Abstract
Major Depressive Disorder (MDD) is a widespread mental illness that causes considerable suffering, and neuroimaging studies are trying to reduce this burden by developing biomarkers that can facilitate detection. Prior fMRI- and neurostimulation studies suggest that aberrant subgenual Anterior Cingulate (sgACC)—dorsolateral Prefrontal Cortex (DLPFC) functional connectivity is consistently present within MDD. Combining the need for reliable depression markers with the electroencephalogram’s (EEG) high clinical utility, we investigated whether aberrant EEG sgACC–DLPFC functional connectivity could serve as a marker for depression. Source-space Amplitude Envelope Correlations (AEC) of 20 MDD patients and 20 matched controls were contrasted using non-parametric permutation tests. In addition, extracted AEC values were used to (a) correlate with characteristics of depression and (b) train a Support Vector Machine (SVM) to determine sgACC–DLPFC connectivity’s discriminative power. FDR-thresholded statistical maps showed reduced sgACC–DLPFC AEC connectivity in MDD patients relative to controls. This diminished AEC connectivity is located in the beta-1 (13–17 Hz) band and is associated with patients’ lifetime number of depressive episodes. Using extracted sgACC–DLPFC AEC values, the SVM achieved a classification accuracy of 84.6% (80% sensitivity and 89.5% specificity) indicating that EEG sgACC–DLPFC connectivity has promise as a biomarker for MDD.
Subject terms: Depression, Predictive markers, Psychology
Introduction
Major Depressive Disorder (MDD) is a severe, widespread and often recurring psychiatric illness that is primarily characterized by a loss of experiencing pleasure, persistent sad mood, sleep disturbances, changes in appetite and impaired concentration1. The lifetime prevalence of MDD is estimated around 20% and 30% for men and women respectively2. In addition, the societal and economic burden of MDD is considerable due to an increase in absenteeism, alcohol- and drug related issues, suicide attempts and general illness comorbidity, making MDD a leading cause of disability3. It is therefore imperative to focus research efforts on the development of reliable and practical biomarkers of MDD that can be applied in clinical settings.
In the past decade, research investigating neural correlates of MDD has shifted focus from identifying specific dysfunctional brain regions to examining intrinsic neural networks implicated in depression4. One of these networks is the cognitive control network (CCN) which comprises the dorsolateral prefrontal cortex (DLPFC), the anterior cingulate cortex (ACC) and the parietal cortex5–7. The CCN’s main function is to regulate cognition and behavior in the pursuit of internal goals by way of directing attention towards task relevant stimuli while simultaneously inhibiting task irrelevant stimuli8,9. In patients with depression the CCN’s connectivity is diminished, resulting in difficulties with both sustained attention and the downregulating of negative emotions1,10,11. There is mounting evidence showing the involvement of the CCN in regulating both positive and negative emotions through indirect top-down connections with limbic regions in which the subgenual ACC (sgACC) serves as a gatekeeper between the cognitive- and emotional network12–17. In short, the sgACC has dense connections with the amygdala and other regions of the limbic system18–20, constituting a brain network involved in emotional processing4,21,22. Moreover, the sgACC seems to project information from this affective network to the CCN’s frontal cortical regions1,12,13, resulting in top-down emotion regulation.
Abnormalities in the DLPFC and sgACC have been consistently found in MDD patients. Specifically, the sgACC seems hyperactive in depression and successful treatment leads to a normalization of this hyperactivity23–25. In contrast, the DLPFC has been found to be hypoactive in MDD patients26–29 and restoring DLPFC activity seems to elicit an antidepressant response29,30. As a result, both the left- and right DLPFC have become popular targets for noninvasive neurostimulation techniques such as Transcranial Magnetic Stimulation (TMS) in the treatment of severe depression31–36. Fox et al. posit that the left DLPFC and the sgACC are intrinsically anticorrelated during rest and that this anticorrelation is exacerbated in MDD24. Consistent with this notion, a neurostimulation study from Baeken and colleagues found stronger sgACC–DLPFC anticorrelations in responders before high frequency TMS treatment while observing a normalization in sgACC–DLPFC connectivity after remission23. Interestingly, applying low frequency TMS to the right DLPFC also seems to normalize sgACC hyperactivity34 and a meta-analysis that compared the clinical efficacy of both treatments found them equally effective31. Consistent with these neurostimulation findings, functional Magnetic Resonance Imaging (fMRI) studies reported reduced functional connectivity between the DLPFC and the dorsal ACC in late-life depression patients during an executive-control task37 and when at rest38. Taken together, aberrant sgACC–DLPFC connectivity seems to be a robust component of MDD, making it a promising candidate as a biomarker for depression.
A reliable biomarker should be an objective measurement of the physiologic or pathologic processes underlying a biological trait or illness39. Moreover, a biomarker that is being applied in diagnostics or treatment outcome should be relatively accessible for it to have any clinical relevance. Indeed, a biomarker has little clinical value if the operational costs are too high or the equipment necessary for measuring it is too scarce. For example, even though MDD biomarkers that are based on fMRI studies produce valuable information regarding the neurobiological underpinnings of the disease, they are seldom used in clinical practice. Since acquiring and operating MRI’s is expensive, hospitals will simply prioritize more urgent clinical matters than an MDD diagnosis. In contrast, the electroencephalogram (EEG) is a relatively affordable, time-efficient, and commonly available neuroimaging tool that is already routinely used in psychiatric and neurologic departments during a patient’s hospital admission40. Furthermore, EEG’s temporal resolution is superior when compared to most other neuroimaging techniques. For example, fMRI’s Blood Oxygenation level-dependent (BOLD) functional connectivity is based on the relatively slow hemodynamic response, restricting its temporal resolution to around 1 Hz. EEG can measure the brains electrical activity with a precision of milliseconds, resulting in a temporal resolution of more than 1000 Hz depending on the sampling rate capabilities of the amplifier. This allows researchers to estimate the functional connectivity of neural oscillations in real time over an extensive frequency range which can reveal unique electrophysiologic frequency signatures of neural processes.
One notable disadvantage of EEG is the low spatial precision which arises from the diffusion of the electrical signals caused by volume conduction of the skull41. In brief, electrical sources in the brain are projected on the scalp which are then measured by the EEG’s electrodes. However, volume conduction and the mixing of electrical signals introduces spatial artifacts, distorting scalp projections. For instance, a relatively small source localized in the occipital cortex could present as a large frontal projection42. These spatial artifacts are especially problematic when estimating functional connectivity since, diffused electrical signals measured by different electrodes could originate from the same neural source which would result in spurious connectivity values43. Fortunately, great strides have been made to minimize spatial artifacts stemming from volume conduction. Advancements in source estimation techniques such as the development of realistic head models44,45, high-density EEG (whole-head electrode coverage)46 and more reliable linear inverse solutions47 substantially improve the spatial accuracy of electrophysiological source models48. Consequently, numerous methods to estimate EEG resting state functional connectivity have been developed49. A study from Colclough and colleagues reports that out of 12 electromagnetic connectivity measures, amplitude envelope correlation (AEC) and partial correlation measures have the best intra-subject and between group consistency50; while another study found AEC to best mirror connectivity results obtained using fMRI51, making AEC an excellent measure of connectivity that can be compared across modalities.
While some EEG studies have looked at general spectral signatures of aberrant network functional connectivity in depression52,53, none have investigated whether disturbances in resting state sgACC–DLPFC functional connectivity could be utilized as a potential biomarker for MDD. Therefore, the aim of the current EEG study was twofold. Firstly, to replicate fMRI and neurostimulation studies that observed disturbances in connectivity between the DLPFC and the sgACC in depression and secondly, to evaluate whether this EEG sgACC–DLPFC functional connectivity has any reliable biomarker capabilities. The latter goal was attempted by training a support vector machine (SVM) on the estimated sgACC–DLPFC connectivity values and subsequently test the SVM performance to reliably distinguish individual MDD patients from healthy controls based on these connectivity values. Compared to other machine learning methods, SVM has some unique properties that are advantageous in the context of identifying psychiatric biomarkers such as the ability to analyze high-dimensional datasets with small sample sizes54. We chose to include a supervised machine learning approach, since accurately identifying MDD patients should be an essential attribute of any clinically relevant biomarker of depression.
Methods
Participants
The study sample is comprised of 40 participants including 20 MDD patients (13 females; mean age: 36.6, sd: 13.1) and 20 healthy controls (15 females; mean age: 41.25, sd: 14.64). The sample was originally collected for an ERP study performed by Vanderhasselt and colleagues in which they examined inhibitory control performance during MDD episodes55. Although the current study uses the EEG resting state of the same participants, it has no further relation with the ERP study beyond the concurrent collection of the data. The MDD patients were recruited from a psychiatric care facility where they were screened by a licensed psychiatrist using the Mini-International Neuropsychiatric Interview (MINI)56 and a structured clinical interview. Furthermore, all of the patients met the DSM-IV-TR diagnostic criteria of unipolar major depression and MDD severity was assessed with both the 17-item Hamilton Depression Rating Scale (HDRS)57 and the 21-item Beck Depression Inventory (BDI-II)58. Depression severity was repeatedly verified one week before- and at the time of testing, confirming the presence of MDD during data collection. Patient exclusion criteria included comorbid mood disorders with the exception of anxiety disorders, history of psychotic episodes or use of anti-psychotic medications, tricyclic anti-depressants and/or long-lasting benzodiazepines, a history of neurological conditions such as loss of consciousness for more than 5 min, head injuries, epilepsy, history of electroconvulsive therapy, past or present alcohol/substance abuse, and learning disorders. All patients were taking either Selective Serotonin Reuptake Inhibitors (SSRI’s) or Selective Noradrenalin Reuptake Inhibitors (SNRI’s) during data collection. Healthy controls were recruited through newspaper advertisements and had no history of depression or other psychiatric disorders. Additionally, all healthy controls were medication free, and all had a BDI-II score of < 14 and a HDRS score of < 7 during EEG data acquisition. All 40 participants received renumeration for participating and signed informed consent. Lastly, the medical ethics committee of the Ghent University Hospital approved of the study’s aim, methods, purposes, and the data collection was performed in accordance with the Ghent University Hospital ethics committee guidelines.
EEG procedure and preprocessing
The EEG resting state data were acquired using a 128-channel Biosemi Active Two system (http://www.biosemi.com) within an electrically and acoustically shielded room. The EEG amplifier sampled the data at 512 Hz and employed an analogue filter with a bandwidth of 0.01–100 Hz. The data were referenced to the Common Mode Sense (CMS) active electrode and the Driven Right Leg (DRL) passive electrode. The EEG resting state was collected as 12-min segments (6 min eyes-closed and 6 min eyes-open, counterbalanced across subjects). Only eyes-closed segments were selected for analysis since the processing of visual scenes can have a confounding effect on the participants resting state data59.
A semi-automatic preprocessing pipeline was applied in MATLAB (version 2020b, The MathWorks, inc., Natick, MA) which utilized functions from the signal processing toolbox and the EEGLAB toolbox60. Digital offline filtering involved a 1 Hz high-pass and a 250 Hz low-pass filter. 50 Hz line noise was removed from the timeseries using the cleanline function which utilizes statistical thresholding to substract line noise estimations from the original signal61. Channels containing artifacts were identified using the clean_artifacts function using the following 3 parameters: (a) channel flatline lasting longer than five seconds, (b) channel noise exceeding four standard deviations relative to its own signal and (c) a correlation < 0.85 with its neighboring channels. General data cleaning was performed using the validated Artifact Subspace Reconstruction (ASR) method62. ASR decomposes the timeseries data into principal components and detects artifactual components by comparing specific components with components from the data’s cleanest segments. ASR then removes the artifactual components and reconstructs the timeseries with the remaining non-artifactual components. Before computing Independent Component Analysis (ICA), each subject’s EEG data was re-referenced to the average63 and a data rank correction was implemented. Following ICA decomposition, a Multiple Artifact Rejection Algorithm (MARA) was applied on the ICA components. MARA is a supervised machine learning algorithm that flags prevalent artifactual ICA components that represent eyeblink-, muscle-, noise- and electrocardiogram artifacts64. ICA components flagged by MARA were first visually inspected prior to removal. Lastly, omitted channels were interpolated using the spline interpolation method65 and both the preprocessed timeseries and its EEG frequency power spectra were visually inspected prior to data analysis.
Preliminary analysis
A preliminary analysis was conducted in R (version 4.1.1) on the subjects’ demographic- and clinical questionnaire data. Chi-square and student t-tests were performed to examine if the study groups differed in age, sex, education, and depression severity.
EEG source-space functional connectivity analysis
EEG functional connectivity was computed in source-space using the MATLAB toolbox Brainstorm66. The USCBrain atlas67 was selected as the shared brain anatomy template across participants. The USCBrain anatomy template (http://brainsuite.org/uscbrain-description/) is a high-resolution single-subject atlas that was created utilizing both anatomical- and functional data for cortex parcellation. Human connectome fMRI data68 from 40 subjects was used for the functional sub-parcellation which resulted in 65 regions of interest (ROIs) per hemisphere. Moreover, the Boundary Element Method was applied on the USCBrain anatomy template using OpenMEEG44 to produce a realistically shaped head model. This resulted in a head model that was identical for each subject. Individual sensor noise was estimated by calculating a noise covariance matrix on the resting state data of each participant. Only the diagonal elements of the noise covariance matrix were used as inputs to estimate sensor noise for the source localization model. Current density maps with unconstrained dipole orientations were generated using Minimum Norm69 as the source estimation method.
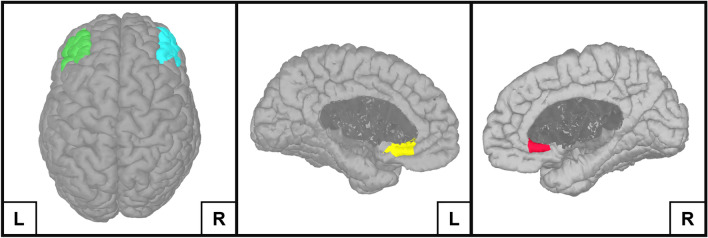
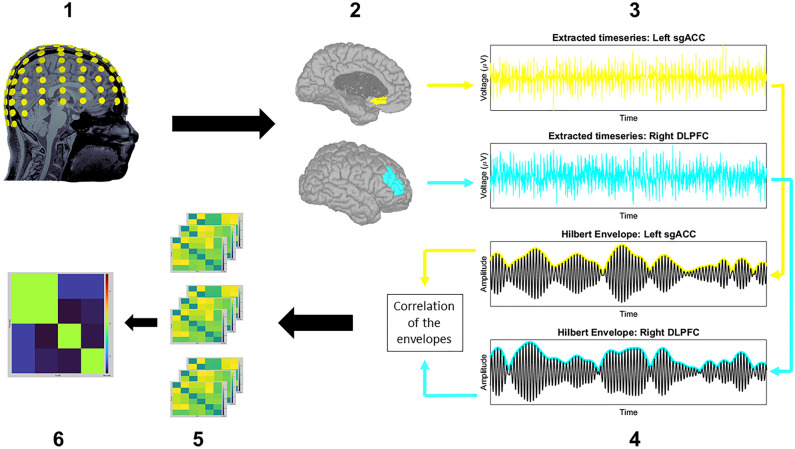
Based on the growing neuroimaging literature that demonstrates a disunion between sgACC–DLPFC activity in MDD23–25,29 and the brain network model for depression4, the left/right DLPFC and left/right sgACC of the USCBrain atlas were chosen as ROIs for the source-space functional connectivity analysis (Fig. 1). Functional connectivity was estimated between these four ROIs using the AEC method described by Brooks and colleagues51 (Fig. 2). In brief, AEC values are calculated by applying a Hilbert transform to the ROI-extracted timeseries data, resulting in band-pass filtered analytical signals. The magnitude is taken from these signals and is used to compute power envelopes. Before these power envelopes are calculated, a symmetric orthogonalization procedure removes instantaneous signals that are shared between the different ROIs. This results in ROI-extracted power envelopes that have been corrected for spatial leakage artifacts which are a major source of spurious connectivity values43. Finally, linear correlations are calculated between the power envelopes of the different ROIs, resulting in a matrix that contains the averaged connectivity values for each prespecified frequency band. Since the frequency signatures of MDD are insufficiently understood, we opted to take a broadband approach in our analysis. Based on the findings of prior factor-analyses70,71, we investigated the following EEG frequency bands: theta (4–8 Hz), alpha (8–12 Hz), beta-1 (13–17 Hz), beta-2 (18–24 Hz), beta-3 (25–30 Hz) and gamma (30–100 Hz).
Figure 1.
The four ROIs that were used in the source-space functional connectivity analysis performed in Brainstorm. The four ROIs include the left Dorsolateral Prefrontal Cortex (green), right Dorsolateral Prefrontal Cortex (turquoise), left subgenual Anterior Cingulate Cortex (yellow) and the right subgenual Anterior Cingulate Cortex (red).
Figure 2.
Estimating EEG source-space functional connectivity with the orthogonalized Amplitude Envelope Correlation method. The computation and statistical evaluation of orthogonalized Amplitude Envelope Correlations can be described in six steps: The EEG resting state timeseries data were transformed from sensor-space to source-space using the Minimum Norm source estimation method (1). Hypothesis driven regions of interest (ROIs) were selected from the USCBrain atlas (2) and their timeseries extracted (3) so that predefined band-pass filters together with an orthogonalization procedure could be applied. A Hilbert Transform was subsequently used to generate the power envelopes of these band-pass filtered signals (4). Correlations were then calculated between the power envelopes of different ROIs for each time sample. The average of these correlations was taken for each ROI pair which produced connectivity matrices for each frequency band of interest per subject (5). Non-parametric permutation tests were then performed, resulting in statistical maps that were thresholded using the false discovery rate (6).
Differences between the two groups source-space functional connectivity values were statistically evaluated by performing two-tailed non-parametric permutation t-tests on the connectivity matrices. These resulting t-value maps were thresholded using the Benjamini and Hochberg’s false discovery rate (FDR) method72, allowing us to correct for multiple comparisons introduced by the multiple ROIs and frequency bands.
Correlating EEG source-space functional connectivity values with MDD characteristics
The AEC values of the ROI pair with the strongest effect size were extracted and subsequently correlated with characteristics of depression, namely age of onset, lifetime number of episodes and duration of the current episode. The correlations were calculated in R using Monte Carlo permutations since AEC values are non-normally distributed. The association between MDD characteristics and AEC values was statistically evaluated using two-tailed, FDR-corrected tests of the Pearson’s correlation coefficient (r). In addition, a post-hoc analysis of covariance (ANCOVA) was used to examine if age, education level, sex and/or BDI-II scores had an influence on the association between connectivity values and MDD characteristics.
Evaluating the Predictive power of functional connectivity data on MDD diagnosis using a SVM classifier
A SVM was used (the MATLAB fitcsvm function) to test the predictive power of left/right sgACC—left/right DLPFC connectivity on MDD diagnosis. The SVM classifier was trained using the extracted connectivity values of 4 ROI pairs (left/right sgACC—left/right DLPFC) to distinguishes MDD patients from healthy controls according to prespecified dichotomic labels. We used a Gaussian kernel and the Sequential Minimal Optimization solver with a box constraint (the regularization parameter) of 3. All other parameters were kept on their default values. Furthermore, the performance was evaluated using the ‘leave-one-out’ cross-validation method to avoid the overfitting issue.
Results
Participant demographic and clinical characteristics
As expected, MDD patients scored significantly higher on BDI-II and HAM-D scores when compared to healthy controls, demonstrating an ongoing episode of MDD at the time of testing. In contrast, the analysis revealed no significant differences between MDD patients and healthy controls regarding age, sex, and education level. The results and test-statistics of the participants’ demographic and clinical characteristics are outlined in Table 1.
Table 1.
Participant demographic and clinical characteristics.
| HC (n = 20) | MDD (n = 20) | Test value | df | p value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Female, N (%) | 15 (75) | 13 (65) | χ2 = 0.12 | 1 | 0.73 |
| Age in years, mean (SD) | 41.25 (14.6) | 36.6 (13.1) | t = 1.06 | 37.55 | 0.297 |
| Education level, N (%) | HS = 4 (20) | HS = 8 (40) | χ2 = 2.93 | 2 | 0.231 |
| BA = 9 (45) | BA = 9 (45) | – | – | – | |
| MA = 7 (35) | MA = 3 (15) | – | – | – | |
| Symptomatology | |||||
| BDI-II | 1.45 (3.71) | 33.45 (11.41) | t = − 11.37 | 20.22 | < .001 |
| HAM-D | 0.2 (0.52) | 27.56 (5.31) | t = − 21.78 | 17.3 | < .001 |
Numerical data entries are in the form: mean (SD). Statistical evaluation was conducted with chi-square tests (χ2) and student t-tests (t).
HC healthy controls, MDD major depression disorder, df degrees of freedom, HS High School, BA Bachelor degree, MA Master degree, BDI-II Beck Depression Inventory II, HAM-D Hamilton Depression Rating Scale.
EEG source-space AEC functional connectivity differences between MDD patients and healthy controls
The two-tailed non-parametric permutation t-tests generated statistical maps that revealed significant differences in AEC functional connectivity between MDD patients and healthy controls within the beta-1 (13–17 Hz) and beta-2 (18–24 Hz) bands (p < 0.01, uncorrected for multiple comparisons). Both frequency bands showed diminished AEC functional connectivity for depressed patients and while this was limited to the left sgACC–right DLPFC ROI pair for the beta-2 band, all of the ROI pairs exhibited reduced connectivity within the beta-1 band. To control for the number of ROI pairs and frequency bands, an FDR threshold was applied (q < 0.05, adjusted p value = 0.0056) which yielded a thresholded connectivity map showing decreased AEC connectivity for MDD patients within the beta-1 band for the following three ROI pairs: left sgACC–right DLPFC, right sgACC–right DLPFC and left sgACC–right sgACC (Fig. 3).
Figure 3.
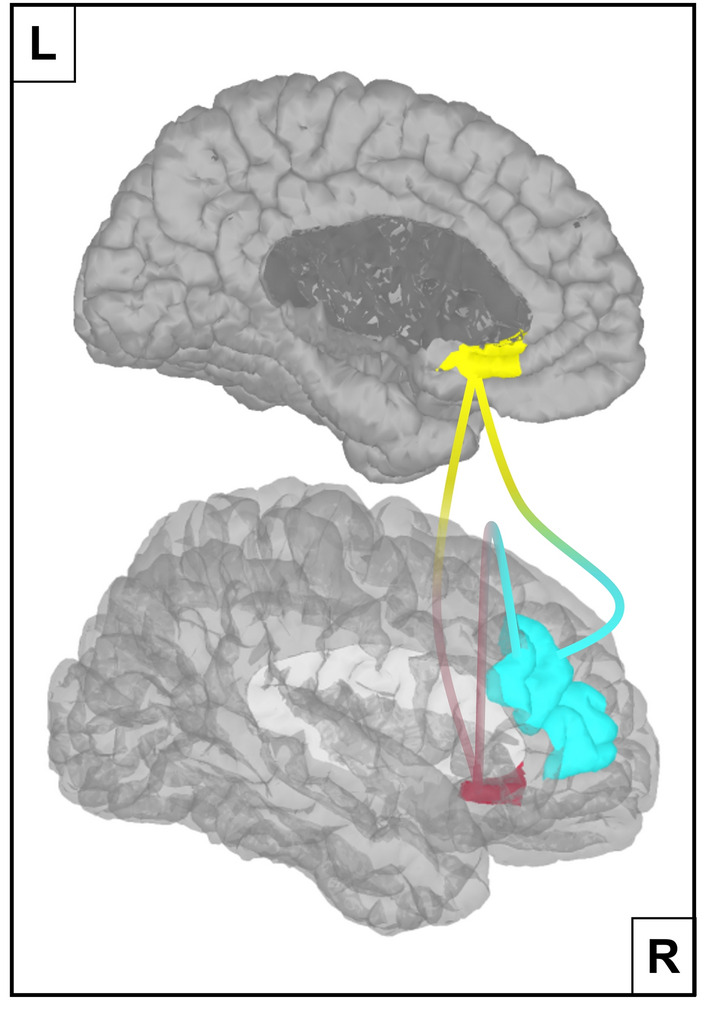
Diminished Amplitude Envelope Correlation connectivity in MDD patients. Depressed patients show reduced functional connectivity in the beta-1 (13–17 Hz) band for the following three ROI pairs: left sgACC (yellow)—right DLPFC (turquoise), right sgACC (red)—right DLPFC and left sgACC—right sgACC. This result was obtained after FDR thresholding. MDD: Major Depressive Disorder, sgACC: subgenual Anterior Cingulate Cortex, DLPCC: Dorsolateral Prefrontal Cortex, FDR: False Discovery Rate.
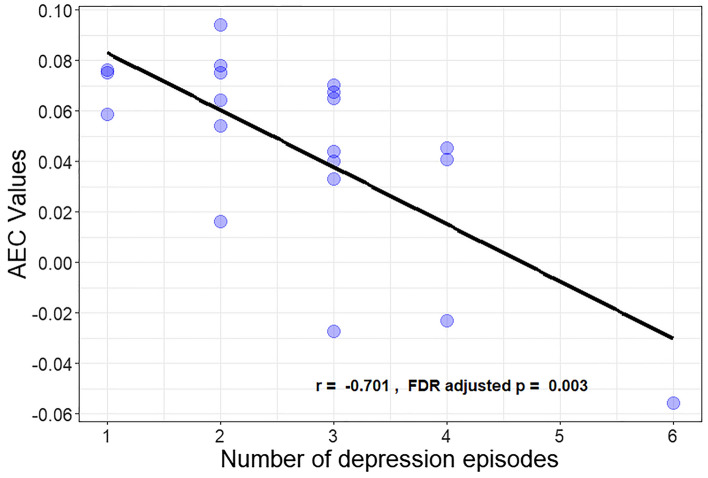
Association between AEC connectivity values and depression characteristics
Since the left sgACC—right DLPFC AEC values had the strongest effect size, they were extracted and subsequently correlated with MDD patients’ age of depression onset, lifetime number of episodes and duration of the current episode. The analysis revealed no significant correlation between patients age of depression onset and their extracted AEC values. However, a marginal negative association was found between current MDD duration and resting state functional connectivity (r = − 0.19, adjusted-p = 0.048, N = 20). More interestingly, reduced sgACC-DLPFC connectivity seems to be strongly correlated with the number of depressive episodes a patient has had in his/her lifetime (r = − 0.71, adjusted-p = 0.001, N = 20) (Fig. 4). Furthermore, this negative association remained significant after controlling for age, sex, education level and BDI-II scores (F(5,12) = 4.72, p = 0.014), suggesting that age and current MDD severity are not the driving factors between diminished sgACC–DLPFC connectivity and the total number of MDD episodes. This finding potentially signifies that reduced sgACC–DLPFC connectivity in the EEG resting state is a marker of a vulnerability to recurrent depressive episodes.
Figure 4.
Scatterplot demonstrating the negative association between the number of depression episodes and sgACC–DLPFC Amplitude Envelope Correlation connectivity for depressed patients. Amplitude Envelope Correlation connectivity values from left sgACC–right DLPFC were correlated with the number of depression episodes a patient has had in his/her lifetime, using non-parametric Pearson correlation coefficients. sgACC: subgenual Anterior Cingulate Cortex, DLPCC: Dorsolateral Prefrontal Cortex.
Reduced sgACC–DLPFC connectivity as a diagnostic marker for MDD
Using the ‘leave-one-out’ cross validation method, the SVM classifier was able to successfully identify 80% of MDD patients (model sensitivity) employing the EEG resting state connectivity values between the left/right sgACC and the left/right DLPFC (4 features). Moreover, 89.5% of healthy controls (model specificity) were accurately identified, resulting in an overall model accuracy of 84.6%.
Discussion
In order to minimize the extensive human- societal- and economic costs associated with depression, identifying usable markers of MDD will be an essential step toward that goal. Unfortunately, due to the infamous spatial issues associated with EEG in the past, research investigating EEG functional connectivity markers of depression is still in its infancy. To date, no EEG study has looked at aberrant sgACC–DLPFC connectivity within the resting state of MDD patients and thus our study addressed the question whether the EEG resting state contains disrupted sgACC–DLPFC connectivity within a sample of MDD patients and whether this aberrant connectivity has the potential to be utilized as a marker for depression.
Our findings revealed abnormal EEG resting state functional connectivity between the sgACC and the DLPFC in depressed patients. More concretely, depressive patients displayed diminished beta-1 (13–17 Hz) band AEC connectivity between the left/right sgACC and the right DLPFC when compared to healthy controls (Fig. 3). In addition, this reduced sgACC–DLPFC connectivity seems to be related to the total number of depressive episodes experienced during a patient’s lifetime (Fig. 4), even when age and current depression severity were taken into account. Lastly, sgACC–DLPFC connectivity within the EEG resting state seems to be a promising marker of depression when applying a SVM classifier, considering the relatively high model accuracy (i.e. 84.6%) based on only 4 features (left/right sgACC—left/right DLPFC) in this preliminary approach.
These results support the hypothesis of a negative association between DLPFC and sgACC activity in MDD patients when compared to healthy controls. Diminished sgACC–DLPFC connectivity in depression is likely to represent the inability of the CCN to inhibit a hyper-active limbic system which results in impaired top-down emotion regulation4,10,11. Furthermore, the DLPFC has been widely recognized to be an effective target site for noninvasive neurostimulation treatments with the aim to normalize DLPFC activity and to inhibit excessive sgACC reactivity which in turn reduces depression symptoms73–75. The DLPFC and sgACC seem to be inversely correlated in depression24 and successful TMS treatment normalizes this negative correlation23. Nevertheless, most studies report reduced functional connectivity between the left DLPFC and the sgACC while our results show diminished functional connectivity between the right DLPFC and the sgACC. Surprisingly, reducing the FDR threshold slightly (q = 0.06, adjusted p-value = 0.009) also reveals reduced connectivity between the left DLPFC and both left/right sgACC in the current study. It is possible that both the left- and right DLPFC have impaired connectivity with the sgACC in depressed patients, and this notion is supported not only by our own findings but by a meta-analysis that did not find a difference in clinical efficacy between TMS stimulation of either the left- or right DLPFC for treating depression31. In addition, Kito and colleagues reported reduced sgACC activity after successful low frequency TMS stimulation of the right DLPFC in treatment resistant depression patients34, demonstrating an overlap of therapeutic working mechanisms with high frequency TMS stimulation of the left DLPFC. Furthermore, the current data demonstrate that reduced EEG sgACC–DLPFC functional connectivity seems to be related to the total number of depressive episodes within a patient’s life. Remarkably, one of the few EEG studies that looked at disrupted network connectivity in depression found an association between the frequency of depressive episodes and hyperconnectivity between the Default Mode Network (DMN) and the CCN, also within the beta-1 band53. This between-network beta-1 band hyperconnectivity may reflect a weakened CCN being “hijacked” by an overactive DMN76,77, sharing an electrophysiologic signature similar to the CCN’s inability to regulate the sgACC hyperactivity. Although, this interpretation is compatible with our current finding, it is unable to address if aberrant EEG CCN connectivity reflects a biological vulnerability for a recurrent illness course or if recurrent depressive episodes increase the intensity of network abnormalities. Further support for an association between impaired cognitive control and the lifetime number of depression episodes can be found in a 2009 ERP study78. The authors observed an inverse correlation between the amplitude of a cognitive control-related ERP (N450) and the number of prior MDD episodes in a sample of remitted depression patients. These results not only corroborate the cumulative nature of cognitive control impairments in depression but also demonstrate that these deficits seem to persist, even when patients are in remission. This association between impaired cognitive control and recurrent depressive episodes is consistent with the kindling hypothesis of recurrent depression79. The kindling hypothesis states that psychosocial stressors become less relevant for depression onset with each subsequent depressive episode and that the onset of successive MDD episodes are increasingly independent of environmental influences and events. It is entirely plausible that impairments in cognitive control and difficulties regulating negative emotions increase the likelihood of future depression episodes through a reduction in an individual’s ability to cope with life events, big or small.
Some fMRI studies report divergent findings in which sgACC–DLPFC connectivity is enhanced in MDD patients. One study found a positive correlation between posterior sgACC–DLPFC connectivity and BDI-II scores in subjects with subclinical depression80, while Davey and colleagues observed increased pregenual ACC—left DLPFC connectivity in the fMRI resting state of depressed patients81. A plausible explanation for these inconsistent findings is that the DLPFC can become hyperactive in depression in an attempt to downregulate sgACC hyperactivity24. This hypothesis might seem inconsistent with our own observations but can potentially be explained by the differences in temporal resolution between EEG and fMRI. Since EEG measures neural activity directly, it is able to detect the instantaneous high frequency (> 4 Hz) discordant activity patterns between the sgACC and the DLPFC. In contrast, the slower and indirect BOLD signal of fMRI measures a low frequency (< 0.15 Hz) timeseries that could represent DLPFC activity that lags behind the hyperactive sgACC, resulting in increased connectivity values between the two regions. Similarly, a TMS-EEG study from Hadas and colleagues reported decreased effective connectivity between the left DLPFC and the sgACC after successful TMS treatment82. In addition, the reduction in left DLPFC–sgACC connectivity was associated with MDD symptoms improvement. This result seems to directly contradict our own observations of reduced DLPFC–sgACC connectivity within a sample of MDD patients. A possible reason for these divergent findings could result from methodological differences with respect to how connectivity was estimated. Our own study applied AEC on band-pass filtered signals to compute functional connectivity, whereas the study from Hadas and colleagues applied a form of effective connectivity on (broadband) current source density values (i.e. source localized voltage values). This explanation seems plausible, since other EEG resting state functional connectivity studies did observe increased beta-band connectivity between the DLPFC and the sgACC within MDD patients after either a successful TMS-83, or pharmacological treatment52, corroborating the current findings. In addition, Hadas and colleagues looked at DLPFC—sgACC connectivity changes within depression patients almost immediately after TMS stimulation (i.e. within a 500 ms interval), while our study examined EEG functional connectivity between depressed- and healthy participants at rest.
Lastly, sgACC–DLPFC AEC functional connectivity seems to have potential as a diagnostic marker for MDD within a machine learning framework. The model performance of 80% to accurately classify depression patients based on left/right sgACC—left/right DLPFC connectivity values is promising. Nevertheless, some considerations need to be kept in mind when interpreting machine learning classification results. The current study’s sample size is small and as a result overfitting can become a problem84. This is especially problematic in neuroimaging research that employs a large number of features85,86. In order to address this issue a SVM classifier was used which can be considered suitable when working with limited sample sizes. Additionally, the total amount of features was constrained through selecting hypothesized connectivity differences between patients and controls. One advantage of this approach is that it is less likely that model’s performance is based on irrelevant features specific to the current dataset. Another advantage of using a hypothesis driven feature selection approach is the increase in reliability and interpretability of the results. The disadvantage is that potentially valuable features (such as connectivity with other relevant regions) could have been excluded.
Future studies should therefore expand on these findings by attempting to replicate the current study in sizeable datasets. Besides increasing the validity of potential diagnostic markers, it would significantly improve the signal-to-noise ratio as well. This increase in signal power would allow researchers to reliably compute measures of dynamic functional connectivity such as estimating AEC values within predefined sliding time windows. These kind of EEG analyses can offer valuable insight into the temporal aspects of disrupted neural networks in MDD. Furthermore, an excellent signal-to-noise ratio would make it possible to develop certain spatial- and temporal filters that could drastically reduce the number of electrodes needed to reveal markers of depression, further boosting clinical applicability.
In conclusion, our results revealed diminished sgACC–DLPFC connectivity within a sample of MDD patients when compared to a sample of healthy controls. In addition, this reduced sgACC–DLPFC connectivity was associated with the total number of depression episodes a patient has experienced at the time of testing. The diagnostic power of this aberrant connectivity was evaluated using a SVM classifier which resulted in a model performance of 80% sensitivity, 89.5% specificity and 84.6% accuracy, suggesting that sgACC–DLPFC functional connectivity could be a potential diagnostic marker of depression.
Acknowledgements
The authors would like to thank Tasha Poppa and Gilles Pourtois for their assistance and feedback on the writing of the manuscript.
Author contributions
L.B. substantially contributed to the conception, analysis, interpretation and drafting of the main manuscript. G.V. and J.L. substantially contributed to the analysis and interpretation of the work. R.M.L substantially contributed to the conception and analysis of the work. M.A.V substantially contributed to the design, acquisition and interpretation of the work. C.B substantially contributed to the conception and interpretation of the work. All of the authors critically revised the manuscript and gave their final approval of the version being published.
Funding
Funding was provided by National Institutes of Health (Grant No. R01 EB026299) and Universiteit Gent (Grant No. BOFSTA2017002501).
Data availability
The data used in this manuscript are available from the corresponding author (Lars Benschop) on suitable request. The data is not publicly available due to the privacy and ethical restrictions regarding Ghent University Hospital’s patients privacy protection policy.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Marie-Anne Vanderhasselt and Chris Baeken.
References
- 1.Gotlib IH, Hamilton JP. Neuroimaging and depression: Current status and unresolved issues. Curr. Dir. Psychol. Sci. 2008;17:159–163. doi: 10.1111/j.1467-8721.2008.00567.x. [DOI] [Google Scholar]
- 2.World Health Organization . Depression and Other Common Mental Disorders: Global Health Estimates. World Health Organization; 2017. [Google Scholar]
- 3.Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 4.Li BJ, et al. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther. 2018;24:1004–1019. doi: 10.1111/cns.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 6.Niendam TA, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braver TS. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn. Sci. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Baene W, Brass M. Switch probability context (in)sensitivity within the cognitive control network. Neuroimage. 2013;77:207–214. doi: 10.1016/j.neuroimage.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Goeleven E, De Raedt R, Baert S, Koster EH. Deficient inhibition of emotional information in depression. J. Affect. Disord. 2006;93:149–157. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Wolkenstein L, Plewnia C. Amelioration of cognitive control in depression by transcranial direct current stimulation. Biol. Psychiatry. 2013;73:646–651. doi: 10.1016/j.biopsych.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 13.Greicius MD, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- 15.Koush Y, et al. Brain networks for engaging oneself in positive-social emotion regulation. Neuroimage. 2019;189:106–115. doi: 10.1016/j.neuroimage.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 16.Palomero-Gallagher N, et al. Functional organization of human subgenual cortical areas: Relationship between architectonical segregation and connectional heterogeneity. Neuroimage. 2015;115:177–190. doi: 10.1016/j.neuroimage.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scharnowski F, et al. The role of the subgenual anterior cingulate cortex in dorsomedial prefrontal-amygdala neural circuitry during positive-social emotion regulation. Hum. Brain Mapp. 2020;41:3100–3118. doi: 10.1002/hbm.25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen-Berg H, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy H, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- 22.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baeken C, et al. Accelerated HF-rTMS in treatment-resistant unipolar depression: Insights from subgenual anterior cingulate functional connectivity. World J. Biol. Psychiatry. 2014;15:286–297. doi: 10.3109/15622975.2013.872295. [DOI] [PubMed] [Google Scholar]
- 24.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Baxter LR, Jr, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 27.Biver F, et al. Frontal and parietal metabolic disturbances in unipolar depression. Biol. Psychiatry. 1994;36:381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- 28.Galynker II, et al. Hypofrontality and negative symptoms in major depressive disorder. J. Nucl. Med. 1998;39:608–612. [PubMed] [Google Scholar]
- 29.Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald PB, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: A meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210:1260–1264. doi: 10.1016/j.psychres.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald PB, Daskalakis ZJ. A practical guide to the use of repetitive transcranial magnetic stimulation in the treatment of depression. Brain Stimul. 2012;5:287–296. doi: 10.1016/j.brs.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Kito S, Fujita K, Koga Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology. 2008;58:29–36. doi: 10.1159/000154477. [DOI] [PubMed] [Google Scholar]
- 34.Kito S, Hasegawa T, Koga Y. Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Clin. Neurosci. 2011;65:175–182. doi: 10.1111/j.1440-1819.2010.02183.x. [DOI] [PubMed] [Google Scholar]
- 35.Lefaucheur JP, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018) Clin. Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: A meta-analysis. Psychol. Med. 2009;39:65–75. doi: 10.1017/S0033291708003462. [DOI] [PubMed] [Google Scholar]
- 37.Aizenstein HJ, et al. Altered functioning of the executive control circuit in late-life depression: Episodic and persistent phenomena. Am. J. Geriatr. Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexopoulos GS, et al. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesko LJ, Atkinson AJ., Jr Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: Criteria, validation, strategies. Annu. Rev. Pharmacol. Toxicol. 2001;41:347–366. doi: 10.1146/annurev.pharmtox.41.1.347. [DOI] [PubMed] [Google Scholar]
- 40.Boutros NN. A review of indications for routine EEG in clinical psychiatry. Hosp. Community Psychiatry. 1992;43:716–719. doi: 10.1176/ps.43.7.716. [DOI] [PubMed] [Google Scholar]
- 41.Da Silva FL, Schomer DL. Niedermeyer's Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins; 2018. [Google Scholar]
- 42.Smith EE, Reznik SJ, Stewart JL, Allen JJB. Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 2017;111:98–114. doi: 10.1016/j.ijpsycho.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. Oxford University Press; 2006. [Google Scholar]
- 44.Gramfort A, Papadopoulo T, Olivi E, Clerc M. OpenMEEG: Opensource software for quasistatic bioelectromagnetics. Biomed. Eng. Online. 2010;9:45. doi: 10.1186/1475-925X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vorwerk J, et al. A guideline for head volume conductor modeling in EEG and MEG. Neuroimage. 2014;100:590–607. doi: 10.1016/j.neuroimage.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 46.Song J, et al. EEG source localization: Sensor density and head surface coverage. J. Neurosci. Methods. 2015;256:9–21. doi: 10.1016/j.jneumeth.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Baillet S, Mosher JC, Leahy RM. Electromagnetic brain mapping. IEEE Signal Proc. Mag. 2001;18:14–30. doi: 10.1109/79.962275. [DOI] [Google Scholar]
- 48.Michel C, He B. EEG mapping and source imaging. Niedermeyer's Electroencephalogr. 2012;6:1179–1202. [Google Scholar]
- 49.Cohen MX. Analyzing Neural Time Series Data: Theory and Practice. The MIT Press; 2014. [Google Scholar]
- 50.Colclough GL, et al. How reliable are MEG resting-state connectivity metrics? Neuroimage. 2016;138:284–293. doi: 10.1016/j.neuroimage.2016.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brookes MJ, et al. Measuring functional connectivity using MEG: Methodology and comparison with fcMRI. Neuroimage. 2011;56:1082–1104. doi: 10.1016/j.neuroimage.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olbrich S, Trankner A, Chittka T, Hegerl U, Schonknecht P. Functional connectivity in major depression: Increased phase synchronization between frontal cortical EEG-source estimates. Psychiatry Res. 2014;222:91–99. doi: 10.1016/j.pscychresns.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Whitton AE, et al. Electroencephalography source functional connectivity reveals abnormal high-frequency communication among large-scale functional networks in depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2018;3:50–58. doi: 10.1016/j.bpsc.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neurosci. Biobehav. Rev. 2012;36:1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Vanderhasselt M-A, et al. Abnormal proactive and reactive cognitive control during conflict processing in major depression. J. Abnorm. Psychol. 2014;123:68–80. doi: 10.1037/a0035816. [DOI] [PubMed] [Google Scholar]
- 56.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 57.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 59.Fingelkurts AA, Fingelkurts AA. Altered structure of dynamic electroencephalogram oscillatory pattern in major depression. Biol. Psychiatry. 2015;77:1050–1060. doi: 10.1016/j.biopsych.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Bigdely-Shamlo N, Mullen T, Kothe C, Su KM, Robbins KA. The PREP pipeline: Standardized preprocessing for large-scale EEG analysis. Front Neuroinform. 2015;9:16. doi: 10.3389/fninf.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang CY, Hsu SH, Pion-Tonachini L, Jung TP. Evaluation of artifact subspace reconstruction for automatic artifact components removal in multi-channel EEG recordings. IEEE Trans. Biomed. Eng. 2020;67:1114–1121. doi: 10.1109/TBME.2019.2930186. [DOI] [PubMed] [Google Scholar]
- 63.Makeig, S., Bell, A. J., Jung, T.-P. & Sejnowski, T. J. Independent component analysis of electroencephalographic data, in Proceedings of the 8th International Conference on Neural Information Processing Systems 145–151 (MIT Press, 1995).
- 64.Winkler I, et al. Robust artifactual independent component classification for BCI practitioners. J. Neural Eng. 2014;11:035013. doi: 10.1088/1741-2560/11/3/035013. [DOI] [PubMed] [Google Scholar]
- 65.Perrin F, Pernier J, Bertrand O, Giard MH, Echallier JF. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr. Clin. Neurophysiol. 1987;66:75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- 66.Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011;2011:879716. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joshi, A., et al. A whole brain atlas with sub-parcellation of cortical gyri using resting fMRI (2017). [DOI] [PMC free article] [PubMed]
- 68.Glasser MF, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baillet, S., Mosher, J. C. & Leahy, R. M. Electromagnetic brain imaging using brainstorm, in 2004 2nd IEEE International Symposium on Biomedical Imaging: Macro to Nano, vols. 1 and 2, 652–655 (2004).
- 70.Kubicki S, Herrmann WM, Fichte K, Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsychiatr. Neuropsychopharmakol. 1979;12:237–245. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- 71.Ogawa T, et al. Developmental characteristics of the beta waves of EEG in normal healthy children. No To Hattatsu. 1989;21:424–429. [PubMed] [Google Scholar]
- 72.Yekutieli D, Benjamini Y. Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. J. Stat. Plann. Inference. 1999;82:171–196. doi: 10.1016/S0378-3758(99)00041-5. [DOI] [Google Scholar]
- 73.Leyman L, De Raedt R, Vanderhasselt MA, Baeken C. Influence of high-frequency repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex on the inhibition of emotional information in healthy volunteers. Psychol. Med. 2009;39:1019–1028. doi: 10.1017/S0033291708004431. [DOI] [PubMed] [Google Scholar]
- 74.O'Reardon JP, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol. Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 75.Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp. Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 76.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Northoff G. How do resting state changes in depression translate into psychopathological symptoms? From 'Spatiotemporal correspondence' to 'Spatiotemporal Psychopathology'. Curr. Opin. Psychiatry. 2016;29:18–24. doi: 10.1097/YCO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 78.Vanderhasselt MA, De Raedt R. Impairments in cognitive control persist during remission from depression and are related to the number of past episodes: An event related potentials study. Biol. Psychol. 2009;81:169–176. doi: 10.1016/j.biopsycho.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am. J. Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 80.Philippi CL, Motzkin JC, Pujara MS, Koenigs M. Subclinical depression severity is associated with distinct patterns of functional connectivity for subregions of anterior cingulate cortex. J. Psychiatr. Res. 2015;71:103–111. doi: 10.1016/j.jpsychires.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol. Med. 2012;42:2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- 82.Hadas I, et al. Subgenual cingulate connectivity and hippocampal activation are related to MST therapeutic and adverse effects. Transl. Psychiatry. 2020;10:392. doi: 10.1038/s41398-020-01042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kito S, et al. Transcranial magnetic stimulation modulates resting EEG functional connectivity between the left dorsolateral prefrontal cortex and limbic regions in medicated patients with treatment-resistant depression. J. Neuropsychiatry Clin. Neurosci. 2017;29:155–159. doi: 10.1176/appi.neuropsych.15120419. [DOI] [PubMed] [Google Scholar]
- 84.Arbabshirani MR, Plis S, Sui J, Calhoun VD. Single subject prediction of brain disorders in neuroimaging: Promises and pitfalls. Neuroimage. 2017;145:137–165. doi: 10.1016/j.neuroimage.2016.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whelan R, Garavan H. When optimism hurts: Inflated predictions in psychiatric neuroimaging. Biol. Psychiatry. 2014;75:746–748. doi: 10.1016/j.biopsych.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 86.Vabalas A, Gowen E, Poliakoff E, Casson AJ. Machine learning algorithm validation with a limited sample size. PLoS ONE. 2019;14:e0224365. doi: 10.1371/journal.pone.0224365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this manuscript are available from the corresponding author (Lars Benschop) on suitable request. The data is not publicly available due to the privacy and ethical restrictions regarding Ghent University Hospital’s patients privacy protection policy.