Abstract
Environmental iron concentrations coordinately regulate transcription of genes involved in iron acquisition and virulence via the ferric uptake regulation (fur) system. We identified and sequenced the fur gene and flanking regions of three Bartonella species. The most notable difference between Bartonella Fur and other Fur proteins was a substantially higher predicted isoelectric point. No promoter activity or Fur autoregulation was detected using a gfp reporter gene fused to the 204 nucleotides immediately upstream of the Bartonella fur gene. Bartonella henselae fur gene expression complemented a Vibrio cholerae fur mutant.
Bartonella, an extremely fastidious gram-negative bacillus, causes cat scratch disease, bacillary angiomatosis, and other syndromes (2, 13, 15, 29). Few data exist regarding the pathogenic mechanisms of this hemophilic bacterium (5), which can occupy two alternate niches: the iron-rich gut of obligately hematophagous arthropods and the iron-restricted bloodstream of mammals (11, 32). Acquisition of iron and expression of many virulence factors are under transcriptional regulation by the fur gene product, the ferric uptake regulation (Fur) protein, and its homodimeric complex (7). At sufficient intracellular iron levels, the corepressors Fur and Fe2+ form an active Fur-Fe2+ complex that binds a consensus sequence (“iron box,” a 19-bp hyphenated dyad repeat [22] or three repeats of 6 bp of the sequence NAT[A,T]AT [7]) in the promoter region of genes regulated by Fur, down-regulating genes encoding iron-scavenging proteins (7, 22). We hypothesized that Bartonella species possess a fur gene homolog with a gene regulatory system influenced by iron levels.
Bacterial strains.
Strains and plasmids used in this study are listed in Table 1. All Bartonella strains were used at low passage numbers (passes 1 through 3). B. henselae and B. quintana strains were grown on fresh chocolate agar (14), which provided a replete iron source. B. bacilliformis was grown on fresh heart infusion agar supplemented with 5% defibrinated rabbit blood (Hemostat Labs, Dixon, Calif.). Plates were incubated at 34°C (B. henselae and B. quintana) or 29°C (B. bacilliformis) in an enriched CO2 environment for 5 to 7 days. Iron availability to B. henselae and B. quintana was restricted by adding the ferric-specific chelating agent EDDHA (ethylene diamine dihydroxy-o-phenylacetic acid) (The Complete Green Company, El Segundo, Calif.) (25) or by decreasing the hemoglobin (Hb) concentration in agar.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Bartonellae | ||
| JK9R | B. henselae; human isolate from cutaneous bacillary angiomatosis lesion | J. E. Koehler |
| JK31 | B. quintana; human isolate from cutaneous bacillary angiomatosis lesion | J. E. Koehler |
| BBL | B. bacilliformis; human isolate from blood | Generous gift of Robert Gilman, Lima, Peru |
| V. cholerae | ||
| MBG40 | O395 irgA::TnphoA Smr Kmr | 20 |
| CML13 | O395 irgA::TnphoA fur::pCML13 Smr Kmr Apr | 20 |
| Plasmids | ||
| pANT3 | oriRSF1010 mob+ Kmr Smr; promoterless gfpmut3 | 18 |
| pSYP1 | pANT3 backbone; putative Bartonella fur promoter upstream from gfpmut3 | This study |
| pSYP2 | pANT3 backbone; inverted putative Bartonella fur promoter upstream from gfpmut3 | This study |
| pANT4 | oriRSF1010 bla+mob+laclq::Kmr; ptac promoter upstream from gfpmut3 | 18 |
| pSYP3 | pANT4 backbone; ptac promoter upstream from B. henselae fur | This study |
| pACYC184 | orip15A Cmr Tcr | New England BioLabs, Inc., Beverly, Mass. |
| pSYP4 | pACYC184 backbone; ptac promoter upstream from B. henselae fur cloned in place of tet gene | This study |
Vibrio strains were grown overnight in Luria-Bertani medium with the appropriate selective antibiotic(s), with or without the iron chelator 2,2-dipyridyl (Sigma-Aldrich, Inc., St. Louis, Mo.), as previously described (8). Because of limited growth at 37°C by CML13(pSYP4), all strains were grown at 30°C and then incubated at 37°C for 60 to 90 min before assays. Selective antibiotics were added to growth media as required, at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 50 μg/ml; streptomycin, 100 μg/ml; tetracycline, 25 μg/ml.
Identification and analysis of Bartonella Fur.
A 94-bp internal fragment of the B. henselae fur gene was amplified from Bartonella genomic DNA using degenerate primers designed from highly conserved fur gene sequences of other bacteria: FURMID-5′R, 5′-GGA ATT CCA (C,T)CA (C,T)GA (C,T)CA (C,T)(A,C)T (A,C,G,T)AT (A,C,T)GA-3′; FUREND-3′B, 5′-GGG ATC C(G,A)T A(A,C,G,T)A (G,A)(C,T)T C(A,C,G,T)A (G,A)(A,C,G,T)C G(G,A)T G-3′ (Operon Technologies Inc., Alameda, Calif.). The B. henselae fur gene was identified by probing a B. henselae genomic DNA library with the B. henselae fur gene fragment.
To generate fur gene fragment probes for screening of B. quintana and B. bacilliformis genomic DNA libraries, the fur gene open reading frame (ORF) was amplified by PCR from B. quintana and B. bacilliformis genomic DNA. The amplified fur gene fragments were then used to probe B. quintana or B. bacilliformis genomic DNA libraries. An ORF of 417 bp was identified for all three Bartonella species.
The deduced amino acid sequences of the Bartonella Fur proteins (Fig. 1) revealed that they each have a predicted length of 138 amino acids and are highly homologous (B. henselae and B. quintana, 89% identity; B. henselae and B. bacilliformis, 82% identity; B. bacilliformis and B. quintana, 79% identity). The B. henselae Fur protein had 38% amino acid identity with Escherichia coli and Vibrio cholerae Fur and 68% identity with Brucella abortus Fur. The Bartonella Fur amino acid sequence is rich in histidine residues, as are other Fur proteins (3, 4, 26), and 6 of the 42 invariant residues shown in Fig. 1 are histidines. Histidine is the primary amino acid involved in the binding of iron in heme (26, 28). The three Bartonella Fur proteins contain His-His-Asp-His, part of another suggested iron-binding motif, His-His-His-X-His-X2-Cys-X2-Cys, located at positions 86 to 96 of the E. coli and V. cholerae Fur proteins (17). The theoretical isoelectric point of Bartonella Fur is higher (8.1) than that of other Fur proteins (Brucella abortus, 6.1; V. cholerae, 5.5; E. coli, 5.7).
FIG. 1.
Alignment of Bartonella species Fur proteins with those of other gram-negative bacteria. The Fur amino acid sequences of three Bartonella species and three other gram-negative bacterial species were aligned using the Clustal W program from the European Bioinformatics Institute (Cambridge, United Kingdom). There are 42 conserved amino acid positions (shaded). Degenerate oligonucleotide primers (arrows), based on two of the conserved regions (boxes), were designed and utilized in the initial amplification of a B. henselae fur gene fragment. Compared with other gram-negative species, B. henselae Fur had the greatest homology with Brucella abortus.
Confirmation of Bartonella fur genes by Southern blot analysis.
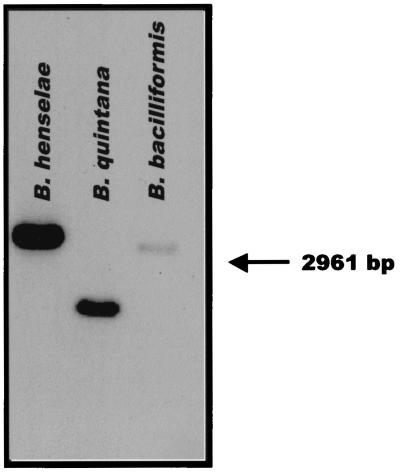
Bartonella genomic DNA (approximately 2 μg each) was digested with HindIII and probed with approximately 250 ng of gel-purified, 417-bp B. henselae fur gene ORF. The B. henselae fur gene probe hybridized to a single band in each lane (in proportion to the homology with the B. henselae fur probe [Fig. 2]), indicating that each species likely contains a single fur gene, as in other bacterial species (1, 19, 21, 30, 31).
FIG. 2.
Southern hybridization analysis of Bartonella species genomic DNA using a B. henselae fur gene probe. B. henselae, B. quintana, and B. bacilliformis DNAs were digested with HindIII. The fragments were separated in a 0.8% agarose gel, transferred to a nylon membrane, and probed with the 417-bp B. henselae fur gene. There was a single band in each lane of HindIII-digested genomic DNA: an approximately 4,200-bp fragment of B. henselae DNA, an approximately 2,000-bp fragment of B. quintana DNA, and an approximately 3,600-bp fragment of B. bacilliformis DNA.
Complementation studies.
Functional homology of Bartonella Fur with the Fur proteins of other gram-negative bacteria was confirmed by complementation studies with a V. cholerae fur mutant. For these studies, the B. henselae fur gene was cloned into pACYC184. The plasmid pSYP3, in which the B. henselae fur ORF replaces the gfp cassette at the BamHI and SphI sites downstream from a constitutive ptac promoter in the pANT4 plasmid, was used as template to amplify the ptac promoter-B. henselae fur ORF sequence. The ptac-fur ORF was ligated into pACYC184, creating pSYP4. Clones were selected for chloramphenicol resistance and tetracycline sensitivity and confirmed by sequence analysis. pSYP4 and pACYC184 were introduced into V. cholerae strains. Expression of the Bartonella fur gene in the transformed V. cholerae fur mutant, CML13(pSYP4), was confirmed by separating proteins from whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown) (16).
The V. cholerae strain MBG40 has TnphoA inserted into the iron-regulated irgA gene in the chromosome such that alkaline phosphatase reporter activity, determined by spectrophotometric measurement of hydrolysis of p-nitrophenyl phosphate by permeabilized cells (8, 19, 20), is strongly regulated by iron and Fur. In a fur mutant of this strain, CML13, alkaline phosphatase activity is not repressed by Fur despite changes in iron concentration (20). The ability of the Bartonella fur gene product to complement the V. cholerae fur mutant was studied by expressing Bartonella fur in this Vibrio strain, CML13(pSYP4), and determining alkaline phosphatase activity. Experiments were performed in triplicate, on four different days. Results were stratified by day and analyzed using a two-sided Mann-Whitney U test.
We observed that the iron-mediated regulation of reporter gene expression could be complemented by constitutive expression of the B. henselae fur gene in the V. cholerae fur mutant (Table 2). As shown previously (20), we found that the V. cholerae strain MBG40, producing a wild-type V. cholerae Fur protein, repressed reporter gene expression when grown on high-iron medium (induction ratio 56 [Table 2]). In contrast, the V. cholerae fur mutant CML13 had partially derepressed alkaline phosphatase reporter gene expression in the presence of high iron concentrations (induction ratio 10 [Table 2]). Plasmid pSYP4 complemented the fur mutation in strain CML13, restoring iron regulation very nearly to the level of strain MBG40. Repression of alkaline phosphatase activity may not have been complete because of the limited amino acid homology between V. cholerae Fur and B. henselae Fur (38%), the difference in pI, or both, which may result in reduced affinity of the Bartonella Fur-Fe2+ homodimer for the Vibrio Fur binding sequence. The absence of wild-type Vibrio Fur in CML13, CML13(pACYC184), and CML13(pSYP4) was confirmed by immunoblot analysis with polyclonal anti-Vibrio Fur antibody (data not shown). For reasons that are not clear, the vector plasmid pACYC184 partially restored iron regulation in strain CML13; nevertheless, the level of alkaline phosphatase expression in CML13(pACYC184) in high-iron media remained significantly higher (P < 0.001) than in either CML13(pSYP4) or MBG40. When iron was depleted, all four strains demonstrated derepression of reporter gene activity, although derepression was greatest in the V. cholerae fur mutant strain, a finding consistent with those of Litwin and Calderwood (20). Interestingly, the V. cholerae fur mutant CML13 did not produce equivalent alkaline phosphatase activity when grown under iron-replete compared with iron-depleted conditions. There was apparently some degree of regulation mediated by iron despite the absence of a functional Fur protein, as previously noted (20). Although the Bartonella Fur protein functionally complemented a V. cholerae fur mutant, there may be substantial structural differences compared with V. cholerae Fur. This is corroborated by the failure of a polyclonal anti-V. cholerae Fur antibody (33) to recognize Bartonella Fur (wild type or overexpressed) by immunoblotting (data not shown).
TABLE 2.
Complementation of a V. cholerae fur mutant by Bartonella fur
| V. cholerae strain | Alkaline phosphatase activity (U/OD600 unit)a
|
Induction ratiob | |||
|---|---|---|---|---|---|
| High-iron medium
|
Low-iron medium
|
||||
| Median | Range | Median | Range | ||
| MBG40 | 16 | 12–20 | 889 | 750–1,339 | 56 |
| CML13 | 119 | 82–179 | 1,200 | 1022–2,099 | 10 |
| CML13(pACYC184) | 34 | 23–42 | 1,022 | 748–1,256 | 30 |
| CML13(pSYP4) | 17 | 10–23 | 881 | 502–1,272 | 52 |
Data were generated from triplicate experiments performed on four separate days. Data were stratified by day and analyzed using a two-sided Mann-Whitney U test. All comparisons were statistically significant (P < 0.001) except CML13(pSYP4) versus MBG40 (P = 0.66).
Induction ratio = medianlow Fe/medianhigh Fe.
Studies of the fur upstream region fused to a gfp reporter gene.
The 204-bp region immediately upstream of the B. henselae fur gene ORF was amplified from genomic DNA and cloned into pANT3 just upstream of the gfp gene to create the plasmids pSYP1 (forward fur upstream sequence) and pSYP2 (inverted fur upstream sequence).
Plasmids were transferred into Bartonella strains by conjugation. B. henselae(pSYP1) and B. quintana(pSYP1) were grown on agar containing different concentrations of available iron and then scraped and suspended in phosphate-buffered saline for FACS analysis (FACScalibur; Becton Dickinson, Franklin Lakes, N.J.) (18). Chocolate plates were prepared with final EDDHA concentrations of 250, 300, 325, and 350 μM or with final Hb concentrations of 0.5, 1, 10, 20, and 30 mg/ml. (B. henselae growth is restricted on chocolate agar containing ≥400 μM EDDHA, and B. quintana growth is restricted at ≥350 μM EDDHA. Growth of both species is restricted at Hb concentrations of ≤1 mg/ml, and growth is optimal on chocolate agar plates containing 10 mg of Hb/ml.) The wild-type B. henselae and B. quintana strains were streaked on chocolate agar plates containing 10 mg of Hb/ml as negative fluorescence controls. Bartonella species containing a plasmid with a constitutively expressed gfp reporter gene (pANT4) or containing the upstream fur region in an inverted position (pSYP2) also were grown on agar containing different concentrations of available iron.
B. quintana(pSYP1), B. henselae(pSYP1), and the control B. henselae(pSYP2) demonstrated no change in GFP (green fluorescent protein) expression regardless of whether they were grown on iron-replete or iron-deficient medium. Wild-type B. henselae exhibited a small amount of autofluorescence, and B. henselae(pANT4) demonstrated stable fluorescence at an average of 1,000-fold higher than wild-type strains. B. henselae(pSYP2) and B. quintana(pSYP2) did demonstrate a small amount of GFP activity, but this activity did not vary with changes in iron concentration (data not shown) and probably represents artifactual activity.
Studies of other bacteria, e.g., E. coli (6, 7, 22), indicate that fur transcription is autoregulated in the presence of iron. We were unable to identify a Fur binding sequence or “iron box” that fulfilled the consensus of a 19-bp hyphenated dyad repeat (22) or two directed and one inverted 6-bp repeats (7) in the 204-bp region upstream of the Bartonella fur gene. However, there is one 19-bp sequence (positions 83 to 101) that conserves all four of the invariant nucleotides of the consensus (positions 6 and 14 to 16) but not the dyad repeat structure of the 19-bp sequence. This sequence is located within an ORF immediately upstream of fur, and functional, iron-regulated binding of this DNA sequence by Bartonella Fur was not detected by our gfp reporter assay. An autoregulatory binding sequence also is absent in the regions upstream of fur in Bradyrhizobium japonicum (9) and V. cholerae (19).
For other gram-negative bacteria, a promoter is located directly upstream of the fur gene; for some bacteria, a Fur binding region is located within the promoter region. The E. coli fur gene can be transcribed and regulated from its own promoter, or, in response to oxidative stress, it can be transcribed as part of an operon including the upstream fldA gene in addition to the downstream fur gene (35). We were unable to detect any promoter activity (regardless of iron availability) or autoregulatory activity in the 204-bp region upstream of the Bartonella fur gene. Because there are fewer than 35 bp between the upstream ORF and the Bartonella fur gene, and no promoter activity was detected in the upstream 204-bp region, it is possible that the Bartonella fur gene lacks its own promoter and that it is transcribed exclusively as part of an operon, which would be an unusual arrangement.
Conclusions.
Bartonella has an obligate heme requirement (27, 34) and alternates between iron-rich and iron-restricted environments. In this study, we identified, cloned, and sequenced a fur gene in B. henselae, B. quintana, and B. bacilliformis. The deduced amino acid sequences of the three Bartonella fur genes demonstrate a high degree of homology among each other and substantial homology with the closest phylogenetic relative, Brucella abortus (24). To date, multiple attempts to generate a Bartonella fur mutant have been unsuccessful. Loss of the fur gene may be a lethal mutation in Bartonella, as occurs in some other gram-negative bacteria (1, 9, 23). Study of gene regulation by Fur in Bartonella will be critical to elucidating the mechanisms of iron acquisition, virulence gene expression, and Bartonella pathogenesis.
Nucleotide sequence accession numbers.
The fur gene sequences of the three Bartonella species used in this study have been deposited in GenBank under accession numbers AF388196 (B. henselae fur), AF388197 (B. quintana fur), and AF388198 (B. bacilliformis fur).
Acknowledgments
We thank Peter Bacchetti for assistance with statistical analysis and Stanley Falkow for valuable advice.
This work was supported by funds received by J.E.K. as a Pew Scholar in the Biomedical Sciences and by S.Y.P. from the Pediatric Infectious Diseases Society Fellowship Grant through Eli Lilly and Company.
REFERENCES
- 1.Berish S A, Subbarao S, Chen C-Y, Trees D L, Morse S A. Identification and cloning of a fur homolog from Neisseria gonorrheae. Infect Immun. 1993;61:4599–4606. doi: 10.1128/iai.61.11.4599-4606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer K M. Bartonella (cat-scratch disease) In: Feigin R D, Cherry J D, editors. Textbook of pediatric infectious disease. II. Philadelphia, Pa: W. B. Saunders Company; 1998. pp. 1512–1517. [Google Scholar]
- 3.Coy M, Doyle C, Besser J, Neilands J B. Site-directed mutagenesis of the ferric uptake regulation gene of Escherichia coli. Biometals. 1994;7:292–298. doi: 10.1007/BF00144124. [DOI] [PubMed] [Google Scholar]
- 4.Coy M, Neiland J B. Structural dynamics and functional domains of the Fur protein. Biochemistry. 1991;30:8201–8210. doi: 10.1021/bi00247a016. [DOI] [PubMed] [Google Scholar]
- 5.Dehio C, Sander A. Bartonella as emerging pathogens. Trends Microbiol. 1999;7:226–228. doi: 10.1016/s0966-842x(99)01523-1. [DOI] [PubMed] [Google Scholar]
- 6.De Lorenzo V, Herrero M, Giovannini F, Neilands J B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988;173:537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- 7.Escolar L, Perez-Martin J, de Lorenzo V. Opening the iron box: transcriptional metalloregulation by the fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg M B, Dirita V J, Calderwood S B. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990;58:55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamza I, Hassett R, O'Brian M R. Identification of a functional fur gene in Bradyrhizobium japonicum. J Bacteriol. 1999;181:5843–5846. doi: 10.1128/jb.181.18.5843-5846.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 11.Ito S, Vinson J W. Fine structure of Rickettsia quintana cultivated in vitro and in the louse. J Bacteriol. 1965;89:481–495. doi: 10.1128/jb.89.2.481-495.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler B, de Lorenzo V, Timmis K. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol Gen Genet. 1992;233:293–301. doi: 10.1007/BF00587591. [DOI] [PubMed] [Google Scholar]
- 13.Koehler J E. Bartonella: an emerging human pathogen. In: Scheld W M, Armstrong D, Hughes J M, editors. Emerging infections 1. Washington, D.C.: ASM Press; 1998. pp. 147–163. [Google Scholar]
- 14.Koehler J E, Quinn F D, Berger T G, LeBoit P E, Tappero J W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 15.Koehler J E, Tappero J W. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus. Clin Infect Dis. 1993;17:612–624. doi: 10.1093/clinids/17.4.612. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lam M S, Litwin C M, Carroll P A, Calderwood S B. Vibrio cholerae fur mutations associated with loss of repressor activity: implications for the structural-functional relationships of Fur. J Bacteriol. 1994;176:5108–5115. doi: 10.1128/jb.176.16.5108-5115.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee A K, Falkow S. Constitutive and inducible green fluorescent protein expression in Bartonella henselae. Infect Immun. 1998;66:3964–3967. doi: 10.1128/iai.66.8.3964-3967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litwin C M, Boyko S A, Calderwood S B. Cloning, sequencing, and transcriptional regulation of the Vibrio cholerae fur gene. J Bacteriol. 1992;174:1897–1903. doi: 10.1128/jb.174.6.1897-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litwin C M, Calderwood S B. Analysis of the complexity of gene regulation by Fur in Vibrio cholerae. J Bacteriol. 1994;176:240–248. doi: 10.1128/jb.176.1.240-248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litwin C M, Calderwood S B. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J Bacteriol. 1993;175:706–715. doi: 10.1128/jb.175.3.706-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litwin C M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prince R W, Cox C D, Vasil M L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993;175:2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Relman D A, Loutit J S, Schmidt T M, Falkow S, Tompkins L S. The agent of bacillary angiomatosis: an approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 25.Rogers H J. Iron-binding catechols and virulence in Escherichia coli. Infect Immun. 1973;7:445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaffer S, Hantke K, Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;200:110–113. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- 27.Schwartzman W A, Nesbit C A, Baron E J. Development and evaluation of a blood-free medium for determining growth curves and optimizing growth of Rochalimaea henselae. J Clin Microbiol. 1993;31:1882–1885. doi: 10.1128/jcm.31.7.1882-1885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith A, Hooper N I, Shipulina N, Morgan W T. Heme binding by a bacterial repressor protein, the gene product of the ferric uptake regulation (fur) gene of Escherichia coli. J Protein Chem. 1996;15:575–583. doi: 10.1007/BF01908539. [DOI] [PubMed] [Google Scholar]
- 29.Spach D H, Koehler J E. Bartonella-associated infections. Emerg Infect Dis. 1998;12:137–155. doi: 10.1016/s0891-5520(05)70414-1. [DOI] [PubMed] [Google Scholar]
- 30.Staggs T M, Perry R D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991;173:417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thupvong T, Wiideman A, Dunn D, Oreschak K, Jankowicz B, Doering J, Castignetti D. Sequence heterogeneity of the ferripyoverdine uptake (fpvA), but not the ferric uptake regulator (fur), genes among strains of the fluorescent pseudomonads Pseudomonas aeruginosa, Pseudomonas aureofaciens, Pseudomonas fluorescens and Pseudomonas putida. Biometals. 1999;12:265–274. doi: 10.1023/a:1009270302536. [DOI] [PubMed] [Google Scholar]
- 32.Vinson J W. In vitro cultivation of the rickettsial agent of trench fever. Bull W H O. 1966;35:155–164. [PMC free article] [PubMed] [Google Scholar]
- 33.Watnick P I, Eto T, Takahashi H, Calderwood S B. Purification of Vibrio cholerae Fur and estimation of its intracellular abundance by antibody sandwich enzyme-linked immunosorbent assay. J Bacteriol. 1997;179:243–247. doi: 10.1128/jb.179.1.243-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong M T, Thorton D C, Kennedy R C, Dolan M J. A chemically defined liquid medium that supports primary isolation of Rochalimaea (Bartonella) henselae from blood and tissue specimens. J Clin Microbiol. 1995;33:742–744. doi: 10.1128/jcm.33.3.742-744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng M, Doan B, Schneider T D, Storz G. OxyR and SoxRS regulation of fur. J Bacteriol. 1999;181:4639–4643. doi: 10.1128/jb.181.15.4639-4643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]