Introduction
Chronic kidney disease of unknown etiology (CKDu) is an emerging form of CKD that predominantly affects working-age adults belonging to agricultural communities living in discrete geographic areas. Disease hotspots have been identified in several countries, including El Salvador, Nicaragua, Guatemala, Mexico, India, Egypt, and Sri Lanka.1 Despite its widespread distribution, the main clinicopathological features of the disease are similar among the different communities.2
In Sri Lanka, CKDu is prevalent in the north-central region and the most affected communities are involved in paddy farming. The prevalence of the disease in this region range from 6% to 15%.3,4 Similar environmental, socioeconomic, and occupational risk factors for CKDu are prevalent in other areas of Sri Lanka, specifically among the sugarcane and paddy-farming communities living in the south-eastern dry zone. Nevertheless, these populations have not been a common focus of epidemiological studies on CKDu.
Although the exact etiology of CKDu is yet to be explained, it is considered a disease of multifactorial origin primarily resulting from environmental factors.5 Renal damage can progress even in the absence of continuous exposure to the risk factors. Therefore, community screening is of utmost importance in the detection and management of CKDu. The conventional markers, serum creatinine (sCr), and urine albumin creatinine ratio (uACR), are not sensitive and specific enough for the diagnosis of CKD or CKDu in their asymptotic early stages. On the contrary, novel markers such as urinary β2 microglobulin (uβ2M), could provide better specificity and sensitivity in the early diagnosis.
A cross sectional study was conducted in 6 selected Grama Niladhari divisions located within the Buttala Divisional Secretariat in Moneragala district in Sri Lanka (Supplementary Figure S1 and S2) (Supplementary Methods) to assess the prevalence of CKD and CKDu, the value of uβ2M as a biomarker in the early diagnosis of CKD or CKDu, and to investigate the association of heavy metals such as cadmium, lead, and arsenic with CKD or CKDu.
Results
Of the 352 individuals included, 194 (55.1%) were males. The median age was 47 years (interquartile range 36–57; Range = 12–83). In addition, 81% were farmers with the majority involved with either paddy or sugarcane cultivation or both (Supplementary Table S1). Deep (36.6%) and shallow (38.6%) wells were the main sources of drinking water with only 15.6% having access to tap water. Approximately two-thirds of the study population have used pesticides (67.4%) or fertilizer (67.7%). Of the participants, 26.7%, 25.8%, and 39.8% were involved with alcohol consumption, smoking, and betel chewing, respectively.
Twenty-seven (7.7%) had diabetes and 31 (8.8%) had hypertension. In addition, 47 (13.4%) had CKD with 2.6%, 1.4%, 4%, 3.1%, 0.9%, and 1.4% in CKD stages 1, 2, 3A, 3B, 4, and 5, respectively. Among the individuals with CKD, 6 (12.7%) had diabetes and 11 (23.4%) had hypertension. Suspected CKDu cases represented 30 of 47 (63.8 %) of the total CKD cases. The suspected CKDu prevalence within the study population was 30 of 352 (8.5 %). There was a male predominance (73.3%) in the suspected CKDu population (Chi-squared test; P = 0.0003).
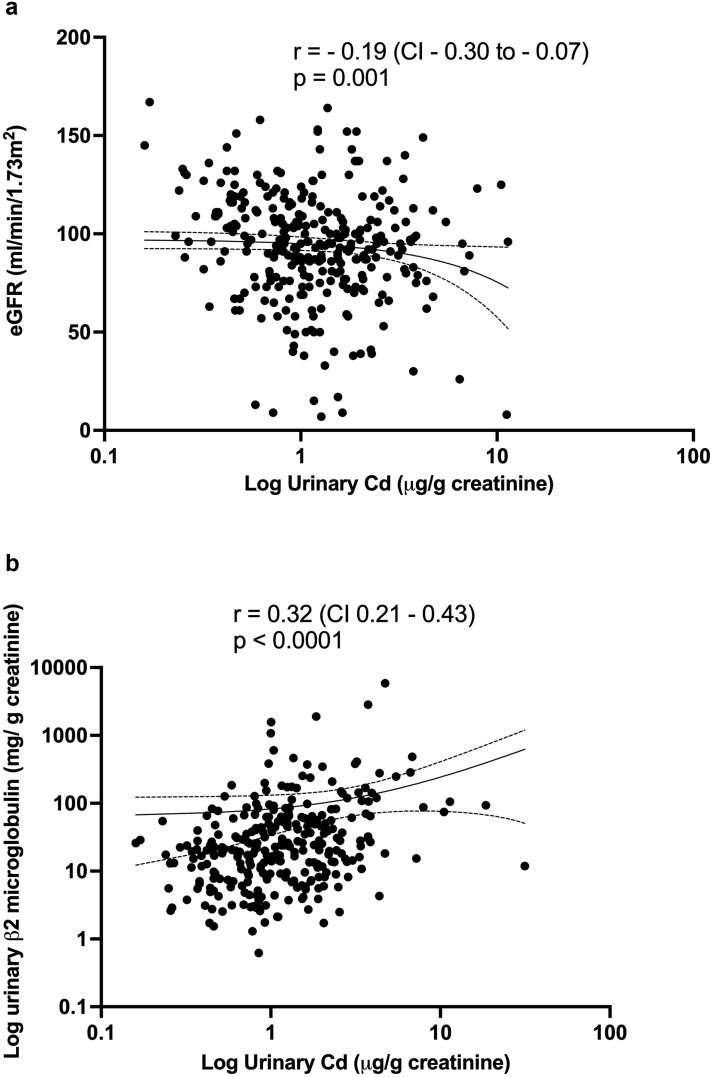
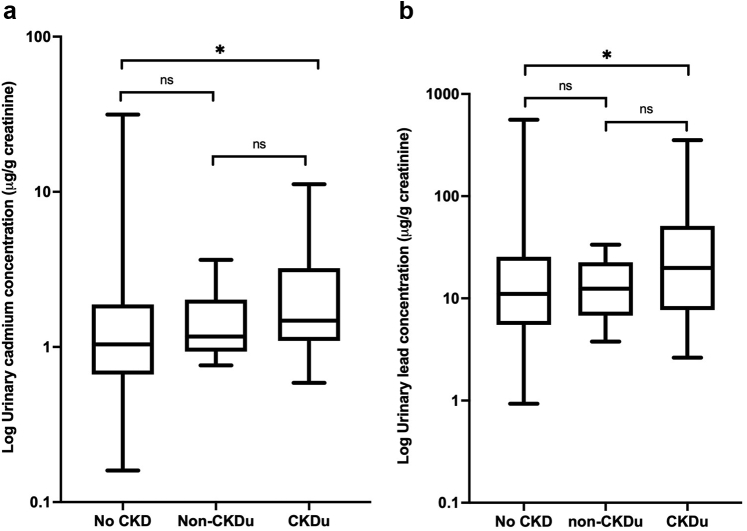
Median urinary cadmium and lead excretion was significantly higher among individuals with suspected CKDu compared to individuals without CKD (Figure 1) (Supplementary Table S2). No such increase in the median urinary cadmium and lead excretion was observed among individuals with CKD presumed to be secondary to diabetes or hypertension (non-CKDu). There was a progressive increase in the median urinary cadmium excretion from the individuals without CKD, to individuals with CKD (non-CKDu), and to individuals with suspected CKDu (Kruskal-Wallis statistic = 9.646, P = 0.008) (Figure 1). There was no significant difference in median urinary arsenic excretion between individuals with suspected CKDu, CKD (non-CKDu) and without CKD (Supplementary Table S2). There was a significant negative correlation of eGFR with urinary cadmium excretion (Spearman r = −0.19, confidence interval [CI] −0.3 to −0.07, P = 0.001) (Figure 2a) but not with urinary arsenic or lead excretion.
Figure 1.
(a) Urinary cadmium and (b) lead excretion and its association with CKD and CKDu. Median, interquartile distance and range is illustrated for each data set. Cd, cadmium; eGFR, estimated glomerular filtration rate. ∗Denotes statistical significance (P < 0.05) in comparison to the group with no CKD.
Figure 2.
Correlation of urinary cadmium excretion with (a) eGFR and (b) urinary β2 microglobulin excretion. CKD, chronic kidney disease; CKDu, chronic kidney disease of unknown etiology.
Median uβ2M level was significantly elevated among individuals with suspected CKDu compared to individuals without CKD (153.9 mg/g creatinine vs. 20.4 mg/g creatinine, P < 0.0001). uβ2M performed well in discriminating individuals with suspected CKDu from those without CKD with an AUC-ROC value of 0.790 (95% CI 0.689−0.891) (Supplementary Figure S3). Nevertheless, both sCr and uACR performed better than uβ2M in discriminating individuals with CKDu from those without CKD with AUC-ROC values of 0.854 and 0.861 respectively. Uβ2M levels were higher in CKD stages 3B to 5 compared to CKD stages 1, 2 and 3A (Supplementary Table S3). There was a significant positive correlation between uβ2M level and urinary cadmium excretion (Spearman r = 0.32, P < 0.0001) (Figure 2b).
Increased age (odds ratio = 1.07; 95% CI 1.04–1.10), lower body mass index (odds ratio = 0.87; 95% CI 0.79–0.96), betel chewing (odds ratio = 2.28; 95% CI 1.35–4.95) and tubular dysfunction, as defined by uβ2M more than or equal to 300 mg/g (odds ratio = 9.40; 95% CI 3.58–24.63), were significantly associated with suspected CKDu (Supplementary Figure S4).
Discussion
This study provides new evidence that CKDu is present in areas outside the traditional CKDu endemic areas in the country. There were significantly high levels of cadmium and lead in urine among individuals having suspected CKDu. Furthermore, urinary cadmium level had a significant negative correlation with the eGFR suggesting a possible cadmium associated kidney damage.
The urinary cadmium level is an indicator of total body burden of cadmium. The critical urinary cadmium level associated with renal injury is considered as 2 μg/g to 10 μg/g creatinine, which corresponds with a renal cortical cadmium concentration of approximately 150 μg/g to 200 μg/g renal tissue.6 Nevertheless, a more recent study has shown that even a lower urinary cadmium level of more than 0.5 μg/g creatinine could be associated with CKD.7 Therefore, cadmium exposure could be considered as a potential risk factor for CKD in our population.
We observed a significant correlation between urinary cadmium and uβ2M level in the study population. Similar observations have been made in other populations exposed to cadmium.S1–S3 Β2-microglobulin is a low molecular weight protein that is freely filtered by the glomerulus and almost completely reabsorbed and catabolized by proximal tubular cells.8 Elevated levels of uβ2M suggest renal tubular damage and have been observed in the setting of nephrotoxin associated renal injury.8,S4 In a previous Sri Lankan study, significantly higher uβ2M levels were observed in CKDu subjects compared to the healthy individuals and uβ2M with a receiver operating characteristic-area under the curve value of 0.853 has shown a high sensitivity and a low specificity in diagnosing individuals with CKDu.9
Although uβ2M performed well in discriminating individuals with CKDu from those not having CKD in our study, sCr and uACR performed better. Considering that both sCr and uACR are part of the criteria used to diagnose CKDu, assessing uβ2M against histopathological changes of CKDu would have given a more accurate in understanding of its true value as a biomarker in the diagnosis of CKDu. Furthermore, uβ2M showed a significant positive correlation with the urinary cadmium excretion in our study, suggesting that it could be a useful biomarker in the setting of cadmium associated CKD.
One of the key limitations of the present study was the classification of CKD based on a single measurement of sCr and/ or uACR, rather than 2 measurements 3 months apart as recommended by the KDIGO guidelines. It is also possible that some of the individuals classified as CKD due to hypertension or diabetes could actually be having CKDu.
Our study provides useful insights into the prevalence, distribution and risk factors of CKDu in a non-CKDu endemic area in Sri Lanka. It is clear that the disease is not limited to the traditional endemic areas of Sri Lanka, and is found in other parts of the country that share similar geographical, environmental, and socioeconomic characteristics to those of the north-central province. Our findings also support the possible association of CKDu with exposure to heavy metals, such as cadmium and lead. It also suggests that uβ2M could be used as a potential biomarker for early diagnosis of CKDu.
Disclosure
All the authors declared no competing interests.
Author Contributions
ESW, EPSC, SSJ, TR, SJ, MCSDS were responsible for the conception, design, collection, analysis, interpretation of data, drafting, and final approval of the article. WAGT, EMDE, TDKSG were involved in data collection, clinical investigation, analysis, and data interpretation. ESW received the funding and all authors approved contents of the article.
Acknowledments
We would like to convey our sincere thanks to all the participants, medical and technical staff and government officials of the study areas for their valuable cooperation throughout the study. We would also like to thank the International Society of Nephrology for providing the funding for the study.
Funding
The study was funded by a grant awarded to ESW under International Society of Nephrology Clinical Research Program (16-02-37).
Footnotes
Supplementary Methods.
Supplementary References.
Figure S1. GN divisions within Buttala Divisional Secretariat. GN divisions selected for the study are shown with respect to distribution of cultivations within the study area. The map was created based on the data from the Department of Survey, Sri Lanka.
Figure S2. Flow diagram of the sampling strategy. ∗Population of Moneragala district and Buttala DS in 2017 estimated based on the 2012 census data considering annual percentage increase of 1.21%.
Figure S3. Receiver operator characteristic curves for biomarkers of CKDu.
Figure S4. Risk factors associated with suspected CKDu.
∗Indicates significant associations.
Table S1. Demographic, clinical and exposure related data of the study population.
Table S2. Median urinary cadmium, arsenic and lead excretion in individuals with CKDu, non-CKDu and without CKD.
Table S3. Urine albumin and β2 microglobulin excretion according to CKD stage in individuals with suspected CKDu.
STROBE Statement.
Supplementary Material
Supplementary Methods.
Supplementary References.
Figure S1. Grama Niladhari divisions within Buttala Divisional Secretariat. GN divisions selected for the study are shown with respect to distribution of cultivations within the study area. The map was created based on the data from the Department of Survey, Sri Lanka.
Figure S2. Flow diagram of the sampling strategy.∗Population of Moneragala district and Buttala Divisional Secretariat in 2017 estimated based on the 2012 census data considering annual percentage increase of 1.21%.
Figure S3. Receiver operator characteristic curves for biomarkers of CKDu
Figure S4. Risk factors associated with suspected CKDu.∗Indicates significant associations.
Table S1. Demographic, clinical and exposure related data of the study population.
Table S2. Median urinary cadmium, arsenic and lead excretion in individuals with CKDu, non-CKDu and without CKD.
Table S3. Urine albumin and β2 microglobulin excretion according to CKD stage in individuals with suspected CKDu
STROBE Statement.
References
- 1.Pearce N., Caplin B., Gunawardena N., Kaur P., O’Callaghan-Gordo C., Ruwanpathirana T. CKD of unknown cause: a global epidemic? Kidney Int Rep. 2019;4:367–369. doi: 10.1016/j.ekir.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunawardena S., Dayaratne M., Wijesinghe H., Wijewickrama E. A systematic review of renal pathology in chronic kidney disease of uncertain etiology. Kidney Int Rep. 2021;6:1711–1728. doi: 10.1016/j.ekir.2021.03.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayatilake N., Mendis S., Maheepala P., Mehta F.R., CKDu National Research Project Team Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruwanpathirana T., Senanayake S., Gunawardana N., et al. Prevalence and risk factors for impaired kidney function in the district of Anuradhapura, Sri Lanka: a cross-sectional population-representative survey in those at risk of chronic kidney disease of unknown aetiology. BMC Public Health. 2019;19:763. doi: 10.1186/s12889-019-7117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soderland P., Lovekar S., Weiner D.E., Brooks D.R., Kaufman J.S. Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis. 2010;17:254–264. doi: 10.1053/j.ackd.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Navas-Acien A., Tellez-Plaza M., Guallar E., et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferraro P.M., Costanzi S., Naticchia A., Sturniolo A., Gambaro G. Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999–2006. BMC Public Health. 2010;10:304. doi: 10.1186/1471-2458-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyata T., Jadoul M., Kurokawa K., Van Ypersele de Strihou C. Beta-2 microglobulin in renal disease. J Am Soc Nephrol. 1998;9:1723–1735. doi: 10.1681/ASN.V991723. [DOI] [PubMed] [Google Scholar]
- 9.Wanigasuriya K., Jayawardene I., Amarasiriwardena C., Wickremasinghe R. Novel urinary biomarkers and their association with urinary heavy metals in chronic kidney disease of unknown aetiology in Sri Lanka: a pilot study. Ceylon Med J. 2017;62:210–217. doi: 10.4038/cmj.v62i4.8568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.