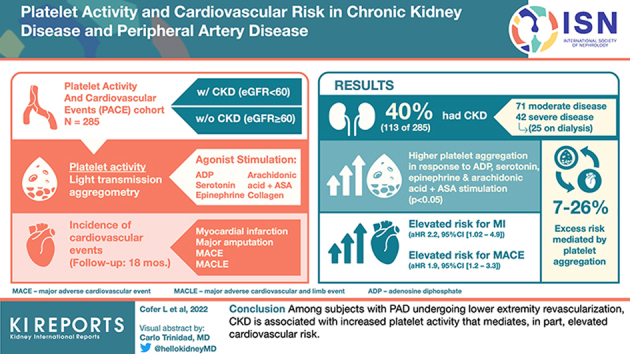
Abstract
Introduction
Platelet dysfunction and cardiovascular risk are well-recognized features of chronic kidney disease (CKD). Platelets drive the development and progression of cardiovascular disease (CVD). The relationships between kidney function, platelet activity, and cardiovascular risk are poorly defined.
Methods
We compared platelet activity and incident cardiovascular events by CKD status (estimated glomerular filtration rate [eGFR] < 60 ml/min per 1.73 m2) using data from the Platelet Activity and Cardiovascular Events study, a prospective cohort study that enrolled adults with peripheral artery disease (PAD) undergoing lower extremity revascularization. Platelet activity was measured using light transmission aggregometry (LTA) in response to submaximal dose agonist stimulation, and the subjects were followed for incident adverse cardiovascular events for a median of 18 months.
Results
Overall, 113 of 285 (40%) subjects had CKD. Subjects with, versus without, CKD had higher platelet aggregation in response to stimulation with adenosine diphosphate (ADP), serotonin, epinephrine, and arachidonic acid (AA) + ex vivo aspirin (P < 0.05 for each). Following multivariable adjustment, subjects with CKD had elevated risk for myocardial infarction (MI) (adjusted hazard ratio 2.2, 95% confidence interval [1.02–4.9]) and major adverse cardiovascular events (MACE) (1.9 [1.2–3.3]) compared to those without CKD. Platelet aggregation in response to submaximal dose agonist stimulation mediated 7% to 26% of the excess risk for cardiovascular events associated with CKD.
Conclusion
Among subjects with PAD undergoing lower extremity revascularization, CKD is associated with increased platelet activity that mediates, in part, elevated cardiovascular risk.
Keywords: CKD, CVD, PAD, Platelet activity
Graphical abstract
See Commentary on Page 2126
CKD is associated with accelerated atherosclerosis and increased cardiovascular risk.1 Patients with CKD are more likely to suffer cardiovascular events than progress to end-stage kidney disease (ESKD).2,3 PAD is highly prevalent in the CKD population, and is itself associated with significant cardiovascular morbidity and mortality.4,5 Patients with comorbid PAD and CKD are at greater risk for cardiovascular events and death than those with either disease alone.6, 7, 8
Platelets mediate inflammation and thrombosis, thereby driving the progression of CVDs, including PAD.9,10 Platelet activation is crucial to both chronic atherogenesis and acute thrombosis.11,12 Clinical evidence suggests that platelet activation increases with degree of kidney impairment.13,14 In experimental models, heightened platelet reactivity in CKD has been associated with thrombogenicity.15 In individuals with coronary artery disease, elevated platelet reactivity during treatment with antiplatelet agents is more frequently observed in those with CKD.16, 17, 18, 19 Nevertheless, the platelet phenotype in CKD is a source of conflicting and indeterminate clinical influence.20, 21, 22, 23 Better characterization of the relationships between kidney function, platelet activity, and cardiovascular risk would facilitate improved clinical management of individuals with CKD.
We hypothesized that prevalent CKD is associated with elevated platelet activity and increased cardiovascular risk in individuals with PAD. To test our hypothesis, we explored the associations between CKD status, platelet activity measured using LTA (the gold standard test for measuring platelet activity), and incident adverse cardiovascular events in a cohort of subjects with PAD who were undergoing lower extremity revascularization.
Methods
Study Design
The Platelet Activity and Cardiovascular Events study was a prospective cohort study approved by the New York University Langone Medical Center Institutional Review Board and conducted at New York University Langone Health, Bellevue Hospital, and the Manhattan Veterans Administration Hospital (URL: https://www.clinicaltrials.gov; Unique identifier: NCT02106429).24,25 The Platelet Activity and Cardiovascular Events study enrolled nonpregnant adults older than 21 years with symptomatic PAD and undergoing nonemergent lower extremity revascularization. Subjects were excluded if they had any of the following: hemoglobin <8 g/dl, hemorrhagic diathesis, platelet count >500×109/l or <100×109/l, or use of any nonsteroidal anti-inflammatory drug (other than aspirin) within 72 hours. Upon enrollment and prior to undergoing lower extremity revascularization, each subject was interviewed and they completed questionnaires soliciting clinico-demographic information. Venous blood drawn prior to revascularization using standard aseptic phlebotomy technique was used to measure platelet activity using LTA. Enrolled subjects were followed up with during regular clinic visits or contacted by telephone at 30 days, then every 6 months for a maximum of 3.5 years, until study closure or death. During follow-up visits, subjects were asked about the occurrence of clinically manifest cardiovascular events, which were adjudicated by an independent, blinded committee based on prespecified study definitions.
Study Groups
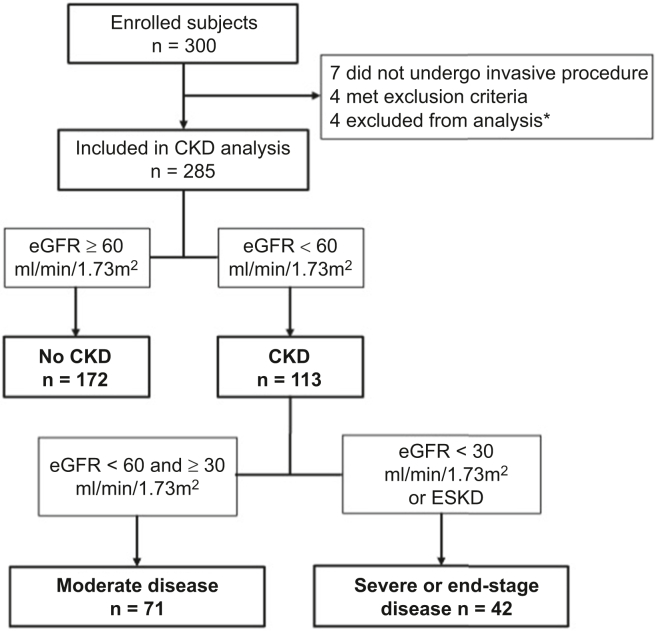
Of the 300 enrolled subjects, 7 did not undergo a procedure, 4 met an exclusion criterion and were not followed up with, and 4 were excluded from this analysis (3 due to history of prior kidney transplant and 1 for lack of a recorded baseline serum creatinine value). To define primary groups based on the presence of CKD, we calculated the eGFR using the CKD-EPI equation.26 We defined CKD by eGFR <60 ml/min per 1.73 m2 and no CKD by eGFR ≥60 ml/min per 1.73 m2. For secondary analyses, we stratified the CKD group into 2 subgroups based on disease severity. A moderate disease group included subjects with stage 3 CKD (eGFR <60 and ≥30 ml/min per 1.73 m2), and a severe or end-stage disease group included those with stage 4 or 5 CKD (eGFR <30 ml/min per 1.73 m2) or who indicated the presence of ESKD on the enrollment questionnaire.8,27 The creation of study groups from enrolled subjects is summarized in Figure 1.
Figure 1.
Study group flowchart. ∗3 were excluded for history of prior kidney transplant and 1 was excluded for lack of a recorded baseline serum creatinine. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease.
LTA
LTA is considered the gold standard test of platelet function in CVD.28 Platelet aggregation in response to submaximal agonist stimulation on LTA has been used to identify individuals with platelet hyperreactivity.29,30 In our study, LTA was performed within 2 hours of phlebotomy on an AggRAM light transmission aggregometer (Helena Laboratories, Beaumont, TX) according to the manufacturer’s specifications. Sodium citrate blood tubes were spun at 200g for 10 minutes to generate platelet-rich plasma for collection. These same tubes were then spun again at 2500g for 10 minutes to generate platelet-poor plasma. Platelet aggregation was measured in response to submaximal concentrations of ADP (0.4, 1, 2 μM), collagen (0.2, 1 μg/ml), serotonin (10 μM), epinephrine (0.1, 0.4, 2 μM), AA; 1600 μM, and AA 1600 μM with ex vivo aspirin (AA 1600 μM + ex vivo aspirin [ASAev]). All reported values represent platelet aggregation percentage measured 3 minutes after agonist exposure.
Clinical Endpoints
The primary clinical endpoint was MI. Other endpoints included major unplanned amputation (any above-ankle amputation, referred to as “amputation” hereafter), death, a composite of MACE, and a composite of major adverse cardiovascular and limb events. MACE was defined as MI, stroke, or death. Major adverse cardiovascular and limb events was defined as MACE, amputation, or major reintervention (new bypass graft, thrombectomy or thrombolysis, or jump/interposition graft of the index limb only).
Mediation Analysis
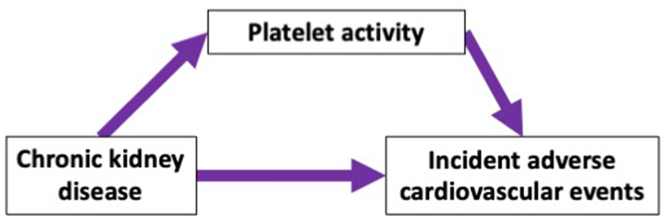
We followed the Baron and Kenny method for testing mediation as per the conceptual model depicted in Figure 2.31 After demonstrating independent associations between CKD status and both platelet activity and clinical cardiovascular risk, we used the following formula to calculate clinical hazard mediation percentages attributable to platelet activity: , in which HRu is the unadjusted clinical hazard ratio and HRa is the hazard ratio adjusted for the variable being evaluated for mediation.32,33 The 95% confidence intervals of the mediation estimates were obtained by performing bootstrap resampling 1000 times. For these analyses, we included only subjects with complete datasets for all clinical outcomes and platelet aggregation assays.
Figure 2.
Proposed mediation model. Conceptual model of platelet-mediated cardiovascular risk in chronic kidney disease.
Statistics
Data are presented as mean ± SD or median [interquartile range] according to the distribution. Differences in group clinico-demographic characteristics were assessed using χ2 tests of independence or Fisher’s exact tests for categorical variables, and 2-tailed independent samples t-tests for continuous data. Platelet activity data were compared between groups using Mann–Whitney U tests. Linear regression was used to assess for associations between platelet activity and CKD group before and after adjustment for clinico-demographic variables. As LTA data are non-normally distributed, prior to performing regression we log-transformed the data to meet the normality assumption of linear regression. To assess for correlation between eGFR and platelet aggregation, we first assigned eGFR as 5 ml/min per 1.73 m2 for each subject with ESKD. Next, Pearson’s correlation coefficient was used to assess for correlation between the polynomial eGFR and platelet activity. Clinical endpoint incidence proportions were compared using χ2 test or Fisher’s exact tests. Cox proportional-hazards models were used to calculate clinical endpoint hazard ratios before and after adjusting for clinico-demographic variables. The proportional hazards assumption was tested using the Schoenfeld test. For all tests, P < 0.05 was the threshold for statistical significance. Analyses were performed using R Version 3.5.2 (R Core Team).
Results
Demographic and Clinical Characteristics of the Study Groups
Among the 285 subjects for whom eGFR was calculated, there were 113 (40%) with CKD. Complete clinical and demographic characteristics are listed in Table 1. Subjects with CKD, compared to those without CKD, were older, more likely to be female and Hispanic, and more likely to have diabetes, heart failure, and critical limb ischemia. Subjects with CKD were less often current smokers and on statin therapy (P < 0.05 for each). There were no significant differences between groups with respect to prevalent coronary artery disease or use of antiplatelet therapy. Among the 113 subjects with CKD, 71 (63%) had moderate disease and 42 (37%) had severe or end-stage disease, of whom 25 (60%) were receiving maintenance dialysis. Baseline characteristics stratified by CKD severity are presented in Supplementary Table S1.
Table 1.
Demographic and clinical characteristics stratified by the presence of CKD
| Characteristic | No CKD (n = 172) | CKD (n = 113) | P value |
|---|---|---|---|
| Demographic variables, n (%) | |||
| Age, yr, mean±SD | 68.2 ± 10.6 | 71.8 ± 10.6 | 0.005 |
| Female sex | 46 (26.7) | 49 (43.4) | 0.005 |
| Race | 0.27 | ||
| African-American | 36 (20.9) | 30 (26.5) | |
| Asian-American | 4 (2.3) | 1 (0.9) | |
| White | 112 (65.1) | 63 (55.8) | |
| Other | 20 (11.6) | 19 (16.8) | |
| Hispanic ethnicity | 25 (14.7) | 28 (25.0) | 0.04 |
| Clinical variables, n (%) | |||
| Hypertension | 148 (86.0) | 103 (91.2) | 0.27 |
| Diabetes | 72 (41.9) | 78 (69.0) | <0.001 |
| Current tobacco use | 41 (23.8) | 8 (7.1) | 0.001 |
| Body mass index, kg/m2, mean±SD | 26.5 ± 5.2 | 27.3 ± 5.7 | 0.17 |
| Family CVD history | 52 (30.8) | 28 (25.5) | 0.41 |
| Coronary artery disease | 92 (53.5) | 66 (58.4) | 0.49 |
| Prior MI | 42 (24.4) | 33 (29.2) | 0.45 |
| Prior stroke or TIA | 34 (19.8) | 18 (15.9) | 0.51 |
| Prior critical limb ischemia | 122 (70.9) | 101 (89.4) | <0.001 |
| Heart failure | 25 (14.5) | 30 (26.5) | 0.02 |
| Antiplatelet therapy | 157 (91.3) | 97 (85.8) | 0.21 |
| Aspirin | 142 (82.6) | 90 (79.6) | 0.64 |
| Clopidogrel | 76 (44.2) | 42 (37.2) | 0.29 |
| Statin therapy | 150 (87.2) | 82 (72.6) | 0.003 |
| ACE inhibitor/ARB therapy | 93 (54.1) | 61 (54.0) | 1 |
| Beta blocker therapy | 97 (56.4) | 74 (65.5) | 0.16 |
| Revascularization procedure type | 0.01 | ||
| Endovascular | 64 (37.2) | 53 (46.9) | |
| Hybrid | 20 (11.6) | 8 (7.1) | |
| Open | 81 (47.1) | 39 (34.5) | |
| No intervention | 7 (4.1) | 13 (11.5) |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; CVD, cardiovascular disease; MI, myocardial infarction; TIA, transient ischemic attack.
Platelet Activity is Increased in Individuals With CKD
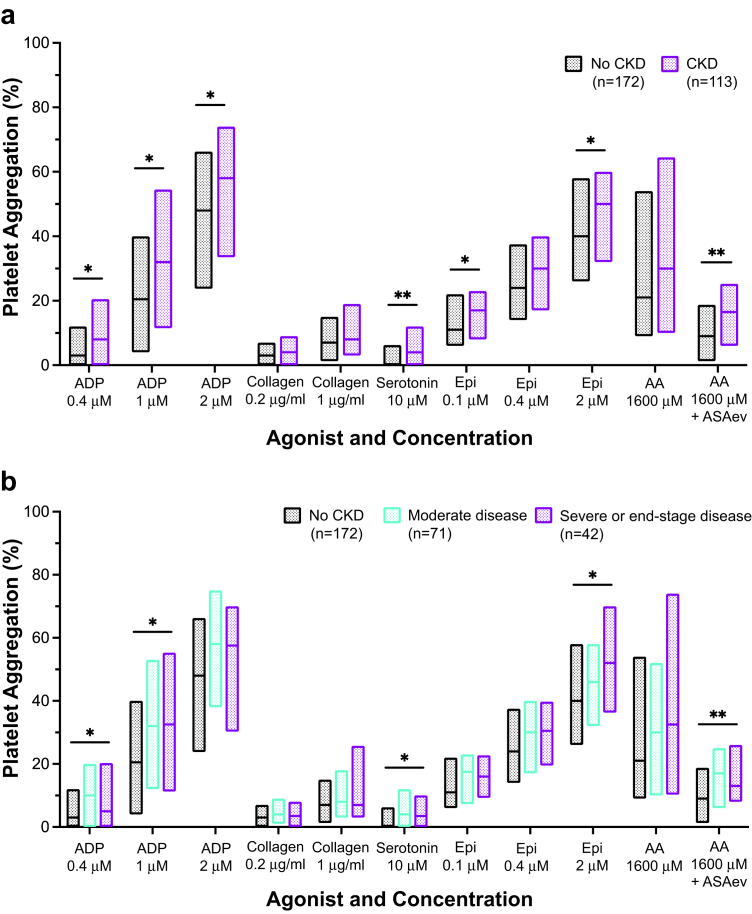
To determine the influence of CKD on platelet activity, we compared LTA results between groups. For each LTA assay, median platelet aggregation trended higher in subjects with CKD than subjects without CKD. Median aggregation was significantly higher in the CKD versus the non-CKD group for submaximal agonist stimulation with ADP (0.4 μM, 1 μM, 2 μM), serotonin (10 μM), and epinephrine (0.1 μM, 2 μM). After incubating platelet-rich plasma with aspirin ex vivo, subjects with CKD had higher platelet aggregation in response to AA 1600 μM + ASAev; Figure 3a. When stratified by CKD severity, platelet aggregation was significantly elevated in subjects with severe or ESKD in comparison to those without CKD in response to stimulation with ADP (0.4 μM, 1 μM), serotonin (10 μM), epinephrine (2 μM), and AA (1600 μM + ASAev) (Figure 3b).
Figure 3.
Platelet aggregation by (a) the presence of CKD and (b) the presence and severity of CKD. Platelet aggregation percentage (y-axis) on aggregometry in response to agonist-dose combinations (x-axis) by study group. Floating bars show median and interquartile range. ∗indicates P < 0.05; ∗∗indicates P < 0.01. ADP, adenosine diphosphate; Epi, epinephrine; AA, arachidonic acid; ASAev, ex vivo aspirin; CKD, chronic kidney disease.
We performed linear regression to investigate the association between CKD and platelet aggregation after adjustment for key variables. In unadjusted models, prevalent CKD was significantly associated with increased platelet aggregation on the following assays: ADP 0.4 μM, ADP 1 μM, ADP 2 μM, serotonin 10 μM, epinephrine 2 μM, and AA 1600 μM + ASAev. After adjustment for age, sex, race, and ethnicity, CKD status was independently associated with higher platelet aggregation in response to serotonin 10 μM, epinephrine 2 μM, and AA 1600 μM + ASAev. There was a borderline association between CKD and platelet aggregation to ADP 0.4 μM (P = 0.05) (Supplementary Table S2).
To better characterize the relationship between declining kidney function and increased platelet aggregation on key LTA assays, we assessed whether eGFR and platelet aggregation were correlated on the 4 assays for which we found platelet aggregation to be robustly associated with prevalent CKD (serotonin 10 μM, epinephrine 2 μM, AA 1600 μM + ASAev, and ADP 0.4 μM). This analysis revealed significant negative correlations between eGFR and platelet aggregation on all 4 assays (Supplementary Table S3).
CKD is Associated With More Incident Cardiovascular Events
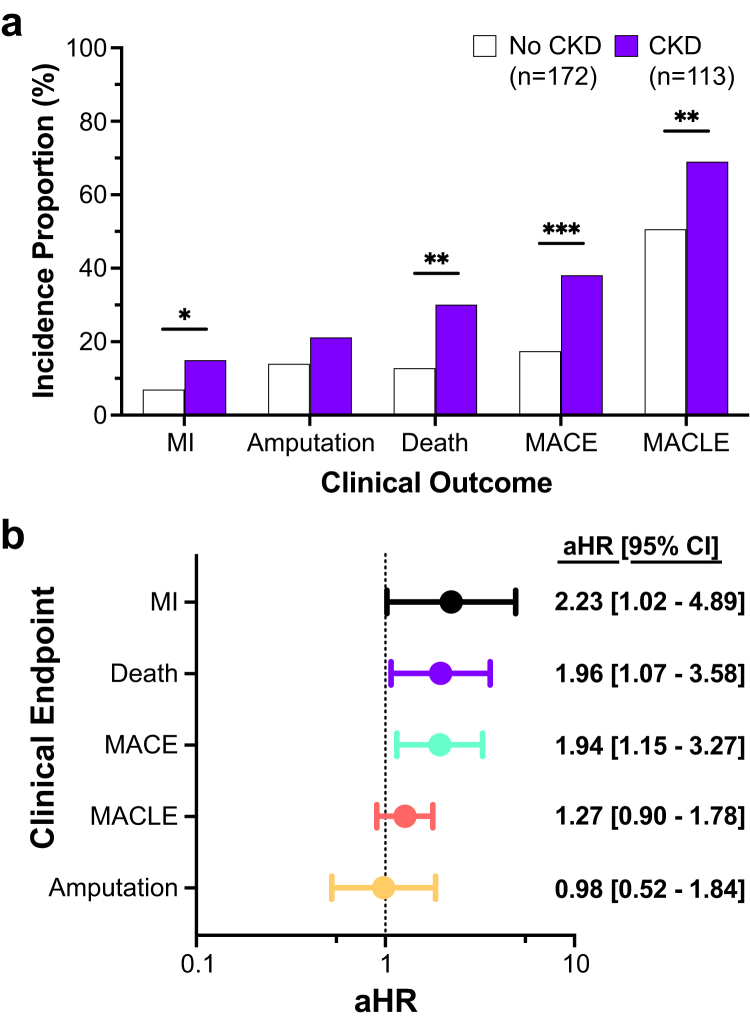
To evaluate the influence of CKD on cardiovascular risk, we compared the incidence of cardiovascular events in subjects with and without CKD over a median follow-up of 18 months. Subjects with CKD had a higher incidence of MI, death, MACE, and major adverse cardiovascular and limb events than subjects without CKD (Figure 4a). After adjustment for age, sex, race, and ethnicity, there was a significant association between CKD status and hazard for cardiovascular events (Supplementary Table S4). Following further adjustment for current tobacco use, prevalent diabetes and coronary artery disease, prior MI and critical limb ischemia, mode of revascularization, and use of antiplatelet and statin therapy, CKD status remained positively associated with risk of MI, death, and MACE (Figure 4b). In secondary analyses, clinical outcomes increased in a stepwise fashion with increasing CKD disease severity (Supplementary Figure S1). After adjustment for age, sex, race, and ethnicity, subjects with severe or ESKD were at heightened risk of MI, death, amputation, MACE, and major adverse cardiovascular and limb events compared to those without CKD (Supplementary Figure S2).
Figure 4.
Cardiovascular outcomes during follow-up. (a) Incidence rates during follow-up (y-axis) of outcomes (x-axis) by the presence of CKD. ∗indicates P < 0.05; ∗∗ indicates P < 0.01; ∗∗∗indicates P < 0.001. (b) Adjusted hazard ratios (circles) with 95% confidence intervals (bars) for outcomes (y-axis) in the CKD group versus the no CKD group. Models include adjustment for age, sex, race, ethnicity, current tobacco use, prevalent diabetes and coronary artery disease, prior MI and CLI, mode of revascularization, and antiplatelet and statin therapy use. CI, confidence interval; MI, ; MACE, major adverse cardiovascular events; MACLE, major adverse cardiovascular and limb event.
Platelet Activity Mediates Cardiovascular Risk in CKD
We next evaluated whether the observed heightened platelet activity among subjects with CKD mediated any of their increased cardiovascular risk. Focusing on the 4 LTA assays for which we had previously established robust associations between CKD status and platelet aggregation, we calculated the percentage of excess cardiovascular risk that platelet aggregation on each assay, and a composite of all 4, mediated. Platelet aggregation to ADP, serotonin, epinephrine, and AA mediated 7% to 18%, 8% to 26%, 7% to 18%, and 9% to 16% of the excess risk associated with having CKD for cardiovascular events, respectively. Platelet aggregation to all 4 assays mediated up to 26% of the risk associated with having CKD for cardiovascular events (Table 2).
Table 2.
Clinical hazard mediation by platelet activity in subjects with CKD
| Platelet variable adjustment | Outcome |
||||
|---|---|---|---|---|---|
| MI | Death | MACE | MACLE | ||
| Unadjusted model | HR [95% CI] | 2.38 [1.09, 5.20] | 2.31 [1.29, 4.12] | 2.32 [1.40, 3.84] | 1.56 [1.11, 2.19] |
| ADP 0.4 μM |
aHR [95% CI] | 2.15 [0.98, 4.73] | 2.18 [1.22, 3.91] | 2.22 [1.34, 3.69] | 1.46 [1.03, 2.06] |
| % mediation [95% CI] | 17% [−25 −85%] | 10% [−15 −61%] | 7% [−11 −29%] | 18% [−32 −113%] | |
| Serotonin 10 μM |
aHR [95% CI] | 2.17 [0.99, 4.76] | 2.21 [1.23, 3.96] | 2.22 [1.34, 3.68] | 1.42 [1.00, 2.00] |
| % mediation [95% CI] | 16% [−33 −101%] | 8% [−16 −41%] | 8% [−6 −33%] | 26% [−20 −150%] | |
| Epinephrine 2 μM |
aHR [95% CI] | 2.28 [1.04, 4.98] | 2.17 [1.21, 3.88] | 2.23 [1.35, 3.69] | 1.45 [1.03, 2.05] |
| % mediation [95% CI] | 8% [−24 −36%] | 11% [−12 −42%] | 7% [−7 −25%] | 18% [−22 −102%] | |
| AA 1600 μM + ASAev |
aHR [95% CI] | 2.24 [1.02, 4.93] | 2.19 [1.22, 3.94] | 2.20 [1.32, 3.67] | 1.47 [1.04, 2.08] |
| % mediation [95% CI] | 11% [−50 −47%] | 9% [−22 −41%] | 9% [−11 −29%] | 16% [−24 −79%] | |
| All 4 variables | aHR [95% CI] | 2.19 [0.99, 4.86] | 2.16 [1.20, 3.89] | 2.21 [1.33, 3.68] | 1.41 [1.00, 2.00] |
| % mediation [95% CI] | 14% [−81 −104%] | 11% [−36 −56%] | 9% [−22 −40%] | 26% [−88 −152%] | |
AA, arachidonic acid; ADP, adenosine diphosphate; aHR, adjusted hazard ratio; ASAev, ex vivo aspirin; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; MACE, major adverse cardiovascular event; MACLE, major adverse cardiovascular and limb event.
Discussion
The Platelet Activity and Cardiovascular Events study was designed to investigate the role of platelet activity in PAD and its association with incident cardiovascular events. Because CKD is frequently coprevalent with PAD, we compared platelet aggregation and cardiovascular events by CKD status in this well-phenotyped population. We identified significantly increased platelet aggregation in subjects with CKD compared to those without CKD, despite a similar prevalence of antiplatelet therapy between the groups. There were strong positive associations between CKD status and cardiovascular outcomes, including MI, death, and MACE, that persisted after adjustment for key clinico-demographic variables and appeared to be mediated, in part, by increased platelet aggregation.
The presence of platelet dysfunction in CKD is well documented, but data describing the nature of this dysfunction are conflicting. A number of previous studies demonstrated heightened platelet aggregation in response to ADP stimulation in subjects with CKD who are not on antiplatelet therapy.20, 21, 22 In contrast, other prior works summarized in a recent meta-analysis demonstrated reduced platelet aggregation to ADP in CKD, albeit with high enough study heterogeneity that the authors suggested definitive conclusions were premature.23 We identified associations between CKD status and platelet aggregation in response to ADP that were borderline significant after adjustment. That these associations were less robust to adjustment than those for other agonists appears consistent with the meta-analysis. In addition, the meta-analysis concluded that platelet aggregation to collagen stimulation is significantly reduced in subjects with CKD. Similarly, we did not identify differences in platelet response to collagen between individuals with and without CKD. Of note, the meta-analysis only included studies comparing platelets from subjects with CKD to those from healthy volunteers and excluded subjects on hemodialysis or antiplatelet therapy. We assessed a different and more at-risk cohort by including individuals with ESKD on maintenance dialysis as well as those with established CVD on antiplatelet agents. We did not observe a dose-response relationship between worsening kidney function and platelet activity in secondary analyses, however it may not be possible to define the true nature of this relationship with our data given the small number of subjects with ESKD in our cohort.
Prior data describe CKD-related platelet dysfunction in subjects with coronary artery disease, often during the peri-coronary intervention period. These works specifically focus on platelet activity in response to ADP and AA agonism during thienopyridine and aspirin therapy. As a whole, they strongly suggest increased on-antiplatelet therapy platelet reactivity in subjects with CKD.16, 17, 18, 19 Consistently, in our cohort with high antiplatelet therapy use, we observed elevated platelet response to both ADP and AA with ex vivo aspirin in individuals with CKD, suggesting incomplete P2Y12 and COX-1 inhibition in the group. High on-treatment platelet reactivity in CKD has been further associated with elevated risk for ischemic thrombosis.34,35 Our results extend upon those findings and suggest that heightened platelet aggregation in response to submaximal ADP partially mediates the well-described excess risk for cardiovascular events in individuals with CKD.
There are several possible explanations for the inconsistent descriptions of platelet activity in CKD in the literature. Prior studies vary considerably by design, including, importantly, the chosen platelet function assay. Point-of-care methods have been widely used but are not the tests of choice to assess global platelet activity. A study compared point-of-care methods to LTA, and found that point-of-care measurements consistently overestimated platelet reactivity.36 Another study compared 9 different tests for assessing platelet responsiveness to 3 different doses of aspirin in patient with diabetes, and found the tests to be differentially effective in measuring aspirin’s antiplatelet effect and poorly correlated with one another.37 Our study, which uses LTA with a broad panel of agonists, provides a more complete assessment of platelet activity than do prior studies. Separately, CKD patients present a heterogenous population; intracohort differences related to disease etiology and extent likely account for some of the noted inconsistencies. Other patient-related factors, such as age, sex, smoking, and diabetes, are known to influence platelet activity. Thus, CVD is likely to manifest heterogeneously in individuals with CKD, depending on the balance of different pathologic forces (e.g., proatherosclerotic, proarteriosclerotic). Our cohort, with established PAD, likely best represents the CKD subgroup with predominantly atherosclerotic CVD and an associated hyperreactive platelet phenotype.
The mechanisms underlying platelet dysfunction in CKD are poorly understood. Abnormal interaction between platelets and platelet-endothelium in the setting of kidney impairment may play a role.38 In CKD, endothelial cell activation and elevated levels of von Willebrand Factor, a key platelet adhesion protein, have been observed, though it is unclear whether this contributes to increased thrombotic risk.39,40 Imbalance among platelet modulators such as ADP, serotonin, and cAMP, reduction in release of thromboxane A2, and increased intracellular calcium have been shown to cause activation defects in CKD, though inconsistently.38,40 Accumulation of uremic toxins is hypothesized to play a role in modulating platelet activity in CKD, though how changing concentrations of stimulatory and inhibitory toxins influences risk for bleeding and thrombosis remains unclear.39, 40, 41 Finally, decreased bioavailability of nitric oxide, a potent vasodilator and inhibitor of platelet adhesion and aggregation, accompanied by accumulation of inflammatory mediators such as asymmetric dimethylarginine likely contributes to proatherosclerotic platelet dysfunction in patients with CKD.42, 43, 44
Antiplatelet therapies are the current standard of care for prevention and treatment of atherosclerosis and its complications in the general population. Nevertheless, evidence regarding the optimal regimen of antiplatelet therapy in CKD is lacking. Current evidence is largely extrapolated from post hoc analyses of trials, with some studies suggesting that benefits of antiplatelet therapy are outweighed by bleeding hazards in certain settings.45 A meta-analysis analyzing the use of aspirin in the nondialysis dependent CKD population reported no clear benefit for primary prevention of cardiovascular events or mortality.46 Thus, treatment of platelet hyperreactivity in CKD presents a therapeutic challenge; balancing antithrombotic benefits of dual antiplatelet therapy with the prospect of paradoxically increasing bleeding risk remains difficult. Our findings that platelet aggregation to several, but not all, agonists is increased in CKD and that increased aggregation partially mediates adverse cardiovascular outcomes emphasize the importance of adequately inhibiting platelets in patients with comorbid CKD and vascular disease. Conversely, the varying effects of CKD status on platelet responsiveness to different agonists suggest that individualizing treatment based upon platelet function assays has potential to improve outcomes in individuals with CKD.
Several limitations of this study should be considered. We analyzed a group of subjects with CKD and PAD; generalization of our results to individuals with CKD and nonatherosclerotic CVD (e.g., cardiomyopathy, arteriosclerosis) requires confirmation. Further, our cohort featured heterogeneous manifestations of CKD, so our findings address CKD broadly rather than specific CKD subpopulations. Our modest sample size, especially in the ESKD subgroup, limited statistical power. Thus, negative findings should be cautiously interpreted. Wide confidence intervals surrounding the mediation estimates limit our conclusions and require larger studies for validation. Finally, associations between biomarkers in CKD such as proteinuria, and platelet aggregation and cardiovascular events could not be explored.
In summary, we present evidence that platelet activity is increased in CKD, although differentially across agonists of platelet aggregation, and that increased platelet activity contributes to elevated risk for cardiovascular complications in individuals with CKD, including those with ESKD. We analyzed a unique cohort, including subjects with PAD across the CKD spectrum, assessed platelets using the gold standard test of platelet function across a broad range of agonists, and conducted clinical follow-up over a long period. Our results highlight a need for well-designed, larger clinical trials to assess the role of platelet aggregation testing using novel techniques and optimized antiplatelet therapeutic regimens to mitigate cardiovascular morbidity and mortality in individuals with CKD.
Disclosure
DMC reports consulting for Eli Lilly/Boehringer Ingelheim, Janssen (steering committee), PLC medical (clinical events committee), Astra Zeneca, Allena Pharmaceuticals (DSMB), Fresenius, Gilead, Novo Nordisk, GSK, Medtronic, Merck, and Amgen; research funding from Medtronic (clinical trial support), Bioporto (clinical trial support), Gilead, Fiftheye, NovoNordisk, and Amgen; and expert witness fees related to proton-pump inhibitors. JSB reports consulting for Janssen and Amgen; and research funding from NHLBI, AHA, and Astra Zeneca. All other authors declared no competing interests.
Acknowledgments
Funding
This work was supported by funding provided by the National Heart, Lung, and Blood Institute of the National Institute of Health (R01HL114978 and R35HL144993 to JSB).
Footnotes
Figure S1. Cardiovascular outcome incidence proportions by the presence and severity of CKD.
Figure S2. Adjusted hazard for cardiovascular events in subjects with severe or end-stage kidney disease.
Table S1. Demographic and clinical characteristics stratified by the presence and severity of CKD.
Table S2. Linear regression of platelet activity on CKD status.
Table S3. Correlation between platelet aggregation and kidney function.
Table S4. Crude and adjusted hazard for cardiovascular events in subjects with CKD. STROBE Checklist.
Supplementary Material
Figure S1. Cardiovascular outcome incidence proportions by the presence and severity of CKD.
Figure S2. Adjusted hazard for cardiovascular events in subjects with severe or end-stage kidney disease.
Table S1. Demographic and clinical characteristics stratified by the presence and severity of CKD.
Table S2. Linear regression of platelet activity on CKD status.
Table S3. Correlation between platelet aggregation and kidney function.
Table S4. Crude and adjusted hazard for cardiovascular events in subjects with CKD.
STROBE Checklist.
References
- 1.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Keith D.S., Nichols G.A., Gullion C.M., Brown J.B., Smith D.H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 3.Foley R.N., Murray A.M., Li S., et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. Clin J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 4.O’Hare A.M., Glidden D.V., Fox C.S., Hsu C.Y. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination survey 1999–2000. Circulation. 2004;109:320–323. doi: 10.1161/01.CIR.0000114519.75433. DD. [DOI] [PubMed] [Google Scholar]
- 5.Criqui M.H., Langer R.D., Fronek A., et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 6.O’Hare A.M., Feinglass J., Sidawy A.N., et al. Impact of renal insufficiency on short-term morbidity and mortality after lower extremity revascularization: data from the Department of Veterans Affairs’ National Surgical Quality Improvement Program. J Am Soc Nephrol. 2003;14:1287–1295. doi: 10.1097/01.ASN.0000061776.60146.02. [DOI] [PubMed] [Google Scholar]
- 7.Liew Y.P., Bartholomew J.R., Demirjian S., Michaels J., Schreiber M.J., Jr. Combined effect of chronic kidney disease and peripheral arterial disease on all-cause mortality in a high-risk population. Clin J Am Soc Nephrol. 2008;3:1084–1089. doi: 10.2215/CJN.04411007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hare A.M., Bertenthal D., Shlipak M.G., Sen S., Chren M.M. Impact of renal insufficiency on mortality in advanced lower extremity peripheral arterial disease. J Am Soc Nephrol. 2005;16:514–519. doi: 10.1681/ASN.2004050409. [DOI] [PubMed] [Google Scholar]
- 9.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 10.Koupenova M., Clancy L., Corkrey H.A., Freedman J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. 2018;122:337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davì G., Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 12.Ruggeri Z.M. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 13.Landray M.J., Wheeler D.C., Lip G.Y., et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43:244–253. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Thijs A., Nanayakkara P.W., Ter Wee P.M., Huijgens P.C., van Guldener C., Stehouwer C.D. Mild-to-moderate renal impairment is associated with platelet activation: a cross-sectional study. Clin Nephrol. 2008;70:325–331. [PubMed] [Google Scholar]
- 15.Song T.J., Kwon I., Piao H., et al. Increased thrombogenicity in chronic renal failure in a rat model induced by 5/6 ablation/infarction. Yonsei Med J. 2018;59:754–759. doi: 10.3349/ymj.2018.59.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gremmel T., Müller M., Steiner S., et al. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant. 2013;28:2116–2122. doi: 10.1093/ndt/gft103. [DOI] [PubMed] [Google Scholar]
- 17.Park K.W., Park J.J., Jeon K.H., et al. Clinical predictors of high posttreatment platelet reactivity to clopidogrel in Koreans. Cardiovasc Ther. 2012;30:5–11. doi: 10.1111/j.1755-5922.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- 18.Angiolillo D.J., Bernardo E., Capodanno D., et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Rubin G.A., Kirtane A.J., Chen S., et al. Impact of high on-treatment platelet reactivity on outcomes following PCI in patients on hemodialysis: an ADAPT-DES substudy. Catheter Cardiovasc Interv. 2020;96:793–801. doi: 10.1002/ccd.28577. [DOI] [PubMed] [Google Scholar]
- 20.Jain N., Li X., Adams-Huet B., et al. Differences in whole blood platelet aggregation at baseline and in response to aspirin and aspirin plus clopidogrel in patients with versus without chronic kidney disease. Am J Cardiol. 2016;117:656–663. doi: 10.1016/j.amjcard.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain N., Wan F., Kothari M., et al. Association of platelet function with depression and its treatment with sertraline in patients with chronic kidney disease: analysis of a randomized trial. BMC Nephrol. 2019;20:395. doi: 10.1186/s12882-019-1576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang M.J., Wei R.B., Wang Y., et al. Blood coagulation system in patients with chronic kidney disease: a prospective observational study. BMJ Open. 2017;7:e014294. doi: 10.1136/bmjopen-2016-014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baaten C.C., Sternkopf M., Henning T., et al. Platelet function in CKD: a systematic review and meta-analysis. J Am Soc Nephrol. 2021;32:1583–1598. doi: 10.1681/ASN.2020101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dann R., Hadi T., Montenont E., et al. Platelet-derived MRP-14 induces monocyte activation in patients with symptomatic peripheral artery disease. J Am Coll Cardiol. 2018;71:53–65. doi: 10.1016/j.jacc.2017.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman J.D., Cornwell M.G., Zhou H., et al. Gene expression signature in patients with symptomatic peripheral artery disease. Arterioscler Thromb Vasc Biol. 2021;41:1521–1533. doi: 10.1161/ATVBAHA.120.315857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eknoyan G., Lameire N., Eckardt K., et al. Notice. Kidney Int. 2013;3:5–14. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 28.Michelson A.D. Platelet function testing in cardiovascular diseases. Circulation. 2004;110:489–493. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 29.Berger J.S., Becker R.C., Kuhn C., Helms M.J., Ortel T.L., Williams R. Hyperreactive platelet phenotypes: relationship to altered serotonin transporter number, transport kinetics and intrinsic response to adrenergic co-stimulation. Thromb Haemost. 2013;109:85–92. doi: 10.1160/TH12-03-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montenont E., Echagarruga C., Allen N., Araldi E., Suarez Y., Berger J.S. Platelet WDR1 suppresses platelet activity and is associated with cardiovascular disease. Blood. 2016;128:2033–2042. doi: 10.1182/blood-2016-03-703157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron R.M., Kenny D.A. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 32.Hafeman D.M. Proportion explained”: a causal interpretation for standard measures of indirect effect? Am J Epidemiol. 2009;170:1443–1448. doi: 10.1093/aje/kwp283. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Woodward M., Perkovic V., et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail. 2020;8:57–66. doi: 10.1016/j.jchf.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Morel O., El Ghannudi S., Jesel L., et al. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol. 2011;57:399–408. doi: 10.1016/j.jacc.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Htun P., Fateh-Moghadam S., Bischofs C., et al. Low responsiveness to clopidogrel increases risk among CKD patients undergoing coronary intervention. J Am Soc Nephrol. 2011;22:627–633. doi: 10.1681/ASN.2010020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roule V., Ardouin P., Repessé Y., et al. Point of care tests VerifyNow P2Y12 and INNOVANCE PFA P2Y compared to light transmittance aggregometry after fibrinolysis. Clin Appl Thromb Hemost. 2018;24:1109–1116. doi: 10.1177/1076029618772354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison P., Bethel M.A., Kennedy I., Dinsdale R., Coleman R., Holman R.R. Comparison of nine platelet function tests used to determine responses to different aspirin dosages in people with type 2 diabetes. Platelets. 2019;30:521–529. doi: 10.1080/09537104.2018.1478402. [DOI] [PubMed] [Google Scholar]
- 38.Boccardo P., Remuzzi G., Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579–589. doi: 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 39.Addi T., Dou L., Burtey S. Tryptophan-derived uremic toxins and thrombosis in chronic kidney disease. Toxins (Basel) 2018;10:412. doi: 10.3390/toxins10100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabelink T.J., Zwaginga J.J., Koomans H.A., Sixma J.J. Thrombosis and hemostasis in renal disease. Kidney Int. 1994;46:287–296. doi: 10.1038/ki.1994.274. [DOI] [PubMed] [Google Scholar]
- 41.Linthorst G.E., Avis H.J., Levi M. Uremic thrombocytopathy is not about urea. J Am Soc Nephrol. 2010;21:753–755. doi: 10.1681/ASN.2009111181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galbusera M., Remuzzi G., Boccardo P. Treatment of bleeding in dialysis patients. Semin Dial. 2009;22:279–286. doi: 10.1111/j.1525-139X.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 43.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Ren Physiol. 2008;294:F1–F9. doi: 10.1152/ajprenal.00424.2007. [DOI] [PubMed] [Google Scholar]
- 44.Chen J.Y., Ye Z.X., Wang X.F., et al. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed Pharmacother. 2018;97:423–428. doi: 10.1016/j.biopha.2017.10.122. [DOI] [PubMed] [Google Scholar]
- 45.Palmer S.C., Di Micco L., Razavian M., et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;156:445–459. doi: 10.7326/0003-4819-156-6-201203200-00007. [DOI] [PubMed] [Google Scholar]
- 46.Major R.W., Oozeerally I., Dawson S., Riddleston H., Gray L.J., Brunskill N.J. Aspirin and cardiovascular primary prevention in non-end stage chronic kidney disease: a meta-analysis. Atherosclerosis. 2016;251:177–182. doi: 10.1016/j.atherosclerosis.2016.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.