Abstract
Coexistence between closely related species can lead to intense competition for resources. Stable isotope analysis (SIA) is a reliable tool to estimate the extent of species competition. We employed SIA to evaluate niche partitioning among two syntopic species of Galápagos land iguanas: Conolophus subcristatus and C. marthae. Samples were collected on Wolf Volcano, Isabela Island, where C. marthae is endemic and syntopic with C. subcristatus. We determined δ13C and δ15N ratios and described the isotopic niche of each species using corrected standard ellipse area (SEAc). We tested for differentiation between the isotopic niches, while controlling for sex, body size, spatial location of samples and mean annual primary productivity at capture points, using bivariate linear models. Despite the extensive overlap of the isotopic niches, we found species and sex to be a significant, interacting predictor of a sample’s location in the δ13C, δ15N space, indicating the existence of niche partitioning mechanisms acting between species and sexes. We also found that body size and productivity at the capture points, compounded with yet undetermined spatial effects, explain ca. 75% of the differences observed between species and sexes, providing evidence for differential microhabitat and food-items usage. Our study provides essential baselines for evaluating conservation actions for C. marthae, such as the potential translocation to a sanctuary area free of competition from C. subcristatus.
Subject terms: Conservation biology, Stable isotope analysis
Introduction
Competition is a critical determinant of population dynamics and community structure1–3. Closely related species that diverged in allopatry may retain their common ancestral niche and be expected to competitively exclude each other when they come into secondary contact4. Under these circumstances and given appropriate conditions5, natural selection acting on one or both species may favor traits that reduce niche-overlap and, therefore, competition6. This mechanism has been often called into question to explain patterns of coexistence between syntopic (sensu Rivas7) closely related species competing for limited resources8–12. On the other hand, neutral models of community evolution (“community drift”)13–15 may also explain niche divergence and, ultimately, coexistence between sister species. These neutral mechanisms may be expected to be particularly important where community structures are strongly limited by incomplete dispersal, as is typically the case for communities of terrestrial vertebrates living in oceanic islands16. Such communities are shaped by complex patterns of extinction and recolonization17, and species that happen to coexist on one island may have well spent large portions of their evolutionary past confined into different islands, experiencing different biotic and abiotic conditions and, hence, selective pressures17.
Beyond its fundamental importance as a key concept of animal ecology, a better understanding of competitive dynamics between co-occurring, closely related species can contribute important insights to the conservation of interacting endangered species. One such case involves two species of Galápagos land iguanas: Conolophus subcristatus and C. marthae. While the former is found on seven islands across the Galápagos Archipelago, namely Santa Cruz, Plaza Sur, Isabela, Fernandina, Seymour Norte (introduced), Baltra (repatriated) and Santiago (reintroduced;18), the latter is only found in a very small high-altitude area (ca. 25 km2) on Wolf Volcano, Isabela Island19. At this site, the only known population of C. marthae is syntopic with a population of C. subcristatus20. Conolophus marthae has been recently described to science21 and, shortly after, assessed as Critically Endangered in the IUCN Red List of Threatened Species19. The main justifications for this assessment include small population size, scarce recruitment, introduced predators, limited distribution, and the potential for intense competition with C. subcristatus19–21.
While new and important information on the ecology and biology of C. marthae are constantly accreting, very little is known about its dietary habits and trophic preferences, but some preliminary evidence, obtained indirectly from analyzing the chemical composition of femoral pore secretions, suggest they could be partially different from those of C. subcristatus22, for which a generalist trophic behavior has been described23,24. Moreover, field observations indicate differential microhabitat use, hinting towards the use of different trophic resources25. Molecular phylogeny indicates that the divergence of C. marthae and C. subcristatus started ca. 1.5 million years ago26, when the island of Isabela had not yet formed, and it is still unknown when these two species colonized Isabela and how long they existed in sympatry. As intense competition for trophic resources may lead to the extinction of the least competitive species27,28, a thorough exploration of the trophic ecology of C. marthae and C. subcristatus populations in syntopy is essential for guiding conservation actions29,30.
Stable isotope analysis (SIA) is an effective and widely applicable tool to characterize the trophic niche of syntopic species31–33. The isotopic composition of animal tissues is known to reflect the isotopic composition of their trophic resources34–36, with differences in diet composition translating into differences in the stable isotope composition31,37. In this work, we used isotope ratios to evaluate the dietary overlap between the syntopic populations of C. marthae and C. subcristatus. We determined δ13C and δ15N isotopic compositions in nail-keratin sampled from individuals of these two species on Wolf Volcano. We first estimated the degree of overlap between the isotopic spaces occupied by individuals of the two species by computing standard ellipse areas (SEAc) and assessed trophic niche segregation while controlling for individuals’ sex. On the one hand, measuring the extent of isotopic niche overlap contributes to the evaluation of the potential for competitive interaction between C. marthae and C. subcristatus. On the other hand, rejecting the null hypothesis of no differentiation in isotopic composition between species, would strongly argue for some mechanisms of niche partitioning to act between the two species. We then used a variance partitioning approach to assess how much ecologically relevant predictors such as body size and mean primary productivity at the capture points, compounded with unmeasurable variables linked to spatial location itself, explained variation in isotopic profiles and the differences between species and sexes.
Materials and methods
Study area and sampling
We captured 60 individuals of C. marthae (38 males, 22 females) and 58 individuals of C. subcristatus (33 males, 25 females) from June 14 to June 23 2014. We sampled the northwestern slope of Wolf Volcano at an altitude of 1488–1659 m asl, within an area no larger than 2 km2. Individuals were hand-grabbed or captured using noosed telescopic poles. June is a transitional month, between the warm/wet season (typically occurring between January and May) and the cold/dry season (approximately from June to December38,39).
For each captured individual, we collected nail-keratin samples and recorded GPS coordinates of the capture point, snout-to-vent length (SVL; in cm), mass (in kg) and sex. According to the habitat classification in40, 114 collected individuals (96.6% of the total) were captured in patches of “deciduous shrubland”, while only 2 C. marthae and 2 C. subcristatus were captured in a different habitat (evergreen seasonal forest and shrubland). Other habitat types in this area include deciduous forest, tallgrass vegetated areas, and evergreen forest on the western side of the volcano40. All captured individuals were classified as adults based on morphological characteristics. In compliance with the Galápagos National Park regulation, we minimized the nail-keratin amount sampled, taking three to four toenails from each individual, using sterilized bolt cutters. Nail-keratin samples were immediately placed into 1.5 ml polypropylene collection tubes. Sixty individuals were identified as having been captured and tagged in previous field expeditions by our research group19–21. All other captured iguanas were assigned a new ID and were tagged with a passive integrated transponder (PIT). To counter the illegal trade of Galápaganian wildlife41, in agreement with the Galápagos National Park, we decided not to disclose the exact GPS coordinates of the capture points here and avoid publishing a detailed map of our sampling.
Stable isotopes analysis (SIA)
In animal ecology, SIA is performed by analyzing the ratios between the two main stable isotopes of carbon (12C and 13C) and nitrogen (14N and 15N) respectively, which can then be compared among individuals, species, or populations34–36. As animal tissues are generally enriched in 13C by only ≈ 1‰ relative to their food34,36, the distribution of δ13C in a sample of individuals is typically used to estimate between-individuals variation in the diet of the population they belong to. In contrast, the tissues of animals are enriched in 15N by ≈ 3–4‰ compared to their food source, and δ15N is widely used to estimate the relative trophic level of individuals and species35,36. Intuitively, the differences between the isotopic composition of food sources and that of animal tissues, indicated through the Trophic Discrimination Factor (TDF), can vary between species, and the values provided above should be considered indicative42. The turnover rates of stable isotopes vary largely among tissues, with metabolically more active tissues reflecting diet within a few weeks, and less active tissues being informative of dietary habits of the last several months43,44. Differences in δ15N and δ13C from different tissues from a single iguana individual have been described and interpreted as informative on diet composition in different time periods45. Inert tissues, such as the keratin in hair or nails, provide long-term information on consumed food sources46. For example, it has been demonstrated that, for turtles’ claws, a change in diet requires about 6 and 12 months to be reflected by a change in δ15N and δ13C, respectively47, while it takes 3 to 4 months in endothermic Sauropsida (birds48).
To determine the isotopic composition of carbon and nitrogen, toenail samples were cleaned using distilled water to remove any sources of contamination. Nail-keratin fragments were freeze-dried (− 55 °C) and then ground into a fine, homogeneous powder with an agate pestle and mortar. They were then placed into small tin capsules (4 × 6 mm). For each individual, a pair of samples of 0.2–0.3 mg was analyzed, using continuous-flow isotope ratio mass spectrometry (Thermo Flash 1112 Elemental Analyzer coupled to a Finnigan Delta Plus mass spectrometer) at IGAG (Istituto di Geologia Ambientale e Geoingegneria, CNR). Isotopic compositions were expressed in the usual δ notation, which represents the relative deviation in part per thousand of the heavy isotope/light isotope ratios of the sample from the same ratio of a reference standard. Isotope data were normalized to the V-PDB (Vienna Pee-Dee Belemnite) scale for carbon and AIR (atmospheric air) scale for nitrogen using IAEA standards (CH-6, CH-7 USGS-24 for carbon, and N-1, N-2, USG25 for nitrogen). Based on repeated measurements of laboratory standards, the analytical error was < 0.3 ‰ for both carbon and nitrogen.
Data analysis
To characterize the isotopic niche of each species and sex, we calculated standard ellipse areas corrected for small sample size (SEAc), as described by Jackson and colleagues49. SEAc was developed to represent the core of the isotopic niche of a sampled population, and we used these metrics to measure niche overlap between species and sexes as the proportion of overlap between ellipses. The entire procedure was carried out using the “SIBER” R-package49.
We tested for differences in carbon and nitrogen stable isotope profiles between species and sexes by fitting a bivariate linear model, with δ13C and δ15N as joint response variables and species, sex and their interaction as predictors. To evaluate the effect of individual morphology, habitat features and spatial distribution of the samples, we fitted Generalized Additive Models (GAMs) including parametric terms for body-size and mean primary productivity at the capture point, and a spatial tensor product on the X and Y coordinates (UTM/WGS-84 Zone 15S) of the capture points accounting for unmeasured variables related to the spatial distribution of samples. We expressed body size as snout-to-vent-length (SVL). We chose SVL rather than mass to express body size because the former represents a more stable measure, not strongly affected by reproductive status, fat storage and digestive state of the animal50–54. We measured primary productivity as Normalized Difference Vegetation Index (NDVI), using Copernicus Sentinel-2 10 m resolution images, freely available on the cloud service Google Earth Engine (https://earthengine.google.com/). We calculated the mean NDVI for a 50-m radial buffer around the capture point of each individual averaged on a 3-year period (2019–2021), after masking for clouds (i.e., excluding from the averaging all pixels where clouds were detected in a given image). Before fitting our models, both SVL and NDVI were standardized to zero mean and unit standard deviation using the scale() function from package “base”55 in order to obtain more easily interpretable coefficients. We preliminarily investigated whether these two variables differed between species and sexes by fitting two linear models with scaled SVL and NDVI as response variables and species and sex as predictors. To assess spatial aggregation of the samples, we conducted a multiple regression on distance matrices using the MRM() function in the “ecodist” R package56 with the Euclidean distance matrix computed from X and Y coordinates of the capture points as response variable and the dissimilarity matrices of species and sex as predictors (where 0 indicates matching and 1 indicates not-matching).
To investigate the contribution of ecologically-relevant variables (size, productivity and space) in defining the isotopic differences observed between species and sexes, we used a variance partitioning approach based on partial correlation analyses57, which we implemented by adapting the ecospat.varpart() function from package “ecospat”58. To this end, we fitted two additional models: first, a “full” bivariate generalized additive model (GAM) with δ13C and δ15N as joint response variables and species, sex, body size and mean productivity at the capture points as parametric predictors and a tensor product smooth interaction of geographic coordinates to adjust for the spatial effect of samples distribution; second, an analogous model excluding species and sex from predictors. We then compared these models with the previously described model considering species and sex only and computed the portions of deviance (i) uniquely explained by species + sex, (ii) uniquely explained by size + productivity + spatial tensor product, (iii) jointly explained by the two sets of predictors, and (iv) left unexplained59.
We fitted all models using the R function gam() from package “mgcv”60. For each model, we also checked that distribution of residuals was consistent with assumptions of normality and homoscedasticity. We used Likelihood Ratio Test (LRT) as implemented in the R function anova() from package “stats”55 to test for the significance of individual predictors in GAM models and F as test statistics to compute P-values for linear models with SVL and NDVI as response variables. Non-significant interactions were removed from our final models. We checked for model stability by re-fitting each model 200 times on random sub-samples with 90% of the data and visually assessing the robustness of the coefficient estimates. We did not find evidence of instability for any of our models (for details see SM Fig. 1; SM Fig. 2; SM Fig. 3).
Study design
This study was designed, carried out and reported following the ARRIVE guidelines (https://arriveguidelines.org) according to Percie du Sert78.
Ethics approval
The handling and sampling of the animals were carried out according to a protocol that minimized the stress of the animals. These techniques were approved by the University of Rome “Tor Vergata” ethics and animal handling protocol and following the guidelines of the European Community and with the approval of the Directorate of the Galápagos National Park (GNPD). The GNPD does not have a specific ethics committee. However, the GNPD is responsible for the administration of the research (including the issuing of research permits) carried out in the protected areas of the Galápagos, as administrator of these areas and representative of the National Environmental Authority. The GNPD has granted a research permit to G.G. for this project. The samples were exported and imported with GNPD and CITES permits issued to G.G.
Results
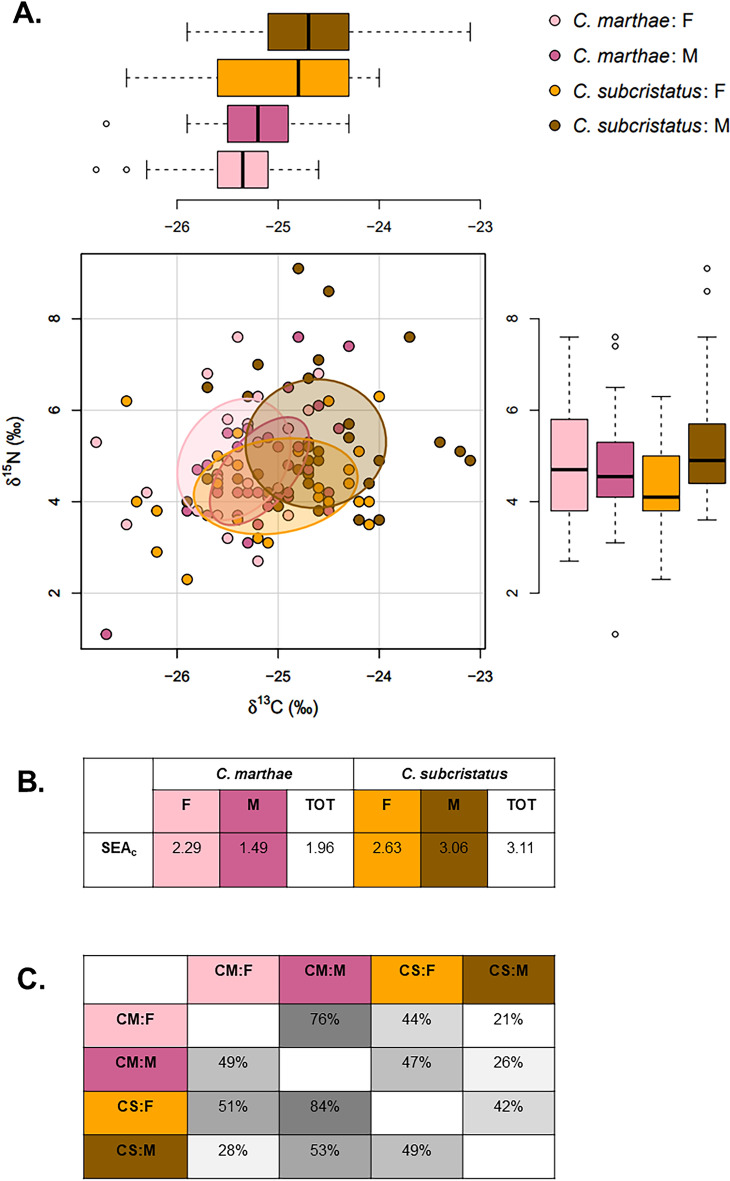
We found that SEAc was larger for C. subcristatus than for C. marthae (Fig. 1A–B). The overlapping portions of SEAc were 65% and 41% for C. marthae within C. subcristatus and C. subcristatus within C. marthae, respectively. Both sexes of C. subcristatus had larger SEAc than the corresponding sex of C. marthae (Fig. 1A–B). At intraspecific level, we found higher levels of overlap between sexes for C. marthae (76% for males and 49% for females; Fig. 1C) than for C. subcristatus (42% for males and 49% for females; Fig. 1C).
Figure 1.
Carbon and nitrogen isotope niche of C. subcristatus and C. marthae males and females. In (A) each dots represents an individual. Solid lines enclose standard ellipse areas with sample size correction (SEAc). Marginal boxplots show the distribution of δ 13C and δ 15 N for each combination of sex and species. Color codes are shown in the top right corner. (B) Shows estimated SEAc for each species and sex. (C) Shows pairwise overlap (expressed as percentage) between each combination of sex and species (CM:F = C. marthae females, CM:M = C. marthae males, CS:F = C. subcristatus females, CS:M = C. subcristatus males). Note that overlap is not symmetrical because values indicate the percentage of overlapping area over each ellipse. Intensity of grey is proportional to overlap.
Results of models fitted using δ13C and δ15N as response variables are reported in Table 1. Model 1 (Table 1), which only included species, sex and their interaction as predictors, indicated that species and sex independently influenced δ13C, with values in C. marthae being lower than those in C. subcristatus and higher in males than females (Fig. 1A), while δ15N depended on the interaction between sex and species, with similar values for males and females in C. marthae and clearly higher values for males than females in C. subcristatus (Fig. 1A). In Model 2 (Table 1), where size, productivity and space were added, only species showed a statistically significant effect on δ13C, while species and sex had no effect on δ15N, and body size and primary productivity at capture points strongly influenced the relative position of each individual in the δ13C, δ15N bi-plot space. The spatial tensor did not provide a statistically significant contribution to the model for δ13C, but it did for δ15N. The same pattern was found with Model 3 (Table 1).
Table 1.
Estimated models coefficients for the two response variables, δ13C and δ15N.
| Estimate | SE | χ2 | Z | df | P | |
|---|---|---|---|---|---|---|
| Model 1: (δ13C, δ15N) ~ Species + Sex | ||||||
| δ13C | ||||||
| Intercept | − 25.483 | 0.109 | – | – | – | – |
| Species (C. subcristatus) | 0.499 | 0.114 | 19.130 | 4.374 | 1 | << 0.001 |
| Sex (male) | 0.325 | 0.117 | 7.780 | 2.789 | 1 | 0.005 |
| δ15N | ||||||
| Intercept | 4.903 | 0.255 | – | – | – | – |
| Species (C. subcristatus) | − 0.549 | 0.348 | – | − 1.580 | – | – |
| Sex (male) | − 0.223 | 0.319 | – | − 0.700 | – | – |
| Species (C. subcristatus): Sex (male) | 1.126 | 0.444 | 6.423 | 2.530 | 1 | 0.011 |
| Model 2: (δ13C, δ15N) ~ Species + Sex + SVL + NDVI + te (X, Y) | ||||||
| δ13C | ||||||
| Intercept | − 25.266 | 0.103 | – | – | – | – |
| Species (C. subcristatus) | 0.243 | 0.119 | 4.167 | 2.041 | 1 | 0.041 |
| Sex (male) | 0.174 | 0.105 | 2.733 | 1.653 | 1 | 0.098 |
| SVL | 0.307 | 0.055 | 31.089 | 5.576 | 1 | << 0.001 |
| NDVI | − 0.120 | 0.056 | 4.578 | − 2.140 | 1 | 0.032 |
| te (X, Y) | – | – | 4.171 | – | 3 | 0.339 |
| δ15N | ||||||
| Intercept | 5.011 | 0.239 | – | – | – | – |
| Species (C. subcristatus) | − 0.612 | 0.348 | – | − 1.760 | – | – |
| Sex (male) | − 0.166 | 0.284 | – | − 0.590 | – | – |
| Species (C. subcristatus): Sex (male) | 0.729 | 0.408 | 3.188 | 1.790 | 1 | 0.074 |
| SVL | 0.356 | 0.103 | 11.906 | 3.450 | 1 | 0.001 |
| NDVI | − 0.234 | 0.107 | 4.833 | − 2.200 | 1 | 0.028 |
| te (X, Y) | – | – | 25.833 | – | 3 | << 0.001 |
| Model 3: (δ13C, δ15N) ~ SVL + NDVI + te (X, Y) | ||||||
| δ13C | ||||||
| Intercept | − 25.041 | 0.051 | – | – | – | – |
| SVL | 0.365 | 0.051 | 50.472 | 7.100 | 1 | << 0.001 |
| NDVI | − 0.135 | 0.057 | 5.683 | − 2.380 | 1 | 0.017 |
| te (X, Y) | – | – | 5.725 | 3 | 0.126 | |
| δ15N | ||||||
| Intercept | 4.814 | 0.094 | – | – | – | – |
| SVL | 0.340 | 0.095 | 12.756 | 3.570 | 1 | < 0.001 |
| NDVI | − 0.258 | 0.105 | 6.033 | − 2.460 | 1 | 0.014 |
| te (X, Y) | – | – | 30.947 | – | 3 | << 0.001 |
The table shows the output of the three fitted models: Model 1 is the bivariate linear regression with species and sex as predictors; Model 2 is the bivariate generalized additive model (GAM) with species, sex, body size (scaled SVL), productivity at the capture points (scaled NDVI) and a spatial tensor product smooth (te (X, Y)) as predictors; Model 3 is the bivariate generalized additive model (GAM) including only body size, productivity of the capture point and the spatial tensor product smooth as predictors. The table reports the estimated model value (Estimate), standard error (SE), degrees of freedom (df), the χ2 statistic for LRT with the corresponding P-values (P) and the z-score (z). The interaction between Species and Sex was not a significant predictor of δ13C (χ2 = 0.386, P = 0.535) and was therefore removed from the final models. P-values for intercept and for main effects included in an interaction do not have a meaningful interpretation and are not shown.
Results of variance partitioning analysis are illustrated in Fig. 2. Our predictors collectively explained 36% of the deviance in the response (i.e., 64% of the total deviance was left unexplained by our analysis). Species and sex together explained only 12% of the response deviance, while size, productivity, and the spatial tensor, as a whole, explained 33%. Importantly, 9% of the deviance was jointly explained by the two sets of predictors. The latter result shows that the three quarters (9% on 12%) of the variation in isotopic composition observed between species and sexes may be explained by differences in size, productivity of the habitat they exploit, and some yet unmeasured spatial variable.
Figure 2.
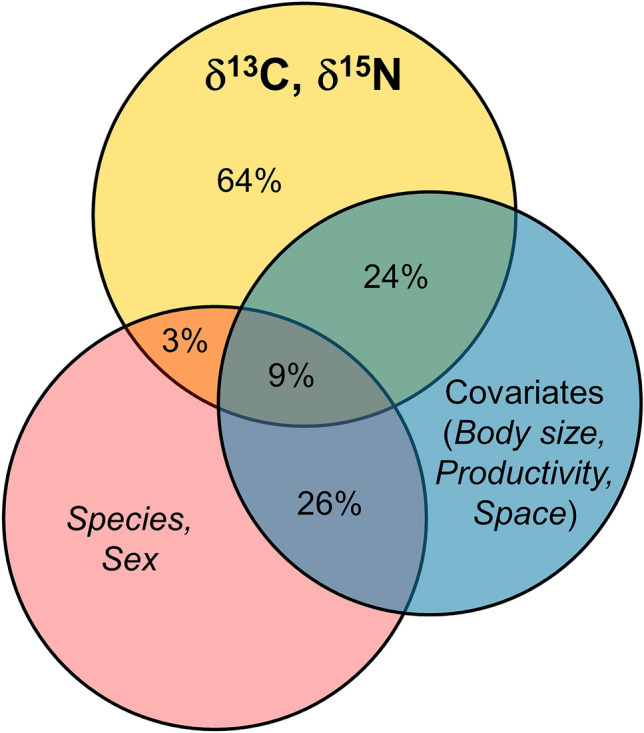
Venn diagram illustrating explained deviance estimated by variance partitioning. The yellow circle on top represents the total deviance of the response variables, whereas the red (bottom left) and blue (right) circles represent sets of predictors (species + sex and body size + productivity + space, respectively). The total deviance of each circle is set to unity. The area of the intersection between each of the predictors’ circles (red and blue) and the response (yellow) circle represents the deviance in the response variables that is explained by each set of predictors. The intersection of all circles is the variance jointly explained by the two sets of predictors. The intersection between the red and the blue circles represents the correlation between the two sets of predictors. The area of the yellow circle not overlapped by any other circle shows the proportion of response deviance not explained by the predictors.
Consistently, results of univariate linear models with scaled SVL and NDVI as response variables (Table 2) showed that body size strongly differed between species and sexes (P < 0.001; P = 0.006 respectively), with C. marthae being, on average, smaller than C. subcristatus and males being, on average, larger than females (SM Fig. 4). The productivity of the capture points was higher for C. marthae than for C. subcristatus (P = 0.018; Table 2; SM Fig. 4) while it did not differ between sexes (Table 2; SM Fig. 4). Multiple regression on distance matrices indicated that species were slightly aggregated (P = 0.002) whereas we did not find evidence of spatial aggregation of individuals of the same sex (P = 0.939).
Table 2.
Estimated coefficients for the two univariate linear models with SVL and NDVI, respectively, as response.
| Estimate | SE | F | df | P | |
|---|---|---|---|---|---|
| SVL | |||||
| Intercept | − 0.613 | 0.163 | – | – | – |
| Species (C. subcristatus) | 0.643 | 0.171 | 14.098 | 1 | < 0.001 |
| Sex (male) | 0.493 | 0.175 | 7.936 | 1 | 0.006 |
| NDVI | |||||
| Intercept | 0.117 | 0.172 | – | – | – |
| Species (C. subcristatus) | − 0.435 | 0.181 | 5.796 | 1 | 0.018 |
| Sex (male) | 0.161 | 0.185 | 0.759 | 1 | 0.386 |
The table shows the estimated model value (Estimate), standard error (SE), degrees of freedom (df), the F statistic and corresponding P-values (p).
Discussion
In this study, we used stable isotopes analysis to evaluate the potential for trophic competition between the only known population of the Galápagos Pink Land Iguana, C. marthae, and the syntopic population of the closely related and more widely distributed C. subcristatus on Wolf Volcano, Isabela Island. Our results rejected the null hypothesis of no difference in carbon and nitrogen stable isotopes profiles between species (Table 1—Model 1), indicating some degree of trophic niche partitioning. We also found that C. marthae occupies a smaller isotopic niche than C. subcristatus, with 65% of the standard ellipse area falling within the SEAc of C. subcristatus. The similar δ15N recorded for the two species (Fig. 1A, Table 1—Model 1) indicate that they occupy similar trophic levels, consistently with previous reports indicating that both species primarily feed on vegetation, as typical of most large iguanas23,24,61,62. Importantly, males and females show larger isotopic space overlap in C. marthae than in C. subcristatus (Fig. 1A–C, Table 1—Model 1), suggesting that differentiation between the isotopic niches of males and females contributes to the larger overall niche occupied by C. subcristatus. Comparing the standard ellipses obtained from raw data (Fig. 1A) and those from the residuals of a model accounting for size, productivity and space (SM Fig. 5), it appears that, while the difference between sexes in residual δ13C of C. subcristatus is much smaller than in the raw data, the difference in δ15N is similar, implying that potential variation in trophic resource consumption between male and female C. subcristatus is not explained by differences in size or occupied habitat and may directly reflect sex-specific resource selection. A detailed characterization of δ13C and δ15N content of different plant species from the top of the volcano would be necessary to better investigate this pattern.
Previous data hinting at trophic resource partitioning between C. marthae and C. subcristatus were provided by a recent study indicating that differences in chemical composition of femoral pore secretions extracted from these two species could be associated with a difference in species diet22. Trophic niche-partitioning might be achieved by two non-mutually exclusive mechanisms. First, individuals of the two species could feed on different trophic resources in the overlapping distribution area. Second, they could feed in different habitats or microhabitats and consequently use different types of food1–3.
Both strategies are supported by our data. On the one hand, we found that (i) the capture locations of the two species were spatially aggregated, (ii) the two species were captured at locations differing in primary productivity (Table 2; SM Fig. 4) (iii) productivity at capture points is a significant predictor of both δ13C and δ15N (Table 1—Model 2 and Model 3) and (iv) unmeasured features of the space (measured by the spatial tensor product) have a significant effect on δ15N (Table 1), indicating selection of different microhabitats as a likely niche partitioning mechanism. Recent field observations also support a differential microhabitat use for these two species, with C. marthae that seems to preferentially occupy more shaded areas (Gentile, unpublished data), and different use of habitats or microhabitats by the two species was proposed as a potential mechanism contributing to prevent interspecific hybridization between C. marthae and C. subcristatus on Wolf Volcano25. On the other hand, resource selection may also contribute to differences in isotope niches. For example, while C3 plants have a δ13C ranging from − 22‰ to − 30‰, C4 show values ranging from − 10‰ to − 14‰63, and CAM (Crassulacean Acid Metabolism) from − 10 to − 20‰64. Lower δ13C values in C. subcristatus (Fig. 1A) could therefore indicate that the diet of this species includes a marginally larger portion of C4 and/or CAM plants than the diet of C. marthae. Consistent with this view, Opuntia cactus, which were previously reported to constitute a large portion of the diet of C. subcristatus and C. pallidus65,66 during the dry season, is a constitutive CAM species67, relatively rare inside the area of distribution of C. marthae, but abundant on the eastern side of Wolf Volcano. While this area is outside the core area of C. marthae, it is inhabited by C. subcristatus. It is not uncommon that when two species compete for common resources, the dominant species (often the larger one) could displace the less competitive one to a suboptimal trophic niche68. Therefore, the current situation on Wolf Volcano could be the result of a competitive process that has led C. marthae to use a suboptimal array of trophic resources. We foresee that estimates of the time at which these two species colonized Isabela and then reached Wolf Volcano to establish their current syntopy (which can be obtained from population genomics analyses) will help to clarify the nature of the interaction we are currently observing.
Admittedly, the absence of information about the trophic discrimination factor (TDF) of species may introduce inaccuracy in the interpretation of isotope data, in particular for δ13C42. The TDF-δ13C and to a lesser extent TDF-δ15N can be affected by metabolic routing, heterogeneity in isotope ratios and differences in macronutrient content among food sources69. Mean TDF-δ13C and TDF-δ15N estimates provided for the skin of three species of Cyclura ranged from + 2.8 to + 5.5‰ and + 5.8 to + 6.2‰, respectively45. If TDF-δ13C and TDF-δ15N of Conolophus species are similar to those described in Cyclura, we note that differences in δ13C and δ15N between the two Conolophus species are well within the range of variation of TDFs. Conceivably, despite differences in macronutrients and metabolic routing ultimately lead back to dietary characteristics42, we cannot rule out a possible role of unknown genetic or environmental factors.
Our data hint at resource partitioning operating at an intraspecific level too, with males of both species showing higher δ13C than females (Fig. 1A) and C. subcristatus males showing higher δ15N than their conspecific females (Fig. 1A). Intraspecific niche partitioning mechanisms are widespread in animal populations and can be interpreted as a strategy to reduce intersexual competition for food resources70. The isotopic pattern observed in our data is likely to reflect behavioral differences between sexes. It is well known that, during the reproductive season, approximately between January and May, on Fernandina Island, C. subcristatus males exhibit a territorial behavior, defending areas that constitute the core part of the mating area, while the females of the species shift between territories defended by different males71. Males reduce feeding during the mating season and food resources inside or near their territories are primarily necessary for females71. After mating, females leave these areas to reach suitable nesting sites, while males remain in their territories if food is still abundant or they disperse, looking for alternative feeding areas71. These observations, therefore, indicate that both temporal and spatial mechanisms act to reduce intersexual competition for trophic resources in C. subcristatus population on Fernandina Island. As δ15N of consumers generally increase with starvation72, if the same behavioral mechanisms acted in C. marthae and C. subcristatus populations on Wolf Volcano, males would show higher δ15N during the period for which our data are informative (which can be very approximately estimated to encompass the months from January to June). Interestingly, our results suggest that this explanation might be reasonable for C. subcristatus population on Wolf Volcano as males of the species showed δ15N values higher than those of conspecific females (Fig. 1A), while this difference was not revealed for C. marthae (males and females were characterized by similar δ15N values; Fig. 1A), suggesting that this behavior may not be shared by the two syntopic populations.
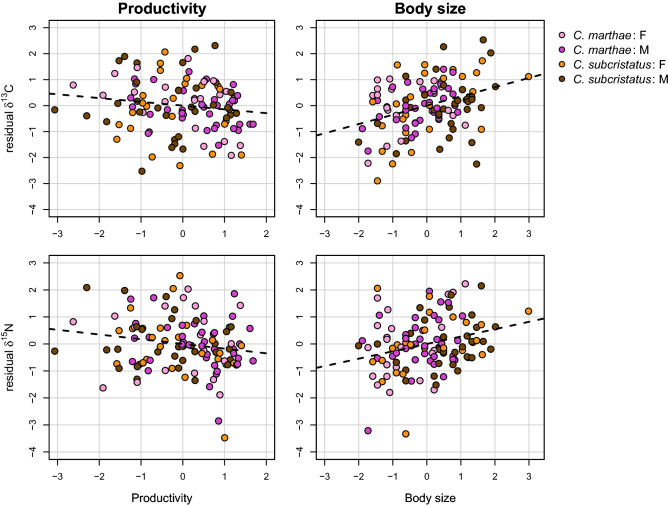
A large portion of the variance observed in δ13C and δ15N between species and sexes can be explained in terms of body size, productivity, and relative location of the capture points (Fig. 2). Moreover, these ecological variables provided the highest independent contribution to explaining δ13C and δ15N recorded in the samples (Fig. 2). We found that, other predictors being equal, larger individuals show higher levels of both δ13C and δ15N (Table 1, Fig. 3), suggesting that they potentially feed on a different sweet of resources. It is not uncommon to find a positive relation between body size and trophic level in natural populations73,74. Larger individuals often have a competitive advantage over smaller ones, allowing them to integrate their diet with trophic resources of animal origin that could not be available for smaller individuals. Accordingly, Hanson and colleagues75 found a positive relationship between 13C and SVL in Crocodylus porosus, suggesting that individuals of different body size are linked to different primary resources. Our data, therefore, also suggest that the most intense competition may happen between individuals of the same age or at least class size, but we lack the appropriate resolution in our data to analyze this aspect in more detail.
Figure 3.
Visualisation of the marginal effects of productivity (left) and body size (right) on δ 13C (top) and δ 15N (bottom). Residuals of reduced models (identical to Model 2 in Table 1, but with the variable of interest excluded) are plotted against the variable of interest. Each dot represents a single individual. Color codes are shown in the top right corner.
We also found a negative relation between the NDVI of samples location and both δ13C and δ15N (Fig. 3). On the one hand, different availability of C3, C4 and CAM plant resources in areas with different productivity may contribute to the differences in δ13C. On the other hand, as animal tissues are enriched in both δ13C and δ15N relative to their diet, a direct explanation of this pattern may involve a higher consumption of animal food by those iguanas that occupy less productive microhabitats compared to those that were captured in more productive habitats. Interestingly, Conolophus marthae individuals were mostly found in areas with higher levels of NDVI, if compared with C. subcristatus (SM Fig. 4). More vegetated areas also offer better shelter opportunities. Evidence in the field suggest that C. marthae may prefer shaded areas whereas C. subcristatus does not show this preference. These observations hint towards an ecological response to C. marthae’s skin depigmentation76. Therefore, this species might be found in more vegetated areas also because, here, iguanas might find a more shelters-enriched environment that could facilitate their basking-regulation behavior77.
Our study outlines that small-scale spatial pattern, both in terms of habitat productivity and of an undetermined spatial effect revealed by the tensor product smooth on capture locations coordinates, did affect the isotopic composition of samples (Table 1—Model 2, Model 3). On the one hand, we argued that this finding supports differential use of microhabitats by these two species. On the other hand, as the isotopic composition recorded for these species should reflect a timescale of a few to several months, this result strongly suggests that, at least in the time of the year for which our data may be informative, the feeding ranges of individuals of these two species may be extremely restricted. More in general, as our study only covered an area of ca. 2 km2, these results highlight the importance of considering the spatial dimension of processes when assessing ecological problems even at relatively small spatial scales.
Conclusions
In this study, we evaluated, for the first time, trophic competition between the critically endangered C. marthae and the syntopic C. subcristatus on Wolf Volcano. Competition was quantitatively estimated, and a mechanism of resource partitioning was described, through which the two congeneric species may coexist. Some trophic niche partitioning is also likely to exist between sexes, especially in C. subcristatus. Differences in isotopic composition between species and sexes are largely (but not entirely) explained by differential use of microhabitats and body size differences between and within species. While the indications of niche partitioning mechanisms between the two species suggest potential for coexistence of these two endemic iguanas, the observed extent of niche overlap raises concern over the impact that interspecific competition may have on the only known population of C. marthae. This urgently calls for a careful evaluation of conservation actions aimed at sustaining the only existing population which may prudentially include the creation of a new, viable population in a sanctuary area, in another suitable area of the archipelago. Finally, this study underlines the importance of considering small-scale spatial mechanisms when working in an ecological framework.
Supplementary Information
Acknowledgements
Data presented here result from a long-term program in collaboration between the Galápagos National Park and the University of Rome “Tor Vergata”, aimed at conserving Galápagos land iguanas. We thank the Galápagos National Park for the support given in this study. We are grateful to the Galápagos National Park Rangers, Martina Fabbri, and Carlos Vera for field and technical assistance. We are grateful for the comments and suggestions made by a panel of 5 reviewers.
Author contributions
G.G. designed the project; G.G., G.C. and C.S. collected samples in the field; M.B. and F.G. carried out laboratory analysis; M.G., G.C., P.G., G.G. and S.M. performed data analyses; M.B. designed the laboratory analysis; M.G. wrote the manuscript with significant input from G.C., P.G., S.M. and G.G. All authors provided feedback.
Funding
This work was supported in part by funding from the University of Rome “Tor Vergata” to G. Gentile.
Data availability
The data are available upon reasonable and documented request to the senior author.
Code availability
Code is available upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21134-2.
References
- 1.Luiselli L, Akani G, Capizzi D. Food resource partitioning of a community of snakes in a swamp rainforest of south-eastern Nigeria. J. Zool. 1998;246(2):125–133. doi: 10.1111/j.1469-7998.1998.tb00141.x. [DOI] [Google Scholar]
- 2.Rouag R, Djilali H, Gueraiche H, Luiselli L. Resource partitioning patterns between two sympatric lizard species from Algeria. J. Arid Environ. 2007;69:158–168. doi: 10.1016/j.jaridenv.2006.08.008. [DOI] [Google Scholar]
- 3.Bergeron R, Blouin-Demers G. Niche partitioning between two sympatric lizards in the Chiricahua Mountains of Arizona. Copeia. 2020;108(3):570–577. doi: 10.1643/CH-19-268. [DOI] [Google Scholar]
- 4.Lucek K, Butlin RK, Patsiou T. Secondary contact zones of closely-related Erebia butterflies overlap with narrow phenotypic and parasitic clines. J. Evol. Biol. 2020;33(9):1152–1163. doi: 10.1111/jeb.13669. [DOI] [PubMed] [Google Scholar]
- 5.Freeman BG. Competitive interaction upon secondary contact drive elevational divergence in tropical birds. Am. Nat. 2015;186(4):470–479. doi: 10.5061/dryad.6qg3g. [DOI] [PubMed] [Google Scholar]
- 6.Schoener TW. Resource partitioning in ecological communities. Science. 1974;185(4145):27–39. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- 7.Rivas LR. A Reinterpretation of the concepts “sympatric” and “allopatric” with proposal of the additional terms “syntopic” and “allotopic”. Syst. Zool. 1964;13(1):42. doi: 10.2307/2411436. [DOI] [Google Scholar]
- 8.Macarthur R, Levins R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967;101(921):377–385. doi: 10.1086/282505. [DOI] [Google Scholar]
- 9.Dayan T, Simberloff D. Ecological and community-wide character displacement: The next generation. Ecol. Lett. 2005;8(8):875–894. doi: 10.1111/j.1461-0248.2005.00791.x. [DOI] [Google Scholar]
- 10.Holomuzki JR, Feminella JW, Power ME. Biotic interactions in freshwater benthic habitats. J. N. Am. Benthol. Soc. 2010;29(1):220–244. doi: 10.1899/08-044.1. [DOI] [Google Scholar]
- 11.Ferretti F, Corazza M, Campana I, Pietrocini V, Brunetti C, Scornavacca D, Lovari S. Competition between wild herbivores: Reintroduced red deer and Apennine chamois. Behav. Ecol. 2015;26(2):550–559. doi: 10.1093/beheco/aru226. [DOI] [Google Scholar]
- 12.Takada H, Yano R, Katsumata A, Takatsuki S, Minami M. Diet compositions of two sympatric ungulates, the Japanese serow (Capricornis crispus) and the sika deer (Cervus nippon), in a montane forest and an alpine grassland of Mt. Asama central, Japan. Mamm. Biol. 2021;101:681–694. doi: 10.1007/s42991-021-00122-5. [DOI] [Google Scholar]
- 13.Hubbel SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press; 2001. [Google Scholar]
- 14.Bell G. Neutral macroecology. Science. 2001;293:2413–2418. doi: 10.1126/science.293.5539.2413. [DOI] [PubMed] [Google Scholar]
- 15.Rosindell J, Hubbel SP, Etienne RS. The unified neutral theory of biodiversity and biogeography at age ten. Trends Ecol. Evol. 2011;26(7):340–348. doi: 10.1016/j.tree.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Cowie RH, Holland BS. Dispersal is fundamental to biogeography and the evolution of biodiversity on oceanic islands. J. Biogeogr. 2006;33:193–198. doi: 10.1111/j.1365-2699.2005.01383.x. [DOI] [Google Scholar]
- 17.Amarasekare P, Nisbet RM. Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. Am. Nat. 2001;158(6):572–584. doi: 10.1086/323586. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, K., Gentile, G. & Grant, T. D. Conolophus subcristatus. The IUCN Red List of Threatened Species 2020, e.T5240A3014082 (2020). 10.2305/IUCN.UK.2020-2.RLTS.T5240A3014082.en
- 19.Gentile, G. Conolophus marthae. The IUCN Red List of Threatened Species 2012, e. T174472A1414375 (2012). 10.2305/IUCN.UK.2012-1.RLTS.T174472A1414375.en
- 20.Gentile G, Marquez C, Snell HL, Tapia W, Izurieta A. Conservation of a New Flagship Species: The Galápagos Pink Land Iguana (Conolophus marthae Gentile and Snell, 2009) In: Angelici FM, editor. Problematic Wildlife: A Cross-Disciplinary Approach. Springer International Publishing; 2016. pp. 315–336. [Google Scholar]
- 21.Gentile G, Snell HL. Conolophus marthae sp. Nov. (Squamata, iguanidae), a new species of land iguana from the Galápagos Archipelago. Zootaxa. 2009;2201:1–10. doi: 10.11646/zootaxa.2201.1.1. [DOI] [Google Scholar]
- 22.Colosimo G, Di Marco G, D’Agostino A, Gismondi A, Vera CA, Gerber GP, Scardi M, Canini A, Gentile G. Chemical signatures of femoral pore secretions in two syntopic but reproductively isolated species of Galápagos land iguanas (Conolophus marthae and C. subcristatus) Sci. Rep. 2020;10(1):14314. doi: 10.1038/s41598-020-71176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson M. Galápagos: A Natural History, Revised and Expanded. University of Calgary Press; 1994. [Google Scholar]
- 24.Traveset A, Nogales M, Vargas P, Rumeu B, Olesen JM, Jaramillo P, Heleno RH. Galápagos land iguana (Conolophus subcristatus) as a seed disperser. Integr. Zool. 2016;11(3):207–213. doi: 10.1111/1749-4877.12187. [DOI] [PubMed] [Google Scholar]
- 25.Di Giambattista L, Fulvo A, Fabiani A, Bonanni J, Carrión JE, Gentile G. Molecular data exclude current hybridization between iguanas Conolophus marthae and C. subcristatus on Wolf volcano (Galápagos islands) Conserv. Genet. 2018;19(6):1461–1469. doi: 10.1007/s10592-018-1114-3. [DOI] [Google Scholar]
- 26.MacLeod A, Rodríguez A, Vences M, Orozco-terWengel P, García C, Trillmich F, Gentile G, Caccone A, Quezada G, Steinfartz S. Hybridization masks speciation in the evolutionary history of the Galápagos marine iguana. Proc. R. Soc. B. 2015;282:1–9. doi: 10.1098/rspb.2015.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gause GF. The Struggle for Existence. Williams and Wilkins Company; 1934. [Google Scholar]
- 28.Hardin G. The competitive exclusion principle. Science. 1960;131(3409):1292–1297. doi: 10.1126/science.131.3409.1292. [DOI] [PubMed] [Google Scholar]
- 29.Ashrafi S, Beck A, Rutishauser M, Arlettaz R, Bontadina F. Trophic niche partitioning of cryptic species of long-eared bats in Switzerland: Implications for conservation. Eur. J. Wildl. Res. 2011;57:843–849. doi: 10.1007/s10344-011-0496-z. [DOI] [Google Scholar]
- 30.Bleyhl B, Arakelyan M, Askerov E, Bluhm H, Gavashelishvili A, Ghasabian M, Ghoddousi A, Heidelberg A, Khorozyan I, Malkhasyan A, Manvelyan K, Masoud M, Moqanaki EM, Radeloff V, Soofi M, Weinberg P, Zazanashvili N, Kuemmerle T. Assessing niche overlap between domestic and threatened wild sheep to identify conservation priority areas. Divers. Distrib. 2019;25(1):129–141. doi: 10.1111/ddi.12839. [DOI] [Google Scholar]
- 31.Newsome SD, del Rio CM, Bearhop S, Phillips DL. A niche for isotopic ecology. Front. Ecol. Environ. 2007;5(8):429–436. doi: 10.1890/060150.1. [DOI] [Google Scholar]
- 32.Riera P, Stal LJ, Nieuwenhuize J. δ13C versus δ15N of co-occurring mollusks within a community dominated by Crassostrea gigas and Crepidula ornicate (Oossterschelde, The Netherlands) Mar. Ecol. Prog. Ser. 2002;240:291–295. doi: 10.3354/meps240291. [DOI] [Google Scholar]
- 33.Page B, McKenzie J, Goldsworthy SD. Dietary resources partitioning among sympatric New Zealand and Australian fur seals. Mar. Ecol. Prog. Ser. 2005;293:283–302. doi: 10.3354/meps293283. [DOI] [Google Scholar]
- 34.DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta. 1978;42(5):495–506. doi: 10.1016/0016-7037(78)90199-0. [DOI] [Google Scholar]
- 35.DeNiro MJ, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta. 1981;45(3):341–351. doi: 10.1016/0016-7037(81)90244-1. [DOI] [Google Scholar]
- 36.Post DM. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology. 2002;83(3):703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2. [DOI] [Google Scholar]
- 37.Crawford K, McDonald RA, Bearhop S. Applications of stable isotope techniques to the ecology of mammals. Mammal. Rev. 2008;38(1):87–107. doi: 10.1111/j.1365-2907.2008.00120.x. [DOI] [Google Scholar]
- 38.Trueman M, d’Ozouville N. Characterizing the Galápagos terrestrial climate in the face of global climate change. Gala Res. 2010;67:26–37. [Google Scholar]
- 39.Paltán HA, Benitez FL, Rosero P, Escobar-Camacho D, Cuesta F, Mena CF. Climate and sea surface trends in the Galápagos Islands. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-93870-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivas-Torres GF, Benítez FL, Rueda D, Sevilla C, Mena CF. A methodology for mapping native and invasive vegetation coverage in archipelagos: An example from the Galápagos islands. Prog. Phys. Geogr. 2018;42(1):83–111. doi: 10.1177/0309133317752278. [DOI] [Google Scholar]
- 41.Gentile G, Ciambotta M, Tapia W. Illegal wildlife trade in Galápagos: Molecular tools help taxonomic identification and guide rapid repatriation of confiscated iguanas. Conserv. Genet. Resour. 2013;5:867–872. doi: 10.1007/s12686-013-9915-7. [DOI] [Google Scholar]
- 42.Stephens RB, Ouimette AP, Hobbie EA, Rowe RJ. Re-evaluating trophic discrimination factors (Δδ13C and Δδ15N) for diet reconstruction. Ecol. Mono. 2022;92:e1525. doi: 10.1002/ecm.1525. [DOI] [Google Scholar]
- 43.Hobson KA, Clark RG. Assessing avian diets using stable isotopes I: Turnover of 13C in tissues. The Condor. 1992;94(1):181–188. doi: 10.2307/1368807. [DOI] [Google Scholar]
- 44.Li C-H, Roth JD, Detwiler JT. Isotopic turnover rates and diet-tissue discrimination depend on feeding habits of freshwater snails. PLoS ONE. 2018;13(7):e0199713. doi: 10.1371/journal.pone.0199713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinitz R, Lemm J, Pasachnik S, Kurle C. Diet-tissue stable isotope (δ13C and δ15N) discrimination factors for multiple tissues from terrestrial reptiles. Rapid Commun. Mass Spectrom. 2016;30(1):9–21. doi: 10.1002/rcm.7410. [DOI] [PubMed] [Google Scholar]
- 46.Ethier DM, Kyle CJ, Kyser TK, Nocera JJ. Variability in the growth patterns of the cornified claw sheath among vertebrates: Implications for using biogeochemistry to study animal movement. Can. J. Zool. 2010;88(11):1043–1051. doi: 10.1139/Z10-073. [DOI] [Google Scholar]
- 47.Aresco, M. J. & James, F. C. Ecological relationships of turtles in northern Florida lakes: A study of omnivory and the structure of a lake food web. Florida Fish and Wildlife Conservation Commission (2005). https://www.semanticscholar.org/paper/ECOLOGICAL-RELATIONSHIPS-OF-TURTLES-IN-NORTHERN-A-A-Aresco-James/f6d59265eb6494aa19cfde7d2d80bb165e6432ac
- 48.Lourenço PM, Granadeiro JP, Guilherme JL, Catry T. Turnover rates of stable isotopes in avian blood and toenails: Implications for dietary and migration studies. J. Exp. Mar. Biol. Ecol. 2015;472:89–96. doi: 10.1016/j.jembe.2015.07.006. [DOI] [Google Scholar]
- 49.Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER—Stable isotope Bayesian ellipses in r. J. Animal Ecol. 2011;80(3):595–602. doi: 10.1111/j.1365-2656.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- 50.Wikelski M, Romero LM. Body size, performance and fitness in Galápagos marine iguanas. Integr Comp Biol. 2003;43(3):376–386. doi: 10.1093/icb/43.3.376. [DOI] [PubMed] [Google Scholar]
- 51.Iverson J, Smith G, Pieper L. Factors Affecting Long-Term Growth of the Allen Cays Rock Iguana in the Bahamas. In: Alberts A, Carter R, Hayes W, Martins E, editors. Iguanas: Biology and Conservation. University of California Press; 2004. pp. 176–192. [Google Scholar]
- 52.Smith GR, Iverson JB. Effects of tourism on body size, growth, condition, and demography in the Allen Cay Iguana. Herpetol. Conserv. Biol. 2016;11:214–221. [Google Scholar]
- 53.Wikelski M, Carrillo V, Trillmich F. Energy limits to body size in a grazing reptile, the Galápagos Marine Iguana. Ecology. 1997;78(7):2204–2217. doi: 10.2307/2265956. [DOI] [Google Scholar]
- 54.Bulakhova NA, Böhme W, Clasen A, Orlova VF, Carretero MA, Kuranova VN, Ljubisavljevic K, Roitberg ES, Shamgunova RR, Fokt M. Inter-observer and intra-observer differences in measuring body length: A test in the common lizard, Zootoca vivipara. Amphibia-Reptilia. 2011;32(4):477–484. doi: 10.1163/156853811X601636. [DOI] [Google Scholar]
- 55.R Development Core Team. R: A language and environment for statistical computing (2021). https://cran.r-project.org
- 56.Goslee SC, Urban DL. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 2007;22(7):1–19. doi: 10.18637/jss.v022.i07. [DOI] [Google Scholar]
- 57.Randin CF, Jaccard H, Vittoz P, Yoccoz NG, Guisan A. Land use improves spatial predictions of mountain plant abundance but not presence–absence. J. Veg. Sci. 2009;20:996–1008. doi: 10.1111/j.1654-1103.2009.01098.x. [DOI] [Google Scholar]
- 58.Broennimann, O., Di Cola, V. & Guisan, A. ecospat: Spatial Ecology Miscellaneous Methods. R package version 3.2.1 (2022) https://CRAN.R-project.org/package=ecospat
- 59.Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 1992;73(3):1045–1055. doi: 10.2307/1940179. [DOI] [Google Scholar]
- 60.Wood SN. Generalized Additive Models: An Introduction with R. 2. Chapman and Hall/CRC; 2017. [Google Scholar]
- 61.Van Marken Lichtenbelt WD. Optimal foraging of a herbivorous lizard, the green iguana in a seasonal environment. Oecologia. 1993;95:246–256. doi: 10.1007/BF00323497. [DOI] [PubMed] [Google Scholar]
- 62.Pasachnik SA, Martin-Velez V. An evaluation of the diet of Cyclura iguanas in the Dominican Republic. Herpetol. Bull. 2017;140:6–12. [Google Scholar]
- 63.Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR. Global vegetation change through the Miocene/Pliocene boundary. Nature. 1997;389(6647):153–158. doi: 10.1038/38229. [DOI] [Google Scholar]
- 64.O’Leary MH. Carbon isotopes in photosynthesis. Bioscience. 1988;38(5):328–336. doi: 10.2307/1310735. [DOI] [Google Scholar]
- 65.Snell HL, Tracy CR. Behavioral and morphological adaptations by Galapagos land iguanas (Conolophus subcristatus) to water and energy requirements of eggs and neonates. Am. Zool. 1985;25(4):1009–1018. doi: 10.1093/icb/25.4.1009. [DOI] [Google Scholar]
- 66.Christian K, Tracy CR, Porter WP. Diet, digestion, and food preferences of Galápagos land iguanas. Herpetologica. 1984;40(2):205–212. [Google Scholar]
- 67.Mallona I, Egea-Cortines M, Weiss J. Conserved and divergent rhythms of crassulacean acid metabolism-related and core clock gene expression in the cactus Opuntia ficus-indica. Plant Physiol. 2011;156:1978–1989. doi: 10.1104/pp.111.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.San Sebastián O, Navarro J, Llorente GA, Richter-Boix Á. Trophic strategies of a non-native and a native amphibian species in shared ponds. PLoS ONE. 2015;10(6):1–17. doi: 10.1371/journal.pone.0130549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perga ME, Grey J. Laboratory measures of isotope discrimination factors: Comments on Caut, Angulo & Courchamp (2008, 2009) J. Appl. Ecol. 2010;47(4):942–947. doi: 10.1111/j.1365-2664.2009.01730.x. [DOI] [Google Scholar]
- 70.Freeman B. Sexual niche partitioning in two species of new Guinean Pachycephala whistlers. J. Field Ornithol. 2014;85(1):23–30. doi: 10.1111/jofo.12046. [DOI] [Google Scholar]
- 71.Werner DI. Social Organization and Ecology of Land Iguanas, Conolophus subcristatus, on Isla Fernandina, Galápagos. In: Burghardt GM, Rand AS, editors. Iguanas of the World: Their Behavior, Ecology, and Conservation. Noyes Publications; 1982. pp. 342–365. [Google Scholar]
- 72.Doi H, Akamatsu F, González AL. Starvation effects on nitrogen and carbon stable isotopes of animals: An insight from meta-analysis of fasting experiments. R. Soc. Open Sci. 2017;4(8):170633. doi: 10.1098/rsos.170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Persaud A, Dillon P, Molot L, Hargan K. Relationships between body size and trophic position of consumers in temperate freshwater lakes. Aquat. Sci. 2012;74(1):203–212. doi: 10.1007/s00027-011-0212-9. [DOI] [Google Scholar]
- 74.Keppeler FW, Olin JA, López-Duarte PC, Polito MJ, Hooper-Bùi LM, Taylor SS, Rabalais NN, Fodrie FJ, Roberts JR, Turner RE, Martin CW, Jensen OP. Body size, trophic position, and the coupling of different energy pathways across a saltmarsh landscape. Limnol. Oceanogr. Lett. 2021;6(6):360–368. doi: 10.1002/lol2.10212. [DOI] [Google Scholar]
- 75.Hanson JO, Salisbury SW, Campbell HA, Dwyer RG, Jardine TD, Franklin CE. Feeding across the food web: The interaction between diet, movement and body size in estuarine crocodiles (Crocodylus porosus) Austral. Ecol. 2015;40(3):275–286. doi: 10.1111/aec.12212. [DOI] [Google Scholar]
- 76.Gustavino, B., Terrinoni, S., Paglierani, C. & Gentile, G. Conolophus marthae vs. Conolophus subcristatus: Does the skin pigmentation pattern exert a protective role against DNA damaging effect induced by UV light exposure? Analysis of blood smears through the micronucleus test. Paper presented at the Galápagos Land and Marine Iguanas Workshop, IUCN SSC Iguana Specialist Group Meeting, Puerto Ayora, 28–29 October 2014.
- 77.Di Giacomo C, Pucillo L, Sevilla C, Fucci G, Massoud R, Bernardini S, Fraziano M, Gentile G. 25–Hydroxivitamin D plasma levels in natural populations of pigmented and partially pigmented land iguanas from Galápagos (Conolophus spp.) Hind. 2022;2022:1–9. doi: 10.1155/2022/7741397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18(7):e3000411. doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon reasonable and documented request to the senior author.
Code is available upon reasonable request.