinuation will be summarized by treatment, primary system organ class and preferred term. For vital signs, electrocardiogram, and laboratory data, summary statistics and graphical displays where appropriate will be provided by treatment and visit.
Sample Size Calculation
We calculated the sample size to ensure enough power for testing superiority of iptacopan versus placebo on proteinuria reduction (the primary endpoint of log-transformed ratio to baseline in UPCR at month 6). Assuming a reduction in UPCR of 60% in the iptacopan group versus 20% in the placebo group (i.e., a relative reduction vs. placebo in UPCR of 50%) and a SD of 0.69 (on the log-scale) based on an interim analysis of the C3G Phase II and extension studies, a sample size of 34 participants per group (total 68 participants) provides at least 98% power at a 1-sided significance level of 0.025. Assuming a reduction in UPCR of 50% in iptacopan group versus 20% in placebo group (i.e., a relative reduction vs. placebo in UPCR of 37.5%), a sample size of 68 participants provides at least 80% power at the 1-sided 0.025 significance level.
Discussion
APPEAR-C3G is a pivotal Phase III study designed to evaluate the potential benefits of a small molecule inhibitor of FB in patients with C3G. Despite significant improvements in our understanding of this rare disease, kidney survival continues to be low with a high recurrence rate post-transplantation, and no approved targeted therapies are available to slow or halt disease progression. The lack of novel therapies for C3G treatment reflects the challenges in designing effective complement inhibitors, an incomplete understanding of the nuances of several key aspects of complement activity (for example, the role of intermediate compounds in the formation of both the C3 and C5 convertases), the rarity and heterogeneity of C3G (which affect trial design), and the natural history of this disease (which is incompletely understood).
Despite the potential merits of anticomplement therapeutics in C3G, few studies have entered clinical trials for its treatment and fewer still have progressed beyond Phase I.23,24 In addition to this study of iptacopan, which targets FB, Phase II clinical trials for C3G include investigations of inhibitors of factor D (BCX9930; NCT05162066), C3 (pegcetacoplan; NCT04572854), and the C5a receptor (avacopan; NCT03301467) (Figure 1)25,26 at the time of writing this manuscript. Each trial is unique in its choice of targets and presumed effects. Iptacopan targets FB, an AP-specific serine protease that complexes with C3b to drive the catalytic activity of the AP C3 and C5 convertases. Therefore, iptacopan is expected to prevent pathological drivers of C3G from activating the AP. Indeed, in both native and recurrent patients, a sustained inhibition of the AP (Wieslab) and normalization of C3 levels were observed following treatment with iptacopan and an increase in serum C3 levels of approximately 200% was reported in patients with native C3G.21,22 In comparison, inhibiting downstream complement components such as C5 inhibition (with either anti-C5 antibodies or C5a inhibitors) is unlikely to be as effective, as has been demonstrated in mice with targeted deletion(s) of AP components and in patients treated with eculizumab.17,27 In addition, inhibition of all C5 convertase activity blocks downstream effects, which may potentially result in a higher risk of infection with Neisseria meningitidis.28 In contrast, iptacopan, does not fully block the generation of membrane attack complex initiated by the C5 convertases of the classical and lectin pathways (C4b2aC3b) and therefore infection risk is theoretically lower. This may be important in immunized individuals such that membrane attack complex-dependent killing of bacteria, such as Neisseria meningitidis, is maintained.28, 29, 30
Efforts have been made recently to identify the potential value of proteinuria as a surrogate endpoint for kidney failure. In APPEAR-C3G, proteinuria and eGFR were chosen as surrogate endpoints of disease progression given that proteinuria is a marker for kidney damage and a risk factor for progression to kidney failure in other glomerular diseases.31,32 Level of proteinuria is considered a reliable predictor of treatment effect on long-term outcome in other chronic kidney diseases such as diabetic kidney disease, immunoglobulin A nephropathy, focal segmental glomerulosclerosis and idiopathic membranous nephropathy. Though data are scarce to date in C3G, emerging data support the use of proteinuria as a surrogate endpoint in C3G.21,22,33,34 A recent analysis conducted by the GLOSEN (Spain) group showed that a doubling of proteinuria levels was associated with a 2.5-fold increase in the risk of kidney failure and a ≥50% reduction in proteinuria significantly lowered this risk of disease progression.34 Similarly, an analysis of patients in a C3G registry at the University of Iowa (USA) indicated that a 30% to 50% reduction in UPCR was associated with 4.6% to 9% relative improvement in eGFR over 1 year, supporting the premise that reductions in proteinuria are associated with a more stable eGFR in native kidney C3G.35 Consistent with these observations, in a proof-of-concept Phase II study, treatment with iptacopan 200 mg twice daily in patients with native or recurrent C3G post-transplant was well tolerated and resulted in a statistically significant and clinically important 45% reduction in proteinuria in patients with native C3G, demonstrating the potential therapeutic value of using a FB inhibitor for treatment of complement-mediated diseases and providing a basis for its clinical development. In APPEAR-C3G, measurement of proteinuria response over time may help to establish the trajectory of treatment response, thus allowing clinicians to dynamically monitor kidney outcomes.
Assessment of eGFR is the key secondary endpoint in this trial, which is supported for use as a surrogate endpoint by the National Kidney Foundation in other glomerular diseases and is being investigated by the Kidney Health Initiative.36,37 Though it should be noted that there are very few studies that have examined the correlation of eGFR with predicted kidney outcomes in C3G, eGFR (which had been declining prior to the study) stabilized following treatment with iptacopan in a short Phase II study.21 One unique aspect of this study is the collection of 2 years of historical eGFR data, which will permit long-term or chronic slope analysis with estimation of the change in slope after the commencement of iptacopan.
At the time of writing, there are 3 active Phase II studies and 1 Phase III study testing 2 novel compounds suspected to play an important role in C3G pathogenesis.
Three studies investigating the safety and efficacy of pegcetacoplan, a C3 inhibitor, which unlike iptacopan is administered via subcutaneous injection, have chosen proteinuria and eGFR as their primary and secondary endpoints, respectively.25,38,39 These studies consist of a Phase II basket study assessing multiple glomerulopathies (NCT03453619) as well as a Phase II (NCT04572854) and a Phase III (NCT05067127) study, each recruiting patients with both C3G and immune complex-MPGN. Patients with post-transplant recurrent disease are being recruited for the Phase II study, whereas the Phase III study is pooling native and transplant patients. Similarly, a Phase II study investigating the therapeutic potential of an oral factor D inhibitor BCX9930 in C3G, immunoglobulin A nephropathy and primary membranous nephropathy will assess change in proteinuria as a primary endpoint (NCT05162066).
The recent Phase II ACCOLADE study (N = 57) assessing the C5a oral inhibitor avacopan in C3G opted for a primary endpoint of change in C3G activity score (measured by C3G-HI) at 26 weeks (NCT03301467).26 Secondary objectives are comparable between APPEAR-C3G and ACCOLADE (eGFR; progression of fibrosis) and preliminary data from the ACCOLADE study have provided some evidence of improved kidney function.40
Histological changes as assessed by biopsy are key indicators of disease progression and of treatment response. Assessment of biopsies for total activity scores and C3 deposits will allow correlation with changes in kidney function (proteinuria and eGFR) with those at the histological level reflecting attentuation or reversal of pathological changes. In the Phase II study, following iptacopan treatment, patients with C3G recurrence post-transplant had reduced C3 deposit scores at week 12.21 Our study will examine the status of disease progression with biopsy at 6 months to assess the change in disease activity and chronicity score between baseline and 6-months (and 1 year [optional biopsy]) in native C3G. The optional biopsy at 12-months as part of the study design may further validate the observations at 12 weeks and has value as a means of confirming that treatment is effective as a targeted therapy, allowing a direct comparison with end of trial results and highlighting any clinical improvements with treatment.41 Expert opinion considers kidney biopsy to be the benchmark of disease progression because biomarkers may not be exact indicators of complement activation within the kidney.41 It should be noted that this view is not evidence-based, and biopsies are not employed universally. Though a recent kidney biopsy is required in most trials in glomerular disease as part of the inclusion criteria, ensuring diagnosis and complement system activation in the kidneys at trial entry, biopsies are invasive, and risks and benefits of a repeat biopsy must be considered. APPEAR-C3G incorporates C3 deposit score analysis as an exploratory objective, to evaluate whether FB inhibition can reduce glomerular C3 deposition. Disease activity and chronicity will be scored using the C3G-HI. Recently, the ACCOLADE study found that at 26 weeks, treatment with avacopan resulted in a statistically insignificant 2% improvement in primary endpoint C3G-HI disease activity, compared with a 38% worsening in the placebo group (P = 0.4608).40
Almost all patients with C3G who have progressive kidney disease have a markedly diminished quality of health and life. Improving “hard” outcomes like kidney failure are key to treatment. Nevertheless, from a patient’s perspective, improved quality of life (QoL), including life participation, fatigue, anxiety, and family impact, is also a priority.42 This study design is built around patient-centric QoL outcomes, such as the FACIT-Fatigue, EQ-5D5L total, Short Form 36 summary, and patient global impression of severity score to collectively assess various domains of daily activities and function, and to provide insight into efficacy of a therapy from a patient perspective. One of the most commonly reported symptoms among patients with chronic kidney disease and kidney failure is fatigue.43 APPEAR-C3G employs the FACIT-Fatigue score, a comprehensive assessment tool that assesses self-reported fatigue and its effect upon daily activities and function in order to assist management of chronic illness through a series of health-related QoLs (https://www.facit.org). In a recent trial, assessing efficacy of ravulizumab in the complement-mediated kidney disease atypical hemolytic uremic syndrome, FACIT-Fatigue scores were shown to improve with treatment in the initial 183 day evaluation period, and were maintained through the extension period up to day 351.44 Whereas other QoL scores were used in the ACCOLADE C3G trial, to the best of our knowledge the FACIT-Fatigue score has not been employed in many, if any, Phase III C3G trials to date, and this study will provide novel insight into this key aspect of patient QoL.
Another innovative approach to this study design is the use of estimands. Published in 2019, a new addendum to the ICH E9 guideline presented the estimand framework as a systematic approach to ensure alignment among clinical trial objectives, trial execution/conduct, statistical analyses, and interpretation of results.45 Following this approach, in this study we state clearly how intercurrent events (such as patient discontinuations, concomitant medications, etc.) will be dealt with and ensure that the appropriate data needed to address specific scientific questions of interest are estimated. This adds precision to this study design and ensures that the questions of interest are answered as accurately as possible.
Supportive care, focusing on proteinuria and hypertension, form the basis of current management of all glomerulopathies, including C3G.1,7 There is no established standard of care for patients with C3G due to the rare nature of the disease and the lack of evidence-based treatment.20 Consequently, there is a need for a C3G therapy targeting the underlying cause of the disease to improve patient outcomes. Several unique aspects of this trial design, including the use of estimands, surrogate endpoints, histological measures, patient population, and patient-centered outcomes, will not only provide valuable evidence toward efficacy and safety of iptacopan in C3G, but also a valuable framework for complement-mediated kidney disease therapeutic development. By inhibiting FB and preventing overactivation of the AP to slow disease progression, iptacopan has the potential to be one of the first targeted therapies to become available to patients with C3G.
Disclosure
ASB declares consulting honoraria from Achillion, Alexion, Chemocentryx, Novartis, Silence, Catalyst, and Principio. DK is scientific founder of and hold stocks in gyroscope therapeutics. He has received consultancy income from gyroscope therapeutics, Alexion Pharmaceuticals, Novartis, Apellis and Sarepta. His spouse works for GSK. MV declares honoraria for advisory boards and consulting fees, participation in clinical studies sponsored by the following pharmaceutical companies: Achillion, Alexion, Apellis, Bayer, Catalyst, Novartis, Roche, Retrophin/Travere. RJHS declares research funding from NIH, consultant for Novartis. MM, YW, NW and AJT are employees and stockholders of Novartis.
Acknowledgments
The authors thank Lorna Mulvey and Nagabhushana Ananthamurthy of Novartis for providing medical writing and editorial support in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3). This study is funded by Novartis Pharma AG.
Clinical trials registration number: NCT04817618.
Author Contributions
All authors contributed to the design of the study, drafting and critically reviewing the manuscript. YW contributed to the development of statistical analysis plan for the study. All authors have read and approved the manuscript.
Footnotes
Video1. A mechanism of action video for iptacopan in C3G.
CONSORT Checklist.
Supplementary Materials
Video 1. A mechanism of action video for iptacopan in C3G.
CONSORT Checklist.
References
- 1.Smith R.J.H., Appel G.B., Blom A.M., et al. C3 glomerulopathy - understanding a rare complement-driven renal disease. Nat Rev Nephrol. 2019;15:129–143. doi: 10.1038/s41581-018-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caravaca-Fontán F., Lucientes L., Cavero T., Praga M. Update on C3 glomerulopathy: a complement-mediated disease. Nephron. 2020;144:272–280. doi: 10.1159/000507254. [DOI] [PubMed] [Google Scholar]
- 3.Servais A., Noël L.H., Roumenina L.T., et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 4.Noris M., Remuzzi G. Genetics of immune-mediated glomerular diseases: focus on complement. Semin Nephrol. 2017;37:447–463. doi: 10.1016/j.semnephrol.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Donadelli R., Pulieri P., Piras R., et al. Unraveling the molecular mechanisms underlying complement dysregulation by nephritic factors in C3G and IC-MPGN. Front Immunol. 2018;9:2329. doi: 10.3389/fimmu.2018.02329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noris M., Donadelli R., Remuzzi G. Autoimmune abnormalities of the alternative complement pathway in membranoproliferative glomerulonephritis and C3 glomerulopathy. Pediatr Nephrol. 2019;34:1311–1323. doi: 10.1007/s00467-018-3989-0. [DOI] [PubMed] [Google Scholar]
- 7.Iatropoulos P., Daina E., Curreri M., et al. Cluster analysis identifies distinct pathogenetic patterns in C3 glomerulopathies/immune complex–mediated membranoproliferative GN. J Am Soc Nephrol. 2018;29:283–294. doi: 10.1681/ASN.2017030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holle J., Berenberg-Goßler L., Wu K., et al. Outcome of membranoproliferative glomerulonephritis and C3-glomerulopathy in children and adolescents. Pediatr Nephrol. 2018;33:2289–2298. doi: 10.1007/s00467-018-4034-z. [DOI] [PubMed] [Google Scholar]
- 9.Bomback A.S., Santoriello D., Avasare R.S., et al. C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 2018;93:977–985. doi: 10.1016/j.kint.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Lu D.F., Moon M., Lanning L.D., et al. Clinical features and outcomes of 98 children and adults with dense deposit disease. Pediatr Nephrol. 2012;27:773–781. doi: 10.1007/s00467-011-2059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman L.D.B., Bomback A., Nester C. Voice of the patient: report of externally-led patient-focused drug development meeting on: complement 3 glomerulopathy (C3G). National Kidney Foundation, 2018. https://www.kidney.org/sites/default/files/C3G_EL-PFDD_VoP-Report_3-29-18.pdf
- 12.Goodship T.H., Cook H.T., Fakhouri F., et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91:539–551. doi: 10.1016/j.kint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizk D.V., Maillard N., Julian B.A., et al. The emerging role of complement proteins as a target for therapy of IgA nephropathy. Front Immunol. 2019;10:504. doi: 10.3389/fimmu.2019.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harboe M., Ulvund G., Vien L., Fung M., Mollnes T.E. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramadass M., Ghebrehiwet B., Smith R.J., Kew R.R. Generation of multiple fluid-phase C3b:plasma protein complexes during complement activation: possible implications in C3 glomerulopathies. J Immunol. 2014;192:1220–1230. doi: 10.4049/jimmunol.1302288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickering M.C., Cook H.T., Warren J., et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 18.Williams A.L., Gullipalli D., Ueda Y., et al. C5 inhibition prevents renal failure in a mouse model of lethal C3 glomerulopathy. Kidney Int. 2017;91:1386–1397. doi: 10.1016/j.kint.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubart A., Anderson K., Mainolfi N., et al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc Natl Acad Sci U S A. 2019;116:7926–7931. doi: 10.1073/pnas.1820892116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int Suppl. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Wong E.K.S., et al. Iptacopan, a novel oral complement factor B (FB) inhibitor, significantly reduces proteinuria and C3 deposit scores in native and transplanted kidneys C3 glomerulopathy (C3G) patients. J Am Soc Nephrol. 2021;32(B8) [Google Scholar]
- 22.Wong E.K.S., Praga M., Nester C., et al. FC 036IPTACOPAN (LNP023): a novel oral complement alternative pathway factor B inhibitor safely and effectively stabilises eGFR in C3 glomerulopathy. Nephrol Dial Transplant. 2021;36(Suppl 1) doi: 10.1093/ndt/gfab121.005. [DOI] [Google Scholar]
- 23.Ricklin D., Lambris J.D. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan B.P., Harris C.L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov. 2015;14:857–877. doi: 10.1038/nrd4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Study assessing the safety and efficacy of pegcetacoplan in post-transplant recurrence of C3G or IC-MPGN (NOBLE). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04572854
- 26.Controlled trial evaluating avacopan in C3 glomerulopathy (ACCOLADE). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03301467
- 27.Le Quintrec M., Lapeyraque A.L., Lionet A., et al. Patterns of clinical response to eculizumab in patients with C3 glomerulopathy. Am J Kidney Dis. 2018;72:84–92. doi: 10.1053/j.ajkd.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Konar M., Granoff D.M. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood. 2017;130:891–899. doi: 10.1182/blood-2017-05-781450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ispasanie E., Muri L., Schubart A., et al. Alternative complement pathway inhibition does not abrogate meningococcal killing by serum of vaccinated individuals. Front Immunol. 2021;12:747594. doi: 10.3389/fimmu.2021.747594. 747594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muri L., Ispasanie E., Schubart A., et al. Alternative complement pathway inhibition abrogates pneumococcal opsonophagocytosis in vaccine-naïve, but not in vaccinated individuals. Front Immunol. 2021;12:732146. doi: 10.3389/fimmu.2021.732146. 732146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey A.S., Gansevoort R.T., Coresh J., et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: A scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75:84–104. doi: 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Kidney disease: improving global outcomes (KDIGO) Glomerulonephritis work group KDIGO clinical practice guideline for Glomerulonephritis. Kidney Int, Suppl. 2012;2:139–274. doi: 10.1038/kisup.2012.12. [DOI] [Google Scholar]
- 33.Wong EK, Lim WH, Craig JC. When less becomes more: life and losses without the ‘Roids’? J Am Soc Nephrol. 2020;31:6-8. doi:10.1681/ASN.2019111183 [DOI] [PMC free article] [PubMed]
- 34.Caravaca-Fontán F., Díaz-Encarnación M., Cabello V., et al. Longitudinal change in proteinuria and kidney outcomes in C3 glomerulopathy. Nephrol Dial Transplant. 2022;37:1270–1280. doi: 10.1093/ndt/gfab075. [DOI] [PubMed] [Google Scholar]
- 35.Nester C., Breheny P., Hall M., et al. Relationship between UPCR and eGFR in C3 glomerulopathy. Nephrol Dial Transplant. 2021;36(suppl 1) doi: 10.1093/ndt/gfab092.0014. [DOI] [Google Scholar]
- 36.Barratt J., Rovin B., Diva U., et al. Implementing the Kidney Health Initiative Surrogate Efficacy Endpoint in Patients With IgA Nephropathy (the PROTECT Trial) Kidney Int Rep. 2019;4:1633–1637. doi: 10.1016/j.ekir.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene T., Ying J., Vonesh E.F., et al. Performance of GFR slope as a surrogate end point for kidney disease progression in clinical trials: a statistical simulation. J Am Soc Nephrol. 2019;30:1756–1769. doi: 10.1681/ASN.2019010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phase III study assessing the efficacy and safety of pegcetacoplan in patients with C3 glomerulopathy or immune-complex membranoproliferative glomerulonephritis (VALIANT). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT05067127
- 39.Phase II study assessing safety and efficacy of APL-2 in glomerulopathies. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03453619
- 40.Bomback A.S., LCH, Yue H., et al. Effect of avacopan, a selective C5a receptor inhibitor, on C3G histologic index of disease chronicity. J Am Soc Nephrol. 2021;7:S47–S48. doi: 10.1016/j.ekir.2022.01.124. [DOI] [Google Scholar]
- 41.Bomback A.S., Appel G.B., Gipson D.S., et al. Improving clinical trials for anticomplement therapies in complement-mediated glomerulopathies: report of a scientific workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2021;79:570–581. doi: 10.1053/j.ajkd.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 42.Carter S.A., Gutman T., Logeman C., et al. Identifying outcomes important to patients with glomerular disease and their caregivers. Clin J Am Soc Nephrol. 2020;15:673–684. doi: 10.2215/CJN.13101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao C.-T., Huang J.W., Chiang C.K. COGENT (COhort of GEriatric Nephrology in NTUH) study group. Functional assessment of chronic illness therapy-the fatigue scale exhibits stronger associations with clinical parameters in chronic dialysis patients compared to other fatigue-assessing instruments. PeerJ. 2016;4 doi: 10.7717/peerj.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbour T., Scully M., Ariceta G., et al. Long-term efficacy and safety of the long-acting complement C5 inhibitor ravulizumab for the treatment of atypical hemolytic uremic syndrome in adults. Kidney Int Rep. 2021;6:1603–1613. doi: 10.1016/j.ekir.2021.03.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrance R., Degtyarev E., Griffiths P., et al. What is an estimand & how does it relate to quantifying the effect of treatment on patient-reported quality of life outcomes in clinical trials? J Patient Rep Outcomes. 2020;4:68. doi: 10.1186/s41687-020-00218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.