Abstract
Rationale & Objective:
Few studies have investigated racial disparities in acute kidney injury (AKI), in contrast to the large literature on Black-White differences in risk of end-stage kidney disease.
Study Design:
Prospective cohort study
Setting & Participants:
We studied 2720 self-identified Black or White participants in the Chronic Renal Insufficiency Cohort study from July 1, 2013-December 31, 2017.
Exposure:
Self-reported race (Black vs. White)
Outcome:
Hospitalized AKI (≥50% increase from nadir to peak serum creatinine).
Analytical approach:
Cox regression models. We adjusted for demographics (age and sex), pre-hospitalization clinical risk factors (diabetes, blood pressure, cardiovascular disease, estimated glomerular filtration rate, proteinuria, and receipt of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers) and socioeconomic status (insurance status and education level). In a subset of participants with genotype data, we adjusted for apolipoprotein L1 gene (APOL1) high-risk status and sickle cell trait status.
Results:
Black participants (N=1266) were younger but had a higher burden of pre-hospitalization clinical risk factors. Incidence rate of first AKI hospitalization among Black participants was 6.3 (95% CI: 5.5–7.2) per 100 person-years versus 5.3 (95% CI: 4.6–6.1) per 100 person-years among White participants. In an unadjusted Cox regression model, Black participants were at a modestly increased risk of incident AKI (hazard ratio [HR] 1.22; 95% CI: 1.01–1.48) compared with White participants. However, this risk was attenuated and no longer significant after adjusting for pre-hospitalization clinical risk factors (adjusted HR 1.02; 95% CI: 0.83–1.25). There were only 11 AKI hospitalizations among individuals with high-risk APOL1 risk status and 14 AKI hospitalizations among individuals with sickle cell trait.
Limitations:
Participants are limited to research volunteers and are not fully representative of all CKD patients.
Conclusion:
In this multi-center prospective cohort of CKD patients, racial disparities in AKI incidence were modest and were explained by differences in pre-hospitalization clinical risk factors.
Index words: Acute kidney injury (AKI), chronic kidney disease, racial disparities, CRIC, clinical risk factors
Introduction:
Black Americans face a disproportionately higher risk of end stage kidney disease (ESKD) when compared with White Americans.1 This is thought to be due to an interplay of clinical, socioeconomic, and genetic risk factors.2–5 While multiple studies have described various contributors to racial disparities in ESKD,2–5 few studies have rigorously explored racial variation in acute kidney injury (AKI).6,7
Some studies have reported that Black Americans have higher risk of AKI than White Americans.1,6–13 However, these prior studies were limited by inclusion of specialized populations (e.g. patients with diabetes,9 trauma,12 or those undergoing procedures such as percutaneous coronary intervention,7,13 or knee surgery11). Other studies ascertained AKI using only administrative codes,1,6,8–10 which have low sensitivity to detect AKI.14 Most previous studies also did not include data on pre-AKI estimated glomerular filtration rate (eGFR) and proteinuria, which are two critical AKI risk factors. Therefore, they were unable to examine whether differences in AKI risk could be explained by differences in prevalence and severity of pre-existing chronic kidney disease (CKD). Few investigations have examined whether risk of AKI is influenced by apolipoprotein L1 gene (APOL1) renal risk variants6,15 or sickle cell trait (SCT),16–18 which are more common among Black Americans and are associated with kidney disease.19–21
To fill these gaps in the literature, we analyzed data from the Chronic Renal Insufficiency Cohort (CRIC) Study,22–24 to estimate rates of hospitalized AKI, defined by changes in serum creatinine (SCr), and to examine the association of race with hospitalized AKI after adjustment for clinical, socioeconomic, and genetic risk factors among Black and White Americans with CKD.
Methods:
Study population
CRIC22–24 is a multicenter prospective cohort study of racially and ethnically diverse adult patients with CKD in the United States (U.S.). Initial recruitment of 3939 adults aged 21–74 years with eGFR 20–70 ml/min/1.73m2 was completed between 2003 and 2008 at seven U.S. clinical centers encompassing 13 recruitment sites. Individuals who self-identified as Black and those with diabetes were oversampled.25 Major exclusion criteria included diagnosed glomerulonephritis receiving immunosuppressive therapy, New York Heart Association Class III or IV heart failure, cirrhosis, and polycystic kidney disease. Participants were re-consented for extended follow up in subsequent 5-year periods (2008–2013; 2013–2018; 2018–2023). An additional 1560 patients with CKD were enrolled from 2013 to 2015, augmenting representation of older individuals and those with proteinuria and preserved eGFR.25
CRIC participants are contacted every six months by phone and attend annual in-person research study visits during which SCr and urine protein-creatinine ratio (uPCR) are measured, and vital signs, medication usage and interim medical events are comprehensively updated.
The present analysis included self-identified Black and White CRIC participants who were alive, had not withdrawn from the study and did not develop ESKD as of July 1, 2013 (N=2720) (Figure 1). We selected July 1, 2013, as the start date for this analysis since CRIC started comprehensive collection of inpatient laboratory measurements for all hospitalizations only after July 1, 2013, and our aim was to define AKI using observed SCr levels during hospitalization.
Figure 1.
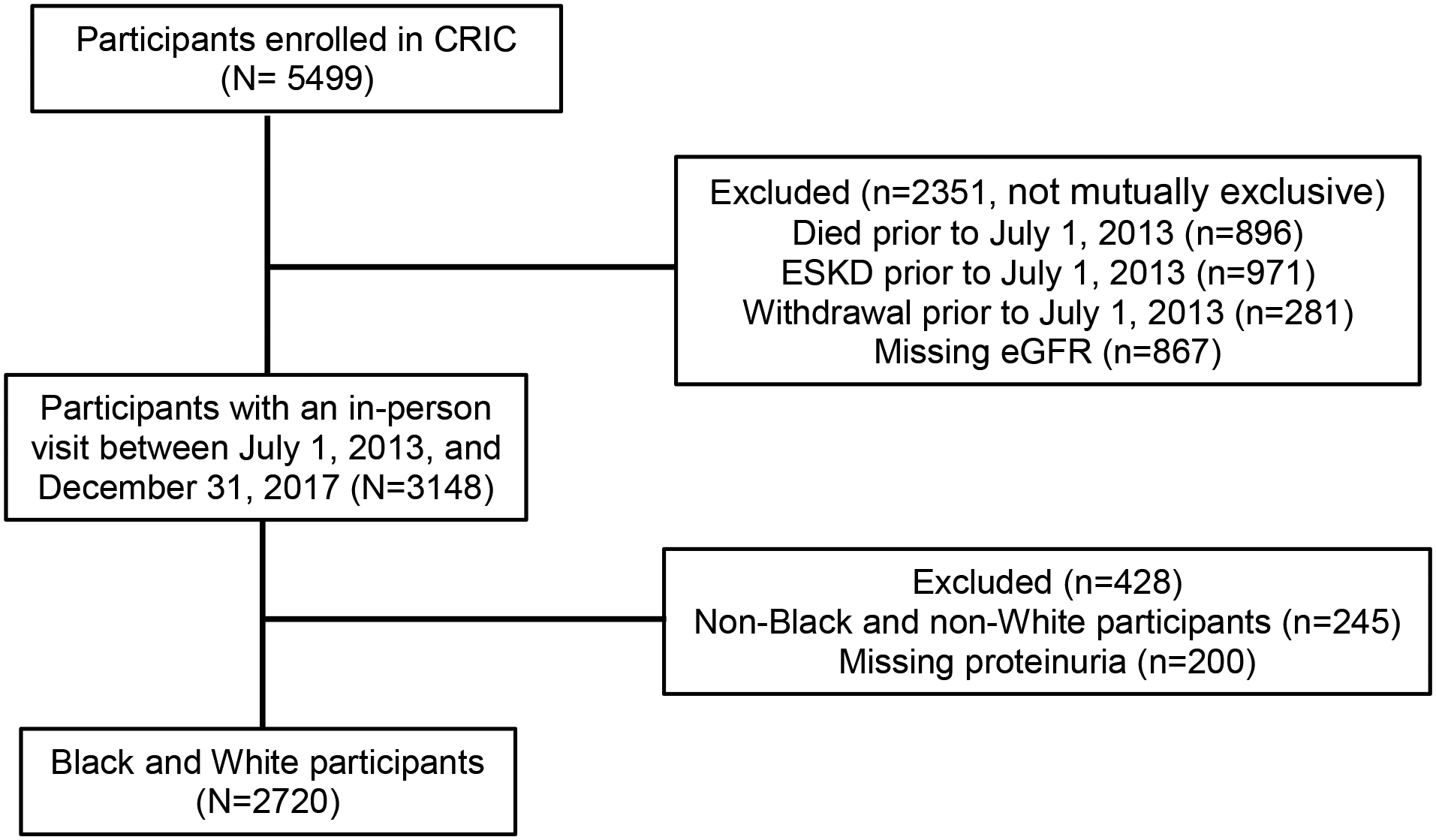
Cohort assembly diagram for eligible CRIC participants.
Institutional review boards at participating institutions approved the study protocol. All study participants provided written informed consent.
Hospitalized AKI
The primary outcome of interest was time to first hospitalized AKI, defined by a ≥50% increase from nadir to peak inpatient SCr measurement. This definition is adapted from the Kidney Disease Improving Global Outcomes (KDIGO) AKI guidelines,26 and is more stringent to reduce false positives and improve specificity.27 We used inpatient SCr measurements only to define hospitalized AKI to reduce the risk of misclassifying progression of CKD as AKI. We staged AKI according to KDIGO guidelines26 and included: Stage 1; peak inpatient SCr 1.5–1.9 times nadir Scr; stage 2; peak inpatient SCr 2.0–2.9 times nadir Scr, and stage 3; peak inpatient SCr ≥3.0 times nadir Scr. AKI Hospitalizations were ascertained through participant self-report and hospital queries and confirmed via medical records review.28,29 SCr values measured during these hospitalizations were systematically abstracted and organized to determine AKI status. Follow-up was through December 31, 2017.
Primary Exposure
The primary exposure was self-reported race. Our objective was to evaluate the risk of AKI among Black and White CRIC participants; therefore, all other races were excluded from this analysis. Hispanic participants were classified as having Hispanic ethnicity regardless of self-identified race.
Covariates
Pre-hospitalization clinical risk factors included diabetes, blood pressure, prior cardiovascular disease, eGFR, uPCR (assessed using either 24-hour urine collections or random spot urine samples)30, and receipt of angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARB), which were all determined at the first in-person CRIC visit after July 1, 2013. We used the 2009 Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) to calculate eGFR.31 We also conducted two sensitivity analyses, one where we adjusted for Hispanic ancestry in our final models, and the other we used the new 2021 race-free CKD-EPI eGFR equation.32
Socioeconomic characteristics included self-reported education attainment (ascertained at the parent CRIC study baseline visit), and health insurance status (ascertained at follow-up visits).
Using diagnosis-related groups for principal discharge codes,33,34 we broadly categorized hospitalizations during the study period as being kidney-related or related to diseases of the nervous, respiratory, circulatory, gastrointestinal, or musculoskeletal systems.
Genetic analysis
Genetic variants of interest included APOL1 risk alleles and SCT. For these, we restricted our analysis to the subset of CRIC participants who were genotyped for the APOL1 renal risk variants35 and had genome wide association studies (GWAS) completed (N=1195).36 High-risk APOL1 status among Black Americans was defined based on having a combination of two risk variants consisting of either G1 or G2 risk alleles (i.e., G1/G1, G1/G2 or G2/G2). Low-risk APOL1 status was defined on the basis of having one or no risk allele (i.e., G0/G0, G0/G1, or G0/G2). TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA) were used to detect the APOL1 risk alleles.35 Genome-wide imputation was used to determine SCT. CRIC Study participants have been genotyped for more than 1 million gene variants on the Illumina Omni-1-Quad beadchip array.36 Genome-wide imputation was completed based on the 1000 Genomes mixed race/ethnicity (ALL) genetic backbone (NCBI build 37, release date March 2012; n=1,093) using IMPUTE2,36 with very high-quality imputation (R2 of 0.90) of the rs334 SNP specific for the hemoglobin S mutation.37,38
Statistical analysis
We compared baseline characteristics among patient subgroups using the t test or Wilcoxon rank sum test for continuous variables and χ2 test for categorical variables. Since hospitalized AKI could only be diagnosed during a hospital admission, we also reported crude rates of all hospitalizations by race.
We performed Cox proportional hazards regression to examine the unadjusted association of race (Black vs. White) with time to first AKI hospitalization (N=2720). After confirming no violation of the proportional hazards assumption, we then conducted a series of nested models that incrementally adjusted for categories of potential explanatory variables: 1) demographic factors (age and sex); 2) clinical risk factors (eGFR, log uPCR, diabetes, blood pressure level, any prior cardiovascular disease, and receipt of ACE inhibitors or ARB); and 3) socioeconomic status (insurance status and level of educational attainment). Consistent with prior literature, we included eGFR and log uPCR as continuous variables in our models.39,40
To explore the potential role of genetic variants, we assembled a cohort that was restricted to the subset of 1195 participants with genetic data. We first examined the unadjusted association of race with time to first AKI hospitalization, and then sequentially adjusted for similar categories of explanatory variables as follows: 1) demographic factors; 2) clinical risk factors; 3) presence of high-risk APOL1 status or SCT; and 4) socioeconomic status. This analytical approach had been used by prior studies exploring reasons for racial disparities in AKI incidence.6
Results:
Main results
Among 2720 CRIC participants included in the main analysis, 1266 (47%) self-identified as Black and 1454 (53%) as White (Figure 1). Black participants were younger, less likely to have graduated from high school, more likely to have diabetes, dyslipidemia, higher blood pressure, higher BMI, and greater amount of proteinuria compared with White participants (Table 1). There was no significant racial difference in having health insurance (95% versus 96%, for Black and White participants, respectively). Baseline characteristics of the 2,720 eligible CRIC participants overall and by self-reported race are described on Table 1.
Table 1.
Baseline characteristics of 2,720 eligible CRIC participants overall and by self-reported race.
| Characteristic | Overall (N=2720) | White (N=1454) | Black (N=1266) | P-values |
|---|---|---|---|---|
| Age, year, mean (SD) | 65.4 (9.3) | 66.0 (9.5) | 64.7 (9.1) | <0.001 |
| Women, n (%) | 1204 (44) | 554 (38) | 650 (51) | <0.001 |
| Hispanic ethnicity, n (%) | 116 (4.3) | 104 (7.2) | 12 (0.9) | <0.001 |
| High school graduate, n (%) | 2378 (88) | 1362 (94) | 1016 (80) | <0.001 |
| Have health insurance, n (%) | 2588 (95) | 1392 (96) | 1196 (95) | 0.1 |
| Current cigarette smokers, n (%) | 263 (10) | 82 (6) | 181 (14) | <0.001 |
| Medical history, n (%) | ||||
| Cardiovascular disease | 993 (37) | 518 (36) | 475 (38) | 0.3 |
| Heart failure | 267 (10) | 129 (9) | 138 (11) | 0.08 |
| Diabetes mellitus | 1439 (53) | 702 (48) | 737 (58) | <0.001 |
| Dyslipidemia | 2226 (82) | 1214 (84) | 1012 (80) | 0.02 |
| Receipt of ACE-I or ARB | 1758 (65) | 919 (64) | 839 (67) | 0.07 |
| Receipt of any antihypertensive medication | 2424 (89) | 1236 (85) | 1188 (94) | <0.001 |
| Baseline laboratory values and vital signs | ||||
| Estimated glomerular filtration rate, mL/min/1.73m2, mean (SD) | 51 (17) | 52 (16) | 52 (17) | 0.7 |
| Urine-Protein-to-Creatine Ratio, g/g, median (IQR) | 0.2 (0.0–0.4) | 0.2 (0.0–0.4) | 0.2 (0.0–0.6) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 32 (7) | 31 (7) | 34 (7) | <0.001 |
| Systolic blood pressure, mmHg, mean (SD) | 126 (19) | 122 (17) | 130 (20) | <0.001 |
| Diastolic blood pressure, mmHg, mean (SD) | 69 (12) | 67 (11) | 71 (12) | <0.001 |
ACE-I; Angiotensin-converting enzyme inhibitor, ARB; Angiotensin-receptor blockers,
During follow-up, Black participants experienced 205 AKI hospitalizations, while White participants experienced 208. The median time between ascertainment of baseline characteristics and development of AKI episode was 1.78 years (interquartile range [IQR] 0.87–2.72). Most AKI events were Stage 1 in severity, including 55% of AKI among Black participants and 59% of AKI among White participants. Stage 2 and 3 AKI accounted for 24% and 20% among Black participants, respectively and 14% and 27% among White participants, respectively. Incidence of first AKI hospitalization among Black participants was 6.3 (95% CI: 5.5–7.2) per 100 person-years versus 5.3 (95% CI: 4.6–6.1) per 100 person-years among White participants (Table 2). These differences in AKI hospitalization rates were similar in magnitude to the differences in rates of all hospitalizations among Black versus White participants (Table 2). Supplementary Table 1 shows potential reasons for hospitalizations, which appeared comparable between Black and White participants.
Table 2:
Unadjusted rates of AKI hospitalizations, overall and stratified by race
| Overall (2720) | White (1454) | Black (1266) | |
|---|---|---|---|
| Number of AKI hospitalizations | 413 (15.2%) | 208 (14.3%) | 205 (16.2%) |
| Unadjusted incidence rate of first AKI hospitalization per 100 person years | 5.8 (95% CI: 5.2–6.3) | 5.3 (95% CI: 4.6–6.1) | 6.3 (95% CI: 5.5–7.2) |
| Unadjusted incidence rate of all hospitalizations per 100 person years | 135 (95% CI: 132–138) | 119 (95% CI: 115–123) | 152 (95% CI: 148–157) |
| Unadjusted incidence rate of first hospitalization per 100 person years | 41 (95% CI: 39–43) | 37 (95% CI: 35–39) | 47 (95% CI: 44–50) |
In the unadjusted Cox regression model, Black participants were at modestly increased risk of incident AKI (hazard ratio [HR] 1.22; 95% CI: 1.01–1.48) compared with White participants (Figure 2). Black participants remained at increased risk of AKI after adjustment for demographics (adjusted HR [aHR] 1.24; 95% CI: 1.02–1.51), but this risk was attenuated and no longer statistically significant after additionally adjusting for pre-hospitalization clinical risk factors (aHR 1.02; 95% CI: 0.83–1.25). Additional adjustment for socioeconomic factors (i.e., educational attainment and health insurance status) did not materially change the results (aHR 1.05; 95% CI: 0.85–1.29).
Figure 2: Cox regression models evaluating the association of race with time to first AKI.
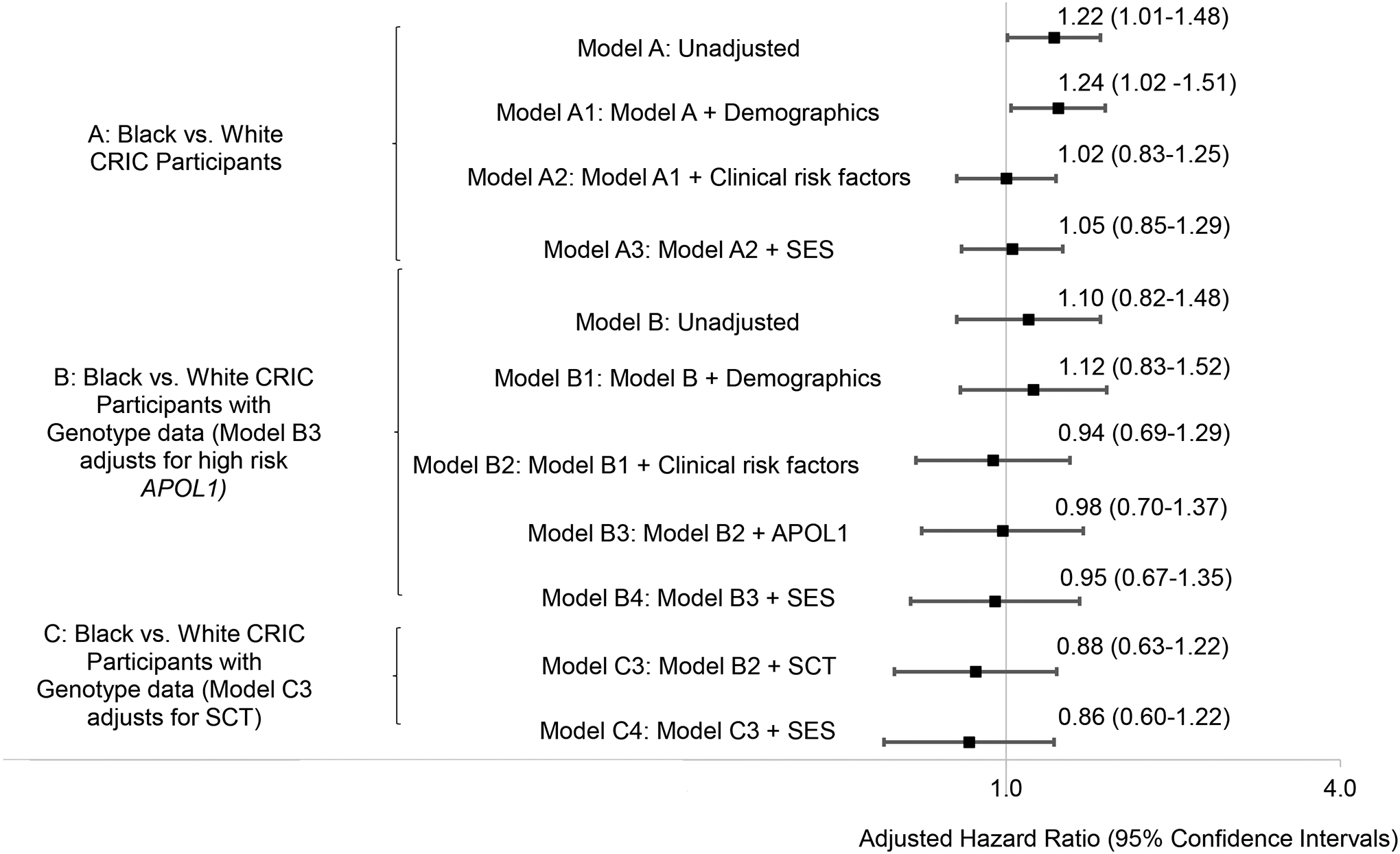
Model A: Unadjusted. Model A1: Model A + demographics (age and sex) (n=2720). Model A2: Model A1 + pre-hospitalization baseline clinical risk factors (diabetes, blood pressure level, prior cardiovascular disease, eGFR, uPCR, and receipt of ACEi or ARB) (n=2720). Model A3: Model A2 + SES (insurance and education level) (n=2720). Model B: Unadjusted. Model B1: Model B + demographics (age and sex) (n=1195). Model B2: Model B1 + pre- hospitalization baseline clinical risk factors (diabetes, blood pressure level, prior cardiovascular disease, eGFR, uPCR, and receipt of ACEi or ARB) (n=1195). Model B3: Model B2 + APOL1 (n=1195). Model B4: Model B3 + SES (insurance and education level) (n=1195). Model C3: Model B2 + SCT (n=1195). Model C4: Model C3 + SES (insurance and education level) (n=1195). APOL1: apolipoprotein L1; SCT: sickle cell trait; SES: socioeconomic status; ACEi: angiotensin-converting enzyme inhibitors; ARB: Angiotensin-receptor blockers; uPCR: urine protein-creatinine ratio; eGFR: estimated glomerular filtration rate
Similar results were seen in our sensitivity analyses after adjusting for ethnicity (Hispanic vs. non-Hispanic) and using the new race-free 2021 CKD-EPI eGFR equation.32 (Supplementary Table 2).
Genetic analysis
Among 499 Black CRIC participants with APOL1 and SCT data, 89 (18%) had high-risk APOL1 status, and 79 (16%) had SCT. Of the participants with high-risk APOL1 status, 11 (12%) had at least one episode of AKI, while 14 (18%) of the individuals with SCT had at least one episode of AKI (Table 3).
Table 3:
AKI hospitalizations among CRIC participants with genotype data
| Events | Unadjusted rate (95% CI) | |
|---|---|---|
| Overall (N=1195) | 177 | 14.8 (12.8–17.0) |
| White participants (N=696) | 101 | 14.5 (12.0–17.4) |
| Black participants (N=499) | 76 | 15.2 (12.1–18.7) |
| High-risk APOL1 status (N=89) | 11 | 12.4 (6.3–21.0) |
| SCT (N=79) | 14 | 17.7 (10.0–27.9) |
APOL1: apolipoprotein L1; SCT: sickle cell trait.
In an unadjusted Cox regression model limited to participants with genetic data, Black race was not significantly associated with AKI (HR 1.10; 95% CI: 0.82–1.48) compared with White participants (Figure 2). In multivariable Cox regression that adjusted for demographic factors, Black race was not significantly associated with AKI (aHR 1.12; 95% CI: 0.83–1.52), with no material change after additionally adjusting for pre-hospitalization clinical risk factors (aHR 0.94; 95% CI: 0.69–1.29). Additional adjustment for APOL1 risk status and socioeconomic status did not substantially change the risk estimates (aHR, 0.98; 95% CI: 0.70–1.37 and aHR, 0.95; 95% CI: 0.67–1.35, respectively). Black race was also not significantly associated with AKI in the SCT-adjusted model (aHR, 0.88; 95% CI:0.63–1.22).
Discussion:
We examined the risk of hospitalized AKI between Black and White participants in a multicenter prospective cohort of CKD patients in the U.S. In unadjusted models, we observed that Black participants had 22% greater hazard for AKI hospitalization than White participants, but this difference was attenuated and no longer significant after adjusting for pre-hospitalization clinical risk factors. These findings highlight the importance of pre-hospitalization baseline risk factors such as diabetes, blood pressure, and proteinuria in influencing the observed modest racial disparities in AKI in the study cohort. Addressing racial disparities in these clinical risk factors for AKI, for example, with tighter control of blood pressure to lower proteinuria, may be a path to mitigate the increased risk of AKI among Black individuals with CKD.
Several studies have reported varying effect sizes when assessing the association of Black race with risk of AKI.6,7,9,12,13 Even after adjustment for potential confounders, Black race was associated with an increased risk of AKI in four hospital-based studies.7,9,12,13 These studies enrolled selected populations, including individuals with diabetes,9 trauma,12 and those admitted for Percutaneous Coronary Intervention (PCI).7,13 Thus, whether these prior findings can be extrapolated to a more diverse population of Black individuals without these conditions is unclear. Furthermore, these earlier studies did not rigorously account for pre-AKI eGFR and proteinuria—two key AKI risk factors which may differ by race in any given study population. In our study, the increased risk of AKI among Black participants was attenuated and no longer significant after adjusting for research protocol ascertained pre-hospitalization baseline risk factors (in fact adjusting for only baseline proteinuria, or only baseline diabetes status, or only baseline blood pressure level, or only prior cardiovascular disease resulted in loss of statistically significant association [Supplementary Table 3]).
In contrast to our findings in CRIC, the Atherosclerosis Risk in Communities (ARIC) study observed an increased risk of AKI associated with Black race that persisted after adjustment for pre-hospitalization baseline clinical risk factors in.6 Potential reasons for this difference include that the ARIC study enrolled individuals with a lower burden of risk factors for AKI, including better baseline kidney function (mean 92 mL/min/1.73m2 among Black participants in ARIC vs. 52 mL/min/1.73m2 in CRIC) and lower proteinuria.6 The increased risk of AKI among Black participants in the ARIC study was attenuated after adjusting for differences in socioeconomic factors (income and health insurance).6 Differences in income and health insurance can affect health outcomes, and although we did not adjust for income due to the amount of missing data, there were no racial differences in health insurance in our study and almost all (≥95%) study participants had health insurance regardless of race.
We only observed 11 AKI hospitalizations among individuals with high-risk APOL1 status, so we were underpowered to evaluate the association of APOL1 and AKI. Prior work by Grams et al., showed that high-risk APOL1 status was not significantly associated with increased risk of AKI (aHR: 1.07; 95% CI, 0.69–1.65).6 However, Privratsky et al, found that among Black patients undergoing cardiac surgery, those with high-risk APOL1 status averaged a more than twofold higher postoperative creatinine.15 The former study was limited by ascertainment of AKI using administrative codes;6,41 and the latter study was limited by lack of adjustment of baseline eGFR and proteinuria.15 There are several recent reports that among patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), high-risk APOL1 status increased risk of AKI.42–44 Therefore, further research is necessary to delineate the potential contribution of APOL1 to risk of AKI.
SCT is another genetic variant that is more common among Black Americans and is associated with CKD.19,20 Several kidney manifestations of SCT have been reported including renal medullary cancer, hyposthenuria, papillary necrosis and asymptomatic hematuria.45 Ischemia and microinfarction are thought to explain some of these observations,46 and ischemia and microinfarction may also predispose SCT carriers to AKI. Two older studies had reported an increased risk of AKI among participants with SCT but they both used administrative codes to define AKI.16,18 A recent study that defined AKI using acute changes in SCr failed to confirm this association.17 We only observed 14 AKI hospitalizations among individuals with SCT in CRIC, and thus were limited in our ability to contribute to the literature.
Our findings have several important implications. First, racial disparities in AKI can be partially explained by differences in pre-hospitalization AKI risk factors. Targeted strategies to reduce the disproportionate burden of these risk factors among Black individuals may alleviate the racial disparities in AKI. Second, although genetic risk factors such as APOL1 risk status and SCT are relevant in CKD, their role in AKI remains unclear.
Strengths of this study include the use of acute changes in SCr to define AKI, which represents an advancement over relying on less accurate administrative codes to ascertain AKI.14 In addition, we rigorously accounted for critical independent predictors of AKI such as pre-hospitalization eGFR and proteinuria,39,47,48 which were ascertained using a well-defined research protocol proximal to AKI hospitalizations. Studies have documented that Black patients are treated differently than White patients in certain clinical settings and conditions such as coronary disease,49 and cancer care,50 and thus reliance on clinical data can lead to ascertainment bias because there may be racial differences in who gets various clinical evaluations.
This study should be interpreted in the context of several limitations. First, we only studied hospitalized AKI and did not consider community-acquired AKI. Second, we did not have biopsies to better define the etiology of AKI, though kidney biopsies are not routinely performed in the setting of AKI in current clinical practice. Third, our sample size for the genetic analysis was modest. Fourth, we did not account for inpatient risk factors for AKI (e.g., presence or absence of sepsis, receipt of nephrotoxic agents). Fifth, although we adjusted for presence of clinical risk factors (such as diabetes mellitus), we were unable to account for severity of these risk factors (for example glycemic control) which may vary by race. Sixth, a majority of AKI were mild and thus we could not assess associations by severity of AKI. Seventh, we lacked robust measures of social determinants of health which are important drivers of racial disparities in health outcomes. However, in our study there was already no differences in risk of AKI by race before we adjusted for any social determinants of health such as health insurance and education, so we think this is unlikely to have biased our results. Lastly, CRIC participants are limited to research volunteers and are not fully representative of all CKD patients.
In conclusion, our study suggests that racial disparities in AKI incidence are modest and can be explained by differences in AKI clinical risk factors. Targeted screening and aggressive management of pre-hospitalization clinical risk factors may reduce the incidence of AKI.
Supplementary Material
Racial disparities in acute kidney injury among Black and White individuals.
Although much is known about racial disparities in end stage kidney disease (ESKD), few studies have investigated Black and White racial differences in acute kidney injury (AKI). We examined whether Black participants in the Chronic Renal Insufficiency Cohort (CRIC) study were at an increased risk of AKI compared with White study participants, after accounting for differences in pre-hospitalization clinical risk factors, socioeconomic status, and genetic risk factors. We found that Black participants had an increased risk of AKI than White participants, however, this risk was modest and was explained by differences in pre-hospitalization clinical risk factors such as proteinuria, hypertension, diabetes, and heart failure. These findings suggest that targeted screening and aggressive management of these clinical risk factors may reduce the risk of AKI.
Acknowledgements:
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902 and U24DK060990). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199.
Support:
ANM is supported by University of California, San Francisco, Dean’s Diversity award (Watson scholar), R01DK114014-01A1S1 diversity supplement and K23DK119562. AS is supported by NIH grant K23DK120811, Kidney Precision Medicine Project Opportunity Pool grant under award U2CDK114886, and core resources from the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY) P30DK114857. CYH is supported by K24 DK92291 and R01 DK114014
The funders had no role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Disclosures:
AS: Personal fees from Horizon Therapeutics PLC, CVS Caremark, AstraZeneca, Bayer, and Tate & Latham (medicolegal consulting).
MME: Received compensation for a one-time session as a member of an advisory panel for Aztra Zeneca related to APOL1.
The remaining authors declare that they have no relevant financial interests.
References:
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. Mar 2019;73(3S1):A7–A8. doi: 10.1053/j.ajkd.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norton JM, Moxey-Mims MM, Eggers PW, et al. Social Determinants of Racial Disparities in CKD. J Am Soc Nephrol. Sep 2016;27(9):2576–95. doi: 10.1681/ASN.2016010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClellan WM, Warnock DG, Judd S, et al. Albuminuria and racial disparities in the risk for ESRD. J Am Soc Nephrol. Sep 2011;22(9):1721–8. doi: 10.1681/ASN.2010101085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. Sep 2005;68(3):914–24. doi: 10.1111/j.1523-1755.2005.00485.x [DOI] [PubMed] [Google Scholar]
- 5.Nicholas SB, Kalantar-Zadeh K, Norris KC. Racial disparities in kidney disease outcomes. Semin Nephrol. Sep 2013;33(5):409–15. doi: 10.1016/j.semnephrol.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grams ME, Matsushita K, Sang Y, et al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol. Aug 2014;25(8):1834–41. doi: 10.1681/ASN.2013080867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunyera J, Clare RM, Chiswell K, et al. Racial Differences in AKI Incidence Following Percutaneous Coronary Intervention. J Am Soc Nephrol. Dec 18 2020;doi: 10.1681/asn.2020040502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. Apr 2006;17(4):1135–42. [DOI] [PubMed] [Google Scholar]
- 9.Mathioudakis NN, Giles M, Yeh HC, Haywood C Jr., Greer RC, Golden SH. Racial differences in acute kidney injury of hospitalized adults with diabetes. J Diabetes Complications. Aug 2016;30(6):1129–36. doi: 10.1016/j.jdiacomp.2016.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grams ME, Sang Y, Ballew SH, et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Age, Race, and Sex With Acute Kidney Injury. Am J Kidney Dis. Oct 2015;66(4):591–601. doi: 10.1053/j.ajkd.2015.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Womble TN, King JD, Hamilton DH, Shrout MA, Jacobs CA, Duncan ST. Greater Rates of Acute Kidney Injury in African American Total Knee Arthroplasty Patients. J Arthroplasty. Jun 2019;34(6):1240–1243. doi: 10.1016/j.arth.2019.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shashaty MG, Meyer NJ, Localio AR, et al. African American race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. J Crit Care. Oct 2012;27(5):496–504. doi: 10.1016/j.jcrc.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Glorioso TJ, Armstrong EJ, et al. Comparative Outcomes After Percutaneous Coronary Intervention Among Black and White Patients Treated at US Veterans Affairs Hospitals. JAMA Cardiol. Sep 1 2017;2(9):967–975. doi: 10.1001/jamacardio.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. Apr 2014;9(4):682–9. doi: 10.2215/CJN.07650713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Privratsky JR, Li YJ, Haynes C, et al. Apolipoprotein L1 (APOL1) Coding Variants Are Associated With Creatinine Rise After Cardiac Surgery. J Cardiothorac Vasc Anesth. Dec 2020;34(12):3314–3320. doi: 10.1053/j.jvca.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Nelson DA, Deuster PA, Marks ES, O’Connor FG, Kurina LM. Sickle cell trait and renal disease among African American U.S. Army soldiers. Br J Haematol. May 2019;185(3):532–540. doi: 10.1111/bjh.15820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olaniran KO, Allegretti AS, Zhao SH, Nigwekar SU, Kalim S. Acute Kidney Injury among Black Patients with Sickle Cell Trait and Sickle Cell Disease. Clin J Am Soc Nephrol. Mar 8 2021;16(3):348–355. doi: 10.2215/cjn.06960520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucknor MD, Goo JS, Coppolino ML. The risk of potential thromboembolic, renal and cardiac complications of sickle cell trait. Hemoglobin. 2014;38(1):28–32. doi: 10.3109/03630269.2013.832689 [DOI] [PubMed] [Google Scholar]
- 19.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. Nov 26 2014;312(20):2115–25. doi: 10.1001/jama.2014.15063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naik RP, Irvin MR, Judd S, et al. Sickle Cell Trait and the Risk of ESRD in Blacks. J Am Soc Nephrol. Jul 2017;28(7):2180–2187. doi: 10.1681/ASN.2016101086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dummer PD, Limou S, Rosenberg AZ, et al. APOL1 Kidney Disease Risk Variants: An Evolving Landscape. Semin Nephrol. May 2015;35(3):222–36. doi: 10.1016/j.semnephrol.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. Jul 2003;14(7 Suppl 2):S148–53. doi: 10.1097/01.asn.0000070149.78399.ce [DOI] [PubMed] [Google Scholar]
- 23.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. Aug 2009;4(8):1302–11. doi: 10.2215/CJN.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. Aug 2011;58(2):214–27. doi: 10.1053/j.ajkd.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denker M, Boyle S, Anderson AH, et al. Chronic Renal Insufficiency Cohort Study (CRIC): Overview and Summary of Selected Findings. Clin J Am Soc Nephrol. Nov 6 2015;10(11):2073–83. doi: 10.2215/CJN.04260415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 27.Lin J, Fernandez H, Shashaty MG, et al. False-Positive Rate of AKI Using Consensus Creatinine-Based Criteria. Clin J Am Soc Nephrol. Oct 7 2015;10(10):1723–31. doi: 10.2215/CJN.02430315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishigami J, Taliercio JT, Feldman HI, et al. Fibroblast Growth Factor 23 and Risk of Hospitalization with Infection in Chronic Kidney Disease: The Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. Aug 2020;31(8):1836–1846. doi: 10.1681/asn.2019101106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrauben SJ, Chen HY, Lin E, et al. Hospitalizations among adults with chronic kidney disease in the United States: A cohort study. PLoS Med. Dec 2020;17(12):e1003470. doi: 10.1371/journal.pmed.1003470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu CY, Hsu RK, Liu KD, et al. Impact of AKI on Urinary Protein Excretion: Analysis of Two Prospective Cohorts. J Am Soc Nephrol. Jul 2019;30(7):1271–1281. doi: 10.1681/ASN.2018101036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. May 5 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inker LA, Eneanya ND, Coresh J, et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. Nov 4 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu RK, McCulloch CE, Heung M, et al. Exploring Potential Reasons for the Temporal Trend in Dialysis-Requiring AKI in the United States. Clin J Am Soc Nephrol. Jan 7 2016;11(1):14–20. doi: 10.2215/CJN.04520415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Medicare & Medicaid Services. Acute Inpatient Prospective Payment System (PPS) (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html?redirect=/AcuteInpatientPPS). Accessed March 18, 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html?redirect=/AcuteInpatientPPS
- 35.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. Dec 2013;369(23):2183–96. doi: 10.1056/NEJMoa1310345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsa A, Kanetsky PA, Xiao R, et al. Genome-Wide Association of CKD Progression: The Chronic Renal Insufficiency Cohort Study. J Am Soc Nephrol. Mar 2017;28(3):923–934. doi: 10.1681/ASN.2015101152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auer PL, Johnsen JM, Johnson AD, et al. Imputation of exome sequence variants into population- based samples and blood-cell-trait-associated loci in African Americans: NHLBI GO Exome Sequencing Project. Am J Hum Genet. Nov 2 2012;91(5):794–808. doi: 10.1016/j.ajhg.2012.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan Q, Liu EY, Auer PL, et al. Imputation of coding variants in African Americans: better performance using data from the exome sequencing project. Bioinformatics. Nov 1 2013;29(21):2744–9. doi: 10.1093/bioinformatics/btt477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. Oct 2010;21(10):1757–64. doi: 10.1681/ASN.2010010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James MT, Grams ME, Woodward M, et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Diabetes Mellitus, and Hypertension With Acute Kidney Injury. Am J Kidney Dis. Oct 2015;66(4):602–12. doi: 10.1053/j.ajkd.2015.02.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Race Demirjian S., class, and AKI. J Am Soc Nephrol. Aug 2014;25(8):1615–7. doi: 10.1681/ASN.2014030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H, Larsen CP, Hernandez-Arroyo CF, et al. AKI and Collapsing Glomerulopathy Associated with COVID-19 and APOL 1 High-Risk Genotype. J Am Soc Nephrol. Aug 2020;31(8):1688–1695. doi: 10.1681/asn.2020050558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shetty AA, Tawhari I, Safar-Boueri L, et al. COVID-19-Associated Glomerular Disease. J Am Soc Nephrol. Jan 2021;32(1):33–40. doi: 10.1681/asn.2020060804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velez JCQ, Caza T, Larsen CP. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol. Oct 2020;16(10):565–567. doi: 10.1038/s41581-020-0332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. Jun 2009;122(6):507–12. doi: 10.1016/j.amjmed.2008.12.020 [DOI] [PubMed] [Google Scholar]
- 46.Statius van Eps LW, Pinedo-Veels C, de Vries GH, de Koning J. Nature of concentrating defect in sickle-cell nephropathy. Microradioangiographic studies. Lancet. Feb 28 1970;1(7644):450–2. doi: 10.1016/s0140-6736(70)90836-6 [DOI] [PubMed] [Google Scholar]
- 47.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. Jul 2008;74(1):101–7. doi: 10.1038/ki.2008.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu RK, Hsu CY. Proteinuria and reduced glomerular filtration rate as risk factors for acute kidney injury. Curr Opin Nephrol Hypertens. May 2011;20(3):211–7. doi: 10.1097/MNH.0b013e3283454f8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. Sep 4 2001;135(5):352–66. doi: 10.7326/0003-4819-135-5-200109040-00012 [DOI] [PubMed] [Google Scholar]
- 50.Pfaendler KS, Chang J, Ziogas A, Bristow RE, Penner KR. Disparities in Adherence to National Comprehensive Cancer Network Treatment Guidelines and Survival for Stage IB-IIA Cervical Cancer in California. Obstet Gynecol. May 2018;131(5):899–908. doi: 10.1097/AOG.0000000000002591 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


