Abstract
Objective:
One of the most disabling aspects of living with chronic epilepsy is the unpredictability of seizures. Cumulative research in the past decades has advanced our understanding of the dynamics of seizure risk. Technological advances have recently made it possible to record pertinent biological signals, including electroencephalogram (EEG), continuously. We aimed to assess whether patient-specific seizure forecasting is possible using remote, minimally invasive ultra-long-term subcutaneous EEG.
Methods:
We analyzed a two-center cohort of ultra-long-term subcutaneous EEG recordings, including six patients with drug-resistant focal epilepsy monitored for 46–230 days with median 18 h/day of recorded data, totaling >11 000 h of EEG. Total electrographic seizures identified by visual review ranged from 12 to 36 per patient. Three candidate subject-specific long short-term memory network deep learning classifiers were trained offline and pseudoprospectively on preictal (1 h before) and interictal (>1 day from seizures) EEG segments. Performance was assessed relative to a random predictor. Periodicity of the final forecasts was also investigated with autocorrelation.
Results:
Depending on each architecture, significant forecasting performance was achieved in three to five of six patients, with overall mean area under the receiver operating characteristic curve of .65–.74. Significant forecasts showed sensitivity ranging from 64% to 80% and time in warning from 10.9% to 44.4%. Overall, the output of the forecasts closely followed patient-specific circadian patterns of seizure occurrence.
Significance:
This study demonstrates proof-of-principle for the possibility of subject-specific seizure forecasting using a minimally invasive subcutaneous EEG device capable of ultra-long-term at-home recordings. These results are encouraging for the development of a prospective seizure forecasting trial with minimally invasive EEG.
Keywords: deep learning, epilepsy, mobile health, seizure forecasting, seizure prediction, subcutaneous EEG
1 |. INTRODUCTION
Uncertainty is at the core of the burden of treatment-resistant epilepsy. The unforeseen, unpredictable times at which seizures occur, together with their potential catastrophic consequences, from injury to death, has wide-ranging limitations in patients’ lives, extending far beyond the short symptomatic (ictal) period.1–3 Approximately one third of all people with epilepsy continue to suffer from repetitive seizures and endure this burden.4
Reliable seizure forecasting could have far-reaching benefits for patients, from allowing treatment modulation according to risk (including fast-acting medication/chronotherapy and neuromodulation) to patient behavior modification and protection, ultimately improving safety and quality of life.2,5
There is, unsurprisingly, high interest from patients and investment from the scientific community in the development of methods for seizure prediction or forecasting.1–3
Significant advances have been made in the past decades in the field,6 including a better understanding of the preictal and proictal states and their systemic/behavioral/neurological correlates; data repositories of long-term recordings,7 including ultra-long-term remote recordings8; better methodological approaches and standardized performance evaluation to improve the generalizability of algorithms; seizure prediction contests opening data analysis to a wider scientific community8,9; and a deepening of the understanding of person-specific seizure risk modulators (sleep, stress, mood levels) and circadian and multiday cycles.10–13
Seizure forecasting has been demonstrated using intracranial ultra-long-term EEG in ambulatory canines9,14 and humans,8,9,15–18 including a prospective trial.15 However, invasive EEG systems have the potential for serious complications,15,19 and may not be appropriate for all patients. More recently, considerable efforts have been made toward forecasting with noninvasive methods, from seizure diaries20 to commercial fitness trackers21 and medical-grade wristwatches.22,23 However, neither seizure diaries nor wearable devices are consistently able to reliability detect and validate seizures, in particular certain seizure types, especially when compared to gold standard video-electroencephalography (EEG).15,24,25 Furthermore, using EEG for forecasting may offer advantages over other systems, given its ability to directly reflect cerebral activity.
Between the infeasibility and signal degradation of scalp EEG systems over long periods and the invasiveness of intracranial EEG, novel subcutaneous EEG (sqEEG) systems offer an attractive trade-off between minimal invasiveness/low risk and robust signal quality.26 One sqEEG device has been approved in Europe for continuous EEG monitoring, and others are at varying stages of development.27,28 We have shown in previous work that signal quality of sqEEG is highly stationary through time, from the beginning of the recording,26 in contrast to implantable intracranial EEG.29 This makes these systems particularly useful for chronic implantation, and for automated analysis, including seizure prediction. The detection of seizure cycles and their potential for forecasting also shows promise for these systems.28,30
This study aimed to assess the feasibility of patient-specific seizure forecasting, using a minimally invasive, two-channel sqEEG device.
2 |. MATERIALS AND METHODS
2.1 |. Patient population
This study included patients with medication-refractory focal epilepsy who recorded ultra-long-term sqEEG from two prospective clinical cohorts, Zealand University Hospital (ZUH), Denmark31 and King’s College London (KCL), UK, from an ongoing observational study (ClinicalTrials.gov NCT04061707). Both studies received institutional review board approval, and all patients provided written informed consent. We considered in the analysis patients with completed and fully annotated recordings as of September 2021 (all nine patients from ZUH and two patients from KCL). At KCL, both recordings were interrupted temporarily due to a now resolved device malfunction. Study recruitment and device implantation were also impeded by COVID-19-related restrictions, resulting in a limited cohort at the time of this analysis. Exclusion criteria were common to both centers, and included other paroxysmal conditions potentially mimicking epileptic seizures (e.g., psychogenic nonepileptic seizures), insufficient evidence for electrographic correlates of seizures, potential clinical risk of device implantation, low seizure frequency (<1 seizure/week in the ZUH study and <2 seizures/month in the KCL study), and planned magnetic resonance imaging during the study period. (See Weisdorf et al.31 and https://clinicaltrials.gov/ct2/show/NCT04061707for full list of eligibility criteria.)
2.2 |. Study procedures
All subjects were recorded with the 24/7 EEG SubQ system. Briefly, the system consists of an implantable three-contact lead wire (yielding two-channel bipolar EEG) with a small ceramic housing, which is placed unilaterally under local anesthesia, over the region of preidentified or presumed ictal EEG changes. Patients then use a small external data logger, which connects via magnetic induction over the scalp to the implant housing, powering the implant and transmitting data. Recordings started 1–3 weeks after implantation. Patients were asked to record (i.e., connect the data logger) as much as possible during their everyday life, except during washing. Patients were also asked to report their seizures on paper (ZUH) or using an electronic diary (KCL, using the Seer app [https://seermedical.com/seer-app/]). Clinical care was not altered by participation in the study; hence, medication and other treatment changes were allowed, including fast-acting medication during seizure clusters. At KCL, monthly visits were also undertaken to review patients’ experiences with the device, their recording adherence, and seizure diaries.
2.3 |. Data preprocessing and labeling
All analyses were performed on retrospective data in a pseudoprospective manner. The two-channel sqEEG signal is recorded at a sampling rate of 207 Hz and bandpass filtered at .5–48 Hz with a finite-impulse-response equiripple design and 40-dB attenuation filter, prior to review. Electrographic seizures were identified by an epileptologist with experience in sqEEG recordings (Dr. Sigge Weisdorf at ZUH, P.F.V. at KCL) and were verified by a board-certified clinical neurophysiologist (T.W.K. at ZUH, J.S.W. at KCL). In both cohorts, patient-specific electrographic seizure patterns (seizure signatures), taken from previous recordings, were taken into consideration during the decision. At ZUH, the EEG was visually inspected based on 10-min time-frequency epochs, and potential seizures were reviewed in the time domain.31 At KCL, the EEG was reviewed in dedicated software (UNEEG Episight viewer v1.11) with an inbuilt 10-min spectrogram (see Figure S1) and a high-sensitivity seizure detector (unpublished). The data review process at this center was as follows: (1) review of events marked by the seizure detector, (2) review of periods around the patient diary reports (within 2 h), and (3) a random sample of 6-h epochs comprising 10% of the whole recording. Finally, the full dataset was reviewed if seizures not previously marked by the seizure detector were encountered on the random 10% review (Subject S02).
Based on confirmed seizures, data epochs were labeled as preictal or interictal as follows. One-hour preictal data segments were defined with a set-back of 5 min before the onset of a lead seizure (i.e., seizure preceded by >4 h without a seizure), so as to avoid the confounding of early seizure detection and theoretically allow reasonable time for potential patient action/behavior modification. Interictal data segments were defined as seizure-free periods at least 1 day apart from any lead seizure. Next, the data were segmented into 1-min epochs and preprocessed, starting with per-segment mean subtraction, low pass filtering at 25 Hz, and downsampling by a factor of 2. The fast Fourier transform (FFT) for each channel was calculated and provided to the classifier as additional input channels. Random white noise-added copies of preictal data segments were generated to compensate for the heavily unbalanced data ratio in training. Finally, the entire dataset was amplitude (z-score) normalized.
2.4 |. Training and testing data
The data cutoff point for splitting into training and testing was chosen at one third of the total recording time, with the initial third of the recording selected for training, to mimic a prospective trial. The prerequisites for including a recording in the analysis were: (1) the training data included a minimum of three 1-h preictal epochs, (2) the testing data included a minimum of four 1-h preictal epochs, (3) the training data included a total of interictal segments at least three times the total of preictal segments, and (4) the testing data included at least as many interictal segments as preictal segments. The training/testing cutoff point was shifted if needed to meet the above requirements.
Overall, the input data consisted of the processed two-channel time series and the FFT of each channel, as well as (for Architectures 2 and 3) the time of day by the 24-h clock, to allow the algorithm to learn circadian periodicities in the subject’s seizures.32
2.5 |. Forecasting algorithms and postprocessing
The three candidate long short-term memory (LSTM) architectures used in this study were optimized after preliminary single subject analysis.32 LSTM networks have shown great promise in various applications in medicine, including in epileptic seizure forecasting.23,28 For a full description of the three different architectures, see Pal Attia et al. (current issue). The output of each architecture was further postprocessed as follows: the raw output was averaged at every nonoverlapping 5-min window, and the maximum of 12 consecutive 5-min averages was taken to obtain an approximately hourly forecast (Figure S2).
2.6 |. Statistical analysis
We based the performance assessment of the forecasting algorithms on Snyder et al.,33 which compares the probability of successful prediction of n of N seizures with that of a Poisson-distributed random predictor with a matched time in warning. We further validated the testing approach by randomizing seizure times for each subject and recalculating the area under the curve (AUC) for the LSTM output, 100 times per subject. Random seizure times were generated such that the total number of seizures and the distribution of intervals between consecutive seizures remained constant.34 Improvement over chance was reported as the mean improvement in sensitivity while holding a constant time in warning.15,18 Periodicity analysis of the forecasts was conducted using the autocorrelation function. Comparison of forecasting horizons between different architectures was done via Wilcoxon rank-sum tests. Python (3.7.8) and MATLAB (MathWorks, R2021b) were used in the analysis.
3 |. RESULTS
3.1 |. EEG recordings
Of the 11 patients considered, six met inclusion criteria and were included in the final analysis. Five patients from the ZUH cohort did not satisfy the data training/testing criteria: four patients had insufficient seizures during monitoring, and one patient had multiple daily seizures with periods of status epilepticus, whereby it was difficult to distinguish between ictal and interictal periods. Of the six patients analyzed (Table 1), recording duration ranged between 46 and 230 days (median = 80 days). The median data capture rate was 76.5%, corresponding to 18 h/day of recorded EEG. Overall, this dataset included >11 000 h of EEG. After visual review, 12–36 electrographic seizures were recorded per subject.
TABLE 1.
Patient demographic, clinical, and recording characteristics
| Subject | Age, years | Sex | Implant location | Seizure types | Antiepileptic drugs | Recording duration, days | Recording adherence, % | Number of EEG seizures | Number of lead seizures (training/testing)a |
|---|---|---|---|---|---|---|---|---|---|
| E02 | 33 | F | LT | FIAS, FBTCS | LMT, CLBb BRVc LEVb PRPb,c | 89 | 70 | 24 | 15 (3/10) |
| E04 | 38 | F | LT | Uncertain | LCM, ESL | 69 | 90 | 12 | 12 (3/6) |
| E06 | 75 | F | LT | FIAS, FBTCS | LCM, LMT | 76 | 62 | 17 | 15 (8/4) |
| E09 | 27 | F | LT | FAS | None | 84 | 80 | 25 | 25 (8/11) |
| S01 | 36 | F | LT | FAS, FIAS | CBZ, LEV | 230 | 84 | 30 | 18 (4/11) |
| S02 | 57 | M | RT | FAS, FIAS | CBZ, BRV, CLBd | 46 | 73 | 35 | 18 (9/5) |
Abbreviations: BRV, brivaracetam; CBZ, carbamazepine; CLB, clobazam; EEG, electroencephalographic; ESL, eslicarbazepine; F, female; FAS, focal aware seizures; FBTCS, focal to bilateral tonic–clonic seizures; FIAS, focal impaired awareness seizures; LCM, lacosamide; LEV, levetiracetam; LMT, lamotrigine; LT, left temporal; M, male; PRP, perampanel; RT, right temporal.
Seizures separated from preceding seizures by at least 4 h. Note that a proportion of seizures from each patient were not available for training or testing, due to insufficient or no preictal recording.
Stopped during monitoring.
Started during monitoring.
Rescue antiseizure medication.
3.2 |. Forecasting performance
Table 2 shows the patient-specific forecasting performance results for the three candidate architectures. Significant results were observed in five of six patients for the BiLSTM architecture, four patients with the 3/200 LSTM architecture, and three patients with the 5/25 LSTM architecture. Two patients (E02, E09) had significant forecasts across all three types of architectures. For Architectures 1–3, respectively, mean (SD) AUC across all patients was .65 (.17), .69 (.08), and .74 (.08). Table 2 also reports sensitivity, time in warning, average daily false alarm rate, and percentage improvement over chance results after selecting a threshold at the top-left-most point of the receiver operating characteristic (ROC) curve. Figure 1 shows an example of a successful forecast using Architecture 3, binarized by the selected threshold from the ROC curve.
TABLE 2.
Seizure forecasting results with the three selected architectures
| Subject | Architecture 1, 3/200 |
Architecture 2, 5/25 |
Architecture 3, BiLSTM |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Se | TIW/FAR | p | IoC | AUC | Se | TIW/FAR | p | IoC | AUC | Se | TIW/FAR | p | IoC | |
| E02 | .83 | 70 | 20.7/5.0 | .001a | 50.3 (21.3) | .72 | 70 | 41.2/9.9 | .045a | 35.0 (35.0) | .74 | 70 | 39.1/9.4 | .033a | 34.9 (34.7) |
| E04 | .76 | 67 | 17.5/4.2 | .008a | 51.3 (17.1) | .65 | 67 | 35.2/8.4 | .095 | n.s. | .59 | 67 | 48.1/11.5 | .258 | n.s. |
| E06 | .71 | 75 | 24.7/6.0 | .040a | 52.8 (18.7) | .64 | 75 | 58.3/14.0 | .393 | n.s. | .78 | 75 | 25.1/6.0 | .041a | 54.3 (21.0) |
| E09 | .71 | 73 | 29.9/7.1 | .003a | 42.7 (18.2) | .59 | 73 | 44.4/10.7 | .039a | 30.2 (21.2) | .64 | 73 | 42.2/10.1 | .027a | 34.4 (20.1) |
| S01 | .50 | 64 | 65.7/15.8 | .614 | n.s. | .81 | 73 | 10.9/2.6 | <.001a | 60.7 (15.0) | .79 | 73 | 13.6/3.3 | <.001a | 61.2 (13.9) |
| S02 | .37 | 40 | 41.0/9.8 | .633 | n.s. | .75 | 60 | 27.2/6.5 | .104 | n.s. | .74 | 80 | 36.3/8.7 | .048a | 50.9 (25.0) |
| Mean (SD) | .65 (.17)b | 71.3 (3.5)c | 23.2 (5.4)/5.6 (1.3)c | – | – | .69 (.08)b | 72.0 (1.7)c | 32.2 (18.5)/7.7 (4.4)c | – | – | .74 (.08)b | 74.2 (3.7)c | 31.3 (11.8)/7.5 (2.8)c | – | – |
Abbreviations: AUC, area under the receiver operating characteristic curve; FAR, mean false alarm rate in hours/day; IoC, improvement over chance: mean (SD) improvement in percentage sensitivity of the forecast compared to 100 randomly generated AUCs after shuffling seizure times, at the same time in warning; n.s., nonsignificant; Se, percentage sensitivity; TIW, time in warning as a percentage of recording in a high-risk state.
p< .05.
Mean (SD) across all subjects.
Mean (SD) of significant forecasts.
FIGURE 1.
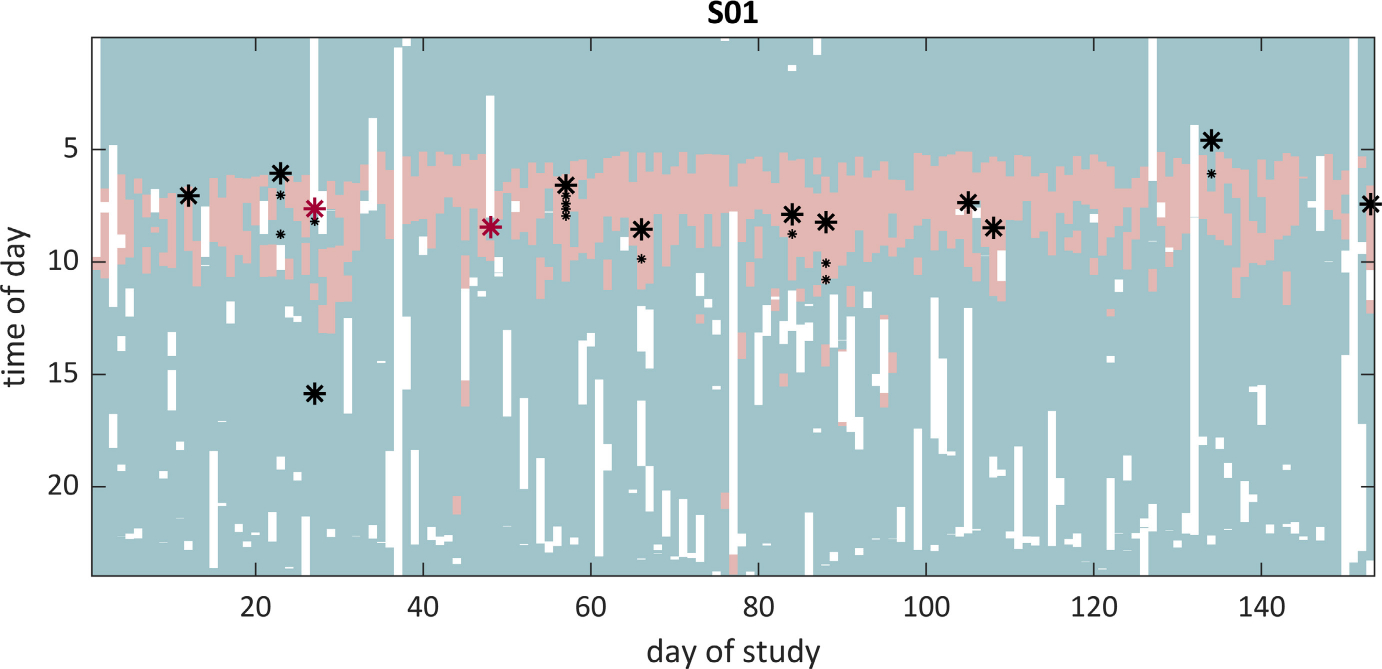
Example of a successful seizure forecast (Subject S01, Architecture 3, bidirectional long short-term memory). Only the testing dataset is depicted (corresponding to two thirds of the full recording). Lead seizures are represented as large asterisks, and clustered seizures as small asterisks. Light red areas depict times of predicted high seizure risk, whereas light green show periods of low seizure risk. White areas show periods when the patient was not recording. Note that two seizures did not have sufficient preictal data (red asterisks) and were removed from the final performance analysis. Also note the high circadian periodicity of the forecast, matching the patient’s preferential seizure times of day. It is also noteworthy that several predicted preictal periods were not followed by a seizure, contributing to increased time in warning
3.3 |. Duration of predicted preictal period
Taking the threshold points as detailed above, we also investigated the duration of preictal periods (as a proxy for preseizure alert timing) by calculating the time between the start of high-risk periods and the onset of each successfully forecasted seizure (Figure 2). Median duration ranged from 16 min (Subject E09, 3/200 architecture) to 2 h and 52 min (Subject E02, 5/25 architecture). A comparison of architectures across patients showed that the 3/200 architecture provided shorter predicted preictal times than the 5/25 and BiLSTM architectures (Figure 2; Wilcoxon rank-sum tests, p =.043 and .012, respectively). In all architectures, however, many predicted preictal periods, long or short, were not followed by a seizure (examples in Figure 1).
FIGURE 2.
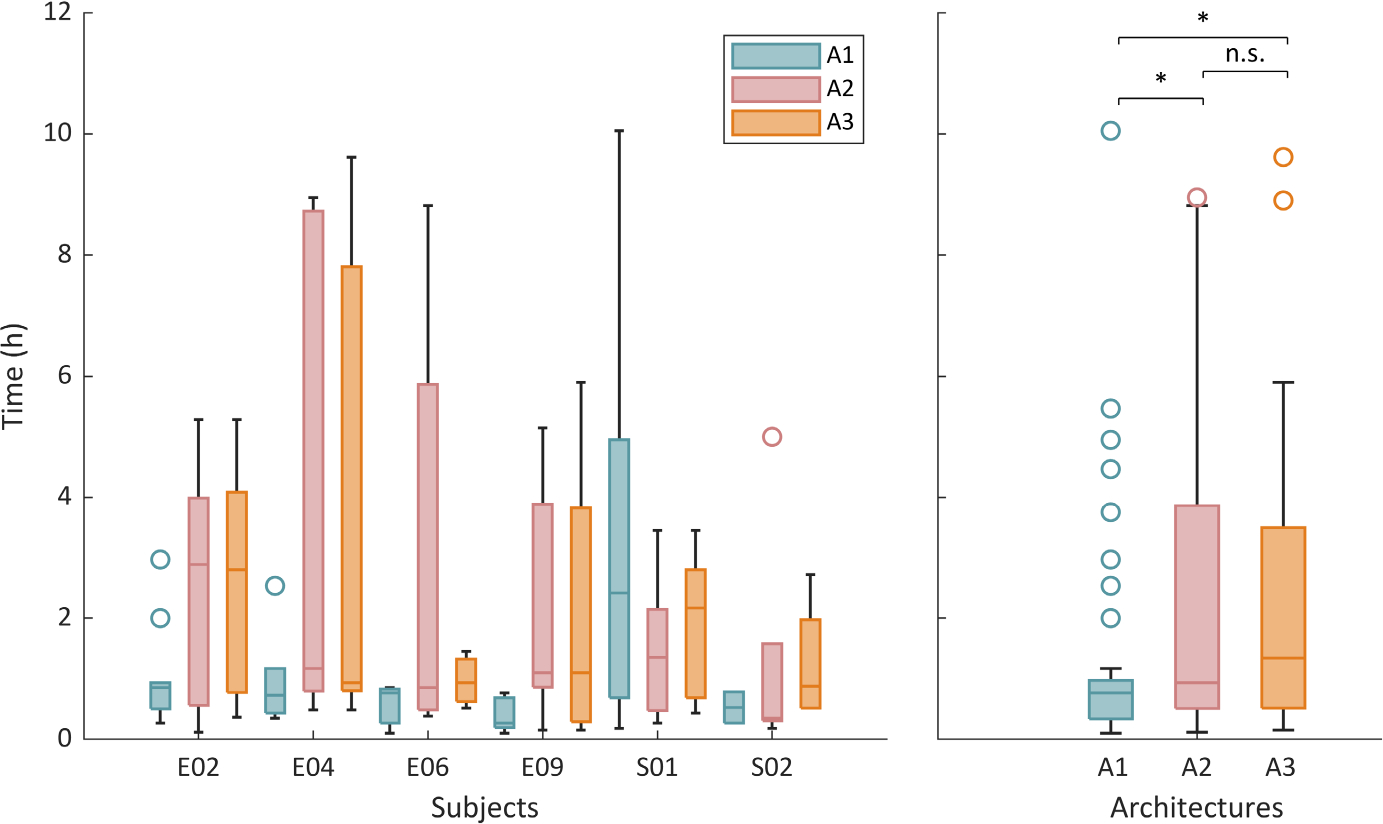
Duration of predicted preictal periods. Box plots represent time from the start of high-risk period to the onset of each successfully forecasted seizure, for different subjects and architectures (left panel) and comparing the three different architectures across patients (right panel). Each architecture is represented by one color, in both plots. *Significant at p < .05; n.s., nonsignificant result (Wilcoxon rank-sum tests)
3.4 |. Periodicity analysis of LSTM forecasts
We aimed to investigate whether LSTM network architectures learned or adapted to patient-specific seizure occurrence patterns. We used an autocorrelation function to assess circadian (all patients, taking average hourly output) and infradian periodicity (one patient, S01, who was recorded for >3 months). Autocorrelation was significant at a 24-h lag for all patients in Architectures 2 and 3 (Figure 3), and for four patients in Architecture 1 (see also Figures S3 and S4). Infradian autocorrelation was statistically significant in the two architectures with longer seizure warning periods (5/25 and 3/200), with long-term periodicity of up to 20 days (Figure 4).
FIGURE 3.
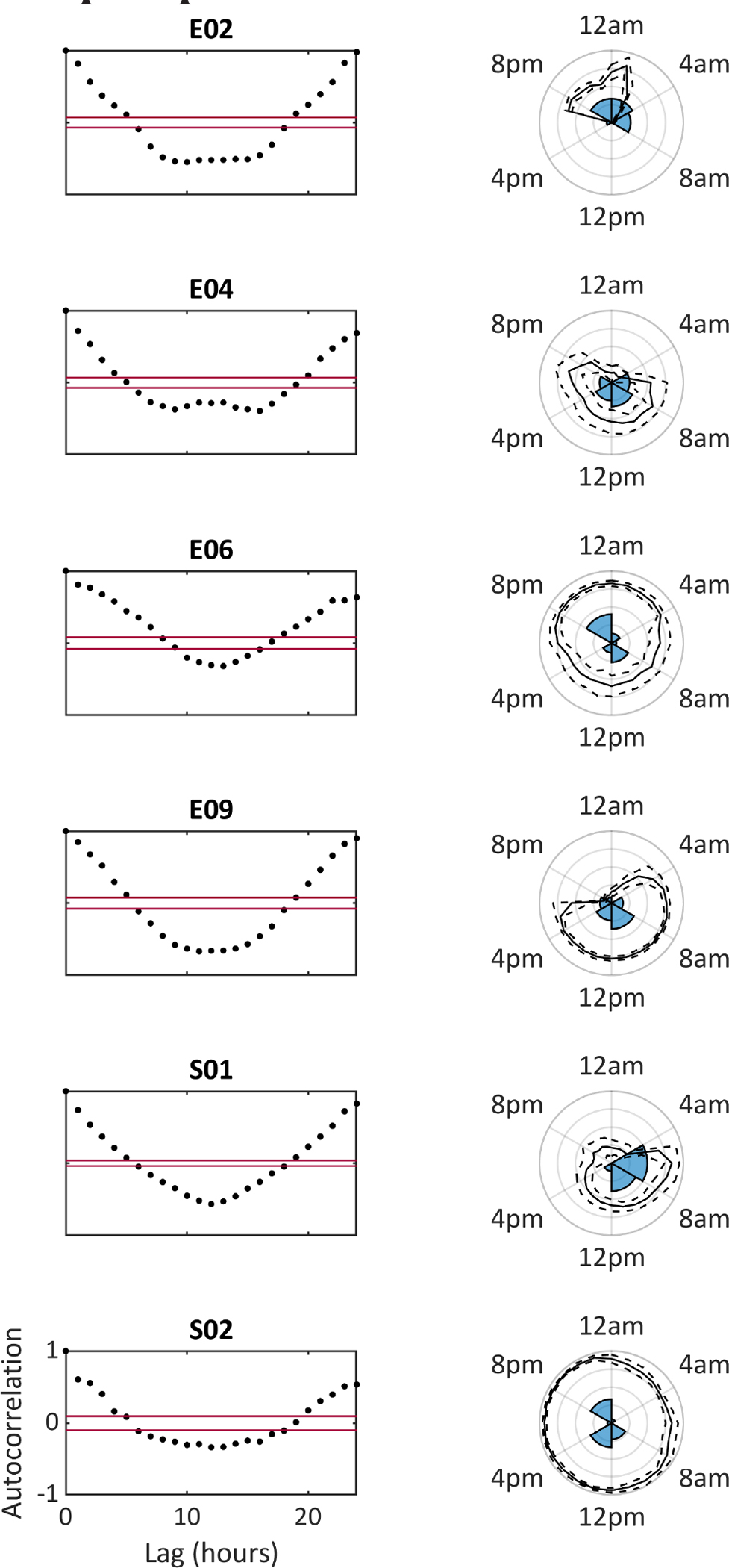
Circadian distribution of seizures and of forecasts (for Architecture 3, bidirectional long short-term memory). Left side shows the results of the autocorrelation function of average hourly forecasts for each subject (red lines delineate 95% confidence interval bounds). Right side polar plots show the time-of-day distribution of seizures (as a percentage histogram) overlaid with the mean (solid lines) and standard deviation (dashed lines) of the forecast output at each time of day. Note the clear 24-h periodicity of the forecast for all subjects, and the overall good correspondence between seizures’ time of day and seizure risk as determined by the forecast
FIGURE 4.
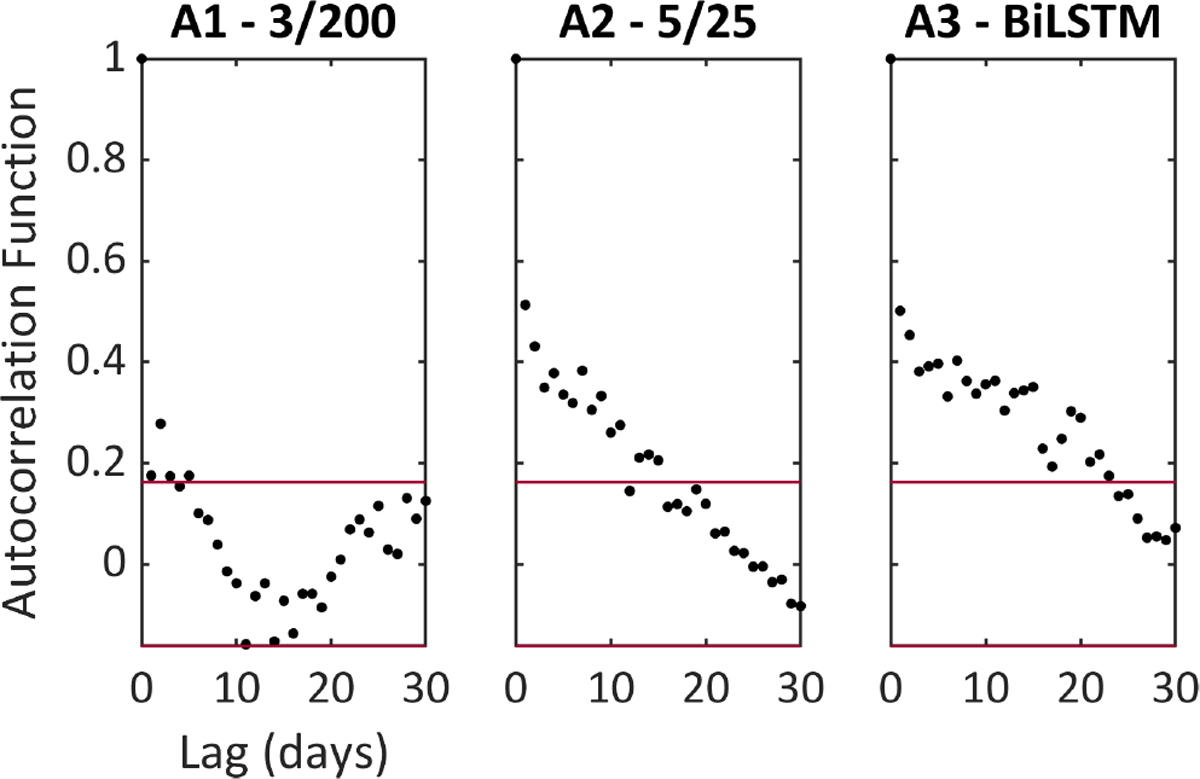
Long-term autocorrelation of seizure forecasts for subject S01, who recorded for 230 days. Each panel shows the results of the autocorrelation function of mean daily outputs of each forecasting architecture (Architectures 1–3, respectively). Red lines indicate 95% confidence interval bounds
4 |. DISCUSSION
In this study, we have demonstrated proof-of-principle and feasibility of seizure forecasting using a mobile, minimally invasive EEG device. To the best of our knowledge, this is the first study to pseudoprospectively forecast seizures with ambulatory EEG, after retrospective forecasting based on seizure cycles and previous seizure times was reported in a single case using a different subscalp device.28
Pooling data across two small cohorts, with recordings lasting from 2 to 9 months, after testing three deep learning LSTM-based architectures, significant forecasting was observed in at least half of the patients using each architecture. Despite limited (preictal) training data and a very unbalanced dataset (typical for seizure detection/forecasting), significant results were achieved. These findings indicate the richness and utility of ambulatory ultra-long-term EEG data for seizure forecasting,15,16 which enables the capture of electrographic patterns spanning a wide variety of daily circumstances and brain states. The algorithms used in this study were computationally efficient enough to run on commercial mobile devices (e.g., smartphones), with the computationally demanding training phase performed in a scalable cloud environment.
Forecasting performance varied between patients and different architectures. In significant forecasts, percentage time in warning was lower in the 3/200 architecture compared to the 5/25 and BiLSTM architectures, whereas sensitivity was similar across architectures. Seizure warning times were also shorter in the 3/200 architecture, which may be preferable in some situations, and suggests it may be possible to ensemble algorithms to provide seizure warnings with long and short time horizons.3 Conversely, the 5/25 and BiLSTM architectures showed significant infradian autocorrelation in the single subject tested, suggesting that they may be able to capture longer timescales of varying seizure risk that are increasingly acknowledged.11–13,30 Future studies with larger datasets should investigate whether certain patient populations are more amenable and/or might prefer shorter timescale prediction compared to longer timescale forecasting. Ultimately, the optimal cutoff values could theoretically be adjusted according to personal preference.18 In all architectures, many predicted high-risk periods, long or short, were not associated with subsequent seizures. These falsely predicted periods, occasionally lasting several hours, contribute to the time in (false) warning that could lead to alert fatigue from patients and caregivers and unnecessary mitigation (including fast-acting medications, neuromodulation). Continuing efforts toward performance improvement are important to minimize these factors.
Circadian periodicity was seen in the output of all architectures and for most patients. This periodicity accompanied patient-specific circadian patterns of seizure occurrence, suggesting that the algorithms can learn circadian patterns directly from the EEG and may not need explicit input, with circadian variation inferred from brain state changes in the sqEEG.26
Previous studies have reported successful forecasting with noninvasive wearable devices and seizure diaries.20,23 sqEEG offers the advantage of simultaneously detecting and documenting seizures directly and objectively, in contrast to wearables and diaries, which require external EEG or other validation of seizures. Ongoing work is focused on improving automated sqEEG seizure detection methods, currently used for data reduction only.30
A limitation in this study was the lack of distinction between clinically impactful seizures and purely electrographic seizures, as well as of differentiation between seizure types. The limited coverage of the EEG device and the lack of semiology make this challenging without an additional monitoring system (e.g., concurrent video, a wearable device). It is possible that some missed seizures may exist in these records undetected by the screening algorithm and outside of the 10% random review subset (KCL, Subject S01), and/or missed seizures upon review of 10-min spectrograms (both cohorts). We believe several measures that were taken minimize this possibility, including thorough review of detected candidate ictal events in the time domain, and extending manual review if the automated detector was observed to have <100% sensitivity (KCL, Subject S02). In addition, the number of seizures per patient was rather low, but future work with more data should expand this analysis. Despite pooling patients from two cohorts in two countries, these patients might not be truly representative of the population of drug-resistant epilepsy, and larger studies are needed to assess the broader application of this technology.
A further limitation is the incomplete data capture across the study. Patients need to keep a logging device connected to record, and the logging device needs to be removed during certain daily activities (e.g., bathing) and to be recharged daily. Therefore, some seizures did not have sufficient preictal data for training or testing (examples in Figure 1). Data capture rate was 78.4% across patients, corresponding to >18 h/day of recording on average, which represents a similar data dropout rate to the NeuroVista study.16 Both studies were strictly observational, and we anticipate that when patients receive clinical feedback on their recordings (including real-time forecasting), adherence may improve. Nonetheless, future work to investigate and improve device usability may be needed.35
Other limitations of this device have been previously described, including the limited spatial coverage and the possibility of missed contralateral or distant seizures. A priori knowledge of the localization of the epileptogenic zone (including the ictal onset zone) is essential for the optimal placement of the device and accuracy of the seizure record.
In conclusion, we have demonstrated that seizure forecasting is possible with ultra-long-term sqEEG. These results support the development of future prospective forecasting trials with these systems, and sqEEG’s clinical potential includes other uses, such as seizure, spike, and sleep monitoring.28,30,31,36,37
Supplementary Material
Key Points.
Patient-specific seizure forecasting with a minimally invasive two-channel subcutaneous EEG device is possible
Significant seizure forecasting was observed in at least 50% of patients
Patient-specific circadian patterns of seizure occurrence provide significant added value to seizure forecasting
ACKNOWLEDGMENTS
This work was supported by the Epilepsy Foundation’s Epilepsy Innovation Institute My Seizure Gauge Project. M.P.R. is supported by the NIHR Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust, the MRC Centre for Neurodevelopmental Disorders (MR/N026063/1), and the RADAR-CNS project funded by the European Commission (www.radar-cns.org, grant agreement 115902). B.H.B. is supported by the Mayo Neurology AI Program and by the NIH (NS UG3 123066). We would like to thank the neurosurgical team (Mr Harishchandra Srinivasan, Mr Harutomo Hasegawa, and Mr Richard Selway) involved in the implantation procedures at King’s College Hospital NHS Foundation Trust. We also acknowledge Dr Sigge Weisdorf for the preparation of seizure annotations in the ZUH cohort. Ultimately, we would like to acknowledge all patients who participated in this study.
Funding information
Epilepsy Foundation’s Epilepsy Innovation Institute My Seizure Gauge Project; NIHR Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust; MRC Centre for Neurodevelopmental Disorders, Grant/Award Number: MR/N026063/1; European Commission, Grant/Award Number: 115902; NIH, Grant/Award Number: NS UG3 123066
Footnotes
CONFLICTS OF INTEREST
JD-H is an employee of UNEEG medical. E.S.N. and D.R.F. are employees and shareholders of Seer Medical. B.H.B. has equity in Cadence Neurosciences and has received research devices from Medtronic at no cost. M.P.R. has been a member of ad hoc advisory boards for UNEEG medical. P.F.V. received a payment from UNEEG medical for data annotation in an unrelated research study. A.S.-B. receives research support from UNEEG medical. None of the other authors has any conflict of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Dumanis SB, French JA, Bernard C, Worrell GA, Fureman BE. Seizure forecasting from idea to reality. Outcomes of the My Seizure Gauge Epilepsy Innovation Institute workshop. eNeuro. 2017;4(6):ENEURO.0349–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grzeskowiak CL, Dumanis SB. Seizure forecasting: patient and caregiver perspectives. Front Neurol. 2021;12:717428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulze-Bonhage A, Sales F, Wagner K, Teotonio R, Carius A, Schelle A, et al. Views of patients with epilepsy on seizure prediction devices. Epilepsy Behav. 2010;18(4):388–96. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365(10):919–26. [DOI] [PubMed] [Google Scholar]
- 5.Baud MO, Rao VR. Gauging seizure risk. Neurology. 2018;91(21):967–73. [DOI] [PubMed] [Google Scholar]
- 6.Kuhlmann L, Lehnertz K, Richardson MP, Schelter B, Zaveri HP. Seizure prediction—ready for a new era. Nat Rev Neurol. 2018;14(10):618–30. [DOI] [PubMed] [Google Scholar]
- 7.Klatt J, Feldwisch-Drentrup H, Ihle M, Navarro V, Neufang M, Teixeira C, et al. The EPILEPSIAE database: an extensive electroencephalography database of epilepsy patients. Epilepsia. 2012;53(9):1669–76. [DOI] [PubMed] [Google Scholar]
- 8.Kuhlmann L, Karoly P, Freestone DR, Brinkmann BH, Temko A, Barachant A, et al. Epilepsyecosystem.org: crowd-sourcing reproducible seizure prediction with long-term human intracranial EEG. Brain. 2018;141(9):2619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkmann BH, Wagenaar J, Abbot D, Adkins P, Bosshard SC, Chen M, et al. Crowdsourcing reproducible seizure forecasting in human and canine epilepsy. Brain. 2016;139(Pt 6):1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karoly PJ, Goldenholz DM, Freestone DR, Moss RE, Grayden DB, Theodore WH, et al. Circadian and circaseptan rhythms in human epilepsy: a retrospective cohort study. Lancet Neurol. 2018;17(11):977–85. [DOI] [PubMed] [Google Scholar]
- 11.Karoly PJ, Freestone DR, Boston R, Grayden DB, Himes D, Leyde K, et al. Interictal spikes and epileptic seizures: their relationship and underlying rhythmicity. Brain. 2016;139(Pt 4):1066–78. [DOI] [PubMed] [Google Scholar]
- 12.Karoly PJ, Rao VR, Gregg NM, Worrell GA, Bernard C, Cook MJ, et al. Cycles in epilepsy. Nat Rev Neurol. 2021;17(5):267–84. [DOI] [PubMed] [Google Scholar]
- 13.Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann BH, Patterson EE, Vite C, Vasoli VM, Crepeau D, Stead M, et al. Forecasting seizures using intracranial EEG measures and SVM in naturally occurring canine epilepsy. PLoS One. 2015;10(8):e0133900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook MJ, O’Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 2013;12(6):563–71. [DOI] [PubMed] [Google Scholar]
- 16.Maturana MI, Meisel C, Dell K, Karoly PJ, D’Souza W, Grayden DB, et al. Critical slowing down as a biomarker for seizure susceptibility. Nat Commun. 2020;11(1):2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proix T, Truccolo W, Leguia MG, Tcheng TK, King-Stephens D, Rao VR, et al. Forecasting seizure risk in adults with focal epilepsy: a development and validation study. Lancet Neurol. 2021;20(2):127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiral-Kornek I, Roy S, Nurse E, Mashford B, Karoly P, Carroll T, et al. Epileptic seizure prediction using big data and deep learning: toward a mobile system. EBioMedicine. 2018;27:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skarpaas TL, Jarosiewicz B, Morrell MJ. Brain-responsive neurostimulation for epilepsy (RNS® System). Epilepsy Res. 2019;153:68–70. [DOI] [PubMed] [Google Scholar]
- 20.Karoly PJ, Cook MJ, Maturana M, Nurse ES, Payne D, Brinkmann BH, et al. Forecasting cycles of seizure likelihood. Epilepsia. 2020;61(4):776–86. [DOI] [PubMed] [Google Scholar]
- 21.Stirling RE, Grayden DB, D’Souza W, Cook MJ, Nurse E, Freestone DR, et al. Forecasting seizure likelihood with wearable technology. Front Neurol. 2021;12:704060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meisel C, El Atrache R, Jackson M, Schubach S, Ufongene C, Loddenkemper T. Machine learning from wristband sensor data for wearable, noninvasive seizure forecasting. Epilepsia. 2020;61(12):2653–66. [DOI] [PubMed] [Google Scholar]
- 23.Nasseri M, Pal Attia T, Joseph B, Gregg NM, Nurse ES, Viana PF, et al. Ambulatory seizure forecasting with a wrist-worn device using long-short term memory deep learning. Sci Rep. 2021;11(1):21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elger CE, Hoppe C. Diagnostic challenges in epilepsy: seizure under-reporting and seizure detection. Lancet Neurol. 2018;17(3):279–88. [DOI] [PubMed] [Google Scholar]
- 25.Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol. 2007;64(11):1595–9. [DOI] [PubMed] [Google Scholar]
- 26.Viana PF, Remvig LS, Duun-Henriksen J, Glasstetter M, Dumpelmann M, Nurse ES, et al. Signal quality and power spectrum analysis of remote ultra long-term subcutaneous EEG. Epilepsia. 2021;62(8):1820–8. [DOI] [PubMed] [Google Scholar]
- 27.Duun-Henriksen J, Baud M, Richardson MP, Cook M, Kouvas G, Heasman JM, et al. A new era in electroencephalographic monitoring? Subscalp devices for ultra-long-term recordings. Epilepsia. 2020;61(9):1805–17. [DOI] [PubMed] [Google Scholar]
- 28.Stirling RE, Maturana MI, Karoly PJ, Nurse ES, McCutcheon K, Grayden DB, et al. Seizure forecasting using a novel sub-scalp ultra-long term EEG monitoring system. Front Neurol. 2021;12:713794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ung H, Baldassano SN, Bink H, Krieger AM, Williams S, Vitale F, et al. Intracranial EEG fluctuates over months after implanting electrodes in human brain. J Neural Eng. 2017;14(5):056011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viana PF, Duun-Henriksen J, Glasstëter M, Dümpelmann M, Nurse ES, Martins IP, et al. 230 days of ultra long-term subcutaneous EEG: seizure cycle analysis and comparison to patient diary. Ann Clin Transl Neurol. 2021;8(1):288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisdorf S, Duun-Henriksen J, Kjeldsen MJ, Poulsen FR, Gangstad SW, Kjaer TW. Ultra-long-term subcutaneous home monitoring of epilepsy—490 days of EEG from nine patients. Epilepsia. 2019;60(11):2204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attia TP, Viana PF, Nasseri M, Richardson MP, Brinkmann BH. Seizure forecasting from subcutaneous EEG using long short term memory neural networks: algorithm development and optimization. In: Huang Y, Kurgan L et al. , editors. 2021 IEEE International Conference on Bioinformatics and Biomedicine. Piscataway, NJ: IEEE; 2021. p. 3599–3602. [Google Scholar]
- 33.Snyder DE, Echauz J, Grimes DB, Litt B. The statistics of a practical seizure warning system. J Neural Eng. 2008;5(4):392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrzejak RG, Mormann F, Kreuz T, Rieke C, Kraskov A, Elger CE, et al. Testing the null hypothesis of the nonexistence of a preseizure state. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;67(1 Pt 1):010901. [DOI] [PubMed] [Google Scholar]
- 35.Bruno E, Viana PF, Sperling MR, Richardson MP. Seizure detection at home: do devices on the market match the needs of people living with epilepsy and their caregivers? Epilepsia. 2020;61(Suppl 1):S11–24. [DOI] [PubMed] [Google Scholar]
- 36.Weisdorf S, Gangstad SW, Duun-Henriksen J, Mosholt KSS, Kjær TW. High similarity between EEG from subcutaneous and proximate scalp electrodes in patients with temporal lobe epilepsy. J Neurophysiol. 2018;120(3):1451–60. [DOI] [PubMed] [Google Scholar]
- 37.Gangstad SW, Mikkelsen KB, Kidmose P, Tabar YR, Weisdorf S, Lauritzen MH, et al. Automatic sleep stage classification based on subcutaneous EEG in patients with epilepsy. Biomed Eng Online. 2019;18(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


