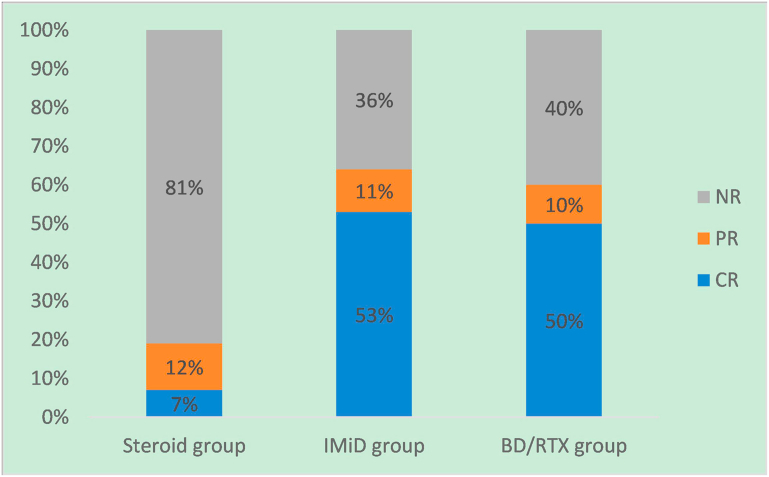Abstract
Introduction
Immunomodulatory drugs (IMiDs) plus dexamethasone are effective for plasma cell dyscrasias, but the treatment efficacy of IMiD in proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID) has been rarely reported.
Methods
We retrospectively analyzed the clinicopathologic data of 64 patients with PGNMID (steroid, IMiD, and bortezomib and dexamethasone/Rituximab [BD/RTX] groups) from January 1, 2010 to December 31, 2020, at the National Clinical Research Center of Kidney Disease in Nanjing. The prognosis of patients receiving different treatment regimens were compared. Factors potentially affecting renal prognosis and renal response were evaluated.
Results
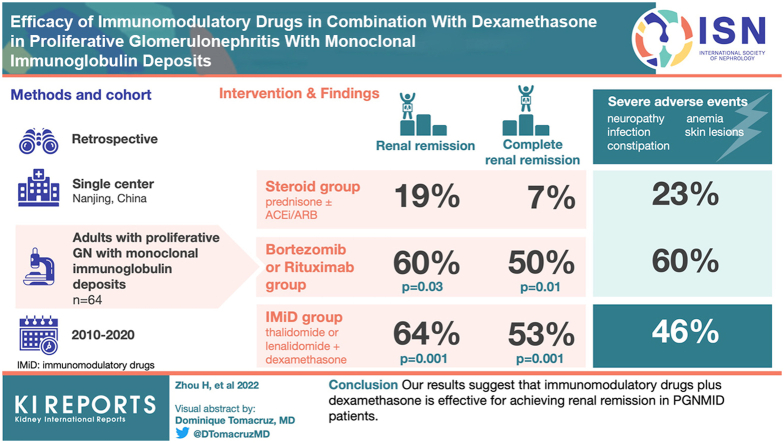
Twenty-eight, 26 and 10 PGNMID patients were divided into IMiD group, steroid group and BD/RTX group respectively. The rate of serum M protein detection was significantly lower in the steroid group than in the other 2 groups. Renal remission (P = 0.001 and P = 0.03, respectively) rates and renal complete remission (CR) (P = 0.001 and P = 0.01, respectively) rates were significantly higher in the IMiD and BD/RTX groups than in the steroid group at the last follow-up. Multivariate logistic analysis identified that hypertension and high serum creatinine (SCr) levels (>1.24 mg/dl) decreased renal remission, whereas low C3 levels, IMiD and BD/RTX treatments were positively associated with renal remission. Multivariate Cox analysis identified IgG3 in renal tissue and high SCr levels as poor renal prognostic indicators. Severe adverse events were more common in the IMiD and BD/RTX groups than in the steroid group (P = 0.072 and P = 0.035, respectively).
Conclusion
Our results suggest that IMiDs plus dexamethasone is effective for achieving renal remission in PGNMID patients.
Keywords: efficacy, immunomodulatory drug, proliferative glomerulonephritis with monoclonal immunoglobulin deposits, renal prognosis
Graphical abstract
PGNMID is a type of monoclonal gammopathy of renal significance that was first reported by Nasr et al.1 in 2004.2 In most PGNMID patient samples, IgG3 deposits with kappa restriction are observable by immunofluorescence, membranoproliferative changes are observable by light microscopy, and electron-dense deposits mainly located in subendothelial and mesangial areas are observable by electron microscopy. Patients with PGNMID typically present with renal insufficiency, proteinuria, and microscopic hematuria. The M protein can be detected in the blood, urine, or bone marrow monoclonal plasma cells of approximately 30% to 37% of patients.3, 4, 5, 6
Though the pathogenesis of PGNMID remains unclear, it is generally thought to be caused by the deposition of nephrotoxic MIg from B cells or plasma cells into the glomerulus. The current options for PGNMID treatment should be determined by the clone or potential clone.7 Gumber et al.4 reported a favorable effect of a clone-directed approach, including bortezomib and RTX on PGNIMID. In addition, Zand et al.8 and Almaani et al.9 reported that daratumumab significantly improved the outcomes of patients with PGNMID.
According to the latest consensus, chemotherapy should be considered as priority in the treatment of PGNMID. Nevertheless, the high cost limits the use of these clone-directed drugs for patients with PGNMID in developing countries. Thalidomide and lenalidomide are IMiDs that are used as one of the main clone-directed approaches and exert pleiotropic antimyeloma effects, because they have immunomodulatory, antiangiogenic, anti-inflammatory, and antiproliferative activity.10,11 Based on their antimultiple myeloma properties, IMiDs are also considered promising for the treatment of PGNMID. Recently, our group demonstrated the therapeutic efficacy of lenalidomide in combination with dexamethasone in 6 patients with PGNMID.12
Herein, we retrospectively analyzed the clinicopathologic characteristics of 64 patients with PGNMID. We further attempted to evaluate and compare the treatment efficacies, outcomes and severe adverse events in the IMiD group and other therapeutic strategy groups.
Methods
Patients
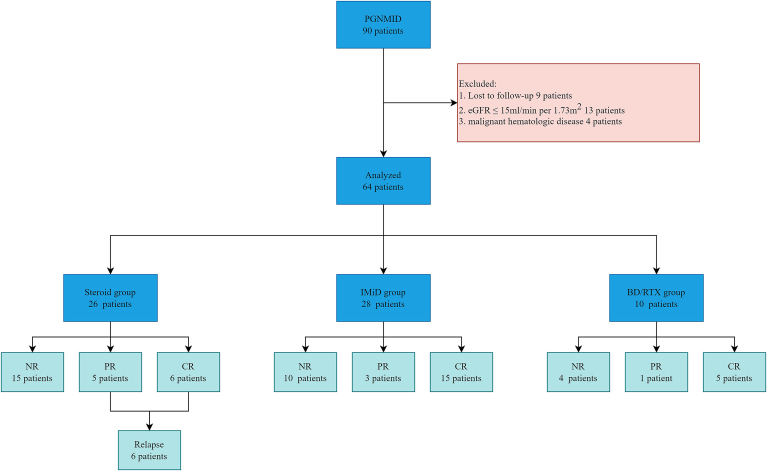
In this study, we observed 90 patients with PGNMID who were registered at our center from January 1, 2010 to December 31, 2020. The diagnosis of PGNMID was determined according to the renal biopsy findings.4 The details of renal pathological diagnosis are available in Supplementary Appendix 1. Patients with cryoglobulinemia and PGNMID associated with renal transplantation were excluded. Patients who were lost to follow-up, patients that had an estimated glomerular filtration rate (eGFR) ≤15 ml/min per 1.73 m2 at renal biopsy and patients with malignant hematologic disorders were also excluded. After the exclusion of 9 patients who were lost to follow-up, 13 patients with an eGFR ≤15 ml/min per 1.73 m2 and 4 patients diagnosed with a malignant hematologic disease, 64 patients, including 6 patients we previous reported on12 were enrolled in our study. The details are shown in Figure 1. This study was approved by the Ethics Committee of Nanjing University. Written consent was not required for this noninvasive study, as determined by the ethics committee.
Figure 1.
Process for the inclusion of PGNMID patients and renal remission in different groups. BD/RTX, Bortezomib and Dexamethasone/ Rituximab; CR, complete remission; eGFR, estimated glomerular filteration rate; NR, no response; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin deposits; PR, partial remission.
Treatment Protocols
Steroid Group
The steroid group was treated with prednisone with or without angiotensin converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs). The initial dose of prednisone was 0.5 to 1 mg/(kg.d), and the maximum dose was 60 to 80 mg/d for 4 to 8 weeks. Once the treatment was determined to be effective, the prednisone dose was reduced by 2.5 to 5.0 mg every 2 to 4 weeks until it reaches the long-term maintenance dose (5 mg/d), which is used for several years. If urine protein level decreased by less than 30% after 4 to 8 weeks of treatment, the prednisone dose was gradually reduced until it was discontinued.13
IMiD Group
The IMiD group was treated with thalidomide in combination with dexamethasone (thalidomide and dexamethasone) and lenalidomide in combination with dexamethasone lenalidomide and dexamethasone).
TD: Modified protocol based on the work of Palladini et al.14 for the treatment of renal amyloidosis was used. The initial dose was 100 mg/d thalidomide and 40 mg/week dexamethasone, which was increased by 25 mg every 1 to 2 weeks to a maximum dose of 200 mg/night thalidomide if no obvious adverse reactions were detected. The thalidomide doses did not need to be adjusted in patients with renal insufficiency.
RD: The lenalidomide dose was adjusted according to renal function as follows: (i) eGFR ≥60 ml/(min.1.73 m2), lenalidomide 25 mg/d; (ii) 30 ≤ eGFR < 60 ml/(min.1.73 m2), lenalidomide 10 mg/d; and (iii) eGFR <30 ml/(min.1.73 m2), lenalidomide 7.5 mg/d. Lenalidomide was administered for 21 days and discontinued for 7 days, and dexamethasone (40 mg/week) was administered on days 1, 8, 15, and 22.15
BD/RTX Groups
RTX: This group was treated with 375 mg/m2 rituximab once every 3 months.16
BD: This group was treated with 1.3 mg/m2 bortezomib and 40 mg/d dexamethasone once a week for 4 weeks for 1 treatment cycle. Cyclophosphamide is not included in this protocol.17
Severe adverse events are defined as those of grade 3 or higher according to the National Cancer Institute Common Terminology Criteria for Adverse Events (4.0) and should be considered for dose reduction or delayed dosing. Discontinuation is required if symptoms persist without relief after dose reduction or deferral.
Hematological Examination
All patients underwent serum immunofixation electrophoresis test and serum free light chain (sFLC) levels and sFLC ratios were in the normal range of 0.37 to 3.1.18 Fifty-two patients underwent bone marrow biopsies, and plasma cell clonality was determined by flow cytometry (n = 30).
Observations and Efficacy Analysis
The demographic, clinical, and laboratory parameters of each patient at the time of renal biopsy were recorded. The follow-up time and treatment information for all patients were also obtained through August 31, 2021. The details of clinical definitions are available in Supplementary Appendix 1.
The definitions of renal remission were as follows:4 CR means urinary protein ≤0.5 g/d, and stable renal function (±25%); partial remission means the urine protein level was decreased by more than 50%. For partial remission, nephrotic range proteinuria was required to be decreased to less than 3.5 g/d, and renal function was required to remain stable (±25%); no response means achievement of neither CR nor partial remission; relapse means worsening SCr or proteinuria levels in the kidneys after a partial remission or CR. Because serum M protein positivity and abnormal sFLC ratios were detected in a small population of patients, only the hematologic changes before and after treatment are described. The endpoint was defined as follows:19 30% decline in eGFR or end-stage renal disease.
Data Analysis
Data were analyzed using SPSS 25.0 statistical software for windows (SPSS Inc., Chicago, IL). Clinical variables are presented as numbers and relative frequencies or medians and interquartile ranges (IQRs). A t test or analysis of variance was used to compare normally distributed continuous variables, and the Mann–Whitney U test or Kolmogorov–Smirnov test was used to compare nonnormally distributed continuous variables. The Kaplan-Meier (K-M) method was used to compare differences in the prognoses of patients receiving different treatment regimens. Factors potentially affecting prognosis were subjected to univariate Cox regression analysis, and those with a P value <0.2 were included in the multivariate Cox regression model. The binary logistic regression model included factors with a P value <0.2 were included in the multivariate logistic regression analysis. P values <0.05 were considered statistically significant.
Results
Clinical Manifestations, Hematological Examination, and Renal Pathology
A total of 64 patients enrolled in this study were divided into 3 groups based on the treatment strategy: the IMiD group (28 patients, 23 received thalidomide, 5 received lenalidomide), the steroid group (26 patients) and the BD/RTX group (10 patients, 1 received RTX, 9 received bortezomib). The male-to-female ratio among all patients was 37:27, and the median follow-up time was 20 (IQR, 10–37) months. The median age was 54 (IQR, 46–62) years, and the median urine protein level was 3.65 (IQR, 2.21–6.35) g/24h. The median SCr concentration was 1.25 (IQR, 0.88–1.56) mg/dl, the median eGFR rate was 60 (IQR, 45–89) ml/min per 1.73 m2 and the median albumin concentration was 33.9 (IQR, 29.5–38.1) g/l. Fifty-three patients had hypertension, and 9 patients had diabetes mellitus. The levels of C3 were low in 30 patients, and 17 patients were anemic.
All patients were subjected to detected M protein in the sera and sFLC. The M protein was detected in the sera of 12 patients (18%), and the sFLC ratio was abnormal in 3 patients. Flow cytometry analysis was performed in 30 patients, and monoclonal plasma cells were found in 12 patients. A total of 19 patients (29%) were found to have a hematological monoclonal presence. The blood and bone marrow examination and distribution are as follows in Supplementary Table S1.
The rate of serum M protein detection in the steroid group was significantly lower than in the IMiD group and the BD/RTX group. The other parameters, including the levels of SCr, proteinuria and eGFR, were comparable among the 3 groups.
All patients were subjected to renal biopsy. Light microscopy analysis revealed membranoproliferative changes in the predominant lesions of 58 patients, and mesangial proliferative changes were found in 6 patients. The mean numbers of sclerotic glomeruli and crescents were similar among the 3 groups. Immunofluorescence revealed 52 patients with κ positivity, 12 patients with λ positivity, 49 patients with IgG3 positivity, 9 patients with IgG1 positivity, 1 patient with IgG2 positivity, 1 patient with IgG4 positivity, 3 patients with IgA positivity, and 1 patient with IgM positivity. The baseline characteristics of the patients in each group are provided in Table 1.
Table 1.
Baseline clinical characteristics, hematological parameters and renal pathologies of PGNMID patients in the different treatment groups
| Characteristics | Steroid group |
IMiD group |
BD/RTX group |
P value | |
|---|---|---|---|---|---|
| (N = 26) | (N = 28) | (N = 10) | |||
| Age (yr) | 52 (42–60) | 59 (48–67) | 53 (46–62) | 0.271 | |
| Male: female | 16:10 | 15:13 | 6:4 | 0.829 | |
| Follow-up time (mo) | 20 (9–44) | 23 (9–36) | 19 (11–35) | 0.854 | |
| Hypertension n, % | 19, 73% | 25, 89% | 9, 90% | 0.232 | |
| Diabetes n, % | 6, 23% | 2, 7% | 1, 10% | 0.224 | |
| Anemia n, % | 7, 26% | 6, 21% | 4,40% | 0.521 | |
| Proteinuria (g/24 h) | 2.75 (1.77–6.34) | 3.65 (2.23–5.84) | 4.85 (2.66–7.20) | 0.331 | |
| Serum creatinine (mg/dl) | 1.17 (0.70–1.64) | 1.22 (0.98–1.46) | 1.44 (0.92–1.77) | 0.434 | |
| eGFR (ml/[min.1.73 m2]) | 60.5 (41.5–97.5) | 61.5 (47.2–78.5) | 50.8 (35.5–80.5) | 0.484 | |
| Serum albumin (g/l) | 35.5 (28.8–39.3) | 34.7 (29.9–37.2) | 30.8 (28.6–33.3) | 0.328 | |
| Low C3 n, % | 9, 34% | 16, 57% | 5, 50% | 0.247 | |
| M spike n, % | 1, 3% | 7, 25%a | 4, 40%b | 0.029a, 0.005b | |
| Abnormal sFLC ratio n, % | 0 | 2, 7% | 1, 10% | 0.319 | |
| Monoclonal plasma cell n, %c | 2/6, 33% | 8/19, 42% | 2/5, 40% | NC | |
| Light microscopy | MPGN | 22 | 27 | 9 | NC |
| MsPGN | 4 | 1 | 1 | ||
| Immunofluorescence | κ-IgG3 | 19 | 17 | 8 | NC |
| λ- IgG 3 | 3 | 2 | 0 | ||
| κ- IgG 1 | 2 | 3 | 1 | ||
| λ- IgG 1 | 1 | 2 | 0 | ||
| λ-IgA | 0 | 2 | 1 | ||
| κ- IgG 2 | 1 | 0 | 0 | ||
| κ- IgG 4 | 0 | 1 | 0 | ||
| λ-IgM | 0 | 1 | 0 | ||
| IFTA | Mild n | 11 | 12 | 5 | NC |
| Moderate n | 10 | 14 | 4 | ||
| Severe n | 5 | 2 | 1 | ||
| Glomerulosclerosis % | 25±21 | 22±21 | 24±17 | 0.804 | |
| Crescents n, % | 8, 30% | 7, 25% | 1, 10% | 0.436 | |
BD/RTX, bortezomib and dexamethasone/rituximab; eGFR, estimated glomerular filtration rate; IFTA, interstitial fibrosis and tubular atrophy; IMiDs, immunomodulatory drugs; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin deposits; NC, not comparable; sFLC, serum free light chain.
Clinical variables are presented as numbers and relative frequencies or medians and interquartile ranges in this table.
Steroid group and IMiD group.
Steroid and BD/RTX groups.
Fifty-two patients underwent bone marrow biopsy. This column shows the positive patients/bone marrow biopsy patients.
Renal Responses and Associated Factors
In the steroid group, the initial dose of steroid was calculated according to body weight and the median dose is 30 (IQR, 27.5–37.5) mg/d and the median treatment duration was 20 (IQR, 9–44) months. The dose of thalidomide is approximately 100 (IQR, 50–200) mg/d and the dose of lenalidomide is about 15 (IQR, 10–25) mg/d with 23 (IQR, 9–36) months in the IMiD group. The median number of treatment cycles in BD/RTX group receiving RTX or bortezomib was 4 (IQR, 2–5) cycles with bortezomib 2.3(1.9–2.4) mg or RTX 700 mg.
At the last follow-up time, the median urinary protein and SCr in the steroid group increased from 2.75 (1.77–6.34) g/24h to 3.58 (1.14–7.57) g/24h, and from 1.17 (0.7–1.64) mg/dl to 1.47 (0.97–2.79) mg/dl, respectively; whereas those of the IMiD group decreased from 3.65 (2.23–5.84) g/24h to 0.62 (0.36–3.44) g/24h, and from 1.22 (0.98–1.46) mg/dl to 1.08 (0.91-1.85) mg/dl, respectively; and those of the BD/RTX from 4.85 (2.66–7.20) g/24h to 1.48 (0.32–8.97) g/24h, 1.44 (0.92–1.77) mg/dl to 1.26 (0.91–1.83) mg/dl, respectively.
During the treatment period, 11 (42%), 18 (64%) and 6 (60%) patients achieved renal remission in the steroid, IMiD and BD/RTX groups, among which 6 (23%), 15 (53%) and 5 (50%) patients achieved renal CR, respectively. The median times to renal remission were 8 (IQR, 2–10) months, 4 (IQR, 2–9) months, and 4 (IQR, 2–14) months in the steroid group, IMiD group and BD/RTX group respectively. The median time to renal CR were 20 (IQR, 9–23) months, 15 (IQR, 11–24) months, and 9 (IQR, 7–24) months in the steroid group, IMiD group and BD/RTX group respectively. The median time to renal remission and renal CR did not significantly differ among the 3 groups. During the follow-up period, 6 patients treated with steroid suffered a relapse, and the median time to relapse was 34 (IQR, 20–47) months. No patients in the IMiD and BD/RTX group relapsed.
The complete renal remission rate in the IMiD group was significantly higher than that in the steroid group (53% vs. 23%, P = 0.022). The remission rates in the IMiD group and BD/RTX group did not significantly differ. The rates of relapse in the steroid group were also significantly higher than those in the IMiD group (P < 0.001) and BD/RTX group (P = 0.04).
The hematologic examination revealed that only 2 patients from BD/RTX group had M protein positivity that turned negative. The sFLC ratio returned to normal in one patient from the IMiD group and in 1 patient from BD/RTX group. Further bone marrow biopsy was not performed for most patients during the follow-up period (Table 2). The renal remission rates in different groups at last follow-up is shown in Figure 2. In addition, the details of the 19 patients with monoclonal presence regarding to the treatment and response in each group is shown in Supplementary Figure S1.
Table 2.
Renal and hematologic remission in the different groups
| Remission | Steroid group (N = 26) | IMiD group (N = 28) | BD/RTX group (N = 10) | P value |
|---|---|---|---|---|
| Renal remission experienced during the treatment period n, % | 11, 42% | 18, 64% | 6, 60% | 0.251 |
| CR n, % | 6, 23% | 15, 53%a | 5, 50% | 0.022a |
| Relapse n, % | 6, 54% | 0a | 0b | <0.001a, 0.04b |
| Renal remission achieved at the last follow-up n, % | 5, 19% | 18, 64%a | 6, 60%b | 0.001a, 0.03b |
| CR n, % | 2, 7% | 15, 53%a | 5, 50%b | 0.001a, 0.01b |
| Median time to renal remission (mo) | 8 (2–10) | 4 (2–9) | 4 (2–14) | 0.874 |
| Median time to renal CR (mo) | 20 (9–23) | 15 (11–24) | 9 (7–24) | 0.969 |
| Median time to relapse (mo) | 34 (20–47) | NC | NC | NC |
| M spike positivity turned negative n | 0/1 | 0/7 | 2/4 | NC |
| Abnormal sFLC ratio returned to normal n, % | 0 | 1/2 | 1/1 | NC |
BD/RTX, bortezomib and dexamethasone/rituximab; CR, complete remission; ImiDs, immunomodulatory drugs; NC, not comparable; sFLC, serum free light chain.
Steroid group and IMiD group.
Steroid group and BD/RTX group.
Figure 2.
Renal remission rates in different groups at last follow-up time. BD/RTX, bortezomib and dexamethasone/rituximab; CR, complete remission; IMiD, immunomodulatory drug; NR, no response; PR, partial remission.
Logistic analysis demonstrated that the SCr level, low C3 level, and treatment variables were associated with renal remission. After multivariate logistic regression analysis, hypertension and a high SCr level (>1.24 mg/dl) were less likely to achieve renal remission. Patients with low C3, IMiD, and BD/RTX protocols were more likely to achieve renal remission (P < 0.05), as detailed in Table 3.
Table 3.
Logistic analysis of factors affecting renal remission
| Factors | Binary logistic |
Multivariate logistic |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Hypertension | 0.406 (0.106–1.556) | 0.188 | 0.103 (0.015–0.705) | 0.021 |
| Serum creatinine >1.24 mg/dl | 0.240 (0.069–0.841) | 0.026 | 0.129 (0.023–0.733) | 0.021 |
| M spike | 2.952 (0.787–11.073) | 0.128 | ||
| Low C3 | 3.316 (1.126–8.738) | 0.029 | 4.058 (1.048–15.714) | 0.043 |
| Sclerotic glomerulia | 0.393 (0.123–1.261) | 0.116 | ||
| Treatment groups | ||||
| Steroid group | 1 | - | ||
| IMiD group | 7.560 (2.178–26.242) | 0.001 | 9.111 (1.537–54.013) | 0.015 |
| BD/RTX group | 6.300 (1.275–31.124) | 0.024 | 12.062 (1.359–107.026) | 0.025 |
BD/RTX, bortezomib and dexamethasone/rituximab; CI, confidence interval; IFTA, interstitial fibrosis and tubular atrophy; ImiDs, immunomodulatory drugs; OR, odds ratio.
Sclerotic glomeruli percentage was classified according to 10%.
Prognosis
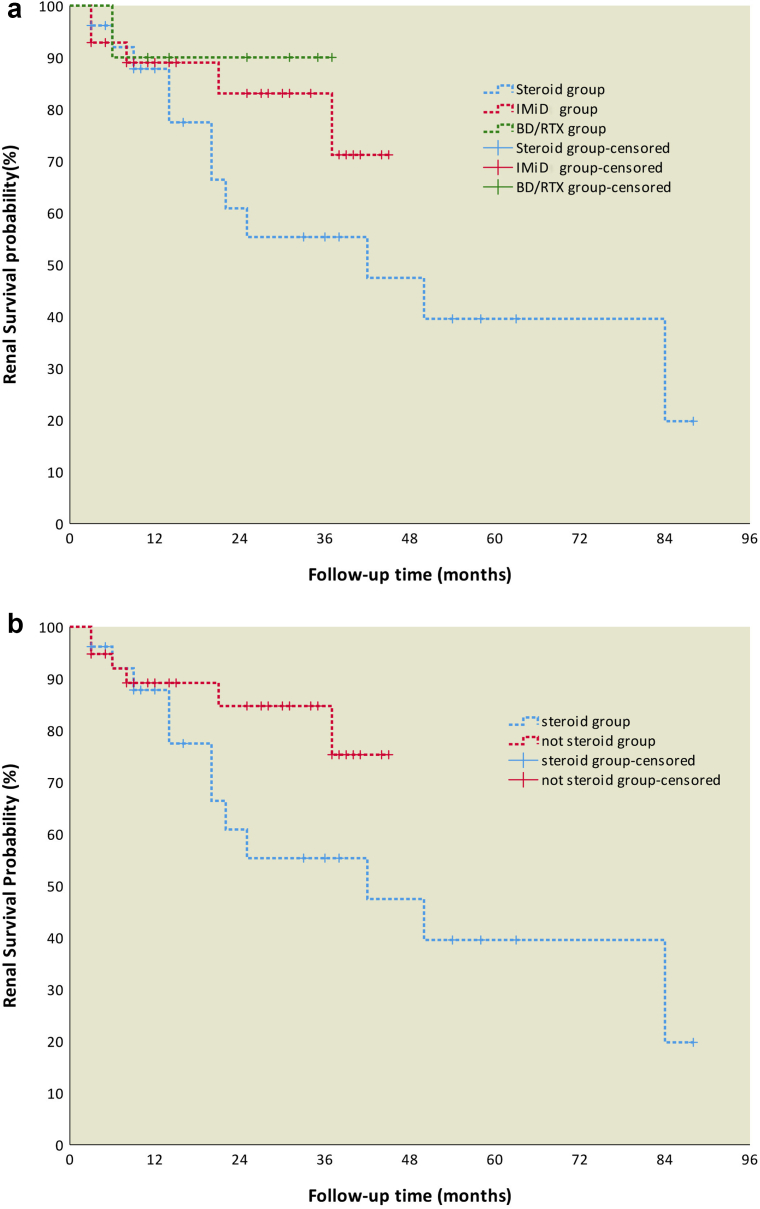
During the follow-up period, 1 patient in the BD/RTX group died, and endpoint event was reported in 12, 5 and 1 patient(s) from the steroid, IMiD and BD/RTX groups, respectively; among these patients, 4, 1, and 1 patient(s), respectively developed end-stage renal disease. One patient from BD/RTX group died from myocardial infarction after he developed end-stage renal disease. The probability of renal survival was calculated using the Kaplan-Meier method, indicating no significant differences in renal survival among the 3 groups (P = 0.245). The renal prognosis of patients in the clone-directed treatment group (IMiDs and BD/RTX) was better than that of patients in the steroid group, but the difference was not statistically significant (P = 0.105) (Figure 3a and 3b).
Figure 3.
(a) Renal survival analysis of patients in the different groups through the study endpoint. (b) Renal survival of patients in the steroid and not steroid groups through the study endpoint. BD/RTX, bortezomib and dexamethasone/rituximab; IMiDs, immunomodulatory drugs.
Univariate Cox analysis revealed that higher SCr level (hazard ratio 2.722, 95% confidence interval 1.446–5.124, P = 0.002) was a factor for progression to endpoint event. Two variables (high SCr level and IgG3) remained independent risk factors for progression to endpoint event after multivariate logistic analysis, as detailed in Table 4.
Table 4.
Cox analysis of factors affecting renal survival
| Factors | Univariate Cox |
Multivariate Cox |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (yr) | 1.026 (0.990–1.063) | 0.163 | ||
| Serum creatinine | 2.605 (1.437–4.722) | 0.002 | 2.844 (1.491–5.424) | 0.002 |
| Ig subtype in renal tissue | ||||
| IgG3 | 1 | - | 1 | - |
| Non-IgG3 | 0.302 (0.067–1.355) | 0.118 | 0.173 (0.035–0.852) | 0.031 |
CI, confidence interval; HR, hazard ratio.
Severe Adverse Events
The incidence rates of severe adverse events were 23%, 46% and 60% in the steroid, IMiD and BD/RTX groups, respectively. The incidence rates of severe adverse events were higher in the IMiD and BD/RTX groups than in the steroid group (P = 0.073 and P = 0.035, respectively) (Table 5). In the IMiD group, peripheral neuropathy, constipation, infection, anemia, and skin lesions were the most common complications. Among them, peripheral neuropathy and constipation were mainly found in patients treated with TD, anemia and skin lesions in patients treated with RD. In most patients, adverse reactions were improved after the dosage of dexamethasone, thalidomide, or lenalidomide was reduced. The IMiD group was 28 patients, including 23 with thalidomide and 5 with lenalidomide. Two patients (2 of 23, 8%) treated with TD were switched to steroid treatment due to peripheral neuropathy intolerance. One patient (20%) with lenalidomide discontinued for severe anemia and the other patient (20%) reduced the dose due to the decreasing trend of hemoglobin. Also, 11 patients (47%) with thalidomide had a dose reduction because of adverse events.
Table 5.
Comparison of severe adverse events among the different treatment groups
| Adverse events | Steroid group | IMiD group | BD/RTX group | P value |
|---|---|---|---|---|
| Total adverse events n, % | 6, 23% | 13, 46% | 6, 60% | 0.035a |
| Infections n, % | 4, 15% | 4, 14% | 4, 40% | 0.172 |
| Herpes n, % | 2, 7% | 1, 3% | 2,20% | 0.251 |
| Peripheral neuropathy n, % | 0 | 6,21% | 2,20% | 0.924b |
| Constipation n, % | 0 | 5,17% | 2, 20% | 0.881b |
| Anemia n, % | 0 | 4, 14% | 0 | NC |
| Skin lesions n, % | 0 | 3, 10% | 0 | NC |
| Acute cerebral infarction n, % | 0 | 0 | 1, 10% | NC |
| Death n, % | 0 | 0 | 1,10% | NC |
BD/RTX, bortezomib and dexamethasone/rituximab; IMiDs, immunomodulatory drugs; NC, not comparable.
steroid group and BD/RTX group.
IMiD group and BD/RTX group.
Infection was most commonly found in the steroid and BD/RTX group. The BD treatment cycle was discontinued in 1 patient after the fourth cycle due to acute cerebral infarction. The adverse reactions improved after symptomatic treatment or dosage reduction.
Discussion
We demonstrated for the first time that IMiDs, such as thalidomide and lenalidomide, in combination with dexamethasone are effective for the treatment of PGNMID, achieving a renal remission rate of 18 of 28 (64%).
Dexamethasone may have played a significant role in rates of remission. In fact, glucocorticoids exert antagonistic effects against plasma cell diseases by indirectly repressing target genes through the inhibitory interaction of glucocorticoid receptor monomers with transcription factors, including nuclear factor κB and activator protein-1.20 The efficacy of dexamethasone alone in the treatment of plasma cell disease is poor. It is used in combination with almost all antiplasmacytosis regimens, usually in pulsed (once daily for 4 days) or once weekly dosing. In addition, Nasr et al.1,3 reported a PGNMID patient with a SCr concentration of 2 mg/dl and a moderate-to-severe degree of interstitial fibrosis, failed to achieve remission after treated with TD. In our study, the median SCr concentration among 28 patients receiving immunomodulatory therapy was 1.22 (IQR, 0.98–1.46) mg/dl, and 26 patients had mild or moderate renal interstitial fibrosis. The renal function and the degree of interstitial fibrosis are favorable to the better renal remission rate in our study. In addition, patients with PGNMID in our study were treated with a low dose of thalidomide, and this dose was gradually increased after no adverse reactions were observed or adverse reactions were tolerated. Due to diverse adverse reactions, thalidomide and lenalidomide treatment may be discontinued due to intolerance, thereby affecting their efficacy. We followed 28 patients with PGNMID for 23 (IQR, 9–36) months, and following more patients for a longer period could lead to more clinically significant results. Our study confirmed that a subset of patients achieved renal remission or renal CR after 4 (IQR, 2–9) months and 15 (IQR, 11–24) months, respectively. Furthermore, ethnic differences may lead to different responses to drugs.
Therapeutic efficacy can be affected by many factors, including hypertension, the SCr, C3 level, and treatment regimen. Low C3 levels are suggestive of dysregulation of an alternative pathway, because lower serum C3 levels have been correlated with worse long-term renal function.21 Nevertheless, our study showed that PGNMID patients with low serum C3 levels had better renal remission rates. This result may be attributed to the patients treated with the clone-directed regimens. Though there was no statistical difference in the number of low C3 in the 3 groups, the ratio of patients with low C3 was numerically higher in the IMiD and BD/RTX group than in the steroid group. IgG3 can activate complement system and is considered to have a strong proinflammatory effect. The surface binding of IgG3 molecules leads to persistent inflammation, which may be one factor accounting for poor renal prognosis.22
In this study, the most common adverse reaction to the TD regimen was peripheral neuropathy. Two patients who achieved CR began receiving steroid treatment because they could not tolerate peripheral neuropathy. Thalidomide is currently believed to cause peripheral neuropathy by inhibiting blood vessel growth, leading to inadequate blood supply to peripheral nerves, inhibiting TNF-α, and blocking the NF-κB pathway, thereby inducing nerve damage.23 For patients exhibiting peripheral neuropathy, a reduction in the dose or discontinuation of the drug, along with the addition of gabapentin, vitamin B and amitriptyline, can effectively relieve symptoms. The most common adverse events in patients who received the RD regimen were anemia and skin lesions. The incidence rates of rashes in clinical trials related to lenalidomide varied widely, with a rash incidence rate of grade 3 and above ranging from 2% to 12%.24 Although the exact mechanism is unknown, lenalidomide has strong T-cell immunomodulatory effects, which may be associated with rash development.25 The administration of steroids or antihistamines is recommended until the symptoms disappear, and the medications need to be stopped if they are not tolerated.
Compared to properties of bortezomib or daratumumab based regimens, it is noticeable that IMiD regimens have some restrictions. Because of the relatively high occurrence rates of severe side effects, thalidomide is now rarely used to treat myeloma. In addition, there is concern about the tolerance of IMiDs in patients with renal insufficiency.26,27 Nevertheless, bortezomib or daratumumab was not affordable due to the high cost especially for patients with PGNMID in developing countries. Based on our results, accurately monitored IMiDs may provide an option for these patients.
Our study has some advantages over others on PGNMID in the literature. We first found that the immunomodulatory treatment approach was associated with the achievement of renal remission in patients with PGNMID. This study has the largest number of patients and a long follow-up period, with a median follow-up of 20 (IQR, 10–37) months. This study also has several limitations. This was a single-center retrospective study that included a relatively small number of patients in the BD/RTX group. The short follow-up time of patients in the IMiD group may not have been sufficient to predict the long-term prognosis. Further prospective multicenter studies are needed to evaluate the efficacy and safety of immunomodulatory therapies for PGNMID patients.
In summary, to the best of our knowledge, this study is the first to report the therapeutic efficacy of IMiDs in combination with dexamethasone for the treatment of PGNMID. Close attention should be paid to related adverse events, and the doses should be monitored individually.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Samples were obtained from the Renal Biobank of the National Clinical Research Center of Kidney Diseases, Jiangsu Biobank of Clinical Resources.
Funding
This study was funded by the Special Funds of the National Natural Science Foundation of China (32141004).
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Author Contributions
Research idea and study design: CZ, TZ; data acquisition: HAZ, MNL; data analysis/interpretation: HAZ, MNL; statistical analysis: HAZ; supervision or mentorship: CHZ, ZHC. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Footnotes
Figure S1. Renal remission rates in the 19 patients with monoclonal presence in different groups at last follow-up time.
Table S1. The details of blood and bone marrow examinations of the 19 patients with monoclonal presence.
Supplementary Appendix 1.
STROBE Statement.
Contributor Information
Ti Zhang, Email: drteezhang@163.com.
Zhen Cheng, Email: chengzhen33@hotmail.com.
Supplementary Material
Figure S1. Renal remission rates in the 19 patients with monoclonal presence in
different groups at last follow-up time.
Table S1. The details of blood and bone marrow examinations of
the 19 patients with monoclonal presence
Supplementary Appendix 1.
STROBE Statement.
Figure S1. Renal remission rates in the 19 patients with monoclonal presence in different groups at last follow-up time.
Table S1. The details of blood and bone marrow examinations of the 19 patients with monoclonal presence.
Supplementary Appendix 1.
STROBE Statement.
References
- 1.Nasr S.H., Markowitz G.S., Stokes M.B., et al. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65:85–96. doi: 10.1111/j.1523-1755.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 2.Bridoux F., Leung N., Hutchison C.A., et al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698–711. doi: 10.1038/ki.2014.408. [DOI] [PubMed] [Google Scholar]
- 3.Nasr S.H., Satoskar A., Markowitz G.S., et al. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumber R., Cohen J.B., Palmer M.B., et al. A clone-directed approach may improve diagnosis and treatment of proliferative glomerulonephritis with monoclonal immunoglobulin deposits. Kidney Int. 2018;94:199–205. doi: 10.1016/j.kint.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Hogan J.J., Alexander M.P., Leung N. Dysproteinemia and the kidney: core curriculum 2019. Am J Kidney Dis. 2019;74:822–836. doi: 10.1053/j.ajkd.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Nasr S.H., Larsen C.P., Sirac C., et al. Light chain only variant of proliferative glomerulonephritis with monoclonal immunoglobulin deposits is associated with a high detection rate of the pathogenic plasma cell clone. Kidney Int. 2020;97:589–601. doi: 10.1016/j.kint.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Leung N., Bridoux F., Nasr S.H. Monoclonal gammopathy of renal significance. N Engl J Med. 2021;384:1931–1941. doi: 10.1056/NEJMra1810907. [DOI] [PubMed] [Google Scholar]
- 8.Zand L., Rajkumar S., Leung N., et al. Safety and efficacy of daratumumab in patients with proliferative GN with monoclonal immunoglobulin deposits. J Am Soc Nephrol. 2021;32:1163–1173. doi: 10.1681/ASN.2020101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almaani S., Parikh S., Satoskar A., et al. Daratumumab in patients with bortezomib-refractory proliferative glomerulonephritis with monoclonal immunoglobulin deposits. Kidney Int Rep. 2021;6:2203–2206. doi: 10.1016/j.ekir.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M., Dimopoulos M., Chen C., et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 11.Warsame R., LaPlant B., Kumar S., et al. Long-term outcomes of IMiD-based trials in patients with immunoglobulin light-chain amyloidosis: a pooled analysis. Blood Cancer J. 2020;10:4. doi: 10.1038/s41408-019-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houan Z., Jingjing C., Manna L., et al. Lenalidomide plus dexamethasone for proliferative glomerulonephritis with monoclonal immunoglobulin deposits. Chin J Nephrol. 2020;36:441–446. [Google Scholar]
- 13.Troyanov S., Wall C.A., Miller J.A., et al. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 14.Palladini G., Perfetti V., Perlini S., et al. The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL) Blood. 2005;105:2949–2951. doi: 10.1182/blood-2004-08-3231. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulosa M.A., Terposa E., Niesvizkyb R. How lenalidomide is changing the treatment of patients with multiple myeloma. Crit Rev Oncol Hematol. 2013;88(suppl 1):s23–s35. doi: 10.1016/j.critrevonc.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Treon S.P., Branagan A.R., Hunter Z., et al. Paradoxical increases in serum IgM and viscosity levels following rituximab in Waldenstrom’s macroglobulinemia. Ann Oncol. 2004;15:1481–1483. doi: 10.1093/annonc/mdh403. [DOI] [PubMed] [Google Scholar]
- 17.Sethi S., Rajkumar S.V. Monoclonal gammopathy-associated proliferative glomerulonephritis. Mayo Clin Proc. 2013;88:1284–1293. doi: 10.1016/j.mayocp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Hutchison C.A., Harding S., Hewins P., et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684–1690. doi: 10.2215/CJN.02290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coresh J., Turin T., Matsushita K., et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burwick N., Sharma S. Glucocorticoids in multiple myeloma: past, present, and future. Ann Hematol. 2019;98:19–28. doi: 10.1007/s00277-018-3465-8. [DOI] [PubMed] [Google Scholar]
- 21.Tsai S., Wu M., Chen C. Low serum C3 level, high neutrophil-lymphocyte-ratio, and high platelet-lymphocyte-ratio all predicted poor long-term renal survivals in biopsy-confirmed idiopathic membranous nephropathy. Sci Rep. 2019;9:6209. doi: 10.1038/s41598-019-42689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L., Ding Z., Heyman B. IgG3-antigen complexes are deposited on follicular dendritic cells in the presence of C1q and C3. Sci Rep. 2017;7:5400. doi: 10.1038/s41598-017-05704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terpos E., Kleber M., Engelhardt M., et al. European myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica. 2015;100:1254–1266. doi: 10.3324/haematol.2014.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreau P., Masszi T., Grzasko N., et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–1634. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 25.Dote S., Ito K., Itakura S., et al. Impact of prior bortezomib therapy on the incidence of lenalidomide-induced skin rash in multiple myeloma: a propensity score-matched multi-institutional cohort study. Leuk Lymphoma. 2019;60:2975–2981. doi: 10.1080/10428194.2019.1608531. [DOI] [PubMed] [Google Scholar]
- 26.Chanan-Khan A., San Miguel J.F., Jagannath S., Ludwig H., Dimopoulos M.A. Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin Cancer Res. 2012;18:2145–2163. doi: 10.1158/1078-0432.CCR-11-0498. [DOI] [PubMed] [Google Scholar]
- 27.Smyth E., Glavey S., Melotti D., et al. Dialysis independence following single-agent daratumumab in refractory myeloma with renal failure. Ir J Med Sci. 2019;188:1079–1080. doi: 10.1007/s11845-018-1951-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.