Abstract
Background
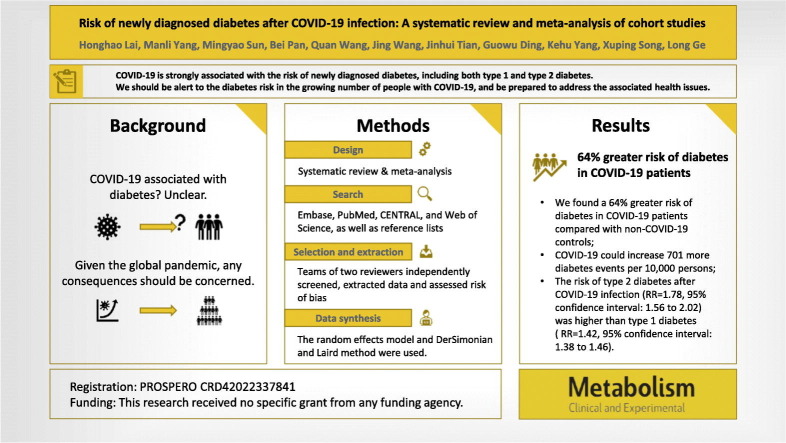
COVID-19 might be a risk factor for various chronic diseases. However, the association between COVID-19 and the risk of incident diabetes remains unclear. We aimed to meta-analyze evidence on the relative risk of incident diabetes in patients with COVID-19.
Methods
In this systematic review and meta-analysis, the Embase, PubMed, CENTRAL, and Web of Science databases were searched from December 2019 to June 8, 2022. We included cohort studies that provided data on the number, proportion, or relative risk of diabetes after confirming the COVID-19 diagnosis. Two reviewers independently screened studies for eligibility, extracted data, and assessed risk of bias. We used a random-effects meta-analysis to pool the relative risk with corresponding 95 % confidence intervals. Prespecified subgroup and meta-regression analyses were conducted to explore the potential influencing factors. We converted the relative risk to the absolute risk difference to present the evidence. This study was registered in advance (PROSPERO CRD42022337841).
Main findings
Ten articles involving 11 retrospective cohorts with a total of 47.1 million participants proved eligible. We found a 64 % greater risk (RR = 1.64, 95%CI: 1.51 to 1.79) of diabetes in patients with COVID-19 compared with non-COVID-19 controls, which could increase the number of diabetes events by 701 (558 more to 865 more) per 10,000 persons. We detected significant subgroup effects for type of diabetes and sex. Type 2 diabetes has a higher relative risk than type 1. Moreover, men may be at a higher risk of overall diabetes than women. Sensitivity analysis confirmed the robustness of the results. No evidence was found for publication bias.
Conclusions
COVID-19 is strongly associated with the risk of incident diabetes, including both type 1 and type 2 diabetes. We should be aware of the risk of developing diabetes after COVID-19 and prepare for the associated health problems, given the large and growing number of people infected with COVID-19. However, the body of evidence still needs to be strengthened.
Keywords: COVID-19, Long COVID, Diabetes, Public health
Graphical abstract
1. Introduction
Coronavirus disease 2019 (COVID-19) is one of the most significant pandemics in human history caused by the virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 has infected >530 million people, of which >6.3 million have died since June 23, 2022 [1]. Recent research has indicated that COVID-19 might be a risk factor for various chronic diseases [2], which means new health problems will be added to the already tough ones. Patients and society must bear more severe public health problems if we do not pay attention.
Diabetes is one of the most widespread and burdensome chronic diseases, and causes a major public health concern worldwide [3], [4], [5]. Unfortunately, evidence suggests a bidirectional relationship between diabetes and COVID-19 [6]. Both pre-existing diabetes, and new-onset diabetes after infection are important factors in increasing the risk of serious adverse outcomes (e.g. acute respiratory distress syndrome, intensive care unit admission, mechanical ventilation use, death) in patients with COVID-19 [6], [7], [8], [9], [10], [11], [12]. Meanwhile, an increased incidence of diabetes has been reported in patients following a COVID-19 diagnosis [13], [14]. Both type 1 diabetes (T1D) and type 2 diabetes (T2D) may be induced by COVID-19, although the underlying mechanisms have not been fully explored [15], [16]. This highlights the extraordinary importance of raising awareness of the risk of diabetes in patients with COVID-19 and identifying potential factors that influence the risk [14].
Therefore, this systematic review and meta-analysis aimed to comprehensively assess the relative risk of diabetes in patients with COVID-19 compared with uninfected populations and to explore the potential influencing factors.
2. Material and methods
2.1. Registration
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17] and Meta-analysis of Observational Studies in Epidemiology (MOOSE) [18] to perform and report the present results. The protocol was registered in the International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022337841).
2.2. Search strategy
A systematic search was carried out using a highly sensitive search strategy that included both MeSH terms and free text words related to “COVID-19,” “diabetes,” and “cohort.” We searched Embase, PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science databases from their inception to June 8, 2022, and the full search strategy is provided in Appendix Table S1. In addition, the references of the relevant articles were checked to identify additional eligible studies. No restrictions were imposed on language and publication states.
2.3. Study selection
Eligible studies were cohort studies that assessed the association between COVID-19 infection and incident diabetes. We excluded studies that did not explicitly report a diagnosis of diabetes or COVID-19; for example, we did not include studies that focused only on hyperglycemia or the pandemic. Conference proceedings, letters and editorials were excluded. When studies were from the same cohort without new information of interest, we included only those with a larger sample size and longer follow-up duration. Appendix Table S2 presents the detailed eligibility criteria.
After pilot testing, teams of two reviewers (HHL and MYS, BP, and JW) independently performed the literature selection, including screening titles and abstracts, using the online literature management software Rayyan [19], and further evaluating the full text for possible eligibility. The reviewers resolved disagreements through discussion or through arbitration by a senior reviewer (LG).
2.4. Data extraction
After two rounds of pilot testing, a standardized data collection form was used to extract data of interest by two reviewers (HHL and MYS) in duplicate. We used the first author's last name and the year of publication to identify each study and cohort. We collected information on study characteristics (type of design, name of cohort), participants (source, age, sex, ethnic origin, country), study design (length of follow-up, method of diagnosing COVID-19, ascertainment of diabetes, variables that were entered into the multivariable model as potential confounders), and the rate or the risk of diabetes (estimates with their 95 % confidence intervals). The pooled effect of diabetes was estimated using the risk for all types of diabetes reported in the primary studies. We used 90 % as a threshold to categorize outcomes as T2D or T1D. For example, if a cohort involved >90 % T2D, we categorized the cohort outcome as T2D. We also extracted data of interest from the subgroup analysis. We first calculated the overall effect using a fixed effects model for cohorts reporting stratified estimates. We preferentially used estimates from comparisons with contemporary general population controls. We contacted the cohort database used by the authors in an attempt to obtain any missing data to support further analysis, but ultimately did not obtain access. Any conflicts in the extraction were resolved by consensus with all authors.
2.5. Risk of bias of individual cohorts
Two reviewers (HHL and MYS) independently assessed the risk of bias (quality) of each cohort using the risk of bias assessment instruments modified by the CLARITY (Clinical Advances Through Research and Information Translation) Group at McMaster University, including 8 items: consistency of exposed and non-exposed cohorts, confidence in exposure assessment, exclusion of interested outcomes, prognostic variables adjusted, confidence in prognostic factors assessment, confidence in outcome assessment, adequacy of follow-up, and similarity of co-interventions [20]. Reviewers rated each item as “definitely/probably low risk of bias” and “probably/definitely high risk of bias” based on a detailed instruction presented in Appendix Table S3. A cohort with two or more “definitely no” and “probably no” would be suspected of having a serious risk of bias.
2.6. Data synthesis
In this meta-analysis, we used relative risks (RRs) and corresponding 95 % confidence intervals (95%CIs) from multivariable models with the most adequate adjustment for potential confounders to assess the risk of diabetes in populations with and without COVID-19. For cohorts that did not report risk estimates, we used the rate ratio or calculated the relative risk by using the matched number of events and the number of total. The random effects model and DerSimonian and Laird method were used to estimate the overall effect in Stata 15.1 [21]. Statistical heterogeneity among cohorts was evaluated by Cochran's Q-test and the proportion of the total variation caused by heterogeneity was quantified using I 2 [22]. In addition, we also visually evaluated the consistency of forest plots to avoid situations where the I 2 statistic might be inflated when the effect estimates from the main study were highly precise [23].
Subgroup analyses were conducted if two or more cohorts were included. We preferentially used subgroup data reported in the original studies, including age (<18 years vs. 18–65 years vs. >65 years, a predefined hypothesis of larger effect in the older group), gender (male vs. female, a predefined hypothesis of larger effect in the female population), the interval between diabetes incidence after COVID-19 diagnosis (>1 day vs. at least 30 days, a predefined hypothesis of larger effect in >1 day), type of diabetes (T1D vs. T2D vs. diabetes, a predefined hypothesis of larger effect in diabetes), type of control (contemporary control vs. historical control, general population vs. specific patients, a predefined hypothesis of larger effect in historical control and general population), location (U.S. vs. Europe, a predefined hypothesis of larger effect in the U.S.), and ethnicity (black vs. white, a predefined hypothesis of larger effect in black). Meta-regression models were fitted to investigate the effect of continuous variables, including mean age, length of follow-up, and the proportion of specific races and females. The P values of the interaction test (P interaction) were calculated using meta-regression to examine the subgroup differences. A significant subgroup effect was detected when P interaction ≤ 0.05. Sensitivity analyses were performed to evaluate each cohort's influence on the overall pooled estimate by sequentially omitting one cohort at a time. Furthermore, absolute risk differences were calculated using baseline risk computed from the median diabetes risk in the non-exposed group of the included cohorts [24]. We evaluated the publication bias through Begg's and Egger's tests as well as a visual inspection of funnel plots recommended by Cochrane Handbook for Systematic Reviews of Interventions [22].
3. Results
3.1. Study selection and characteristics
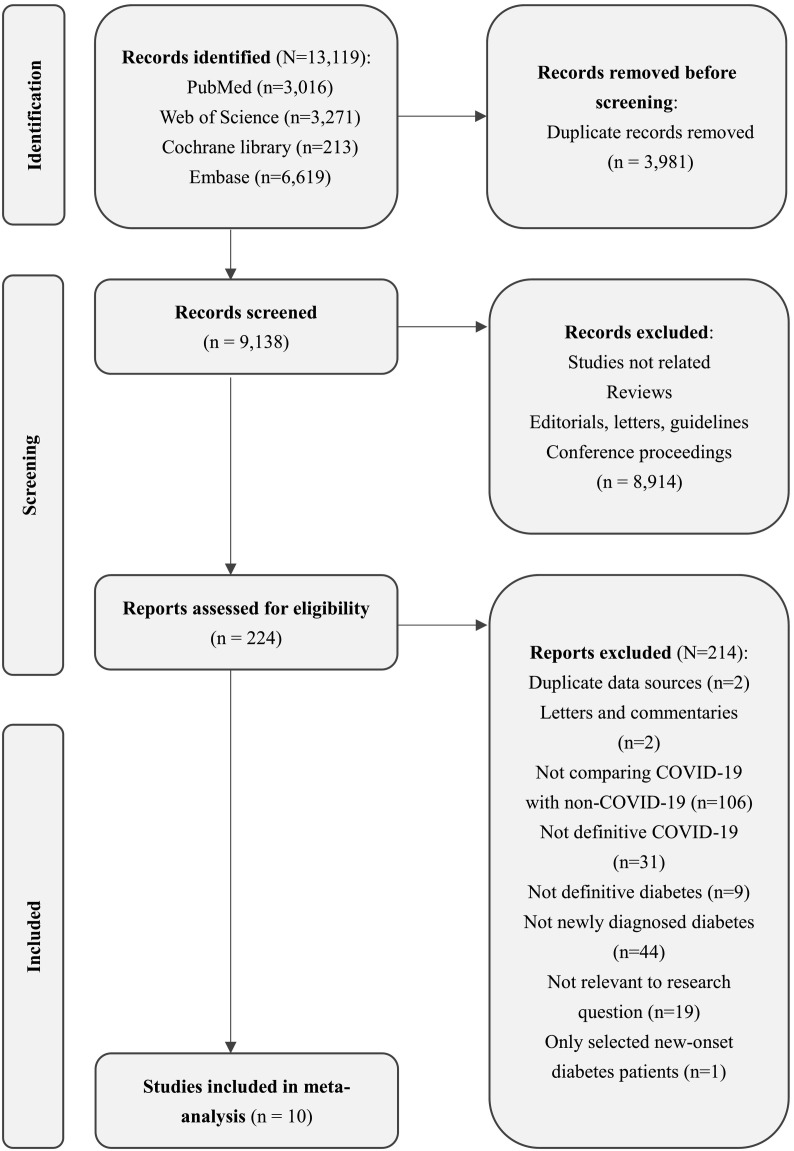
Our electronic search yielded 9138 unique records, of which 224 were potentially eligible (Fig. 1 ). After full text reviews, we excluded 214 (Appendix Table S4 for exclusion reasons): 19 were not relevant to the research question, 44 did not exclude pre-existing diabetes, 106 did not include non-COVID controls, 31 focused on inappropriate exposure, nine focused on inappropriate outcomes, one focused on inappropriate participants, two were letters and commentaries, and two were duplicated data sources. In addition, Wander et al. [25] and Al-Aly [26] assessed populations from the same database as that of Xie et al. [27]. We included Xie's cohort because it had a larger sample size, longer follow-up duration, and more adequate data. Additionally, 10 articles, including 11 cohorts, proved eligible [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], of which two articles were pre-printed [28], [32].
Fig. 1.
Literature screening flow diagram.
Table 1 summarizes the characteristics of all the included cohorts. Seven of the 11 retrospective cohorts included populations from the United States [27], [29], [30], [31], [33], [34], three from Europe (England, Scotland, and Germany) [32], [35], [36], and one from the global population [28]. Among all cohorts, >4.5 million participants with COVID-19 during the pandemic (after December 2019) and >42 million people without either COVID-19 or diabetes were included. Participants in the control group were the general population or patients with specific diseases (Appendix Table S5 shows the diagnostic criteria for COVID-19, diabetes, and specific diseases). The population covered all age groups with a mean age of 41 years and was followed up from 6 months to 22 months. Sex ratios were balanced across most cohorts. Eight of the 11 cohorts reported T2D, and three cohorts reported T1D. Three cohorts included diabetes diagnosed at least a month after a positive COVID-19 record. Two cohorts had a diagnosis interval of 3 weeks. The other five cohorts had only 1 day for the interval.
Table 1.
Characteristics of included cohorts.
| Cohort | Region | Participants source | COVID-19 criteriaa | Diabetes criteriaa | Typec | Control groupa | Participants, n (after matching) | Mean age, years | Female, % | Interval, daysd | Follow-up, monthse |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ayoubkhani, 2021 | U.K. | Hospital Episode Statistics Admitted Patient Care and GDPPR | Medical records, Laboratory test, ICD-10 | Medical records | Type 2 | General population | COVID: 47,780; control: 47,780b | COVID: 64.5; control: 64.5 | 45.1 | NR | 8.4 |
| Barrett, 2022a | U.S. | IQVIA | Medical records, ICD-10 | Medical records, ICD-10 | Type 2 | General population, and historical ARI patients, respectively | COVID: 80,893; control: 404,465 | COVID: 12.3; control: 12.3 | 50.1 | >30 | 12 |
| Barrett, 2022b | U.S. | Health Verity | Medical records, PCR, ICD-10 | Medical records, ICD-10 | Type 2 | General population | COVID: 439,439; control: 439,439b | COVID: 12.7; control: 12.7 | 50.1 | >30 | 16 |
| Birabaharan, 2022 | U.S. | TriNetX | Medical records, PCR, ICD-10 | Medical records, ICD-10 | Type 2 | Historical influenza patients | COVID: 324,360; control: 330,734b | Range: >18 | 50.0 | >1 | 6 |
| Cohen, 2021 | U.S. | United Health Group Clinical Discovery Database | Medical records, PCR, ICD-10 | Medical records, ICD-10 | Type 2 | General, historical population, and historical vLRTI patients, respectively | COVID: 81,850; control: 1,968,898 | COVID: 76.9; control: 75.7 | 58.0 | >21 | 12 |
| Daugherty, 2021 | U.S. | United Health Group Clinical Discovery Database | Medical records, PCR, ICD-10 | Medical records, ICD-10 | Type 2 | General, historical population, and historical vLRTI patients, respectively | COVID: 203,967; control: 8,528,786 | COVID: 41.7; control: 42.4 | 49.8 | >21 | 10 |
| McKeigue, 2022 | U.K. | REACT-SCOT | Medical records, PCR | Medical records | Type 1 | General population | COVID: 365,080; control: 1,484,331 | Range: <35 | 49.7 | >1 | 20.6 |
| Pietropaolo, 2022 | Global | TriNetX | Medical records, PCR, ICD-10 | Medical records, ICD-10 | Type 1 | General population | COVID: 273,807; control: 273,930b | COVID: 18.6; control: 11.2 | 45.0 | >1 | 17 |
| Qeadan, 2022 | U.S. | Cerner Real-World Data | Medical records, Laboratory test | Medical records, ICD-10 | Type 1 | General population | COVID: 2,489,266; control: 24,803,613 | COVID: 44.5; control: 41.1 | 54.1 | >1 | 22 |
| Rathmann, 2022 | Germany | Disease Analyzer | Medical records, ICD-10 | Medical records, ICD-10 | Type 2 | AURI patients | COVID: 35,865; control: 35,865b | COVID: 42.6; control: 42.6 | 45.6 | >1 | 16.7 |
| Xie, 2022 | U.S. | CDW | Medical records, Laboratory test | Medical records, ICD-10 | Type 2 | General, and historical population, respectively | COVID: 181,280; control: 4,278,701 | COVID: 60.9; control: 61.5 | 11.3 | >30 | 11.6 |
vLRTI: viral lower respiratory tract illness; ARI: acute respiratory infection; AURI: acute upper respiratory tract infection; CDW: Corporate Data Warehouse; COVID-19: coronavirus disease 2019; GDPPR: General Practice Extraction Service Data for Pandemic Planning and Research; ICD-10: International Classification of Diseases 10; NR: not report; PCR: polymerase chain reaction; REACT-SCOT: Rapid Epidemiological Analysis of Comorbidities and Treatments as risk factors for COVID-19 in Scotland; SCI-Diabetes: the Scottish Care Information-Diabetes.
Detailed diagnostic criteria for COVID-19, diabetes, and specific diseases are presented in Appendix Table S5.
After 1:1 propensity score matched.
The type of diabetes predominantly reported.
Interval between the first diagnosis of COVID-19 and collection of information on diabetes onset.
Maximum follow-up duration.
3.2. Risk of bias of individual cohorts
Overall, all cohorts had no or one item evaluated as probably/definitely high risk of bias; therefore, we considered that all of them were at low risk of bias. However, there are still some concerns. One cohort might not be sufficiently representative because the participants assessed were U.S. veterans [27]. Another cohort selected patients with influenza diagnosed from 2018 to 2021 as the control group [29]. Three cohorts were not adequately matched or adjusted for prognostic variables [32], [33], [34], which were adjusted for age, sex, and a few other variables. The potential confounding variables adjusted or matched in cohorts are presented in Appendix Table S6. A summary of the assessment with justification is presented in Appendix Table S7.
3.3. Meta-analysis
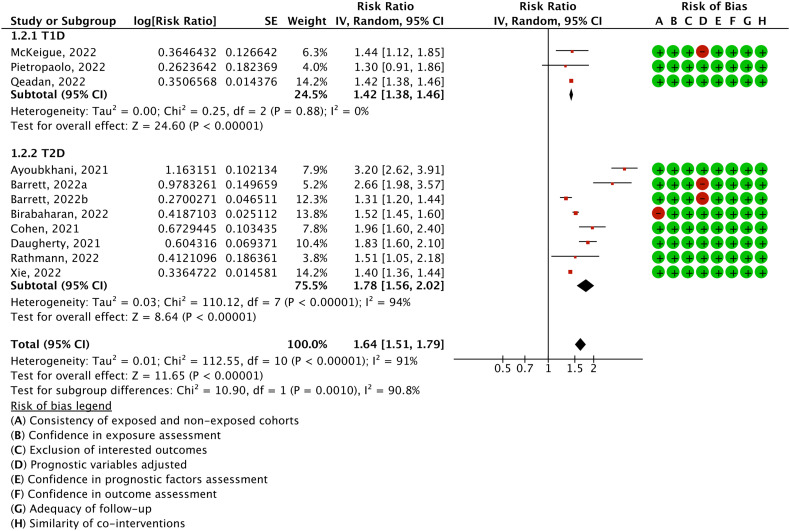
Eleven cohorts including a total of 47.1 million participants (4.5 million patients with COVID-19 and 42.6 million controls) reported on the risk of overall diabetes. A random effects model meta-analysis demonstrated that COVID-19 was associated with a 64 % greater risk of diabetes (RR = 1.64, 95 % confidence interval: 1.51 to 1.79; absolute risk difference = 701 more per 10,000 persons, 558 more to 865 more; I 2 = 91.1 %; Fig. 2 ).
Fig. 2.
Relative risk of diabetes, type 1 diabetes, and type 2 diabetes in patients with COVID-19 compared with those without COVID-19.
Subgroup analyses are summarized in Table 2 . For the different types of diabetes, a stronger association between COVID-19 and T2D was detected with a statistically significant subgroup effect (P interaction = 0.001, Fig. 2). The risk of T2D after COVID-19 infection (RR = 1.78, 95 % confidence interval: 1.56 to 2.02; 1287 more per 10,000 persons, 924 more to 1683 more) was higher than that of T1D (RR = 1.42, 95 % confidence interval: 1.38 to 1.46; 52 more per 10,000 persons, 47 more to 57 more).
Table 2.
Prespecified subgroup meta-analyses of diabetes risk in patients with COVID-19.
| Stratification and categories | Cohorts, n | Pooled relative risk (95 % CI) | Population risk per 10,000 personsa | Risk difference per 10,000 persons (95 % CI) | I2 (%) | Pinteraction |
|---|---|---|---|---|---|---|
| Overall | 11 | 1.64 (1.51 to 1.79) | 1095 | 701 (558 to 865) | 91.1 | – |
| Type of diabetes | ||||||
| Type 2 diabetes | 8 | 1.78 (1.56 to 2.02) | 1650 | 1287 (924 to 1683) | 93.6 | 0.001 |
| Type 1 diabetes | 3 | 1.42 (1.38 to 1.46) | 123 | 52 (47 to 57) | 0 | – |
| Region | ||||||
| U.S. | 7 | 1.54 (1.43 to 1.65) | 1095 | 591 (471 to 712) | 88.2 | 0.483 |
| Europe | 3 | 1.93 (1.09 to 3.42) | 1095 | 1018 (99 to 2650) | 93 | – |
| Global | 1 | 1.30 (0.91 to 1.86) | 1095 | 331 (−97 to 945) | – | – |
| Age | ||||||
| 0–18 | 5 | 1.74 (1.35 to 2.24) | 1095 | 810 (383 to 1358) | 90.5 | 0.375 |
| 18–65 | 5 | 1.44 (1.31 to 1.58) | 1095 | 482 (339 to 635) | 83.3 | – |
| >65 | 3 | 1.50 (1.36 to 1.65) | 1095 | 548 (394 to 712) | 77.8 | – |
| Race | ||||||
| Black | 2 | 1.49 (1.33 to 1.68) | 1095 | 537 (361 to 745) | 85.3 | 0.093 |
| White | 2 | 1.27 (1.1 to 1.47) | 1095 | 296 (110 to 515) | 96.3 | – |
| Sex | ||||||
| Male | 2 | 1.45 (1.37 to 1.53) | 1095 | 488 (405 to 576) | 76.7 | 0.05 |
| Female | 2 | 1.35 (1.30 to 1.41) | 1095 | 388 (331 to 448) | 0 | – |
| Control | ||||||
| Contemporary control | 10 | 1.67 (1.52 to 1.85) | 1095 | 734 (569 to 931) | 91.6 | 0.336 |
| Historical control | 4 | 1.55 (1.38 to 1.74) | 1095 | 602 (416 to 810) | 91 | – |
| Type of control | ||||||
| Compared to specific patients | 5 | 1.49 (1.31 to 1.69) | 1095 | 537 (339 to 756) | 68.2 | 0.148 |
| Compared to general patients | 9 | 1.68 (1.52 to 1.86) | 1095 | 745 (569 to 942) | 92.9 | – |
| Diagnosis interval | ||||||
| At least 3 weeks | 6 | 1.58 (1.34 to 1.85) | 1095 | 635 (372 to 931) | 90.5 | 0.355 |
| Very short interval | 5 | 1.46 (1.39 to 1.52) | 1095 | 504 (427 to 569) | 32.4 | – |
Based on the median baseline risk from the non-exposed group of included cohorts.
With stratification by age, adolescents and children (0–18 years) with COVID-19 had a 74 % greater diabetes risk (1.74, 1.35 to 2.24; 810 more per 10,000 persons, 383 more to 1358 more) compared with general contemporaries. In elderly patients (>65 years), the risk estimate was 1.50 (1.36 to 1.65; 548 more per 10,000 persons, 394 more to 712 more). The risk estimate for younger adults aged 18 to 65 was 1.44 (1.31 to 1.58; 482 more per 10,000 persons, 339 more to 635 more). No statistically significant differences were detected between the age subgroups (P interaction = 0.375, Appendix Fig. S1). With stratification by sex, the risk estimate pooled from two American cohorts indicated a possibly greater risk for males (1.45, 1.37 to 1.53; 488 more per 10,000 persons, 405 more to 576 more) (P interaction = 0.050, Appendix Fig. S2). No statistically significant differences in diabetes risk among regions and races were found (P interaction = 0.483 and 0.093, respectively, Appendix Figs. S3 and S4). Meta-regression found no significant association between mean cohort age, the proportion of black, the proportion of females, and the pooled effect (P interaction = 0.455, 0.216, and 0.586, respectively, Appendix Figs. S5–S7).
Ten cohorts comprised contemporary controls, of which three comprised both contemporary and historical controls. The other comprised historical controls. The relative risk of diabetes did not change significantly when compared to different period controls (P interaction = 0.336, Appendix Fig. S8). Besides, no statistically significant differences were detected between compared to general or patients controls (influenza, viral lower respiratory tract illness or acute upper respiratory tract infections patients) (P interaction = 0.148, Appendix Fig. S9). The onset of diabetes was not included until at least three weeks after the diagnosis of COVID-19 in half of the cohorts. In contrast, other cohorts assessed diabetes onset at any time, 24 h after the patients were diagnosed with COVID-19. The differences between the subgroups were not statistically significant (P interaction = 0.658, Appendix Fig. S10). Further meta-regression models found no significant association between the length of follow-up and pooled estimate (P interaction = 0.147, Appendix Fig. S11). In addition, as suggested by the reviewers, we performed a posteriori comparison of the results from the risk-based studies to those from the rate-based studies. No significant difference was found (P interaction = 0.431).
In the sensitivity analysis, we found that no individual estimate significantly influenced the overall effect (Appendix Fig. S12). The pooled estimate remained robust (P = 0.702) with the exclusion of two preprint cohort studies. In addition, we did not find evidence of publication bias in either Begg's or Egger's tests (P = 0.350 and P = 0.054, respectively).
4. Discussion
4.1. Principal findings
Based on 11 retrospective cohorts involving 47,120,129 participants, primarily from the United States and Europe, we found that COVID-19 was associated with a 64 % increased risk of overall diabetes. Furthermore, the magnitude of the effect of COVID-19 on T2D was significantly greater than that on T1D. The risk was also consistent compared with the historical controls and respiratory infection controls. In summary, there is a significant association between COVID-19 infection and the development of diabetes in the general population. Therefore, the management, prevention, and screening of diabetes in COVID-19 survivors should be strengthened.
4.2. Strengths and limitations
The study was guided by a predefined protocol. The strengths of this meta-analysis mainly include explicit eligibility criteria; a comprehensive literature search; the independent assessments of study eligibility, data extraction, and risk of bias; the large sample size of included cohorts; a transparent statistical analysis based on estimates fully adjusted; the presentation of absolute effects; and the sufficient subgroup factors were explored. We also presented the absolute effects of overall diabetes, T1D, and T2D, respectively, to better understand the results. Sensitivity analyses further supported the robustness of our results.
However, this study had several limitations. First, social, behavioral, and health care practices, such as lockdown, have changed dramatically during the pandemic. Changes in these factors may impact the association between COVID-19 infection and an increased risk of diabetes. The conclusions we draw at the moment may change over time as the pandemic continues, the virus mutates, and treatment strategies improve. Second, diabetes in the included cohort was primarily identified by the International Classification of Diseases (ICD). For instance, using one or more ICD-10 E11 to identify T2D patients showed a 78 % sensitivity in DEpiC (the VA Diabetes Epidemiology Cohort) [37]. Thus, we cannot rule out the possibility that some diabetes cases were missed. Thirdly, this systematic review was based on observational studies. Although we used the results from the fully adjusted models, the residual confounding or other biases may still be present. Fourthly, the number of cohorts for the subgroups was insufficient (e.g., two cohorts for sex, and two cohorts for the race); therefore, the results of these subgroup analyses might be unreliable. Fifthly, although we used a priori specified subgroup analysis and meta-regression, we failed to explore the source of heterogeneity. The limited information reported in included studies is a possible reason, and the residual heterogeneity also increased our uncertainty about the results. Sixthly, although polymerase chain reaction (PCR) tests have been used to diagnose COVID-19 in most cohorts, there are still differences in the diagnostic methods across studies. A few cohorts have specifically declared the use of antigen tests. As the use of antigen tests might be more common, this adds to some of doubts about the certainty of the diagnosis [38]. We might still be unable to establish a strong causal association between COVID-19 infection and increased diabetes risk due to these limitations. Future cohort studies are needed to strengthen the evidence body.
4.3. Comparison with other studies
The magnitude of the apparent effect of COVID-19 on diabetes outcomes echoes the incidence of diabetes in patients with COVID-19 reported or summarized in previous studies [13], [39]. Furthermore, the relative risk and absolute risk difference based on the comparison between people with and without COVID-19 could better describe the adverse effects of COVID-19 on diabetes. Although the incidence of T1D is relatively low, a previous review found a potentially strong association between COVID-19 and T1D incidence [40]. For T2D, the risk estimates and 95 % confidence intervals for all included cohorts indicated a statistically significantly higher risk. Two meta-analyses have addressed this topic [41], [42]. They also concluded that COVID-19 is associated with a higher risk of diabetes than controls without COVID-19. However, these two studies included only half of the cohorts we included (one included 5, and another had 6). We also performed more comprehensive analysis strategies, such as a priori specified subgroup analysis and meta-regression. We used absolute effects to represent risk. These advantages make it more likely that solid conclusions will be drawn.
4.4. Potential interpretations of findings
Current studies have explained the potential mechanism for the association between COVID-19 infection and diabetes risk. A study explored the intricate relationship and underlying mechanisms between COVID-19 and diabetes, and suggested that SARS-CoV-2 can directly induce β cell killing [43]. The development of T1D could be catalyzed when the populations of functional β cells are damaged and depleted [44]. As early as 2009, a study indicated that the binding of SARS coronavirus to its receptor could damage islets and cause acute diabetes [45]. The induction and exacerbation of insulin resistance by SARS-CoV-2 may be the underlying mechanism [46]. In addition to the effects of SARS-CoV-2 infection itself, the lockdown environment during a pandemic might progress T2D in hyperglycemic or other potentially at-risk populations [47]. However, another study concluded that SARS-CoV-2 is not directly cytotoxic to β cells because angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) were not detected in β cells of the human pancreas [48].
In addition, all aspects of health worldwide have been affected by the COVID-19 pandemic. Given the large and growing number of people infected with COVID-19 [1], any consequent adverse effects are too many for patients and society to bear, not to mention diabetes which is already difficult to manage. Furthermore, patients with both COVID-19 and new-onset diabetes had the highest rate of admission to the ICU and had a greater relative risk of all-cause mortality than patients with pre-existing diabetes or hyperglycemia reported in a study in China [11]. The development of diabetes in patients with COVID-19 has complex additive effects on multiple diseases and adverse outcomes. Although relevant issues remain to be further explored and explained, the evidence in this meta-analysis suggests that increased attention and care for diabetes risk in patients with COVID-19 should be addressed in response to a public health problem that may be extremely serious in the future [49].
5. Conclusions
The pooled risk for large cohort studies involving >47.1 million participants demonstrated an apparent association between COVID-19 and the risk of incident diabetes, including both type 1 and type 2 diabetes. Since diabetes has not only long been an important health concern worldwide but also a major factor in adverse outcomes for patients with COVID-19, we should be alert to the diabetes risk in the growing number of people with COVID-19, and be prepared to address the associated health issues.
Funding sources
None.
CRediT authorship contribution statement
HHL conceived the study idea. LG and HHL designed and performed the search strategy. HHL, MYS, PB, and JW screened abstracts and full texts, extracted data, and judged the risk of bias of included studies. HHL and LG designed and performed the data analysis. LG, BP, QW, GWD, and XPS provided opinions, suggestions, technical support or material support. HHL wrote the first draft of the manuscript. LG, XPS, MLY, BP, JHT, and KHY supervised the study. All authors interpreted the data analysis and critically revised the manuscript. HHL is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of competing interest
All authors have no conflicts of interest relevant to this article to disclose.
Acknowledgements
We thank Wenjing Tang for her assistance throughout this review.
Footnotes
Financial support: This research received no specific grant from any funding agency.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metabol.2022.155330.
Appendix A. Supplementary data
Supplementary appendix
Data availability
All data collected for this systematic review and meta-analysis, including search strategies, the review protocol, data extraction sheets, and analytical codes, are available immediately following publication without end date to anyone for any purpose and are either published in the appendices or can be accessed through Mr Lai (email, enenlhh@outlook.com).
References
- 1.Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html
- 2.Scherer P.E., Kirwan J.P., Rosen C.J. Post-acute sequelae of COVID-19: a metabolic perspective. Elife. 2022;11 doi: 10.7554/elife.78200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyu H.H., Bachman V.F., Alexander L.T., et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;i3857 doi: 10.1136/bmj.i3857. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 5.International Diabetes Federation . 2019. IDF Diabetes Atlas 9th Edition.https://diabetesatlas.org [Google Scholar]
- 6.Feldman E.L., Savelieff M.G., Hayek S.S., Pennathur S., Kretzler M., Pop-Busui R. COVID-19 and diabetes: a collision and collusion of two diseases. Diabetes. 2020;69(12):2549–2565. doi: 10.2337/dbi20-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steenblock C., Schwarz P.E.H., Ludwig B., et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamer M., Gale C.R., Batty G.D. Diabetes, glycaemic control, and risk of COVID-19 hospitalisation: population-based, prospective cohort study. Metabolism. 2020;112 doi: 10.1016/j.metabol.2020.154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barron E., Bakhai C., Kar P., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu L., She Z.G., Cheng X., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H., Tian S., Chen T., et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;22(10):1897–1906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathish T., Kapoor N., Cao Y., Tapp R.J., Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. 2021;23(3):870–874. doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubino F., Amiel S.A., Zimmet P., et al. New-onset diabetes in covid-19. N Engl J Med. 2020;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boddu S.K., Aurangabadkar G., Kuchay M.S. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020;14(6):2211–2217. doi: 10.1016/j.dsx.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metwally A.A., Mehta P., Johnson B.S., Nagarjuna A., Snyder M.P. COVID-19-induced new-onset diabetes: trends and technologies. Diabetes. 2021;70(12):2733–2744. doi: 10.2337/dbi21-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1) doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evidence Partners Tool to assess risk of bias in cohort studies. https://www.evidencepartners.com/resources/methodological-resources/tool-to-assess-risk-of-bias-in-cohort-studies-distillersr
- 21.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. 2022. www.training.cochrane.org/handbook Available from. [Google Scholar]
- 23.Rücker G., Schwarzer G., Carpenter J.R., Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8(1):79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siemieniuk R.A., Bartoszko J.J., Zeraatkar D., et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wander P.L., Lowy E., Beste L.A., et al. The incidence of diabetes among 2,777,768 veterans with and without recent SARS-CoV-2 infection. Diabetes Care. 2022;45(4):782–788. doi: 10.2337/dc21-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 27.Xie Y., Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietropaolo M., Hotez P., Giannoukakis N. Incidence of an insulin-requiring hyperglycemic syndrome in SARS CoV-2-infected young individuals: is it type 1 diabetes? Diabetes. 2022 doi: 10.2337/db21-0831. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birabaharan M., Kaelber D.C., Pettus J.H., Smith D.M. Risk of new-onset type 2 diabetes in 600 055 people after COVID-19: a cohort study. Diabetes Obes Metab. 2022;24(6):1176–1179. doi: 10.1111/dom.14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daugherty S.E., Guo Y., Heath K., et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373 doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen K., Ren S., Heath K., et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2022;376 doi: 10.1136/bmj-2021-068414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKeigue P.M., McGurnaghan S., Blackbourn L., et al. bioRxiv; 2022. Relation of incident type 1 diabetes to recent COVID-19 infection: cohort study using e-health record linkage in Scotland. Published online. [DOI] [PubMed] [Google Scholar]
- 33.Qeadan F., Tingey B., Egbert J., et al. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: a nationwide cohort from the US using the Cerner Real-World Data. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0266809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett C.E., Koyama A.K., Alvarez P., et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years - United States, March 1, 2020-June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):59–65. doi: 10.15585/mmwr.mm7102e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayoubkhani D., Khunti K., Nafilyan V., et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathmann W., Kuss O., Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. 2022;65(6):949–954. doi: 10.1007/s00125-022-05670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller D.R., Safford M.M., Pogach L.M. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–B21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 38.Spick M., Lewis H.M., Wilde M.J., Hopley C., Huggett J., Bailey M.J. Systematic review with meta-analysis of diagnostic test accuracy for COVID-19 by mass spectrometry. Metabolism. 2022;126 doi: 10.1016/j.metabol.2021.154922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khunti K., Del Prato S., Mathieu C., Kahn S.E., Gabbay R.A., Buse J.B. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care. 2021;44(12):2645–2655. doi: 10.2337/dc21-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iughetti L., Trevisani V., Cattini U., et al. COVID-19 and type 1 diabetes: concerns and challenges. Acta Biomed. 2020;91(3) doi: 10.23750/abm.v91i3.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ssentongo P., Zhang Y., Witmer L., Chinchilli V.M., Ba D.M. Association of COVID-19 with diabetes: a systematic review and meta-analysis. Res Square. 2022 doi: 10.21203/rs.3.rs-1848430/v1. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee M., Pal R., Dutta S. Risk of incident diabetes post-COVID-19: a systematic review and meta-analysis. Prim Care Diabetes. 2022;16(4):591–593. doi: 10.1016/j.pcd.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C.T., Lidsky P.V., Xiao Y., et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021;33(8):1565–1576.e5. doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roep B.O., Thomaidou S., van Tienhoven R., Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?) Nat Rev Endocrinol. 2021;17(3):150–161. doi: 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal R., Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest. 2020;43(7):1027–1031. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162(108142) doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coate K.C., Cha J., Shrestha S., et al. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 2020;32(6):1028–1040.e4. doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pranata R., Henrina J., Raffaello W.M., Lawrensia S., Huang I. Diabetes and COVID-19: the past, the present, and the future. Metabolism. 2021;121 doi: 10.1016/j.metabol.2021.154814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary appendix
Data Availability Statement
All data collected for this systematic review and meta-analysis, including search strategies, the review protocol, data extraction sheets, and analytical codes, are available immediately following publication without end date to anyone for any purpose and are either published in the appendices or can be accessed through Mr Lai (email, enenlhh@outlook.com).