Abstract
Neural crest (NC) is the primitive neural structure in embryonic stage, which develops from ectodermal neural plate cells and epithelial cells. When the neural fold forms into neural tube, neural crest also forms a cord like structure above the neural tube and below the ectoderm. Neural crest cells (NCC) have strong migration and proliferation abilities. A number of tissue cells differentiate from neural crest cells, such as melanocytes, central and peripheral neurons, glial cells, craniofacial cells, osteoblasts, chondrocytes and smooth muscle cells. The migration and differentiation of neural crest cells are regulated by a gene network where a variety of genes, transcriptional factors, signal pathways and growth factors are involved.
Funding
Financial funds: This work was supported by grants from the National Nature Science Foundation of China (NSFC,81970897).
Sox10 (Sry-box 10) is a key gene in the regulation (SOMMER, 2011). Sox10 gene, as a member of Sox E family, plays an important role in regulating the formation and development of tissues and organs which derive from neural crest in embryonic stage (HERBARTH et al., 1998). During the above process, Sox10 gene also maintains pluripotency, and its mutation leads to neural crest diseases, such as Waardenburg syndrome that is a common congenital deafness syndrome. Therefore, exploring signal pathways and gene regulation network in which Sox10 gene participates in will provide a better understanding of the pathogenesis of neural crest disorders induced by Sox10 gene mutation.
This review elaborates the recent research advance on regulation mechanism of Sox10 gene in the development of cells originated from neural crest, such as glial cells and melanocytes. It will provide a deep understanding of Sox10 gene regulation network and a theoretical basis for researching the pathogenesis of neural crest related disease caused by Sox10 gene mutation. Last, it summaries that molecular mechanisms of inner ear development derived from studies of Sox10 gene mutation, which will facilitate further research on how Sox10 affect the function of inner ear.
1. An overview of Sox10 gene and protein
1.1. Sox10 gene
Sox (Sry-related HMG box) gene, one of a super gene family, of which protein-coding contains highly active component structural motif HMG (high mobility group), is closely related to sex determinant genes (Sex determining region Y, Sry). This motif is highly conserved in eukaryotes, which combines with other transcriptional factors and binding to target DNA in a sequence-specific manner (Gubbay et al., 1990; WEGNER, 1999; BOWLES et al., 2000). Comparing with HMG motifs, Wright (WRIGHT et al., 1993) and Bowles (BOWLES et al., 2000) et al. divided Sox gene family into 8 subfamilies-Sox A-H. Sox10 (Sry-box 10) gene, a member of Sox E family, is located on chromosome 22q13 1, which contains 5 exons as well as exon 03, 04 and 05 encode protein. Sox 10 encode 466 amino acids and a protein weighing 51kD, and its cDNA is 1.4 kb at full length.
1.2. Sox10 protein
The N-terminal of Sox10 protein contains a dimerization domain (DD), which is vital to the protein dimer to bind with target gene. This domain compromises 40 amino acids and is highly conserved in the Sox E family (PEIRANO, 2000). Next to it is a 79-amino acids HMG domain, where includes an intron and a 73-amino acids K2 domain (SCHREINER et al., 2007). The C-terminal contains a transcription activation threshold with 66 amino acids, which is rich in serine, proline and glutamine sequences (PUSCH et al., 1998). It regulates the function of Sox10 protein by binding and interacting with specific molecules. As other members of the family, Sox10 protein specifically recognizes and binds to the target gene sequence 5′-(A/T) (A/T)CAA (A/T)G-3′ with HMG box (HARLEY et al., 1994). Sox10 protein functions as two different DNA response elements. It is unique that the conformation of the DNA is modified by binding monomer or dimer to target genes. Compared to monomer, dimer increases DNA binding affinity and affects DNA bending. Monomer and dimer bind to different DNA promoters of target genes forming optimal conformation (PEIRANO, 2000; SCHLIERF, 2002). Sox10 is an active nucleocytoplasmic shuttle protein, and it contains two nuclear localization signals makingSox10 shuttle between nucleus and cytoplasm, which arecritical for Sox10 mediated transcriptional activation. Besides, Sox10 protein can also bind to the upstream cis acting elements of the target gene directly or indirectly to further regulate the expression of the target gene (SOMMER, 2011; Liu et al., 2014). In turn, post-translational modifications such as methylene, phosphorylation and acetylation also affect the function of Sox10 protein.
1.3. Expression and regulation of Sox10 gene and protein
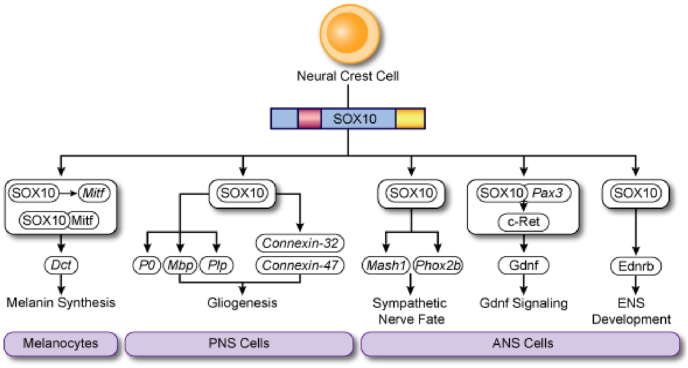
Sox10 gene plays a key role in the maintenance, migration and differentiation of neural crest progenitor cells. Sox10 is first expressed in the dorsal neural tube, and then it is expressed in its derivatives as cell differentiation, which contributes to the formation of the peripheral nervous system (HERBARTH et al., 1998; AOKI et al., 2003). In the group of adult mice and humans, it is expressed at a high level in brain, inner ear, heart and intestinal tract and ata low level in prostate, testis, bladder, pancreas and stomach (Owen et al., 2016). In peripheral nervous system, most neurons and all glial cells originate from the neural crest. Sox10 is no longer expressed in mature neuronal cells but continues to be expressed in mature glial cells (KUHLBRODT et al., 1998). In central nervous system, mainly in oligodendrocytes, Sox10 is crucial to their differentiation, maturation and myelination (LI et al., 2007). As to melanocytes, Sox10 gene significantly and persistently affect its specialization, maturation and maintenance (AOKI et al., 2003). Inthe early stage of inner ear development, Sox10 is widely expressed. After hair cells matured, Sox10 expression is gradually limited to support cells (WATANABE et al., 2000; LOCHER et al., 2013)., It is the gene regulation network, centered on Sox10 gene that plays an important role during the migration of neural crest cells (Owen et al., 2016) (Fig. 1a). Its expression in neural crest is regulated by signal transduction pathways, such as Wnts, BMPs and FGFs (AOKI et al., 2003; KIM et al., 2003; HONORÉ et al., 2003; WERNER et al., 2007).
Fig. 1.1.
Sox10 expression and regulation network during neural crest cell migration (Owen et al., 2016).
Sox10 protein is a key transcription factor for the migration and differentiation of neural crest derived cells, and it function alone or in cooperation with other transcription factors (Liu et al., 2014). Yeast two-hybrid analysis shows that more than a dozen transcription factors, such as Dlx5, Alx4, Hoxa3, BRN-1 and Pax3, regulate the transcriptional activity of Sox10 by binding N-terminal of Sox10 HMG domain with their DNA binding domain (WISSMULLER, 2006).
2. Regulation of Sox10 protein in the development of glial cells
Sox10 protein specifically enhances transcription in glial cells, and it is necessary for the terminal differentiation and maintenance of central nervous glial cells and oligodendrocytes and indispensable in the whole process of migration, survival and differentiation of peripheral glial Schwann cells (WEIDER and WEGNER, 2017).
2.1. Effect of Sox10 protein on the development of schwann cells
Sox10 protein is co-expressed with POU domain proteins tst-1/OCT6/SCIP, Pax3 and Egr2/Krox-20 at some stages of Schwann cell development. They synergistically bind with the target gene at the N-terminal region (KUHLBRODT et al., 1998). Protein zero (P0) gene encoding myelin protein P0 is the direct transcription target of Sox10. Sox10 protein regulates its expression by directly acting on the B, C and F sites of MPZ gene promoter. The expression of heterozygous P0 gene in Sox10 mutant DOM mice decreases, and it is hardly detected in homozygous (PEIRANO et al., 2000). Connexin 32 (Cx32) plays a role in the molecular transport of Schwann cells, which is encoded by GJB1 gene. In the development of Schwann cells, Sox10 activates the promoter of GJB1 gene by acting on S1 and S2 sites synergistically with Pax3, pou3f1 and Egr2 (BONDURAND, 2001). Sox10 directly activates Egr2 or cooperates with Egr2 gene to activate downstream target genes MBP, MAG, DHH, etc. (SRINIVASAN et al., 2012; JANG et al., 2006). Sox10 protein binds to the enhancer site MSE of Krox20 through the K2 domain of HMG and the POU domain of OCT6, to activate the expression of Krox20 (REIPRICH et al., 2010). The encoded Krox20 protein, in turn, activates the expression of target genes together with Sox10. This Sox10 transcription dependent linkage pattern plays an important role in Schwann cell development. Erbb3 is a key gene in the development and differentiation of neural crest derived tissue cells. The encoded protein belongs to a tyrosine kinase receptor of the epithelial growth factor (EGF) receptor family. Erbb3 activates downstream pathways PI3K, MAPK, PKC, JAK stat and PLCG (BRITSCH, 2007). Sox10 regulates expression of Erbb3 by direct combination with enhancer ERBB3-Mcs6 (PRASAD et al., 2011).
At the non-myelinating stage of Schwann cell development, Sox10 protein activates the expression of genes SOX5, Sox6, Notch 1, HMGA2, Hes1, MYCN, ID4 and Id2 that inhibit myelination, preventing premature differentiation of glial cells (GOPINATH et al., 2016). During the development of Schwann cells, Sox10-egr2/Krox-20 pathway and Sox10-ERBB3 pathway regulate in a complex network.
2.2. Effect of Sox10 protein on the development of oligodendrocytes
Sox10 and Sox9 proteins bind to PDGFα receptor of oligodendrocyte precursors, promoting their survival and migration abilities, and keep them in an undifferentiated state. Sox10, Sox5 and Sox6 synergistically activate the PDGFα receptor promoter and keep oligodendrocytes in an immature state (FINZSCH et al., 2008; BAROTI et al., 2016). MYRF (myelin regulatory factor) is an important transcriptional regulator to the formation of myelin in the central nervous system. Sox10 induces the expression of MYRF in oligodendrocytes by combining HMG and DD domains with the enhancer ECR9 at the C-terminal of MYRF, and then cooperates with MYRF to activate the expression of myelin gene. This regulatory network is very important to the differentiation and maturation of oligodendrocytes (HORNIG et al., 2013), which is functionally equivalent to the regulation of Krox20 and Egr2 in Schwann cells. Olig1 and Olig2 are members of the bHLH transcription factor family. Sox10 binds to the bHLH domains of Olig1 and Olig2 with its HMG domain (LI et al., 2007; KÜSPERT et al., 2011), and they jointly activate the expression of Mbp and Plp genes and promote the terminal differentiation of oligodendrocytes. In oligodendrocytes, Sox10 upregulates miR 335 and miR 338. These two microRNAs in turn recognize the 30UTR of Sox9 mRNA, reducing Sox9 expression in oligodendrocytes. This process maintains the balance of the Sox10 and Sox9, contributing to the terminal differentiation of oligodendrocytes (REIPRICH et al., 2017). Activated T-cell nuclear factor 2 (NFAT2) is ubiquitous in mammalian cells. It participates in multiple signal pathways and is closely related to growth and complex cell interactions. Sox10 affects the differentiation and myelination of oligodendrocyte by participating in the calcineurin/NFAT signaling pathway (WEIDER et al., 2018).
3. Effect of Sox10 protein on the development of melanocytes and melanoma cells
Sox10 protein is a key transcription factor to the establishment and maintenance of melanocyte lineage, and that is related to the occurrence, proliferation and invasion of melanoma. Literature (BONDURAND, 2000; POTTERF et al., 2000; ELWORTHY, 2003; LEE et al., 2000; HARRIS et al., 2010) has suggested that MITF is the target gene of Sox10 protein, and Sox10 protein bind to M promoter of MITF to activate transcription directly or cooperate with PAX3. MITF protein is a nuclear transcription factor with basic domain helix-loop-helix leucine zipper (bHLH-Zip) domain. There are four common subtypes, MITF-A, MITF-C, MITF-H and MITF-M, amongwhich MITF-M is expressed in melanocytes and melanoma cells. MITF protein is the main regulator of development and migration of melanocytes derived from neural crest, and its mutation causes abnormalities. Dopachrome tautomerase (DCT), tyrosinase (TYR) and TYR-related protein 1 (TYRP1) are key enzymes to melanin synthesis, which is regulated by MITF and Sox10. Transcriptional activation experiments in vitro determines that Sox10 and MITF synergistically activate DCT (JIAO et al., 2004; LUDWIG et al., 2004). Besides, Sox10 bind to the core enhancers in regulatory elements (DRE) of TYR, and MITF indirectly regulates the transcriptional activation of tyrosinase gene by combining with the E-box sequence of promoter (MURISIER et al., 2007). In conclusion, Sox10-MITF pathway is important in regulating the development of melanocytes. KIT is a tyrosine kinase receptor protein, which is essential for the survival, growth, differentiation and migration of melanocytes. Fufa et al. (FUFA et al., 2015) discover that in melanocytes Sox10 protein inhibits the expression of KIT gene by binding to its homeopathic response element. The results also show that lack of Sox10 haploid upregulate and downregulate the expression of downstream genes at the same time. More importantly, they reveal a new aspect of Sox10 mediated gene regulation, pluripotency (self-renewal) and EMT (epithelial mesen chymal transition), which is related to early development and metastasis of cancer. Consumption of Sox10 leads to cell cycle arrest, aging and inhibition of melanoma. The key point to the occurrence of melanoma is the development of multipotent stem cells with characteristics of melanocytes. Then nestin, cd-271 and cd-133, are expressed. These factors are directly or indirectly regulated by Sox10 gene to inhibit apoptosis and promote the occurrence of melanoma (TUDREJ and EDYTA, 2017). Besides, Sox10 gene also plays an essential role in regulating the invasion of melanoma.
Peripheral myelin protein 2 (PMP2) is the target gene directly regulated by Sox10 in melanoma cells, while Egr2 participates in the regulation as a cofactor and promotes the invasion of melanoma. There are three conserved Sox10 gene binding sites (P1, P2 and P3) in the PMP2 promoter region (GRAF et al., 2019). Melanoma inhibitory activity (MIA) is vital to the migration and invasion of melanoma cells. Sox10 directly regulates MIA, as well as indirectly regulate MIA by cooperating with the binding factor near promoter region (GRAF et al., 2014). Inhibition of Sox10 activity in melanoma cells leads to apoptosis and necrosis, implying that Sox10 protein is vital for the survival and proliferation of melanoma cells.
4. Effect of Sox10 protein on the development of inner ear
The inner ear composes of cochlea and vestibule, and its structure is complex and fine. The cochlear system senses sound and transmit a series of bioelectrical signals to the auditory center after preliminary analysis. The vestibular system senses and transmits balance signals. Therefore, the inner ear is important for auditory generation and balance maintenance. The development of mature inner ear from ear substrate is extremely complex in mammals. The membranous labyrinth cells of inner ear originate from the ear plate of the ectoderm, they invaginate and form the ear sac. After differentiation, they finally form the terminal organ of hearing and balance. This process is developing in an orderly manner and is accurately regulated by a variety of genes. Embryonic development is a chain reaction; different genes are activated successively, with time and space specificity. In the early stage of ear basal plate development, cranial neural crest cells differentiate into forebrain, midbrain and hindbrain. The cranial neural crest cells of the hindbrain continue to differentiate into the rhomboid proto ganglion, the ectoderm outsides continue to form the ear substrate (Liu et al., 2014), then neural crest cells migrate to the inner ear to form the intermediate cells of the stria vascularis and the glial cells of the vestibular cochlear nerve. Sox10 geneplays an important regulatory role in the maintenance, migration and differentiation of neural crest cells, and it is widely expressed in neural crest derived and non-neural crest derived cells in the early development of inner ear (Liu et al., 2014; WATANABE et al., 2000; BREUSKIN et al., 2009; DUTTON et al., 2009). Sox10 gene mutation causes Waardenburg syndrome in human. Clinically, the probability of inner ear malformation in Waardenburg cases with Sox10 gene mutation is significantly higher than that with other gene mutations, and the proportion of vestibular dysplasia is high, which includes vestibular cistern enlargement and absence of semicircular canal (PINGAULT et al., 2015; XU et al., 2016). Sox10 gene mutation animal model also confirmsthat its mutation is associated with abnormal development of inner ear (HERBARTH et al., 1998; BREUSKIN et al., 2009; DUTTON et al., 2009; BRITSCH, 2001; HAI et al., 2017; HAO et al., 2018). However, the regulatory effect of Sox10 gene on the embryonic development of inner ear is not fully understood.
4.1. Effect of Sox10 protein on the development of vestibule and cochlear in the inner ear
Inner ear malformations (IEM) are caused by heredity, drug, virus infection and other factors, which affect the normal development of the inner ear. Inner ear malformation occurs in any part of the membranous labyrinth and bone labyrinth, accounting for 80% and 20% respectively. In membranous labyrinth, hair cells are often involved and imaging examination often cannot detect, while in bone labyrinth, imaging examination can be used to confirm the malformation (SENNAROĞLU and BAJIN, 2017). Vestibular hair cells can sense the change of head position and maintain the balance of the body. In the development of vestibular system from dorsal ear sac, many genes are involved. Otx1, Otx2, PAX2, Fgf8, BMP4 and other genes are all important in the development (BURTON et al., 2004; MORSLI et al., 1998; CANTOS et al., 1170; MERLO et al., 2002; SÁNCHEZ-CALDERÓN et al., 2004; CHANG et al., 2008; DOMÍNGUEZ-FRUTOS et al., 2009). Kirsten Dutton et al. (DUTTON et al., 2009) found that in the early development of zebrafish with Sox10 gene mutation, due to the absence of Sox10 protein, the expression of downstream genes is affected, such as Fgf8 and BMP4, which are essential for the normal development of the vestibule of the inner ear. Therefore, Sox10 gene mutation in zebrafish causes abnormal development of vestibule, such as semicircular canals, cystic plaques and endolymphatic vessels, indicating that Sox10 gene plays a key role in the normal gene expression in the early stage of vestibular system development. Sox10 gene mutation has a more serious impact on the development of the inner ear of Xenopus laevis, which leads to abnormal development of neural crest and loss of ear sac (AOKI et al., 2003; HONORÉ et al., 2003; LANG, 2003). In the established Sox10 gene mutant mouse model and miniature pig model, no obvious abnormalities were found in the general morphology of vestibule during embryonic development (BREUSKIN et al., 2009; HAI et al., 2017; HAO et al., 2018), but the differentiation and development of vestibular sensory epithelium has not further studied. Wnt signaling pathway has been proved significant for the development of vestibular system, especially Wnt1 and Wnt 3a (RICCOMAGNO, 2005; BROWN et al., 2015). Wnt pathway signaling molecules from the dorsal hindbrain region regulate the expression of genes in the dorsal region of the ear sac, such as Dlx5/6, Gbx2 and hmx2/3. These genes play an important regulatory role in the development of the vestibular system (ROBLEDO and LUFKIN, 2006; LIN, 2005; WANG et al., 2004). It is reported that if Wnt1 and Wnt 3a genes were knocked out at the same time, utricle and saccule were unable to develop. The pathological mechanism may be the reduction of the expression of Dlx5 and Gbx2 genes which affects vestibular development (RICCOMAGNO, 2005). Qing Hao et al. (HAO et al., 2018) studied the molecular biological mechanism of inner ear malformation caused by Sox10p.r109w mutant in miniature pig. High throughput transcriptome sequencing and bioinformatics analysis shows that the possible target gene of Sox10 gene regulating inner ear development is Wnt1. In conclusion, Wnt signaling pathway plays a key role in the development of vestibular system.
Sox10 gene mutation could also cause Waardenburg sensorineural hearing loss. In the early stage, it is considered that Sox10 gene affects the expression of melanin related genes such as MITF and DCT, resulting in the deletion of stria vascularis intermediate cells, thus it affects the disturbance of endolymphatic circulation and causes the hypoplasia of Corti organ (TACHIBANA, 1999; TORIELLO, 2011). However, pathological mechanism of abnormal development of inner ear caused by Sox10 gene still needs to be explored. Sox10 gene is widely expressed in the early stage of inner ear development, but it is no longer expressed in differentiated and mature hair cells. So far, for different Sox10 gene mutation animal models, homozygous mutants have embryonic lethality, while heterozygotes have different phenotypes. The research of cochlear malformation caused by Sox10 gene mutation is a hot spot. In Xenopus laevis, the ear sac of is missing; in the homozygous mouse model, the cochlear canal is significantly shortened; in the heterozygous mouse model, the cochlear canal is extended and hair cells and Sertoli cells of Corti's organ are missing. In the miniature pig model, the development of cochlea lag from 28 days on and finally lead to malformation of cochlear and degeneration of Corti's organ, which suggests that Sox10 gene mutation directly affects the early development of cochlear organs.
In conclusion, the above model animals have made important contributions to the study of the effect of Sox10 gene on the development of cochlear organs (BREUSKIN et al., 2009; HAO et al., 2018; LANG, 2003; YANYAN et al., 2014; TACHIBANA et al., 2003). Regarding to signal pathways, Breuskin (BREUSKIN et al., 2009) et al. found that Sox10 protein downregulated the expression of Jagged1 gene in a concentration dependent manner, causing apoptosis of cochlear sensory progenitor cells. Notch signaling pathway mediated by Jagged1 gene regulates the development of sensory progenitor cells, which is indispensable to the regeneration and replacement of Sertoli cells in mammals (KIERNAN et al., 2006; PAN et al., 2010).
In the Sox10 p. r109w mutant miniature pig model, inner ear tissue transcriptome sequencing shows that in the early stage of embryonic development, genes regulated by Sox10 are Wnt1, Pax 6, fezf1, KCNQ4 (BURTON et al., 2004). Sox10 p. r109w mutant miniature pig is a good model to study how Sox10 gene regulates the development of inner ear, but there are also limitations. If cochlear system and vestibular system could be separately studied, it would be more meaningful. Compared with rodents, Sox10 gene is more widely expressed in human fetal and adult tissues, which is not limited within glial cells of the central nervous system, and it plays a more significant role in the human physiological development (PUSCH et al., 1998; Owen et al., 2016; WATANABE et al., 2000; BONDURAND et al., 1998). Rodent models cannot well exhibit the development of human cochlea. Miniature pigs are large mammals, and the development and morphology of cochlea are more similar to humans (GUO et al., 2017; ZHONG et al., 2018), so Sox10p. r109w mutant Waardenburg miniature pig model can better simulate how Sox10 leads to Waardenburg in vivo. For a complex disease, a more detailed understanding of the pathogenesis and what signaling pathways mediate are important for effective treatment (ZhU et al., 2020).
5. Conclusion
Sensorineural deafness and balance dysfunction are common sensory disorders, which seriously affect the quality of people's life and bring a heavy burden to individuals, families and the society. With the progress of research, genetic factors are found more and more important in etiology. Since the discovery of Sox10 gene in 1993, its structure and function have been widely studied. As the main pathogenic factor of Waardenburg, Sox10 gene has been deeply studied in the peripheral nervous system and melanocytes, but its function in inner ear still needs to be explored. Due to special anatomy and important function of inner ear, experiment on pathogenic mechanism cannot be conducted clinically, only case observation and image diagnosis can be carried out.
At present, the research on pathogenic mechanism is mainly conducted in vitro. Therefore, animal models play an irreplaceable role in the discovery, functional verification, diagnosis, prevention and treatment of inner ear pathogenic genes. In the future, by exploring the function of Sox10 protein as a transcription factor at different stages of inner ear development, the ability of Sox10 protein to bind target gene promoters, and verifying them in animal models, especially in miniature pigs, we can better understand the molecular mechanism regulated by Sox10 gene in the development of inner ear.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Aoki Y., Saint-Germain N., Gyda M., et al. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus[J] Dev. Biol. 2003;259(1):19–33. doi: 10.1016/s0012-1606(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Baroti T., Zimmermann Y., Schillinger A., et al. Transcription factors Sox5 and Sox6 exert direct and indirect influences on oligodendroglial migration in spinal cord and forebrain: SoxD Proteins in Oligodendrocyte Progenitors[J] Glia. 2016;64(1):122–138. doi: 10.1002/glia.22919. [DOI] [PubMed] [Google Scholar]
- Bondurand N. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome[J] Hum. Mol. Genet. 2000;9(13):1907–1917. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- Bondurand N. Human Connexin 32, a gap junction protein altered in the X-linked form of Charcot-Marie-Tooth disease, is directly regulated by the transcription factor SOX10[J] Hum. Mol. Genet. 2001;10(24):2783–2795. doi: 10.1093/hmg/10.24.2783. [DOI] [PubMed] [Google Scholar]
- Bondurand N., Kobetz A., Pingault V., et al. Expression of the SOX10 gene during human development[J] FEBS (Fed. Eur. Biochem. Soc.) Lett. 1998;432(3):168–172. doi: 10.1016/s0014-5793(98)00843-6. [DOI] [PubMed] [Google Scholar]
- Bowles J., Schepers G., Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators[J] Dev. Biol. 2000;227(2):239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Breuskin I., Bodson M., Thelen N., et al. Sox10 promotes the survival of cochlear progenitors during the establishment of the organ of Corti[J] Dev. Biol. 2009;335(2):327–339. doi: 10.1016/j.ydbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Britsch S. The transcription factor Sox10 is a key regulator of peripheral glial development[J] Genes Dev. 2001;15(1):66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol 190: 1-65[J]. Advances in Anatomy, Embryology, and Cell Biology. 2007;190:1–65. [PubMed] [Google Scholar]
- Brown A.S., Rakowiecki S.M., Li J.Y.H., et al. The cochlear sensory epithelium derives from Wnt responsive cells in the dorsomedial otic cup[J] Dev. Biol. 2015;399(1):177–187. doi: 10.1016/j.ydbio.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Q., Cole L.K., Mulheisen M., et al. The role of Pax2 in mouse inner ear development[J] Dev. Biol. 2004;272(1):161–175. doi: 10.1016/j.ydbio.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Cantos R, Cole L K, Acampora D, et al. Patterning of the mammalian cochlea[J]. Proced. Nat. Acade. Sci. United States of America, 97(22): 11707–11713. [DOI] [PMC free article] [PubMed]
- Chang W., Lin Z., Kulessa H., et al. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements[J] PLoS Genet. 2008;4(4) doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Frutos E., Vendrell V., Alvarez Y., et al. Tissue-specific requirements for FGF8 during early inner ear development[J] Mech. Dev. 2009;126(10):873–881. doi: 10.1016/j.mod.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Dutton K., Abbas L., Spencer J., et al. A zebrafish model for Waardenburg syndrome type IV reveals diverse roles for Sox10 in the otic vesicle[J] Disease Models and Mechanisms. 2009;2(1–2):68–83. doi: 10.1242/dmm.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elworthy S. Transcriptional regulation of mitfa accounts for the Sox10 requirement in zebrafish melanophore development[J] Development. 2003;130(12):2809–2818. doi: 10.1242/dev.00461. [DOI] [PubMed] [Google Scholar]
- Finzsch M., Stolt C.C., Lommes P., et al. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor expression[J] Development. 2008;135(4):637–646. doi: 10.1242/dev.010454. [DOI] [PubMed] [Google Scholar]
- Fufa T.D., Harris M.L., Watkins-Chow D.E., et al. Genomic analysis reveals distinct mechanisms and functional classes of SOX10-regulated genes in melanocytes[J] Hum. Mol. Genet. 2015;24(19):5433–5450. doi: 10.1093/hmg/ddv267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath C., Law W.D., Rodríguez-Molina J.F., et al. Stringent comparative sequence analysis reveals SOX10 as a putative inhibitor of glial cell differentiation[J] BMC Genom. 2016;17 doi: 10.1186/s12864-016-3167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf S.A., Busch C., Bosserhoff A.-K., et al. SOX10 promotes melanoma cell invasion by regulating melanoma inhibitory activity[J] J. Invest. Dermatol. 2014;134(8):2212–2220. doi: 10.1038/jid.2014.128. [DOI] [PubMed] [Google Scholar]
- Graf S.A., Heppt M.V., Wessely A., et al. The myelin protein PMP2 is regulated by SOX10 and drives melanoma cell invasion[J] Pigment Cell & Melanoma Research. 2019;32(3) doi: 10.1111/pcmr.12760. [DOI] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. [J] .Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Guo W., Yi H., Yan Z., et al. The morphological and functional development of the stria vascularis in miniature pigs[J] Reprod. Fertil. Dev. 2017;29(3):585–593. doi: 10.1071/RD15183. [DOI] [PubMed] [Google Scholar]
- Hai T., Cao C., Shang H., et al. Pilot study of large-scale production of mutant pigs by ENU mutagenesis[J] Elife. 2017;6 doi: 10.7554/eLife.26248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q.-Q., Li L., Chen W., et al. Key genes and pathways associated with inner ear malformation in SOX10 p.R109W mutation pigs[J] Front. Mol. Neurosci. 2018;11:181. doi: 10.3389/fnmol.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley V.R., Lovell-Badge R., Goodfellow P.N. Definition of a consensus DNA binding site for SRY[J] Nucleic Acids Res. 1994;22(8):1500–1501. doi: 10.1093/nar/22.8.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M.L., Baxter L.L., Loftus S.K., et al. Sox proteins in melanocyte development and melanoma: Sox proteins and melanocytes[J] Pigment Cell & Melanoma Research. 2010;23(4):496–513. doi: 10.1111/j.1755-148X.2010.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbarth B., Pingault V., Bondurand N., et al. Mutation of the Sry-relatedSox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease[J] Proc. Natl. Acad. Sci. USA. 1998;95(9):5161–5165. doi: 10.1073/pnas.95.9.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré S.M., Aybar M.J., Mayor R. Sox10 is required for the early development of the prospective neural crest in Xenopus embryos[J] Dev. Biol. 2003;260(1):79–96. doi: 10.1016/s0012-1606(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Hornig J., Fröb F., Vogl M.R., et al. The transcription factors Sox10 and myrf define an essential regulatory network module in differentiating oligodendrocytes[J]. BARRES B A. PLoS Genet. 2013;9(10) doi: 10.1371/journal.pgen.1003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S.-W., Leblanc S.E., Roopra A., et al. In vivo detection of Egr2 binding to target genes during peripheral nerve myelination[J] J. Neurochem. 2006;98(5):1678–1687. doi: 10.1111/j.1471-4159.2006.04069.x. [DOI] [PubMed] [Google Scholar]
- Jiao Z., Mollaaghababa R., Pavan W.J., et al. Direct interaction of Sox10 with the promoter of murine dopachrome tautomerase (dct) and synergistic activation of dct expression with mitf[J] Pigm. Cell Res. 2004;17(4):352–362. doi: 10.1111/j.1600-0749.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- Kiernan A.E., Xu J., Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear[J] PLoS Genet. 2006;2(1):e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lo L., Dormand E., et al. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells[J] Neuron. 2003;38(1):17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K., Herbarth B., Sock E., et al. Sox10, a novel transcriptional modulator in glial cells[J] J. Neurosci. 1998;18(1):237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küspert M., Hammer A., Bösl M.R., et al. Olig2 regulates Sox10 expression in oligodendrocyte precursors through an evolutionary conserved distal enhancer[J] Nucleic Acids Res. 2011;39(4):1280–1293. doi: 10.1093/nar/gkq951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer[J] Hum. Mol. Genet. 2003;12(8):937–945. doi: 10.1093/hmg/ddg107. [DOI] [PubMed] [Google Scholar]
- Lee M., Goodall J., Verastegui C., et al. Direct regulation of the microphthalmia promoter by Sox10 links waardenburg-shah syndrome (WS4)-associated hypopigmentation and deafness to WS2[J] J. Biol. Chem. 2000;275(48):37978–37983. doi: 10.1074/jbc.M003816200. [DOI] [PubMed] [Google Scholar]
- Li H., Lu Y., Smith H.K., et al. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes[J] J. Neurosci. 2007;27(52):14375–14382. doi: 10.1523/JNEUROSCI.4456-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. Gbx2 is required for the morphogenesis of the mouse inner ear: a downstream candidate of hindbrain signaling. J]. Development. 2005;132(10):2309–2318. doi: 10.1242/dev.01804. [DOI] [PubMed] [Google Scholar]
- Liu Y.L., Zhang H., Feng Y. Progress in the study of syndromic hearing loss resulted from neural crest abnormalities[J] Hereditas. 2014;36(11):1131–1144. doi: 10.3724/SP.J.1005.2014.1131. [DOI] [PubMed] [Google Scholar]
- Locher H., Frijns J.H., van Iperen L., et al. Neurosensory development and cell fate determination in the human cochlea[J] Neural Dev. 2013;8(1):20. doi: 10.1186/1749-8104-8-20. 8,1(2013-10-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Rehberg S., Wegner M. Melanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and Mitf transcription factors[J] FEBS (Fed. Eur. Biochem. Soc.) Lett. 2004;556(1–3):236–244. doi: 10.1016/s0014-5793(03)01446-7. [DOI] [PubMed] [Google Scholar]
- Merlo G.R., Paleari L., Mantero S., et al. The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway[J] Dev. Biol. 2002;248(1):157–169. doi: 10.1006/dbio.2002.0713. [DOI] [PubMed] [Google Scholar]
- Morsli H., Choo D., Ryan A., et al. Development of the mouse inner ear and origin of its sensory organs[J] J. Neurosci. Official J. Society. Neurosci. 1998;18(9):3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murisier F., Guichard S., Beermann F. The tyrosinase enhancer is activated by Sox10 and Mitf in mouse melanocytes[J] Pigm. Cell Res. 2007;20(3):173–184. doi: 10.1111/j.1600-0749.2007.00368.x. [DOI] [PubMed] [Google Scholar]
- Owen Signs, Nora G. Smart, Beutler B. Record for Dalmatian, updated May 13, 2016 MUTAGENETIX(TM), B. Beutler and Colleagues, Center for the Genetics of Host Defense, UT Southwestern Medical Center, Dallas, TX. (URL: mutagenetix. utsouthwestern. edu).
- Pan W., Jin Y., Stanger B., et al. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear[J] Proc. Natl. Acad. Sci. U. S. A. 2010;107(36):15798–15803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirano R.I. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences[J] Nucleic Acids Res. 2000;28(16):3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirano R.I., Goerich D.E., Riethmacher D., et al. Protein zero gene expression is regulated by the glial transcription factor Sox10[J] Mol. Cell Biol. 2000;20(9):3198–3209. doi: 10.1128/mcb.20.9.3198-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault V., Faubert E., Baral V., et al. SOX10 mutations mimic isolated hearing loss[J] Clin. Genet. 2015;88(4):352–359. doi: 10.1111/cge.12506. [DOI] [PubMed] [Google Scholar]
- Potterf S.B., Furumura M., Dunn K.J., et al. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3[J] Hum. Genet. 2000;107(1):1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- Prasad M.K., Reed X., Gorkin D.U., et al. SOX10 directly modulates ERBB3 transcription via an intronic neural crest enhancer[J] BMC Dev. Biol. 2011;11(1):40. doi: 10.1186/1471-213X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch C., Hustert E., Pfeifer D., et al. The SOX10/Sox10 gene from human and mouse: sequence, expression, and transactivation by the encoded HMG domain transcription factor[J] Hum. Genet. 1998;103(2):115–123. doi: 10.1007/s004390050793. [DOI] [PubMed] [Google Scholar]
- Reiprich S., Kriesch J., Schreiner S., et al. Activation of Krox20 gene expression by Sox10 in myelinating Schwann cells[J] J. Neurochem. 2010;112(3):744–754. doi: 10.1111/j.1471-4159.2009.06498.x. [DOI] [PubMed] [Google Scholar]
- Reiprich S., Cantone M., Weider M., et al. Transcription factor Sox10 regulates oligodendroglial Sox9 levels via microRNAs: Sox Proteins and microRNAs[J] Glia. 2017;65(7):1089–1102. doi: 10.1002/glia.23146. [DOI] [PubMed] [Google Scholar]
- Riccomagno M.M. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. J]. Genes, Development. 2005;19(13) doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo R.F., Lufkin T. Dlx5 andDlx6 homeobox genes are required for specification of the mammalian vestibular apparatus. J]. genesis. 2006;44(9):425–437. doi: 10.1002/dvg.20233. [DOI] [PubMed] [Google Scholar]
- Sánchez-Calderón H., Martín-Partido G., Hidalgo-Sánchez M. Otx2, Gbx2, and Fgf8 expression patterns in the chick developing inner ear and their possible roles in otic specification and early innervation[J] Gene Expr. Patterns. 2004;4(6):659–669. doi: 10.1016/j.modgep.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Schlierf B. Cooperative binding of Sox10 to DNA: requirements and consequences[J] Nucleic Acids Res. 2002;30(24):5509–5516. doi: 10.1093/nar/gkf690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner S., Cossais F., Fischer K., et al. Hypomorphic Sox10 alleles reveal novel protein functions and unravel developmental differences in glial lineages[J] Development. 2007;134(18):3271–3281. doi: 10.1242/dev.003350. [DOI] [PubMed] [Google Scholar]
- Sennaroğlu L., Bajin M.D. Classification and current management of inner ear malformations[J] Balkan Med. J. 2017;34(5):397–411. doi: 10.4274/balkanmedj.2017.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer L. Generation of melanocytes from neural crest cells: generation of melanocytes from neural crest cells[J] Pigment Cell & Melanoma Research. 2011;24(3):411–421. doi: 10.1111/j.1755-148X.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Sun G., Keles S., et al. Genome-wide analysis of EGR2/SOX10 binding in myelinating peripheral nerve[J] Nucleic Acids Res. 2012;40(14):6449–6460. doi: 10.1093/nar/gks313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Sound needs sound melanocytes to Be heard[J] Pigm. Cell Res. 1999;12(6):344–354. doi: 10.1111/j.1600-0749.1999.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Kobayashi Y., Matsushima Y. Mouse models for four types of Waardenburg syndrome[J] Pigm. Cell Res. 2003;16(5):448–454. doi: 10.1034/j.1600-0749.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Toriello H.V. Pigmentary anomalies and hearing loss[J] Adv. Oto-Rhino-Laryngol. 2011;70:50–55. doi: 10.1159/000322471. [DOI] [PubMed] [Google Scholar]
- Tudrej K.B., Edyta C. SOX10-MITF pathway activity in melanoma cells[J] Arch. Med. Sci. 2017;13(6):1493–1503. doi: 10.5114/aoms.2016.60655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Grimmer J.F., Water T.R.V.D., et al. Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can Be functionally replaced by Drosophila hmx[J] Dev. Cell. 2004;7(3):439–453. doi: 10.1016/j.devcel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Takeda K., Katori Y., et al. Expression of the Sox10 gene during mouse inner ear development[J] Mol. Brain Res. 2000;84(1–2):141–145. doi: 10.1016/s0169-328x(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins[J] Nucleic Acids Res. 1999;27(6):1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weider M., Wegner M. SoxE factors: transcriptional regulators of neural differentiation and nervous system development[J] Semin. Cell Dev. Biol. 2017;63:35–42. doi: 10.1016/j.semcdb.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Weider M., Starost L.J., Groll K., et al. Nfat/calcineurin signaling promotes oligodendrocyte differentiation and myelination by transcription factor network tuning[J] Nat. Commun. 2018 Mar 2;9(1):899. doi: 10.1038/s41467-018-03336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T., Hammer A., Wahlbuhl M., et al. Multiple conserved regulatory elements with overlapping functions determine Sox10 expression in mouse embryogenesis[J] Nucleic Acids Res. 2007;35(19):6526–6538. doi: 10.1093/nar/gkm727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmuller S. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors[J] Nucleic Acids Res. 2006;34(6):1735–1744. doi: 10.1093/nar/gkl105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E.M., Snopek B., Koopman P. Seven new members of the Sox gene family expressed during mouse development[J] Nucleic Acids Res. 1993;21(3):744. doi: 10.1093/nar/21.3.744. 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.Y., Hao Q.Q., Zhong L.L., et al. SOX10 mutation is relevant to inner ear malformation in patients with Waardenburg syndrome[J] Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery. 2016;51(11):832–837. doi: 10.3760/cma.j.issn.1673-0860.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Yanyan M., Simone R., Michael W., et al. Targeted deletion of Sox10 by wnt1-cre defects neuronal migration and projection in the mouse inner ear[J] PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0094580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L.-L., Zhang Y., Liang X.-J., et al. Inner ear structure of miniature pigs measured by multi-planar reconstruction techniques[J] Am J Transl Res. 2018;10(3):709–717. [PMC free article] [PubMed] [Google Scholar]
- ZhU Q.Y., ZhAO G.X., Li Y., et al. Advances in pathogenesis and precision medicine for nasopharyngeal carcinoma. MedComm. 2020;2(2):175–206. doi: 10.1002/mco2.32. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]