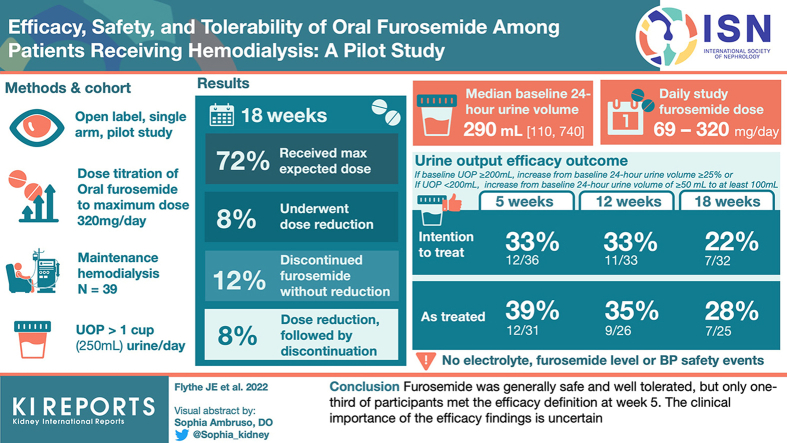
Abstract
Introduction
Diuretic use may reduce volume-related complications in hemodialysis. We evaluated the efficacy, safety, and tolerability of furosemide in patients with hemodialysis-dependent kidney failure.
Methods
We conducted an open label, single-arm, 18-week, dose titration pilot study of oral furosemide (maximum dose 320 mg/day) among patients receiving maintenance hemodialysis who reported at least 1 cup of urine output per day. The primary efficacy outcome was an increase from baseline to a specified threshold of 24-hour urine volume, with the threshold based on baseline urine volume (<200 ml/day vs. ≥200 ml/day). Safety outcomes included hypokalemia and hypomagnesemia, and tolerability was assessed by prespecified patient-reported symptoms.
Results
Of the 39 participants, 28 (72%) received the expected furosemide dose, 3 (8%) underwent dose reduction, 5 (12%) discontinued furosemide without dose reduction, and 3 (8%) underwent dose reduction and subsequently discontinued furosemide. The median (quartile 1, quartile 3) baseline 24-hour urine volume was 290 ml (110, 740), and the maximum, average daily study furosemide dose ranged from 69 mg/day to 320 mg/d. The urine output efficacy outcome was met by 12 (33%), 11 (33%), and 7 (22%) participants at weeks 5, 12, and 18, respectively, in the intention-to-treat analysis, and by 12 (39%), 9 (35%), and 7 (28%) participants at weeks 5, 12, and 18, respectively, in the on-treatment analysis. There were no electrolyte, furosemide level, or patient-reported hearing change safety events.
Conclusion
Furosemide was generally safe and well tolerated, but only one-third of participants met the efficacy definition at week 5. The clinical importance of the efficacy findings is uncertain.
Keywords: diuretic, efficacy, furosemide, hemodialysis, hypervolemia, safety
Graphical abstract
Volume-related factors including extracellular hypervolemia, larger interdialytic weight gains, and higher ultrafiltration rates are modifiable contributors to the high rates of cardiovascular complications in people with hemodialysis-dependent kidney failure.1,2 Nevertheless, over 50% of patients receiving hemodialysis are chronically volume-overloaded,3 more than 35% have interdialytic weight gains exceeding 3.5% of body weight,4 and 40% have average ultrafiltration rates more than 10 ml/h/kg.5 Despite enhanced regulatory and clinical emphasis on volume management in the last decade, volume control remains a major challenge in hemodialysis care.
Oral loop diuretic therapy, a mainstay of advanced chronic kidney disease and peritoneal dialysis treatment regimens, represents a potential strategy to mitigate volume-related complications. Nevertheless, the practice of prescribing diuretics in hemodialysis care is inconsistent. More than 50% of patients in the United States stop diuretic therapy at hemodialysis initiation irrespective of residual urine output, and more than 25% of patients in the United States remain on diuretics 6 months after starting hemodialysis.6,7 In contrast, adjunctive diuretic therapy is common in Europe and Japan, regions with lower interdialytic weight gains and ultrafiltration rates.7,8 Observational studies have suggested benefits of diuretic use versus nonuse among patients with hemodialysis-dependent kidney failure, including lower interdialytic weight gains, fewer hypotensive events, and lower hospitalization and mortality rates,7,9 but potential confounding from residual kidney function limits interpretation of these findings.
Mechanistic data suggest that adaptive renal tubule changes preserve diuretic response even in the setting of substantially impaired kidney function, but higher dosing is typically required.10,11 High-dose loop diuretics can cause electrolyte derangements, ototoxicity, and bullous rashes, among other untoward side effects.10,12 Currently, there are limited clinical trial data on the efficacy, safety, and optimal dosing of oral loop diuretic therapy in patients receiving maintenance hemodialysis. To that end, we conducted a pilot dose titration study of furosemide among patients receiving in-center maintenance hemodialysis.
Methods
Design and Oversight
The study was an open label, single-arm, 18-week, dose titration pilot study designed to provide pilot data on the efficacy, safety, tolerability, and acceptability of oral furosemide. The study protocol (Supplementary Material) was approved by the University of North Carolina at Chapel Hill Institutional Review Board (#19-3550) and registered on clinicaltrials.gov (NCT04622709). We report our findings in accordance with the Consolidated Standards of Reporting Trials 2010 statement: extension to randomized pilot and feasibility trials.13,14 All participants provided written informed consent.
Study Participants and Recruitment
We enrolled participants from 3 outpatient dialysis clinics that are affiliated with the University of North Carolina, between October 2020 and January 2021, with the last follow-up on June 1, 2021. Study inclusion criteria were: aged at least 18 years, treatment with 3-times-weekly maintenance hemodialysis for at least 60 days, and urine volume of at least 1 cup (250 ml) of urine per 24 hours by self-report. Major exclusion criteria included the following: (i) allergy to loop diuretic; (ii) use of a nonloop diuretic; (iii) use of a medication or substance that can interact with loop diuretics (e.g., aminoglycosides, cisplatin, methotrexate, lithium, natural licorice); (iv) predialysis serum potassium less than 3.5 mEq/l, magnesium less than 1 mg/dl, or corrected calcium less than 8 mg/dl in the 30 days before enrollment; (v) cirrhosis; (vi) pregnancy or breastfeeding; (vii) known hearing impairment; (viii) history of poor adherence to hemodialysis; (ix) more than 1 hospitalization in the 30 days before enrollment; and (x) frequent hypotension, defined as a systolic blood pressure less than 80 mmHg during more than 30% of hemodialysis sessions in the 30 days before enrollment.
Intervention and Treatment Algorithm
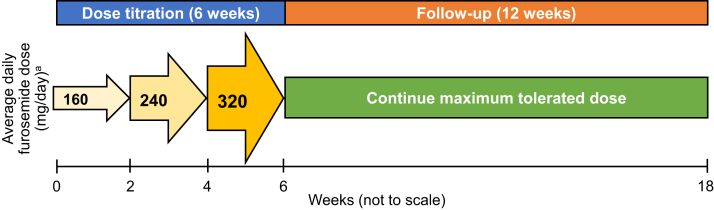
The study consisted of a 6-week dose titration period of oral furosemide and a 12-week follow-up period (Figure 1). During the dose titration period, participants who were not taking a loop diuretic at study enrollment received 80 mg oral furosemide 2 times per day for 14 days and then, if the dose was tolerated, 120 mg 2 times per day for 14 days and then, if the dose was tolerated, 160 mg 2 times per day for 14 days. Participants who were taking a loop diuretic at study enrollment received their baseline furosemide dose (or furosemide-equivalent dose for those taking bumetanide or torsemide) for 14 days. If the starting furosemide dose was tolerated, the dose was then increased by 50% for 14 days, and then, if tolerated, the new dose was increased by 50% for 14 days. The maximum furosemide dose for any participant was 320 mg per day. During the 12-week follow-up period, participants remained on the maximum tolerated furosemide dose achieved during the dose titration period. Furosemide was dispensed in 20 mg, 40 mg, and 80 mg strength tablets by the University of North Carolina Health Investigational Drug Services.
Figure 1.
Study design. aDuring the 6-week dose titration period, participants who were not taking a loop diuretic at study entry received 80 mg furosemide twice a day for 14 days and then, if the dose was tolerated, they received 120 mg oral furosemide twice a day for 14 days and then, if the dose was tolerated, they received 160 mg oral furosemide twice a day for 14 days. During the 6-week dose titration period, participants who were taking a loop diuretic at study entry received their baseline furosemide dose (or furosemide-equivalent dose for those receiving nonfurosemide loop diuretics) for 14 days. If tolerated, the baseline furosemide dose was increased by 50% for 14 days, and then, if the dose was tolerated, the dose was increased by 50% for 14 days. The maximum dose for any participant was 320 mg/day. During the 12-week follow-up period, participants remained on their maximally tolerated dose from the dose titration period.
During the dose titration period there were no further dose increases if any of the following events occurred: (i) predialysis serum potassium less than 3.2 mEq/l; (ii) predialysis serum magnesium less than 0.8 mg/dl; (iii) predialysis serum corrected calcium less than 7 mg/dl; (iv) rash attributed to furosemide; (v) tinnitus attributed to furosemide; (vi) hearing change attributed to furosemide; (vii) at least 10-point decline from baseline in inner effectiveness of auditory rehabilitation (inner EAR) score, a patient-reported outcome correlate for pure tone audiometry, attributed to furosemide;15, 16, 17 and (viii) intradialytic systolic blood pressure less than 80 mmHg attributed to furosemide.18 Upon the occurrence of a dose-limiting event, participants returned to the prior tolerated furosemide dose. If an event occurred at the lowest administered dose, furosemide was discontinued.
Study Visits and Data Collection
All study visits occurred during routine hemodialysis sessions. Medical history, hemodialysis prescription, medications, laboratory values, symptoms, and inner EAR assessments were recorded at baseline. Twenty-four-hour urine collections were performed at baseline, at the end of the dose titration period, and at the midpoint and end of the follow-up period for a total of 4 collections. Laboratory, symptom, and pill count assessments were performed on a weekly basis during the dose titration period. During the follow-up period, laboratory assessments were performed every 4 weeks, and symptom and pill count assessments were performed every 2 weeks. Symptoms were assessed with a questionnaire measuring 12 patient-reported symptoms with 5-point Likert scales (response options: none, mild, moderate, severe, very severe). The inner EAR instrument was administered every 2 weeks during the dose titration period and every 4 weeks during the follow-up period. Information about each hemodialysis session (blood pressures, weights, ultrafiltration volumes, treatment length) and hospitalizations were extracted from the electronic health record.
Laboratory parameters were measured predialysis during the midweek dialysis session, and 24-hour urine collections occurred between the first and second dialysis sessions of the week (i.e., in the 24 hours before the Wednesday session for Monday-Wednesday-Friday patients, and in the 24 hours before the Thursday session for Tuesday-Thursday-Saturday patients). Serum furosemide levels were determined in batched measurements by liquid chromatography mass spectrometry (AB Sciex Triple Quad LC-MS/MS Systems, Framingham, MA).
Outcomes
The primary efficacy outcome was defined as: either (a) an increase from baseline 24-hour urine volume of at least 25% for participants with a baseline 24-hour urine volume of at least 200 ml, or (b) an increase from baseline 24-hour urine volume of at least 50 ml to at least 100 ml for participants with a baseline 24-hour urine volume less than 200 ml. Exploratory efficacy outcomes included interdialytic weight gain, delivered ultrafiltration rate, difference between target weight and postdialysis weight, and predialysis systolic blood pressure.
Safety outcomes included the following: (i) serum potassium less than 3.2 mEq/l, (ii) serum magnesium less than 0.8 mg/dl, (iii) serum corrected calcium less than 7 mg/dl, (iv) serum furosemide level more than 12 mcg/L, (v) dialysis-associated hypotension requiring hospital treatment not attributable to other causes, (vi) rash attributable to furosemide, (vii) tinnitus attributable to furosemide, (viii) hearing change attributable to furosemide, and (ix) at least 10-point decline from baseline in inner EAR score. Tolerability outcomes included cramping, dizziness or presyncope, unusual tiredness or weakness, chest pain, nausea, vomiting, and diarrhea. Acceptability was assessed by the question, “If recommended, would you be willing to stay on the dose of furosemide you have received during the last week?” adherence to furosemide was assessed with pill counts, with “adherent” defined as returning less than 20% of the dispensed tablets.
Statistical Analyses
All statistical analyses were performed using R (3.6.3 R Foundation, Vienna, Austria). Baseline characteristics are presented as count (%) for categorical variables and as mean ± SD or median (quartile 1, quartile 3) for continuous variables. Categorical outcome variables are presented as the number (%) of participants with an event of interest during the specified period. In addition, we determined the number (%) of participants that experienced at least 1 outcome event during the dose titration and follow-up periods, separately, and computed the rate of outcome events per 100 person-weeks during each period. Hemodialysis session-related exploratory efficacy outcomes were reported as the median (quartile 1, quartile 3) of individual participant median values during the baseline, dose titration, and follow-up periods. Analyses were performed as intention-to-treat (i.e., all patients included regardless of study furosemide use) and, where specified, as on-treatment (i.e., patients taking study furosemide at the time of outcome ascertainment).
Results
Participants
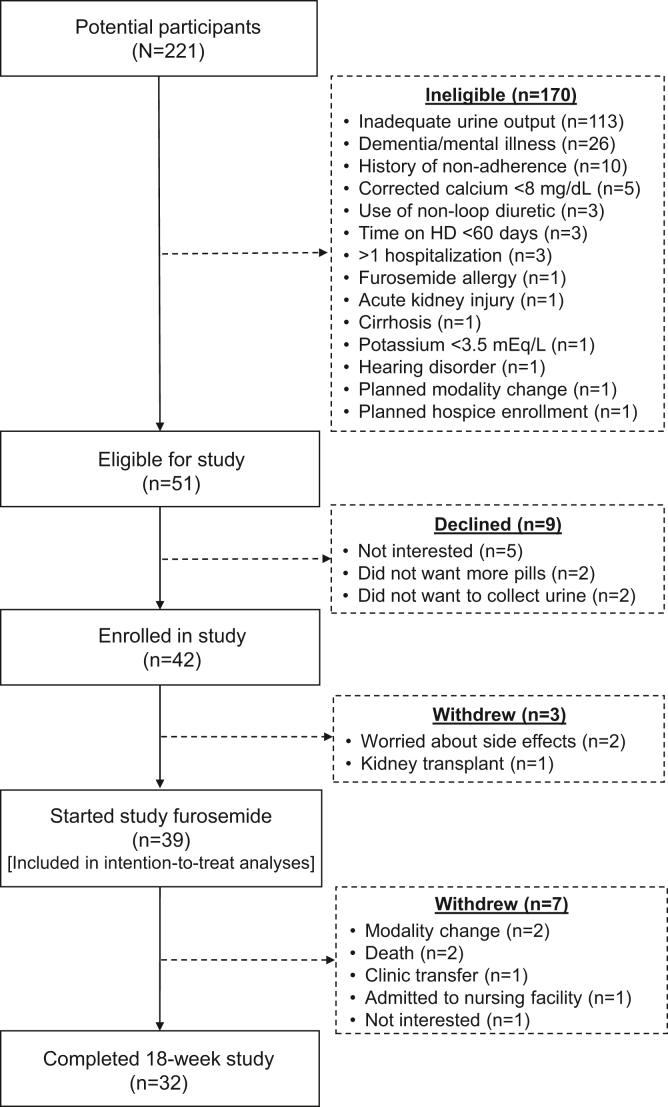
Among 221 patients screened, 51 were eligible, and 42 agreed to participate (Figure 2). The most common reason for ineligibility was urine output of less than 1 cup per day n = 113 (66%). Of the 42 enrollees, 39 (93%) initiated study furosemide and 32 (76%) completed the study. Three patients withdrew consent prior to furosemide initiation, and 7 participants withdrew from the study after furosemide initiation due to death (n = 2), clinic or modality transfer (n = 3), admission to skilled nursing facility (n = 1), and personal choice (n = 1).
Figure 2.
Participant enrollment and follow-up. HD, hemodialysis.
Participant characteristics are presented in Table 1. Participants initiating study furosemide had a median age of 65 years, 26% were women, 59% were Black, 15% were Hispanic, the most common cause of kidney failure was diabetes, and 67% had diabetes, 41% had heart failure, and 90% had hypertension. The median time on dialysis was 1.5 (1, 3) years. The median baseline 24-hour urine volume was 290 ml (110, 740) with volumes ranging from 22 to 1400 ml.
Table 1.
Baseline participant characteristicsa
| Characteristic | Participants (N = 39) |
|---|---|
| Demographic characteristics | |
| Age (yrs) | 65 [58, 75] |
| Female sex | 10 (26%) |
| Black race | 23 (59%) |
| White race | 14 (36%) |
| Other race | 2 (5%) |
| Hispanic ethnicity | 6 (15%) |
| Comorbid medical conditions | |
| Diabetes | 26 (67%) |
| Congestive heart failure | 16 (41%) |
| Coronary artery disease | 17 (44%) |
| Hypertension | 35 (90%) |
| Kidney failure cause | |
| Diabetes | 17 (44%) |
| Hypertension | 16 (41%) |
| Glomerular disease | 2 (5%) |
| Other | 4 (10%) |
| Dialysis treatment characteristics | |
| Dialysis vintage (yrs) | 1.5 [1, 3] |
| Hemodialysis treatment time (mins) | 229 [210, 249] |
| Postdialysis weight (kg) | 88 [68, 102] |
| Interdialytic weight gain (kg) | 2.0 [1.6, 3.0] |
| Predialysis systolic blood pressure (mmHg) | 153 [136, 168] |
| Vascular access type | |
| Fistula | 29 (74%) |
| Graft | 3 (8%) |
| Catheter | 7 (18%) |
| Laboratory measures | |
| Serum potassium (mEq/l) | 4.5 [4.2, 4.9] |
| Serum magnesium (mg/dl) | 2.0 [1.8, 2.2] |
| Serum corrected calcium (mg/dl) | 8.8 [8.4, 9.2] |
| Serum albumin (g/dl) | 4.0 [3.9, 4.3] |
| 24-h urine volume (ml) | 290 [110, 740] |
| Medications | |
| ACE-inhibitor or ARB use | 13 (33%) |
| Beta blocker use | 27 (69%) |
| Calcium channel blocker use | 21 (54%) |
| Loop diuretic use | 22 (56%) |
| Vasodilator use | 2 (5%) |
| Other antihypertensive use | 8 (21%) |
| Patient-reported outcome measures | |
| Inner EAR score | 42 [38, 55] |
| Symptoms reported as severe or very severeb | |
| Cramping | 6 (15%) |
| Dizziness or lightheadedness | 0 (0%) |
| Unusual tiredness | 2 (5%) |
| Unusual weakness | 2 (5%) |
| Chest pain | 1 (3%) |
| New rash | 0 (0%) |
| Nausea or upset stomach | 0 (0%) |
| Vomiting | 0 (0%) |
| Diarrhea | 0 (0%) |
| Tinnitus | 1 (3%) |
| Hearing change | 0 (0%) |
| Numbness or tingling | 2 (5%) |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; EAR, effectiveness of auditory rehabilitation.
Values are presented as number (%) or median [quartile 1, quartile 3].
Symptoms during hemodialysis in the last week reported by the participant as severe or very severe on a 5-point severity Likert scale.
Furosemide Administration
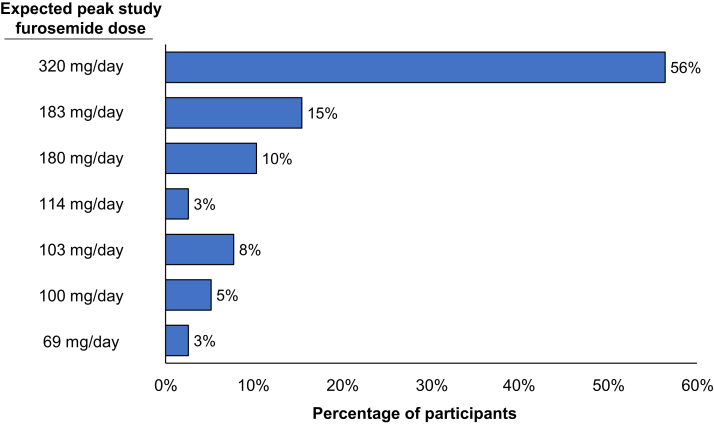
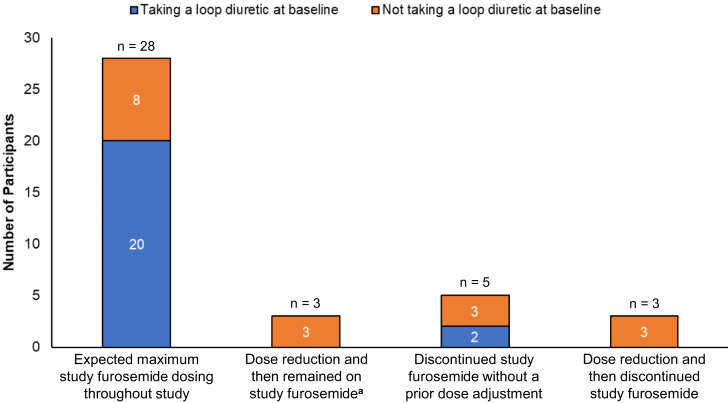
The expected maximum study furosemide dosing for participants are presented in Figure 3 and Supplementary Table S1. Of the 39 participants, 22 (56%) were taking a loop diuretic at baseline (furosemide, n = 16; bumetanide, n = 4; and torsemide, n = 2), with average daily furosemide-equivalent doses ranging from 23 mg/day to 320 mg/day. During the study, 28 (72%) participants achieved and remained on the expected maximum furosemide dose, 3 (8%) underwent dose reduction and remained on study furosemide, 5 (12%) discontinued furosemide without dose reduction, and 3 (8%) underwent dose reduction and subsequently discontinued furosemide. The maximum achieved daily dose of furosemide ranged from 69 mg/day to 320 mg/d. Patterns of furosemide dosing during the study, stratified by baseline loop diuretic use status are presented in Figure 4. Of the 11 participants who reduced and/or discontinued furosemide during the study, 8 (73%) were not taking a loop diuretic at baseline.
Figure 3.
Expected maximum study furosemide dosing among study participants (N = 39).
Figure 4.
Study furosemide dosing patterns stratified by use of a loop diuretic at baseline. aTwo participants reduced study furosemide dose and remained on the lower study furosemide dose for the remainder of the study, and one participant reduced study furosemide dose for one week and then returned to the higher study furosemide dose for the remainder of the study.
Furosemide pill counts demonstrated that participants took, on average, 86% ± 21% of dispensed pills during the dose titration period and 82% ± 21% of dispensed pills during the follow-up period. Among the 26 participants who completed pill count assessments at the end of the dose titration period, 21 (81%) met the adherence definition. Among the 23 participants who completed pill count assessments at the end of the follow-up period, 16 (70%) met the adherence definition. Overall, adherence to study assessments was high with more than 98% adherence to serum laboratory testing, 24-hour urine collections, as well as symptom, hearing, and acceptability questionnaires, and 88% adherence to pill count assessments (Supplementary Table S2).
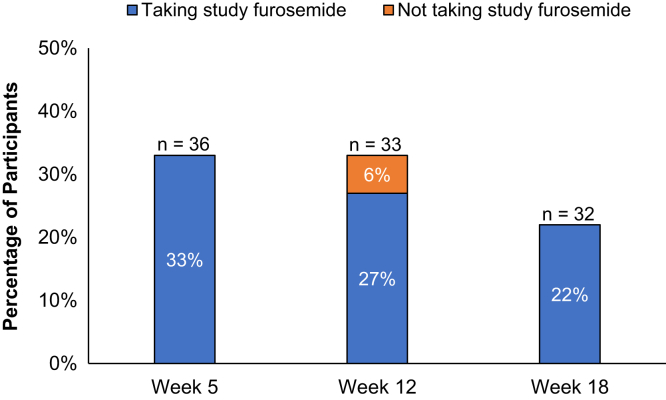
Efficacy
As shown in Figure 5, the urine output efficacy outcome was met by 12 (33%), 11 (33%), and 7 (22%) participants at weeks 5, 12, and 18, respectively, in the intention-to-treat analysis, and by 12 (39%), 9 (35%), and 7 (28%) participants at weeks 5, 12, and 18, respectively, in the on-treatment analysis. The mean (SD) change in urine volume was 39 ± 201 ml (range: −490 to 525 ml) for baseline to week 5 and −17 ± 280 ml (range: −550 to 800 ml) for baseline to week 18 (Supplementary Table S3). There were no changes from baseline evident in the exploratory efficacy outcomes of predialysis systolic blood pressure, interdialytic weight gain, delivered ultrafiltration rate, or difference in postdialysis and target weight (Supplementary Table S4). Changes in serum laboratory values from baseline are shown in Supplementary Table S5, and 24-hour urine sodium values are shown in Supplementary Table S3.
Figure 5.
Percentage of participants meeting the urine output efficacy definition stratified by study furosemide status at the time of 24-hour urine collection (on vs. off study furosemide). aEfficacy for urine output was defined as (a) an increase from baseline 24-hour urine volume of ≥25% among participants with a baseline 24-hour urine volume ≥200 ml and (b) a ≥50 ml increase in 24-hour urine volume to a 24-hour urine volume of at least 100 ml among participants with a baseline 24-hour urine volume <200 ml.
Safety
Safety and tolerability outcomes for both the intention-to-treat and on-treatment populations are presented in Table 2, and adverse events are presented in Supplementary Table S6. There were no safety events meeting the study definitions of hypokalemia, hypomagnesemia, hypocalcemia, elevated serum furosemide level, severe or very severe rash, or severe or very severe hearing change. Severe or very severe tinnitus occurred at rates of 1 and 2 events per 100 person-weeks during the dose titration and follow-up periods, respectively, with all events occurring in a single participant. The participant reported long-standing tinnitus and no change related to study furosemide initiation or titration. At least 10-point decline in the inner EAR score occurred at rates of 0.9 and 3 events per 100 person-weeks during the dose titration and follow-up periods, respectively. None of the safety events necessitated permanent discontinuation of furosemide. Nevertheless, furosemide was discontinued in one participant who developed vertigo and a hearing change not meeting the severity threshold for a safety event.
Table 2.
Safety and tolerability outcomesa
| Outcome | Intention-to-treat |
On-treatment |
||
|---|---|---|---|---|
| Dose titration (N = 39) | Follow-up (N = 36) | Dose titration (N = 38) | Follow-up (N = 31) | |
| Safety | ||||
| Serum potassium <3.2 mEq/l | 0 | 0 | 0 | 0 |
| Serum magnesium <0.8 mg/dl | 0b | 0 | 0b | 0 |
| Serum corrected calcium <7.0 mg/dl | 0 | 0 | 0 | 0 |
| Serum furosemide level >12 mcg/l | 0 | 0 | 0 | 0 |
| Dialysis-associated hypotension | 0 | 0 | 0 | 0 |
| Severe or very severe rash | 0 | 0 | 0 | 0 |
| Severe or very severe hearing change | 0 | 0 | 0 | 0 |
| Severe or very severe tinnitus | ||||
| Events per 100 person-wks | 1 | 2 | 1 | 1 |
| Proportion of participants with ≥1 event | 1 (3%)c | 1 (3%)c | 1 (3%)c | 1 (3%)c |
| ≥10-point decline in inner EAR score | ||||
| Events per 100 person-wks | 0.9 | 3 | 1 | 4 |
| Proportion of participants with ≥1 event | 1 (3%) | 3 (8%) | 1 (3%) | 3 (10%) |
| Tolerability | ||||
| Severe or very severe cramping | ||||
| Events per 100 person-wks | 12 | 11 | 11 | 10 |
| Proportion of participants with ≥1 event | 10 (26%) | 9 (25%) | 10 (26%) | 9 (29%) |
| Severe or very severe dizziness/presyncope | ||||
| Events per 100 person-wks | 3 | 0.5 | 2 | 0.6 |
| Proportion of participants with ≥1 event | 5 (13%) | 1 (3%) | 5 (13%) | 1 (3%) |
| Severe or very severe unusual tiredness | ||||
| Events per 100 person-wks | 0 | 1 | 0 | 1 |
| Proportion of participants with ≥1 event | 0 (0%) | 2 (6%) | 0 (0 %) | 2 (6%) |
| Severe or very severe nausea | ||||
| Events per 100 person-wks | 3 | 2 | 3 | 2 |
| Proportion of participants with ≥1 event | 5 (13%) | 3 (8%) | 5 (13 %) | 3 (10%) |
| Severe or very severe vomiting | ||||
| Events per 100 person-wks | 0.4 | 2 | 0.5 | 1 |
| Proportion of participants with ≥1 event | 1 (3%) | 2 (6%) | 1 (3%) | 2 (6%) |
| Severe or very severe diarrhea | ||||
| Events per 100 person-wks | 0.4 | 2 | 0.5 | 3 |
| Proportion of participants with ≥1 event | 1 (3%) | 4 (13%) | 1 (3%) | 4 (13%) |
EAR, effectiveness of auditory rehabilitation.
Serum laboratory values were assessed every week during dose titration and every 4 weeks during follow-up. Dialysis-associated hypotension was assessed during each treatment throughout the study. Symptoms were assessed every week during dose titration and every 2 weeks during follow-up, each with a 1-week recall period. Hearing was assessed every 2 weeks during dose titration and every 4 weeks during follow-up, each with a 1-week recall period.
One participant was missing serum magnesium values at weeks 3 and 4 (laboratory error).
One participant reported severe tinnitus a total of 9 times during the study, stating that it was a long-standing symptom that was unchanged during the study period. The participant discontinued study furosemide in week 9 because of perceived inefficacy of study medication and concern about falls, and continued to report severe tinnitus after discontinuation of study furosemide.
Tolerability and Acceptability
Severe or very severe cramping was the most commonly reported tolerability event and occurred at rates of 12 and 11 events per 100 person-weeks of follow-up during the dose titration and follow-up periods, respectively. One severe dizziness or presyncope tolerability event led to down-titration of furosemide in 1 participant, and 1 severe nausea and vomiting tolerability event led to study drug discontinuation in another participant. Other reasons for furosemide dose reduction and/or discontinuation not meeting the prespecified tolerability event definitions included, no subjective change in urine output and back pain (reduction), and no subjective change in urine output, foot pain, chest pain, diarrhea, and personal choice (discontinuation).
Discussion
Prescribing diuretics to maximize urine output is a plausible strategy for reducing volume overload, interdialytic weight gains, and ultrafiltration rates and, in turn, mitigating hemodynamic-related symptoms and cardiovascular complications in hemodialysis care. In this open label, single-arm, 18-week, dose titration pilot study among individuals with hemodialysis-dependent kidney failure who reported at least 1 cup urine output per day, we found that oral furosemide appeared to be safe and generally well tolerated and was modestly efficacious for increasing urine output among about one-third of participants. However, the clinical significance and durability of the efficacy findings beyond 18 weeks are uncertain.
Though diuretics promote urine output and may support more consistent volume control in patients with kidney failure and residual kidney function, evidence supporting the effectiveness of loop diuretics in the hemodialysis setting is weak compared to evidence in the settings of advanced chronic kidney disease and peritoneal dialysis.2,6,19 To date, there have been no rigorous interventional studies of loop diuretics in hemodialysis care. Two observational studies found associations between diuretic use (vs. nonuse) and lower rates of volume-related morbidity and mortality in hemodialysis-dependent kidney failure,7,9 but potential confounding from residual kidney function and other factors limit conclusions from these data. There are also concerns about the safety and tolerability of loop diuretics. Furosemide at any dose can lead to hypokalemia, hypomagnesemia, and hypocalcemia, and high-dose furosemide can cause ototoxicity and bullous dermatosis.10,12 Though ototoxicity has not been observed with oral furosemide administered at recommended doses in the absence of interacting medication use (e.g., cisplatin, aminoglycosides),20 uncertainty about optimal dosing remains. The dearth of clinical trial data on furosemide effectiveness and safety is a barrier to the broader use of furosemide in hemodialysis care.
Although our study was small, the findings suggest that oral furosemide, up to doses of 320 mg/day, increases urine output in some patients. Approximately one-third of participants met the study definition of efficacy in the intention-to-treat analysis, with the proportion meeting the efficacy definition declining over the course of the study. Though this decline could reflect decrements in response to furosemide, it is also possible that adherence to the study drug and/or completeness of 24-hour urine collections declined over the 18-week study period. In the on-treatment analysis, compared with the intention-to-treat analysis, a higher proportion of participants met the primary efficacy outcome, suggesting that the observed increase in urine output was potentially attributable to study furosemide use. In exploratory efficacy analyses, we did not detect changes from baseline in mean interdialytic weight gains, delivered ultrafiltration rates, or achievement of target weight postdialysis. These results raise question about whether furosemide, dosed per our study protocol, can induce clinically important changes in volume-related outcomes, and whether such changes can be sustained over time. Nevertheless, these findings should be considered in the context of study furosemide dosing. Because the dose of study furosemide received by participants was contingent on prestudy loop diuretic use and dose, participants achieved a range of maximum average daily study furosemide doses (69 to 320 mg/day) during the study. Almost 20% of participants had a per-protocol maximum average daily study furosemide dose of less than 120 mg/day. The protocol-specified dosing algorithm may have resulted in the administration of furosemide doses that were too low to trigger a physiological response in the setting of dialysis-dependent kidney failure.
In general, furosemide appeared in our study to be safe and generally well-tolerated by patients receiving maintenance hemodialysis. There were no safety events meeting the study definitions of hypokalemia, hypomagnesemia, hypocalcemia, high serum furosemide level, rash, or patient-reported hearing change. Our selection criteria excluding patients with similar events in the 30 days preceding enrollment may have reduced the risk of these events, but confirmation in future studies with broader selection criteria is warranted. One participant experienced severe tinnitus, and 3 participants had clinically important declines (≥10 points) from baseline in inner EAR scores. The participant with tinnitus reported a long-standing history of this issue, and the tinnitus persisted despite discontinuation of furosemide for other reasons. All the participants with inner EAR score declines showed improvement in scores at later timepoints while still taking study furosemide, and none reported subjective changes in their hearing. Though we cannot determine if these hearing-related events were attributable to furosemide, monitoring for furosemide-induced ototoxicity is prudent, particularly when prescribing higher doses (>300 mg/day).20 If hearing changes are reported, patients should be evaluated with audiometry and potentially referred for otolaryngologic evaluation.
In addition to studying safety, we examined furosemide tolerability. Severe or very severe cramping was the tolerability event that occurred most frequently, affecting 10 participants and approximately 10% of study dialysis sessions. Nevertheless, cramping may be precipitated by a range of hemodynamic, electrolyte, and neural factors and is a common intradialytic symptom, affecting approximately 50% of patients in the USA and 7% of hemodialysis sessions.21, 22, 23 Whether furosemide precipitated the reported cramping in our study is unknown, but it is reassuring that the rate of cramping during study treatments was similar to rates reported in the broader hemodialysis population. In one participant, severe dizziness led to reduction of furosemide dose, and severe nausea or vomiting prompted furosemide discontinuation in another participant. In both instances, the symptoms improved after the furosemide was reduced or discontinued. Two participants attributed new back pain and foot pain, symptoms that are not typical of furosemide, to the study drug and opted to reduce (for back pain) and discontinue (for foot pain) furosemide. Given the frequency of dialysis-related symptoms and challenges with distinguishing study drug-induced symptoms from routine, nondrug-related symptoms, we also assessed patient willingness to continue furosemide as a marker of treatment acceptability. Overall, furosemide was largely acceptable to participants with more than 90% of participants on study furosemide indicating willingness to continue furosemide at the end of the 18-week study.
Our study suggests that conducting a larger-scale trial of oral diuretics among patients with hemodialysis-dependent kidney failure may be feasible. Of the 51 patients meeting the eligibility criteria, 82% expressed interest in participating in the study, and 76% began study furosemide. Adherence to study procedures, including furosemide and 24-hour urine collections, was high. In addition, the efficacy findings suggest that some but not all patients who report at least 1 cup of urine output per day may experience increased urine output in response to oral furosemide. Similarly, not all participants tolerated the drug. Notably, more than 70% of dose reduction or discontinuation events occurred among participants who were not taking a loop diuretic at baseline, suggesting that individuals newly initiating furosemide may have a higher likelihood of therapy cessation. Given the modest efficacy findings, it may be important to consider a different dosing scheme and/or use of a diuretic with different pharmacokinetic properties that may have greater efficacy and be more likely to have an important clinical effect. Our results also inform other aspects of the design of future randomized clinical trials. Specifically, the incorporation of a run-in period in which patient tolerance of and urine output response to a loop diuretic are established prerandomization may enhance trial participant retention and facilitate the selection of patients most likely to have an increase in urine output, and thus, benefit clinically from furosemide. In addition, modification of the furosemide dosing algorithm so the target dose is not linked to a pretrial loop diuretic dose may increase the effectiveness of the intervention. Finally, more detailed information about the urine collections, such as the timing of the collection relative to furosemide dosing, as well as consideration of collections that span the entire interdialytic interval may be informative.
Our study has several strengths. The study population was racially and ethnically diverse, composed of 59% Black and 15% Hispanic participants, and baseline 24-hour urine volumes ranged from 22 ml to 1400 ml. Overall, participants were adherent to study procedures, and missing data and patient dropout were minimal. Nevertheless, our findings should be considered in the context of study limitations such as the small sample size, single-arm design, relatively short duration (limiting insights into the durability of the findings), and use of unsupervised 24-hour (vs. entire interdialytic period) urine collections. In addition, as discussed above, some participants received maximum furosemide doses lower than doses expected to be effective in the setting of dialysis-dependent kidney failure. Moreover, the study did not have the statistical power to detect differences in outcomes by furosemide dose nor was it designed to examine volume-related hospitalizations or mortality. Related to this, increases in urine volumes that met the study definition for efficacy may not result in clinically meaningful changes to interdialytic weight gain, ultrafiltration rates, and/or blood pressure. Finally, the study was conducted among patients who reported at least 1 cup urine output per day and were cared for by 1 academic faculty practice in North Carolina and may not generalize to other patients and/or practices.
This study indicates that oral furosemide modestly increases urine output in a subset of patients with hemodialysis-dependent kidney failure who report urine output of at least 1 cup per day. However, the clinical importance of this finding is uncertain. Taken together with the acceptable safety and tolerability data, this finding suggests that larger trials designed to evaluate volume-related clinical outcomes are likely feasible.
Disclosure
In the last 3 years, JEF has received speaking honoraria from the American Society of Nephrology and multiple universities, as well as investigator-initiated research funding unrelated to this project from the Renal Research Institute, a subsidiary of Fresenius Kidney Care, North America. She serves on a medical advisory board for Fresenius Kidney Care, North America as well as a scientific advisory board and Data and Safety Monitoring Committee for the NIDDK. She has received consulting fees from AstraZeneca. In the last 3 years, MMA received investigator-initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America and honoraria from the American Society of Nephrology and the International Society of Nephrology. LMD received compensation from the National Kidney Foundation for her role as Deputy Editor of the American Journal of Kidney Diseases, consulting fees from AstraZeneca, Merck, and Cara Therapeutics, and compensation for serving on Data and Safety Monitoring Boards for the NIDDK, and an Independent Data Monitoring Committee for Vertex. All other authors declare no conflicting interests.
Acknowledgments
This work as well as JEF and MMA were supported by R03 DK124651 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. JEF and MMA are supported by R01 HL152034 from the National Heart, Lung, and Blood Institute of the NIH.
Funding
NIH/NIDDK (R03 DK124651).
Footnotes
Table S1. Baseline loop diuretic and expected maximum study furosemide dosing among study participants (N = 39)
Table S2. Adherence to study assessments
Table S3. Study 24-hour urine volumes and urinary sodium concentrations by participant
Table S4. Exploratory efficacy clinical outcomes, by intention-to-treat and on-treatment status
Table S5. Absolute change from baseline in serum electrolytes, by intention-to-treat and on-treatment status
Table S6. Serious adverse events and study-defined adverse events during the study
Supplemental Item S1. Study protocol
Supplementary Material
Table S1. Baseline loop diuretic and expected maximum study furosemide dosing among study participants (N = 39)
Table S2. Adherence to study assessments
Table S3. Study 24-hour urine volumes and urinary sodium concentrations by participant
Table S4. Exploratory efficacy clinical outcomes, by intention-to-treat and on-treatment status
Table S5. Absolute change from baseline in serum electrolytes, by intention-to-treat and on-treatment status
Table S6. Serious adverse events and study-defined adverse events during the study
Supplemental Item S1. Study protocol
References
- 1.Weiner D.E., Brunelli S.M., Hunt A., et al. Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis. 2014;64:685–695. doi: 10.1053/j.ajkd.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Flythe J.E., Chang T.I., Gallagher M.P., et al. Blood pressure and volume management in dialysis: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020;97:861–876. doi: 10.1016/j.kint.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoccali C., Moissl U., Chazot C., et al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol. 2017;28:2491–2497. doi: 10.1681/ASN.2016121341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrera C., Brunelli S.M., Rosenbaum D., et al. A retrospective, longitudinal study estimating the association between interdialytic weight gain and cardiovascular events and death in hemodialysis patients. BMC Nephrol. 2015;16:113. doi: 10.1186/s12882-015-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assimon M.M., Wenger J.B., Wang L., Flythe J.E. Ultrafiltration rate and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2016;68:911–922. doi: 10.1053/j.ajkd.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sozio S.M., Shafi T., Shafi T., et al. Patterns in blood pressure medication use in US incident dialysis patients over the first 6 months. BMC Nephrol. 2013;14:249. doi: 10.1186/1471-2369-14-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bragg-Gresham J.L., Fissell R.B., Mason N.A., et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the dialysis outcomes and practice pattern study (DOPPS) Am J Kidney Dis. 2007;49:426–431. doi: 10.1053/j.ajkd.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Wong M.M., McCullough K.P., Bieber B.A., et al. Interdialytic weight gain: trends, predictors, and associated outcomes in the international dialysis outcomes and practice patterns study (DOPPS) Am J Kidney Dis. 2017;69:367–379. doi: 10.1053/j.ajkd.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Sibbel S., Walker A.G., Colson C., et al. Association of continuation of loop diuretics at hemodialysis initiation with clinical outcomes. Clin J Am Soc Nephrol. 2019;14:95–102. doi: 10.2215/CJN.05080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison D.H. Clinical Pharmacology in diuretic use. Clin J Am Soc Nephrol. 2019;14:1248–1257. doi: 10.2215/CJN.09630818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voelker J.R., Cartwright-Brown D., Anderson S., et al. Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int. 1987;32:572–578. doi: 10.1038/ki.1987.246. [DOI] [PubMed] [Google Scholar]
- 12.Huang X., Dorhout Mees E., Vos P., et al. Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Ren Physiol. 2016;310:F958–F971. doi: 10.1152/ajprenal.00476.2015. [DOI] [PubMed] [Google Scholar]
- 13.Eldridge S.M., Chan C.L., Campbell M.J., et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thabane L., Hopewell S., Lancaster G.A., et al. Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot Feasibility Stud. 2016;2:25. doi: 10.1186/s40814-016-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohan S., Corrales C.E., Yueh B., Shin J.J. Assessment of disease-specific and general patient-reported outcome measures of hearing health. Otolaryngol Head Neck Surg. 2018;158:702–709. doi: 10.1177/0194599818757998. [DOI] [PubMed] [Google Scholar]
- 16.Yueh B., McDowell J.A., Collins M., et al. Development and validation of the effectiveness of auditory rehabilitation scale. Arch Otolaryngol Head Neck Surg. 2005;131:851–856. doi: 10.1001/archotol.131.10.851. [DOI] [PubMed] [Google Scholar]
- 17.Jessen A., Ho A.D., Corrales C.E., et al. Improving measurement efficiency of the inner ear scale with item response theory. Otolaryngol Head Neck Surg. 2018;158:1093–1100. doi: 10.1177/0194599818760528. [DOI] [PubMed] [Google Scholar]
- 18.Flythe J.E., Xue H., Lynch K.E., et al. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26:724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States Renal Data System. 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2021. [Google Scholar]
- 20.Rybak L.P. Ototoxicity of loop diuretics. Otolaryngol Clin North Am. 1993;26:829–844. doi: 10.1016/S0030-6665(20)30770-2. [DOI] [PubMed] [Google Scholar]
- 21.Moledina D.G., Perry Wilson F. Pharmacologic treatment of common symptoms in dialysis patients: a narrative review. Semin Dial. 2015;28:377–383. doi: 10.1111/sdi.12378. [DOI] [PubMed] [Google Scholar]
- 22.Correa S., Pena-Esparragoza J.K., Scovner K.M., Mc Causland F.R. Predictors of intradialytic symptoms: an analysis of data from the hemodialysis study. Am J Kidney Dis. 2020;76:331–339. doi: 10.1053/j.ajkd.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Flythe J.E., Hilliard T., Lumby E., et al. Fostering innovation in symptom management among hemodialysis patients: paths forward for insomnia, muscle cramps, and fatigue. Clin J Am Soc Nephrol. 2019;14:150–160. doi: 10.2215/CJN.07670618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.