Abstract
Introduction
Glomerular diseases are the leading drivers of nondiabetic chronic kidney disease disability-adjusted life years in resource-limited countries. Proper diagnosis and treatment relies on resources including kidney biopsy, ancillary testing, and access to evidence-based therapies.
Methods
We conducted a cross-sectional internet-based survey cascaded through society mailing lists among nephrologists in countries of Asia, Africa, and Eastern Europe. We collected the data on respondent demographics, their ability to perform and appropriately interpret a kidney biopsy, and their access to complementary investigations and treatment practices.
Results
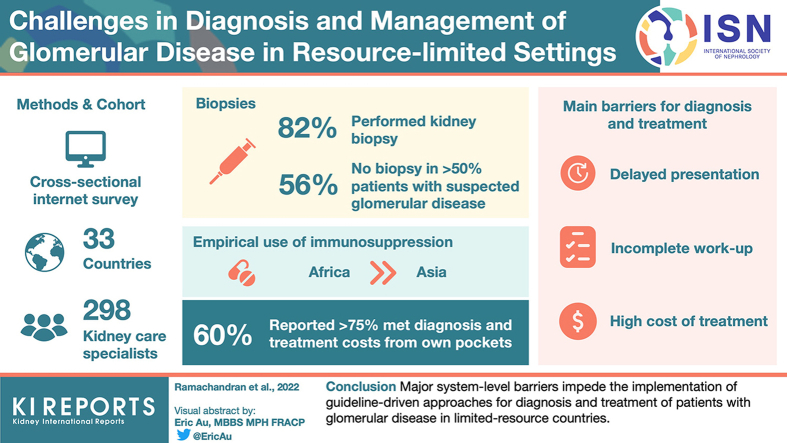
A total of 298 kidney care specialists from 33 countries (53.3% from Asia and 44.6% from Africa; 64% from academic or university hospitals) participated in the survey. Of these specialists, 85% performed kidney biopsy. About 61% of the respondents could not obtain a kidney biopsy in more than 50% of patients with suspected glomerular disease. About 43% of the respondents from Africa had access to only light microscopy. Overall, the inability to undertake and fully evaluate a biopsy and perform ancillary investigations were more profound in Africa than in Asia. Overall, 59% of participants reported that more than 75% of their patients meet the cost of diagnosis and treatment by out-of-pocket payments. Empirical use of immunosuppression was higher in Africa than in Asia. The main barriers for diagnosis and treatment included delayed presentation, incomplete diagnostic work-up, and high cost of treatment.
Conclusion
Major system-level barriers impede the implementation of guideline-driven approaches for diagnosis and treatment of patients with glomerular disease in resource-limited countries.
Keywords: Africa, Asia, glomerular diseases, glomerulonephritis, kidney biopsy, low and middle income countries
Graphical abstract
Glomerular diseases are amongst the leading causes of kidney failure requiring replacement therapy worldwide, especially in low and lower-middle income countries.1,2 According to the global burden of disease study, diabetes3,4 and hypertension are the leading drivers of increased chronic kidney disease disability-adjusted life years worldwide, but glomerulonephritis is the second biggest driver of chronic kidney disease disability-adjusted life years after diabetes in low resource settings.4 The diagnosis of glomerular diseases (apart from childhood nephrotic syndrome) rests on kidney biopsy; its interpretation by appropriate techniques including light microscopy, immunofluorescence (IF), and electron microscopy; and integration with other information collected by context-specific investigations. Molecular and serology testing have improved the accuracy of glomerular disease diagnosis and classification, translating to better and individualized treatment of glomerular disease.5,6
Glomerular disease is among the few potentially treatable cause of chronic kidney disease. Kidney Disease Improving Global Outcomes has laid down guidelines that assist nephrologists worldwide in managing glomerulonephritis using evidence-based approaches.7 Whereas the guidelines have a global remit and aim to reduce variations in practices and improve health outcomes worldwide, their implementation depends on availability of resources to make an accurate diagnosis and access to treatment options. Treatment of glomerular diseases includes nonpharmacological measures to control weight and blood pressure, and the use of angiotensin blocking agents. For patients at high-risk of adverse outcomes, a variety of the following immunosuppressive agents are employed: corticosteroids, alkylating agents, calcineurin inhibitors, mycophenolic acid, and more recently, biological agents that act on B-cells and complement pathway. The ability of clinicians to accurately implement these recommendations in different parts of the world has not been established but has major impact on the ability to deliver evidence-based care.
In a first of its kind assessment, we conducted a survey to ascertain the availability and ease of access to facilities and resources to diagnose and manage glomerular disease in low-resource settings.
Methods
An online survey was conducted between August and November 2021 amongst nephrologists in low-income and middle-income countries of Asia, Africa and Eastern Europe. Participants voluntarily responded to an email distributed via established mailing lists and social media advertisements, with a link to complete the survey. The survey was administered in Google Forms and approved by the Ethics Committee of the George Institute for Global Health India. After creation, the questionnaire (Supplementary File 1) was pilot tested on 15 nephrologists for comprehensibility, clarity, and completeness.
De-identified data was collected. Eligible participants were asked the following: (i) baseline demographic data; (ii) ability to perform and appropriately interpret a kidney biopsy (iii) access to complementary investigations, and (iv) treatment practices.
Statistical Analysis
Descriptive statistics were used to describe the characteristics of the respondents. We expressed the normally distributed continuous variables as mean ± SD (range), non-normally distributed variables as medians with interquartile ranges and categorical data as proportions. Because a majority of responses were from Asia and Africa, we analysed data for these continents separately. We performed all analyses using Graph Pad Prism 9 (GraphPad Software, San Diego, CA; www.graphpad.com).
Results
A total of 298 kidney care providers from 33 countries (Supplementary Table S1) responded to the survey, including 159 (53.3%) from Asia, and 133 (44.6%) from Africa. Twenty-one (7%), 260 (87.3%) and 17 (5.7%) of the responders were from low-income, low-middle-income and upper-middle-income countries, respectively. Characteristics of the survey respondents are described in Table 1. One hundred sixty-nine (63%) were engaged in adult nephrology care, 92 (34%) took care of both adult and pediatric cases, and 8 (3%) were only pediatric nephrologists. A greater proportion of responders from Africa worked in academic centers as compared to Asia (74.8% vs. 54.7%). A total of 115 (38.5%) respondents had received a part or whole of their training from a country different from where they were practicing, with the proportion being higher in the African countries (45.8%) than in Asia (30.8%). The most common reason was an absence of opportunity in their country and a desire to learn new clinical or research skills.
Table 1.
Respondent characteristics
| Total | Africa | Asia | Eastern Europe | |
|---|---|---|---|---|
| Age distribution (yrs) | (N = 269) | (N = 104) | (N = 159) | (N = 6) |
| < 30 | 3 (1.1%) | 0 (0.0%) | 3 (1.9%) | 0(0.0%) |
| 30–39 | 93 (34.6%) | 33 (31.8%) | 58 (36.5%) | 2(33.3%) |
| 40–49 | 98 (36.5%) | 49 (47.1%) | 48(30.2%) | 1(16.7%) |
| 50–59 | 48 (17.8%) | 16 (15.4%) | 30 (18.8%) | 2(33.3%) |
| >60 | 27 (10.0%) | 06 (5.7%) | 20 (12.6%) | 1(16.7%) |
| Work experience in yrs | (N = 269) | (N = 104) | (N =159) | (N = 6) |
| < 5 yrs | 55 (20.4%) | 20 (19.2%) | 34 (21.4%) | 1(16.7%) |
| 5–10 | 89 (33.1%) | 42 (40.4%) | 45 (28.3%) | 2 (33.3%) |
| 11–20 | 68 (25.3%) | 26 (25.0%) | 42 (26.4%) | 0 (0.0%) |
| More than 20 | 57 (21.2%) | 16 (15.4%) | 38 (23.9%) | 3 (50%) |
| Type of practice | (N = 269) | (N = 104) | (N = 159) | (N = 6) |
| Academic/university hospital | 180 (66.9%) | 88 (84.6%) | 87 (54.8%) | 5 (83.3%) |
| Private hospital | 58 (21.6%) | 7 (6.7%) | 50 (31.4%) | 1 (16.7%) |
| Solo practice | 19 (7.0%) | 4 (3.9%) | 15 (9.4%) | 0 (0.0%) |
| Group practice | 12 (4.5%) | 05 (4.8%) | 07 (4.4%) | 0 (0.0%) |
| Who performs kidney biopsy | (N = 269) | (N = 104) | (N = 159) | (N = 6) |
| Nephrologist | 230 (85.5%) | 84 (80.8%) | 145 (91.2%) | 1 (16.6%) |
| Others | 15 (5.6%) | 03 (2.9%) | 08 (5.0%) | 4 (66.8%) |
| Biopsy not performed | 24 (8.9%) | 17 (16.3%) | 06 (3.8%) | 1 (16.6%) |
| Total | Africa | Asia | Eastern Europe |
Kidney Biopsy
Kidney biopsy was not performed at the institutions of 35 (11.8%) responders, whereas for 18 (6.1%) respondents, a non-nephrologist (e.g., a radiologist) conducted the procedure. According to 72 (28.8%) respondents, less than 10% of patients in whom biopsy was indicated for diagnosis of glomerular disease in their practice were able to get it, whereas for 98 (39.2%) respondents the proportion of patients able to undergo the procedure was more than 50% (Table 2). The proportion of patients unable to get their kidney biopsy for diagnosing the kidney disease was higher in Africa, where 50.3% of the respondents could get biopsy only in less than 10% of their patients. The corresponding figure was 4.3% in Asia. The main reasons for biopsies not being done included lack of nephropathology services (40%), lack of training (5.7%), and cost (45.7%).
Table 2.
Kidney biopsy details
| Total | Africa | Asia | Others | |
|---|---|---|---|---|
| Proportion getting kidney biopsy when indicated | (N = 250) | (N = 131) | (N = 113) | (N = 6) |
| <10% | 72 (28.8%) | 66 (50.4%) | 05 (4.5%) | 1 (16.7%) |
| 10–25% | 48 (19.2%) | 30 (22.9%) | 17 (15.0%) | 1 (16.7%) |
| 26–50% | 32 (12.8%) | 14 (10.7%) | 17 (15.0%) | 2 (33.3%) |
| >50% | 98 (39.2%) | 21 (16.0%) | 74 (65.5%) | 2 (33.3%) |
| No of kidney biopsies in a month | (N = 259) | (N = 101) | (N = 153) | (N = 5) |
| Not done | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 0–5 | 117 (45.2%) | 67 (66.3%) | 49 (32.0%) | 1(20%) |
| 5–10 | 62 (23.9%) | 21 (20.8%) | 39 (25.5%) | 2 (40%) |
| More than 10 | 80 (30.9%) | 13 (12.9%) | 65 (42.5%) | 2 (40%) |
| Mean turn-around time (ds) | (N = 257) | (N = 99) | (N = 153) | (N = 5) |
| < 3 | 48 (18.7%) | 05 (5.0%) | 42 (27.4%) | 1(20%) |
| 3–7 | 96 (37.3%) | 17 (17.2%) | 78 (51.0%) | 1(20%) |
| 8–14 | 72 (28.0%) | 42 (42.4%) | 28 (18.3%) | 2 (40%) |
| >14 | 41 (16.0%) | 35 (35.4%) | 05 (3.3%) | 1 (20%) |
| Location of the nephro-pathology service | (N = 253) | (N = 97) | (N = 151) | (N = 5) |
| In my hospital | 106 (41.9%) | 44 (45.4%) | 62 (41.0%) | 0 (0%) |
| In my city | 54 (21.3%) | 11 (11.3%) | 38 (25.2%) | 5 (100%) |
| Another city | 54 (21.3%) | 14 (14.4%) | 40 (26.5%) | 0 (0%) |
| Overseas | 39 (15.5%) | 28 (28.9%) | 11 (7.3%) | 0 (0%) |
| Biopsies processed | (N = 246) | (N = 89) | (N = 152) | (N = 5) |
| LM only | 41 (16.7%) | 38 (42.7%) | 03 (2.0%) | 0 (0%) |
| LM and IF | 98 (39.8%) | 25 (28.1%) | 69 (45.4%) | 4 (80%) |
| LM, IF and EM | 107 (43.5%) | 26 (29.2%) | 80 (52.6%) | 1 (20%) |
| Proportion of biopsies evaluated by IF/IHC | (N = 252) | (N = 94) | (N = 153) | (N = 5) |
| <10% | 59 (23.5%) | 52 (55.4%) | 06 (3.9%) | 1 (20%) |
| 11–50% | 20 (7.9%) | 10 (10.6%) | 10 (6.5%) | 0 (0%) |
| 50–75% | 16 (6.3 %) | 07 (7.4%) | 09 (5.9%) | 0 (0%) |
| >75% | 157 (62.3%) | 25 (26.6%) | 128 (83.7%) | 4 (80%) |
| Proportion of biopsies evaluated by EM | (N = 243) | (N = 90) | (N = 148) | (N = 5) |
| <10% | 167 (68.7%) | 73 (81.2%) | 90 (60.8%) | 4 (80%) |
| 11–50% | 29 (11.9%) | 04 (4.4%) | 25 (16.9%) | 0 (0%) |
| 50–75% | 16 (6.6%) | 03 (3.3%) | 13 (8.8%) | 0 (0%) |
| >75% | 31 (12.8%) | 10 (11.1%) | 20 (13.5%) | 1 (20%) |
EM, electron microscopy; IF, immunofluorescence; IHC, immunohistochemistry; LM, light microscopy.
The number of biopsies carried out every month at survey participants’ centers is shown in Table 2. The volume of biopsies done at responder centers was lower in Africa than in Asia. A total of 160 (64%) responders had access to a nephropathologist in the same city or hospital, and 39 (15.6%) survey participants sent their patient’s biopsy overseas for reporting. The turnaround time for the kidney biopsy reporting was less than 7 days for 144 (57.6%) responders but 41 (16.4%) survey participants had to wait longer than 14 days for a report. The delay was higher in African than in Asian centers (>7 days: 78% vs. 22%). A total of 205 (83.3%) and 107 (43.5%) respondents had access to IF and electron microscopy, respectively. Thirty-eight (42.7%) respondents’ centers in Africa had access to light microscopy only, as compared to 2% in Asia. Among centers performing IF, less than 10% of biopsies were evaluated by this technique in 59 (23.4%) respodents’ centers, whereas 157 (62.3%) were able to get IF study for more than 75% of their biopsies. Fifty-five percent of the centers in Africa reported less than 10% of their patients undergoing IF testing on a regular basis as compared to 3.9% in Asia. Sixty-nine per cent of the responders utilized electron microscopy in less than 10% of the biopsies, with the proportion being similar in Asia and Africa. Details regarding biopsy practices are shown in Table 2.
Serology Testing
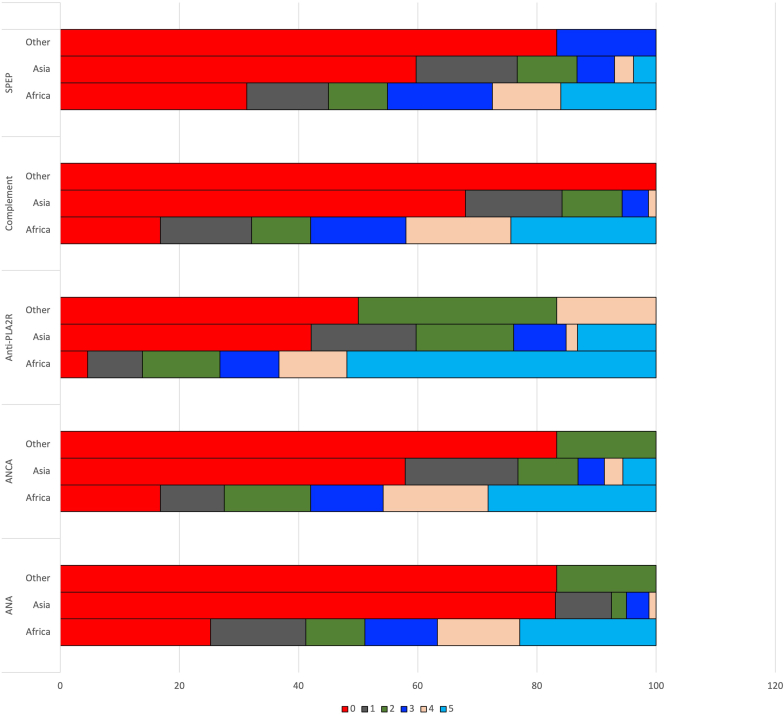
Responders were asked to grade the degree of difficulty in obtaining the common serological assays that help in diagnosis, classification and treatment of glomerular diseases, including antinuclear antibody, antineutrophil cytoplasmic antibody, anti-glomerular basement membrane antibody, and anti-M-type phospholipase A2 receptor antibody on a scale of 0 (no difficulty at all) to 5 (unable to get the test). One hundred seventy-one (57.7%) study participants did not encounter any difficulty performing antinuclear antibody testing, whereas 50 (16.8%) had severe difficulty in getting it. For antineutrophil cytoplasmic antibody, this proportion was 40.2% (no difficulty) and 24.9% (severe difficulty), respectively. Anti-M-type phospholipase A2 receptor testing was easily available to 25.7% of participants whereas 36.4% had severe limitation to access it. The percentage of centers encountering difficulty or inability in getting all the recommended serologic investigation was higher in Africa as compared to Asian countries (Figure 1).
Figure 1.
The degree of difficulty in obtaining serological tests required for diagnosis of glomerular diseases (scale: 0–no difficulty and 5–extreme difficulty, cannot order). ANA, antinuclear antibody; ANCA, anti-neutrophil cytoplasmic antibody; PLA2R, phospholipase A2 receptor; SPEP, serum protein electrophoresis.
Treatment
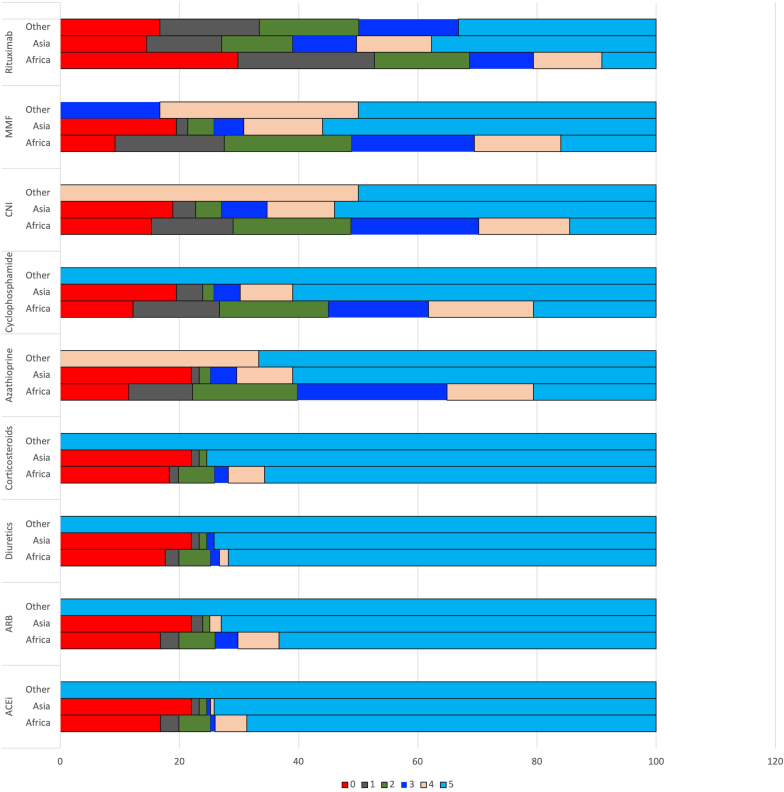
A total of 57 (19.2%) survey participant’s sites did not have unfettered availability of angiotensin-converting enzyme inhibitors and angiotensin-2 receptor blockers, which are central to the management of patients with glomerular disease. Access to diuretics, glucocorticoids, azathioprine, cyclophosphamide, calcineurin inhibitors, mycophenolate mofetil, and rituximab was unavailable or severely limited for 63 (21.3%), 63 (21.3%), 66 (22.3%), 73 (24.7%), 74 (25%), 70 (23.6%) and 114 (38.5%) survey participants, respectively. For all the drugs, a higher proportion of responders from Africa had severely limited access. Access to rituximab was equally limited for countries on the two continents (Figure 2).
Figure 2.
The ease of availability of various medications for managing common glomerular diseases (scale: 0–never available to 5–available to all patients without any problem). ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; CNI, calcineurin inhibitors; MMF, mycophenolate mofetil.
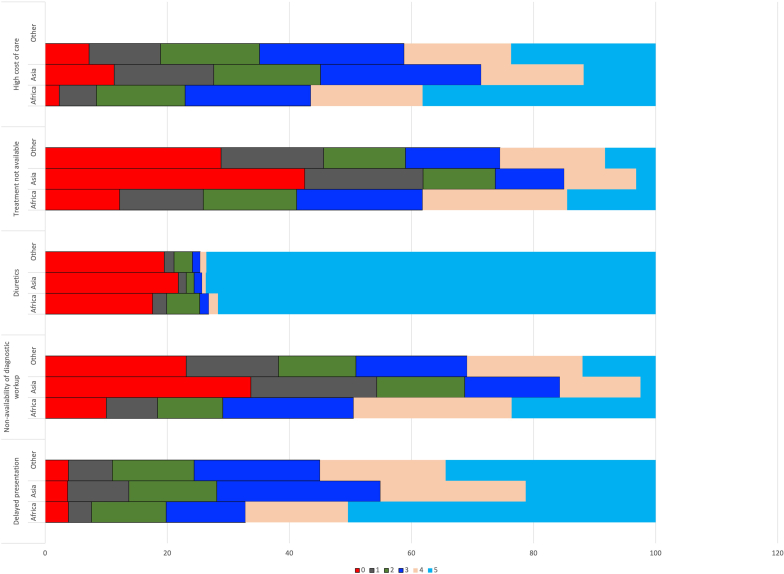
With regard to health seeking behaviours and cost of care, the main barriers (Figure 3) included delayed presentation (frequently encountered by 54.3% responders), diagnostic workup not possible (frequently encountered by 30% responders), expensive treatment (frequently encountered by 40.5% responders), treatment not being available (frequently encountered by 25% responders). and religious or cultural belief (frequently encountered in 8.4%). All the barriers to diagnosis, especially delayed presentation, nonavailability of diagnostic work-up and cost of care were encountered by a greater proportion of respondents in Africa.
Figure 3.
Rating of the importance of common barriers in managing glomerular diseases (scale: 0–not a barrier to 5–a frequently encountered barrier)
According to 175 (59.3%) responders, more than 75% of their patients with glomerular disease met the cost of diagnosis and treatment from their own pockets, whereas this proportion was 50% to 74% for another 44 (14.9%) responders. There was no difference in the frequency of responses regarding out of pocket expenditure between Asia and Africa. A total of 104 (35.6%) respondents treated more than 25% of their patients with oral steroid therapy equivalent to more than 1 mg/kg of oral prednisolone because of limited access to adequate workup. Empiric use of steroid, azathioprine, oral and intravenous cyclophosphamide, calcineurin inhibitors, and mycophenolate are similar in both Asia and Africa and shown in Supplementary Table S2.
Discussion
This first of its kind survey highlights the challenges and identifies factors that limit access to evidence-based diagnosis and management of glomerular disease in resource-limited settings. The barriers include inability of patients to access care in a timely manner, ability to make a diagnosis through biopsy and other ancillary investigations, poor access to treatment, and high cost of care.
With rare exceptions, kidney biopsy is the starting point in the process of providing evidence-based care to patients with glomerular diseases.8 One third of responders in this survey indicated that less than half of patients suspected to have a glomerular disease in their care were able to receive a biopsy. Similarly, over 50% of the survey participants reported moderate-to-severe difficulty in accessing serology testing to aid in diagnosing glomerular disease. Limitations in both of these domains was encountered in significantly greater frequency by respondents from Africa.
Greater than three-fourths of responding nephrologists had received formal training and performed kidney biopsy. The proportion of nephrologists performing kidney biopsies was lower in Africa. Differences in access to formal training programs and infrastructure support may partly account for the aforementioned differences, as suggested by overseas training received by a significant proportion. Okani et al.9 analysed the trend in kidney biopsy requests in Nigeria from 1981 to 2011. Surprisingly, the authors noted a significant reduction in the number of kidney biopsies from 10 to 20 per year pre-1993 to 1 to 10 per year from 1993 to 2011.9 This reduction took place in the background of an incremental improvement of kidney replacement therapy.10 The authors postulated out-of-pocket expense and lack of skilled workforce as the main cause for the decline in kidney biopsies.
Access to nephropathology services was also heterogeneous, including the need to ship the biopsy tissue to another city or country. With the improvement in telemedicine and access to high-speed internet, having an in-house nephropathologist for interpreting the biopsy is less critical now than in the past. Nevertheless, the ability to process biopsies locally is important. Shipping the sample to a different city or country can introduce unacceptable delays in the treatment of patients with rapidly progressive glomerulonephritis or acute kidney allograft dysfunction. Long-distance transport may be unduly affected by exogenous factors like restrictions secondary to local unrest, civil disobedience, natural disasters, or lockdown for pandemics. Telemedicine could be of potential value in addressing the aforementioned challenges for example, a local kidney biopsy processing facility manned by trained technical staff with infrastructure support (including good camera and high-speed internet) would allow images to be shared with a remotely located nephropathologist. Similarly, a nephrologist may be able to connect with a remotely located center of excellence with expertise in treatment of specific glomerular diseases for making crucial treatment decisions where guidelines are ambiguous.
In addition to light microscopy, kidney biopsy tissue almost always needs to be processed by IF for proper diagnosis and classification of glomerular diseases. About one-sixth of the survey participants (one-third in Africa) did not have access to these 2 modalities. This limitation is bound to lead to inappropriate classification of glomerular disease and wrong choice of therapy, including immunosuppressive therapy, which could prove detrimental. Not surprisingly, access to electron microscopy was even more limited. This modality is required for an accurate diagnosis in 20% to 40%11,12 of the kidney biopsies, especially hereditary nephritis (particularly basement membranous disease) and classifying immune-complex mediated diseases like cryoglobulinemic, fibrillary, or immunotactoid glomerulonephritis.12,13
Over the past 2 decades, the diagnosis of glomerular disease has graduated from pattern-based to a pathogenesis-based classification.5,14,15 Study of kidney tissue needs to be supplemented with other tests, such as serology and genetic testing. In some cases, these tests can obviate the need for a biopsy.16 For example, according to the latest Kidney Disease Improving Global Outcomes Guidelines a kidney biopsy is not required to confirm the diagnosis of membranous nephropathy in patients with nephrotic syndrome and a positive antiphospholipase A2 receptor antibody test.7 The survey identified major limitations to access these tests. In addition to the tests included in this survey, tests for C3 nephritic factor, anticomplement factor-H/B, serum immunofixation electrophoresis, free light chain assays, and genetic testing are critical to diagnosis and management of glomerulonephritis. It is reasonable to conclude that patients with conditions that require these tests for diagnosis and/or monitoring will not be identified and treated properly.
Clinical practice guideline recommendations often advocate a risk-based approach to treatment based on these tests, which may need to be repeated.7 In light of these limitations to accurate diagnosis and classification, a significant proportion of patients are treated empirically with potentially toxic immunosuppressive medications, contrary to the essence of evidence-based medicine. Although instituted with good intentions, such low-value care is likely to be detrimental to patient health. The current emphasis in the economically stronger countries has moved to personalised treatment approaches using less toxic regimens increasingly based on even more expensive biological agents. It is likely that the “therapeutic apartheid” between the developed and the less developed countries will become even wider. It is telling that about one-fifth of the respondents’ patients had difficulty in continued access to common drugs like angiotensin-convertor enzyme inhibitors or angiotensin-2 receptor blockers which are available in cheap generic forms.
Finally, we found that a large proportion of patients have to finance care for glomerular diseases from their own resources. Interestingly, the proportion of patients experiencing out-of-pocket expenditure was same in all regions. This reflects an overall neglect leading to failure to include treatment of glomerular diseases in the universal health coverage packages. Three-fourths of the low and middle-income countries spend less than 5% of their gross national product on health care, and struggle with managing multiple competing priorities. Primary and secondary prevention are appropriately prioritized because of greater population benefits. Nevertheless, given the growing burden, the scope of the latter needs to be expanded to include kidney diseases such as the ones discussed here. In the absence of timely diagnosis and treatment, these patients will enlarge the pool of patients with kidney failure requiring expensive kidney replacement therapy that will ultimately cost more to the state.
Our survey specifically focused on diagnosis or treatment of glomerular diseases, and complements the limitations in capacity and resources for care of patients with kidney disease in low and lower-middle income countries highlighted by the Global Kidney Health Atlas.17 The International Society of Nephrology has supported human resource development and capacity strengthening (including for nephropathology) through its capacity building programs over the past 25 years, with particular focus on Africa.18, 19, 20 Brain-drain has been an important problem, however. A significant proportion of those who receive specialist training in centers located abroad either through these programs or on their own initiative do not return to their home countries. Of those who return to their home countries, a substantial proportion go back because they are unable to utilize their newly acquired skills due to lack of resources. Therefore, local investments are necessary for the development and maintenance of sustainable infrastructure. Other novel solutions can be considered, for example use of refurbished biopsy needles or guns, which may be consistently reused after sterilization. This strategy has been used in countries like India, Bangladesh and Nigeria and can enable trained nephrologists to perform kidney biopsies after training in a similar setting.
Our survey received a reasonable response from a large number of countries and provides a snapshot of the current status of the barriers in managing glomerular disease in resource-limited settings. The survey has a few limitations: it was cascaded through network mailing lists, so we have no idea of the denominator. Like all surveys, there exists the possibility of recall bias (where the responses are likely to be guesstimates), participation or non-response bias (participants with specific traits, e.g., interest in glomerular diseases may have responded), and the social desirability bias (providing a more desirable or acceptable answer), and demand characteristics (participants subconsciously changing their behaviour to fit their interpretation of the purpose of the survey). We did not explicitly enquire about complications being a deterrent to performing kidney biopsies. In addition, we were unable to differentiate between multiple responses from the same center; this may have led to a few duplicated responses if more than one respondent works in the same center. Finally, The survey was administered in English and consequently has under-representation from Latin America, Francophone Africa and Eastern European countries.
This survey highlights the large gaps in care for patients with glomerular diseases. Glomerular diseases are classified as rare diseases and suffer from lack of prioritization in health care delivery, research, and funding, which is particularly acute in low-resource settings. Addressing this gap needs a multipronged approach, with collaboration between government, academia, industry, professional societies, and patient community. These may include setting up new services or expanding existing services through public-private partnership, development of robust telemedicine infrastructure, incentivizing investment into infrastructure development, research and capacity building, and sharing of expertise through collaborations. Governments can make therapeutic agents available to the public at reduced prices through strategic purchasing. Private nephropathology services can share services with public sector units. Finally, inclusion of centers from resource-limited countries in cohort studies and randomised clinical trials will facilitate improved clinical care.
In summary, appropriate guideline driven approach to diagnosis and management of glomerular diseases with an expedient reporting of kidney biopsy and use of ancillary investigations remain limited in a club for the chosen few. For advancing kidney care worldwide, it is critical to address the barriers in diagnosis and management of glomerular diseases, which form a substantial chunk of potentially reversible causes of kidney failure, especially in low-resource regions. This requires international co-operation and continued advocacy to strengthen the healthcare system so that these neglected diseases receive due attention.
Disclosure
VJ has received grant funding from GSK, Baxter Healthcare, and Biocon and honoraria from Bayer, AstraZeneca, Boeringer Ingelheim, NephroPlus and Zydus Cadilla, under the policy of all honoraria being paid to the organization.
All the other authors declared no competing interests.
Acknowledgments
This work was funded by the International Society of Nephrology Sister Renal Centre Grant.
Footnotes
Supplementary Methods.
Facility readiness questionnaire.
Table S1. List of countries with number of responders.
Table S2. Frequency of using immunosuppressive therapy.
Supplementary Material
Supplementary Methods.
Facility readiness questionnaire.
Table S1. List of countries with number of responders
Table S2. Frequency of using immunosuppressive therapy
References
- 1.Rajapurkar M.M., John G.T., Kirpalani A.L., et al. What do we know about chronic kidney disease in India: first report of the Indian CKD registry. BMC Nephrol. 2012;13:10. doi: 10.1186/1471-2369-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma M., Doley P., Das H.J. Etiological profile of chronic kidney disease: A single-center retrospective hospital-based study. Saudi J Kidney Dis Transpl. 2018;29:409–413. doi: 10.4103/1319-2442.229297. [DOI] [PubMed] [Google Scholar]
- 3.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y., Bowe B., Mokdad A.H., et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Beck L.H., Jr., Bonegio R.G., Lambeau G., et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumber R., Cohen J.B., Palmer M.B., et al. A clone-directed approach may improve diagnosis and treatment of proliferative glomerulonephritis with monoclonal immunoglobulin deposits. Kidney Int. 2018;94:199–205. doi: 10.1016/j.kint.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Rovin B.H., Adler S.G., Barratt J., et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 2021;100:753–779. doi: 10.1016/j.kint.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Jha V. Nephropathology: a nephrologist’s perspective. AJKD blog. 2013 https://ajkdblog.org/2013/05/09/nephropathology-a-nephrologists-perspective/ [Google Scholar]
- 9.Okani C.O., Ekrikpo U.E., Okolo C.A., Asinobi A.O., Salako B., Akang E.E. Is the art of renal biopsy on the decline in Nigeria? Ann Ib Postgrad Med. 2014;12:38–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Abdu A., Mahmood I.M., Audi K.Y., Umar M.S. Clinical characteristics and outcomes of hemodialysis in a new center in Northern Nigeria. Niger Med J. 2020;61:340–344. doi: 10.4103/nmj.NMJ_148_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokhtar G.A., Jallalah S.M. Role of electron microscopy in evaluation of native kidney biopsy: a retrospective study of 273 cases. Iran J Kidney Dis. 2011;5:314–319. [PubMed] [Google Scholar]
- 12.Haas M. A reevaluation of routine electron microscopy in the examination of native renal biopsies. J Am Soc Nephrol. 1997;8:70–76. doi: 10.1681/ASN.V8170. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita M., LMY H.J., Ren K.Y.M., et al. The continuing need for electron microscopy in examination of medical renal biopsies: examples in practice. Glomerular Dis. 2021;1:145–159. doi: 10.1159/000516831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi S., Fervenza F.C., Zhang Y., et al. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465–473. doi: 10.1038/ki.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feitz W.J.C., van de Kar N., Orth-Holler D., et al. The genetics of atypical hemolytic uremic syndrome. Med Genet. 2018;30:400–409. doi: 10.1007/s11825-018-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobart S.A., Han H., Tehranian S., et al. Noninvasive diagnosis of PLA2R-associated membranous nephropathy: a validation study. Clin J Am Soc Nephrol. 2021 doi: 10.2215/CJN.05480421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qarni B., Osman M.A., Levin A., et al. Kidney care in low- and middle-income countries. Clin Nephrol. 2020;93:21–30. doi: 10.5414/CNP92S104. [DOI] [PubMed] [Google Scholar]
- 18.International Society of Nephrology ISN biennial report 2017-2018. Advancing Kidney Health Worldwide. Together. 2019 https://www.theisn.org/wp-content/uploads/2020/09/ISN_biennial_report_2019.pdf [Google Scholar]
- 19.Oguejiofor F., Kiggundu D.S., Bello A.K., et al. International Society of Nephrology Global Kidney Health Atlas: structures, organization, and services for the management of kidney failure in Africa. Kidney Int Suppl (2011) 2021;11:e11–e23. doi: 10.1016/j.kisu.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feehally J. The International Society of Nephrology (ISN). Roles & challenges in Africa and other resource-limited communities. Clin Nephrol. 2016;86(13):3–7. doi: 10.5414/CNP86S101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.