ABSTRACT
Background
Early‐onset Parkinson's disease (EOPD)/young‐onset Parkinson's disease (YOPD) is defined as Parkinson's disease (PD) with an age at onset (AAO) after age 21 years but before the usual AAO for PD. Consensus is lacking, and the reported maximal age for EOPD/YOPD has varied from 40 to 60 years, leading to a lack of uniformity in published studies and difficulty in harmonization of data. EOPD and YOPD have both been used in the literature, somewhat interchangeably.
Objective
To define the nomenclature and AAO cutoff for EOPD/YOPD.
Methods
An extensive review of the literature and task force meetings were conducted. Conclusions were reached by consensus.
Results
First, the literature has seen a shift from the use of YOPD toward EOPD. This seems motivated by an attempt to avoid age‐related stigmatization of patients. Second, in defining EOPD, 56% of the countries use 50 or 51 years as the cutoff age. Third, the majority of international genetic studies in PD use an age cutoff of younger than 50 years to define EOPD. Fourth, many studies suggest that changes in the estrogen level can affect the predisposition to develop PD, making the average age at menopause of 50 years an important factor to consider when defining EOPD. Fifth, considering the differential impact of the AAO of PD on professional and social life, using 50 years as the upper cutoff for the definition of EOPD seems reasonable.
Conclusions
This task force recommends the use of EOPD rather than YOPD. It defines EOPD as PD with AAO after 21 years but before 50 years.
Keywords: Parkinson's disease, early onset, young onset, age cutoff
Parkinson's disease (PD) is a neurodegenerative disorder characterized by a number of defined clinical features such as bradykinesia with either 1 or both of rest tremor and rigidity as well as a variety of nonmotor symptoms. 1 , 2 , 3 , 4 Early‐onset PD (EOPD), also referred to as young‐onset PD (YOPD), has been defined as PD with an onset of motor symptoms after age 21 but before what is considered the usual age at onset (AAO) for PD. However, consensus is lacking, and the reported maximal age for EOPD/YOPD has varied from 40 to 60, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 whereas onset at or before age 21 defines juvenile PD. 11 , 16 Because of the effect of PD symptoms earlier in life, the interaction of symptoms with potentially more active roles in society, and the longer time horizon of disease, people with EOPD/YOPD face unique challenges compared with those with late‐onset PD. 17 , 18 International Parkinson and Movement Disorder Society (MDS) commissioned the Early Onset Parkinson Disease Task Force with the objectives of defining the best nomenclature for EOPD/YOPD and the AAO cutoff defining this condition as well as identifying unmet needs and unique challenges in patients with EOPD/YOPD to guide the harmonization of research and other initiatives. The task force recommendations were based on the nomenclature, epidemiology, geographic differences, genetics, role of sexual hormones, clinical features, impact, and social perceptions. All conclusions were reached by consensus.
Search Strategies and Selection Criteria
The task force chair was appointed by the MDS. The members of the task force, who are also the authors of the present publication, are PD specialists who were selected based on expertise in EOPD/YOPD, and were appointed by the task force chair. A systematic review of available literature was performed in PubMed up to January 2022 with the search terms early onset OR young onset OR early OR young AND Parkinson AND each of the following: definition, hormon*, menopause, social, and genetic. Articles were included if reporting on EOPD and written in English. Articles were excluded from this review if not available in English or published as editorials or letters. All abstracts were reviewed for relevance to the topic. Of the 2677 abstracts reviewed, 2625 were not considered relevant as the search terms were vague to capture the most abstracts possible and minimize the risk of missing a relevant study. A total of 52 abstracts were retained, and the corresponding full articles were carefully reviewed. Of these, 43 full articles were considered relevant and were included. PubMed links and references from these articles were also scrutinized to identify other relevant studies as suggested by the references' titles. Similar to the initial search, abstracts of potentially relevant references were reviewed and, if considered relevant, the corresponding full articles were reviewed. Thus, an additional 63 studies were included in this article, bringing the total of articles included to 106.
In addition, for the geographic differences section, a list of all countries was created, and then a systematic search in the PubMed and Web of Science databases was conducted using the following search terms: early onset Parkinson* disease AND/OR young onset Parkinson* disease AND age AND the country's name. Articles in English were included. Of the 971 abstracts reviewed, 51 were retained, and the corresponding full articles were carefully reviewed.
Because 12 articles were redundant in both research strategies noted previously, the total number of articles included in this review was 145 (Fig. 1).
FIG. 1.
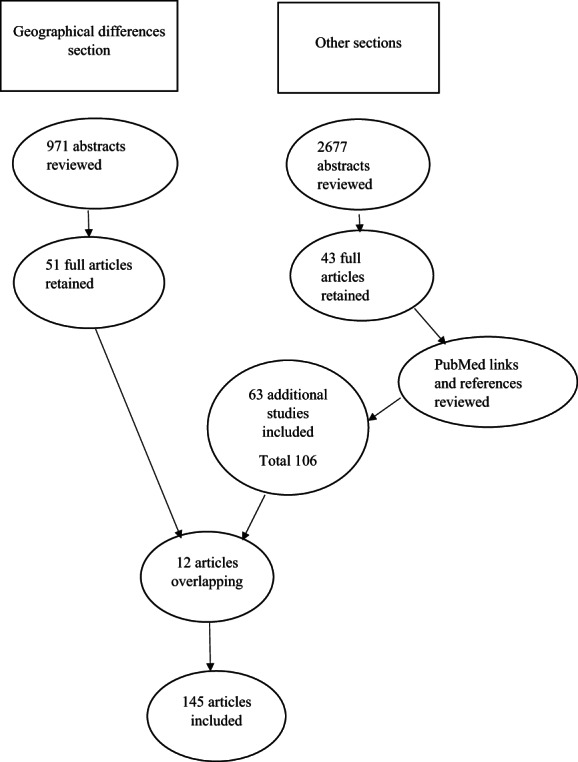
Search strategy.
Nomenclature
Authors who have first developed an interest in, and published on, this subgroup of PD have preferred the use of YOPD. 5 , 6 , 7 , 8 , 19 , 20 However, there has been a shift toward the use of EOPD in the past 2 decades. 9 , 11 , 15 , 21 , 22 The are no clear publications detailing the rationale behind this shift, but it seems motivated by an attempt to avoid age‐related stigmatization (Table 1). Thus, the recommendation of the Early Onset Parkinson Disease Task Force of the MDS is to use the term EOPD to designate PD with an onset of motor symptoms before the specific age that was agreed on.
TABLE 1.
Considerations for nomenclature
Young‐onset Parkinson's disease (YOPD)
|
Early‐onset Parkinson's disease (EOPD)
|
Epidemiology
The prevalence of PD increases sharply with age, reaching 2.6% in people aged 85 to 89 years, 23 , 24 , 25 , 26 with a mean age of onset in the early to mid‐60s in the Western hemisphere. 27 EOPD represents 3% to 7% of PD. 17 , 28 The incidence of EOPD has been reported between 0.29 and 3.3 per 100,000 person‐years. 15 , 20 , 21 , 22 , 29
Geographical Differences
The age cutoff for the definition of EOPD varies from 1 country to another. An extensive review of the literature on this topic is presented in Table 2. Among the countries with a published age cutoff, 38% (n = 20) used a cutoff of 40 to 45 years, 56% (n = 29) used a cutoff of 50 or 51 years, and 6% (n = 3) used a cutoff of 55 years or older. More than 1 age cutoff has been used in some countries in different publications.
TABLE 2.
EOPD age cutoff (in years) by country
| Country | Age cutoff for EOPD |
|---|---|
| Europe | |
| Belarus | 40 30 |
| Belgium | 50 31 |
| Croatia | 40 |
| Czech Republic | 40–45 32 , 33 , 34 |
| Finland | 55 21 |
| Germany | 50 35 |
| Greece | 50 |
| Hungary | 50 36 |
| Iceland | 50 37 |
| Ireland | 50 38 |
| Italy | 40–50 22 , 39 |
| Kazakhstan | 50 40 |
| Luxembourg | 50 41 |
| Netherlands | 40–50 18 |
| Norway | 45 42 |
| Poland | 40 43 |
| Portugal | 50 44 |
| Russia | 40 45 , 46 |
| Serbia | 50 47 |
| Slovakia | 40 48 , 49 |
| Spain | 50 50 |
| Sweden | 50 51 |
| United Kingdom | 40–50 11 |
| Americas | |
| Argentina | 40 52 |
| Brazil | 20–45 53 , 54 |
| Canada | 40 55 |
| Colombia | 50 56 |
| Ecuador | 50 56 |
| Mexico | 45 57 |
| United States | 40–55 14 , 15 , 17 , 25 |
| Asia‐Pacific | |
| Middle East, North Africa, and South Asia: consensus from the MDS Task Force for the Middle East | 50 58 |
| Australia | 50 59 |
| China | 40 60 |
| French Polynesia | 51 61 |
| Guam | 51 61 |
| India | 40–50 62 , 63 , 64 |
| New Zealand | 60 65 |
| South Korea | 40–50 66 |
| Vietnam | 50 67 |
| Japan | 40–50 28 |
| Africa | |
| Morocco | 45 68 |
| Nigeria | 50 69 |
| South Africa | 50 70 |
There were no data published for countries not included in the table.
Abbreviation: EOPD, early‐onset Parkinson's disease.
Genetics
It is beyond the scope of this article to comprehensively review the genetics of PD; however, it has been well recognized that the risk of genetic predisposition may increase with the younger AAO. 71 Indeed, a family history of PD is reported in 20% of patients with EOPD versus 6.9% of patients with later onset PD, and the age‐specific risk of PD is 7.8‐fold higher in the relatives of patients with EOPD compared with 2.9‐fold among the relatives of patients with later onset PD. 14 , 72 Most frequent genetic mutations associated with EOPD are located on parkin (PARK2), PINK1 (PARK 6), DJ‐1 (PARK7), ATP13A2 (PARK9), and PLA2G6 (PARK14). 17 Although some genetic studies use a cutoff of 40 years 73 or 45 years, 74 the vast majority of genetic studies in PD from various parts of the world use the age cutoff of younger than 50 years to define EOPD, 38 , 40 , 67 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 supporting the recommendation to use that age cutoff for the definition of EOPD.
Role of Sexual Hormones
The incidence of PD is 1.5 to 2.0 times higher in men than in women, 83 , 84 with women being less susceptible 85 , 86 to the disease and developing it later in life. 86 , 87 In addition, the incidence and prevalence of PD have been reported as higher in postmenopausal than in premenopausal women of similar age in multiple epidemiological studies. 88 , 89 , 90
Many experimental and human observations suggest that estrogen may have a protective as well as a dopaminergic effect in PD, 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 whereas a smaller number suggests no benefit. 93 , 105 , 106 , 107 One cross‐sectional study of 579 women with PD, 497 of whom developed menopause before PD onset, reported that later age of menopause was associated with older AAO and better on medication Unified Parkinson's Disease Rating Scale motor score. 108 In addition, a population‐based study using the Mendelian randomization method reported that each year of delay in age at menopause was associated with a 7% decrease in PD risk. 109
Regarding estrogen supplementation, a few studies 110 , 111 , 112 have shown that estrogen postmenopausal hormone replacement therapy (HRT) improves motor disability in women with PD, and a metanalysis of 14 observational studies reported that estrogen HRT did not increase the risk of PD. 107 On the other hand, progestin‐only HRT seems to increase the risk of PD 3‐fold in postmenopausal women. 113
Taken together, these studies suggest that changes in the estrogen level can affect the predisposition to develop PD, making the average age at menopause of 50 114 another important factor to consider when defining the upper age cutoff for EOPD.
PD Clinical Features
Clinical features and their underlying biological mechanisms also need to be considered in the definition of the age cutoff for EOPD.
In a large retrospective study assessing the impact of AAO on clinical features and evolution of 593 patients, rigidity as the predominant initial symptom was more frequent in patients with EOPD (AAO < 50), whereas gait instability as the predominant initial symptom was more frequent in patients with older onset PD. 14 There was no statistically significant difference in the frequency of tremor or bradykinesia as the predominant initial symptom among the groups, confirming results of previous studies 115 while contrasting with others whose results indicated both an increased or decreased rate of tremor in patients with earlier onset. 116 , 117 , 118 , 119 Another series of 422 patients reported as well that rigidity was a more frequent presenting symptom in the younger group (AAO < 50). 120 Dystonia has been suggested as a more frequent presenting symptom in EOPD (20% of patients with AAO < 45 vs. 3% of patients with AAO > 64) in a study of 358 patients. Within 2 years of onset, dystonia developed in an additional 11% of EOPD and 1% of late‐onset PD (LOPD). 115 Most studies agree that patients with EOPD tend to have a slower progression of motor symptoms than those with LOPD. 121 , 122 , 123 , 124 , 125 , 126 , 127
In addition, the prevalence of levodopa‐induced dyskinesia (LID) was reported to decrease with advancing AAO, with a higher frequency before age 50. In 1 population‐based study of 91 patients, the frequency of LID after 5 years of levodopa therapy was 40% in patients with PD onset before age 50, decreasing to 35.2% with an AAO between 50 and 69 years and 15.6% in patients with onset after 69 years. 128 Another study suggested that the risk of dyskinesia was reduced by 20% to 30% for each 10 years of older AAO. 129 In yet another series of 109 patients, the frequency of dyskinesia observed within 5 years of treatment was 80%, 26.7%, 22.7%, and 20% for onset between 40 and 49, 50 and 59, 60 and 69, and 70 and 79 years of age, respectively. 130 Finally, in a large retrospective study of 593 patients, dyskinesia developed in 70% of the patients with EOPD (AAO < 50), 34.1% of the patients with middle‐onset PD (AAO 50–69), and 13% of the patients with LOPD (AAO > 69) (P < 0.001). 14 Similarly, treatment‐related dystonia was 2 to 4 times more frequent in patients with PD with AAO < 50 compared with later AAO in that cohort, 14 whereas another cohort of 358 patients suggested that the risk for dystonia was highest with AAO < 48. 115
Regarding nonmotor features, depression was reported twice as frequently in patients with EOPD (AAO < 50) than in patients with LOPD (AAO > 69). 14 Another controlled study similarly reported a higher rate of depression, worse emotional well‐being, and poorer quality of life with AAO ≤ 45 years of age. 131 Finally, 1 Norwegian cohort reported that patients with EOPD (AAO < 50) had a longer survival but a reduced life expectancy compared with patients with later onset PD. 132 However, these data were not confirmed in a North American population study reporting a longer life expectancy in EOPD overall compared with LOPD. 15
These clinical differences between EOPD and LOPD might have an anatomic substrate. Liu et al. 133 compared the striatal patterns of dopaminergic degeneration between 40 EOPD (AAO ≤ 50) and 47 LOPD (AAO > 50), as examined with 11C‐2β‐carbomethoxy‐3β‐(4‐fluorophenyl) tropane positron emission tomography (PET). Although dopamine transporter (DAT) scans from patients with LOPD showed relatively uniform involvement of both the caudate and putamen, the DAT scans of patients with EOPD showed more sparing of the caudate compared with the putamen. Given the involvement of the caudate in complex cognitive functions, 134 , 135 , 136 , 137 this might provide an explanation for the lower prevalence of these nonmotor symptoms in EOPD compared with LOPD. 17 On the other hand, a PET and 18F‐fluorodopa scans study that included 27 patients aged 38 to 79 years reported a higher PD‐induced increase in dopamine turnover compared with the decrease in dopamine synthesis and storage rate in patients of younger age compared with older patients. The authors suggested that this implied greater alteration of dopamine turnover in EOPD that could lead to larger swings in synaptic dopamine levels, which has been suggested as a possible contributor to a greater risk of motor fluctuations. 138
Regarding a potential biochemical substrate separating EOPD from LOPD, a controlled study including 76 patients with PD and 75 sex‐ and age‐matched controls reported that an AAO ≤ 50 was associated with lower levels of cerebrospinal fluid lactate and tau proteins, 139 providing biochemical support for using 50 years as an age cutoff.
Overall, the clinical data and their suggested underlying biological mechanisms support the use of AAO < 50 for the definition of EOPD as well.
Impact of PD on Employment and Social Perception
One last variable to consider in the determination of the upper age limit for EOPD is the impact on social perception and employment. Although the retirement age is usually approximately 65 years in most countries, using that age as the upper limit for EOPD would not be appropriate as the typical AAO of PD is in the early to mid‐60s, 27 thus placing a cutoff of 65 years quite late in the natural history of the disease. In addition, the development of PD 3 years from retirement is unlikely to have the same impact on professional development than a diagnosis made 15 years earlier. Indeed, a study comparing 75 patients with EOPD (AAO < 50) and 66 patients with LOPD (AAO ≥ 50) reported that, among those who retired, 97% of the patients with EOPD retired early versus 73% of those with LOPD (P = 0.003). 19 Other studies have found that 54% of patients with EOPD retire early, and 94% are likely to give up work within 10 years of disease onset, with patients with AAO < 45 years likely to stop working on average 6 to 7 years after diagnosis. 140
In a retrospective cohort review of 88 Irish patients with PD with AAO < 65 years, men aged 55 to 64 years were twice as likely to be unemployed than the general population, whereas unemployment among women was not significantly affected by PD. The authors also found that the median time to loss of employment was 7 years, with 40% still employed after 5 years from onset and 14% after 10 years. Among those who were still working at the time of the study, 77% had made adjustments at work. Of those who had stopped working as a result of PD, 82% were dissatisfied with their unemployment status. 141
The burden of unemployment from EOPD goes beyond a financial cost, including social isolation, feelings of futility, lack of purpose and self‐esteem, and lack of daily structure. Many patients with PD are most bothered by the perception that others might have of their impairment than by their impairment itself. 141 , 142 This higher level of stigma in patients with EOPD 17 , 143 seems to stem essentially from dysarthria, tremor, dyskinesia, and impact of the motor symptoms on activities of daily living such as eating and washing 144 and can manifest as a reluctance to seek help and ask for adjustments at work, which in turn decreases the chances of staying employed.
In a prospective 50‐item survey aimed at clarifying patients' concerns in 222 individuals with PD, patients with EOPD (AAO < 50) reported significantly more concerns about difficulty with speaking (P = 0.003), washing and bathing (P = 0.04), or eating (P = 0.003) as well as with shaking (P = 0.005), dyskinesia (P = 0.001), low and/or depressed mood (P = 0.01), and anxiety and/or panic attacks (P < 0.001)—factors more likely to impact social or professional functioning—as greater concerns than typical‐onset patients with PD. 144
Finally, EOPD may present a challenge to relationships. Marriages or relationships of shorter duration can be more vulnerable to the strain a chronic illness can impose than those of longer duration,145 with significantly worse marital discord scores in couples with EOPD than in those with LOPD in 1 study comparing 75 patients with EOPD (<50 years) and 66 patients with LOPD. 19 The impact of the generational difference on these results cannot be excluded because of the absence of a healthy control. 18 A retrospective study comparing 272 patients with EOPD (AAO < 50) and 690 patients with LOPD (AAO > 70) reported less caregiver strain in the EOPD group. 127 However, LOPD and their frequently similarly aged caregivers are each at greater risk of more medical comorbidities and financial strain, which are big contributors to caregiver strain and could be confounding factors.
In summary, considering the differential impact of the AAO of PD on professional and social life, using onset before age 50 years as the upper cutoff for the definition of EOPD seems reasonable (Fig. 2).
FIG. 2.
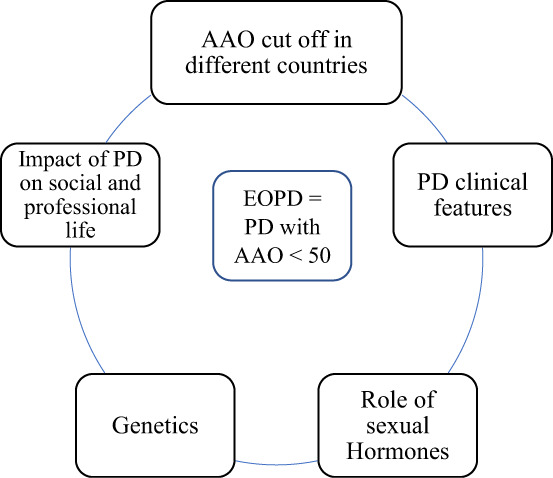
Interplay of factors leading to defining EOPD as AAO < 50. EOPD, early‐onset Parkinson's disease; AAO, age at onset; PD, Parkinson's disease.
Conclusion
After careful review of the available literature and deliberation, the Early Onset Parkinson Disease Task Force created by the MDS recommends the use of early‐onset Parkinson's disease instead of young‐onset Parkinson's disease. Furthermore, after considering hormonal, clinical, and genetic factors as well as the differential impact of the AAO of PD on professional and social life, this task force recommends using an AAO before 50 years for the definition of EOPD. This task force hopes that these recommendations regarding nomenclature and definition will help uniformize future research on the specific challenges and unmet needs of this subset of patients with PD as well as guide and harmonize future research and other initiatives.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
R.M.: 1A, 1B, 1C, 2A, 2B
K.S.: 2A, 2B
J.F.: 2B
B.P.: 2B
T.H.: 2B
M.E.P.P.: 2B
K.R.K.: 2B
V.M.: 2B
B.Z.: 2B
E.‐K.T.: 2B
R.S.: 1A, 1B, 2B
Disclosures
Ethical Compliance Statement: Because this work was a review of the literature and task force deliberation, no approval from an institutional review board was necessary. Similarly, informed patient consent was not necessary for this work. All authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: No specific funding was received for this work, and the authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: Raja Mehanna is on the speaker bureau for TEVA, Adamas Pharmaceuticals, Kyowa Kirin, and Sunovion. He has received research grants from Global Kinetic Corporation, Northera, Neurocrine, and Cerevel. Katarzyna Smilowska has nothing to disclose. Jori Fleisher has received royalties from Wolters Klewer Health/UpToDate and honoraria from the Parkinson's Foundation and the Davis Phinney Foundation. She also has the following research support: National Institute of Neurological Disorders and Stroke (NINDS; 1K23NS097615‐01A1 [principal investigator, PI: Fleisher], 5U01NS100610‐05/737SUB U01NS100610 [PI: Leverenz; role: site PI]), National Institute on Aging (NIA; 5P30AG064200‐02 [PI: Hepburn; role: pilot study PI]), Parkinson's Foundation (PF‐COE‐PC‐920660 [role: site palliative care champion], PF‐CORE_2004 [PI: Fleisher], PF‐CORE‐856255 [PI: Fleisher]), PCORI (15963‐PF PCORI [PI: Schroeder, role: collaborative member]), Rush University (Leslie Nan Burridge Faculty Scholar in Parkinson's Disease Research Endowment [PI: Fleisher]; Rush University Center for Excellence in Aging Pilot Grant [PI: Labuschagne; role: coinvestigator]; Rush University Movement Disorders Pilot Grant [PI: Shah‐Zamori; role: coinvestigator]), and private philanthropic support. Bart Post has nothing to disclose. Taku Hatano is employed at Juntendo University School of Medicine and received a grant from the Japan Agency for Medical Research and Development under grant numbers 20dm0107156, 21wm0425015, 21ak0101112, and 21dk0207055; the Japan Society for the Promotion of Science under grant number 21K07424; a grant from Setsuro Fujii Memorial Foundation; TaNeDS; research funds from Daiichi Sankyo TaNeDS Funding Program; speaker's honoraria from Dainippon Sumitomo; Takeda Pharmaceutical Company Co., Ltd.; FP Pharmaceutical Corporation; Kyowa Kirin Co., Ltd.; Ono Pharmaceutical Co., Ltd.; AbbVie GK; Eisai Co., Ltd.; Sanofi K.K.; and Otsuka Pharmaceutical Co., Ltd. Maria Elisa Pimentel Piemonte has nothing to disclose. Kishore Raj Kumar received honoraria from Seqirus, AbbVie Pty Ltd, Research Review Australia Pty Limited; grant funding from the Paul Ainsworth Family Foundation, The Michael J. Fox Foundation, and GP2 (unrelated to the current study); is employed by New South Wales (NSW) Health; and receives royalties from Oxford University Press for Neurogenetics (What Do I Do Now) first edition. Victor McConvey has nothing to disclose. Baorong Zhang M has nothing to disclose. Eng‐King Tan received honoraria for editorial duties for Clinical Parkinsonism Related Disorders and grant support from the National Medical Research Council, Singapore. Rodolfo Savica received research support from the NIA, the NINDS, and the Mayo Clinic Small Grants Program National Center for Advancing Translational Sciences and an unrestricted research grant from Acadia Pharmaceuticals Inc.
Acknowledgments
We thank all the members of the Early Onset Parkinson Disease Task Force for their contribution to this project. The EOPD task force is composed of Rodolfo Savica, MD, PhD (Chair); Eng‐King Tan, MD (Co‐Chair); Raja Mehanna, MD; Katarzyna Smilowska, MD, PhD; Connie Marras, MD, PHD; Owen Ross, PhD; Kishore Kumar, MBBS, PhD, FRACP; Jori Fleisher, MD, MSCE; Victor McConvey, RN, MACN; Taku Hatano, MD, PhD; Yih‐Ru Wu, MD; Baorong Zhang, MD; Roy Alcalay, MD; Aristide Merola, MD, PhD; Bart Post, MD, PhD; and Mehri Salari, MD.
Relevant disclosures and conflicts of interest are listed at the end of this article.
Contributor Information
Raja Mehanna, Email: raja.mehanna@uth.tmc.edu.
the International Parkinson and Movement Disorder Society Task Force on Early Onset Parkinson's Disease:
Rodolfo Savica, Eng‐King Tan, Raja Mehanna, Katarzyna Smilowska, Connie Marras, Owen Ross, Kishore Kumar, Jori Fleisher, Victor McConvey, Taku Hatano, Yih‐Ru Wu, Baorong Zhang, Roy Alcalay, Aristide Merola, Bart Post, and Mehri Salari
References
- 1. Mehanna R, Jankovic J. Respiratory problems in neurologic movement disorders. Parkinsonism Relat Disord 2010;16:628–638. [DOI] [PubMed] [Google Scholar]
- 2. Obeso JA, Stamelou M, Goetz CG, et al. Past, present and future of Parkinson's disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord 2017;32:1264–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berg D, Adler CH, Bloem BR, et al. Movement disorder society criteria for clinically established early Parkinson's disease. Mov Disord 2018;33:1643–1646. [DOI] [PubMed] [Google Scholar]
- 4. Postuma RB, Poewe W, Litvan I, et al. Validation of the MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2018;33:1601–1608. [DOI] [PubMed] [Google Scholar]
- 5. Quinn N, Critchley P, Marsden CD. Young onset Parkinson's disease. Mov Disord 1987;2:73–91. [DOI] [PubMed] [Google Scholar]
- 6. Gibb WR, Lees AJ. A comparison of clinical and pathological features of young‐ and old‐onset Parkinson's disease. Neurology 1988;38:1402–1406. [DOI] [PubMed] [Google Scholar]
- 7. Golbe LI. Young‐onset Parkinson's disease: a clinical review. Neurology 1991;41:168–173. [DOI] [PubMed] [Google Scholar]
- 8. Butterfield PG, Valanis BG, Spencer PS, Lindeman CA, Nutt JG. Environmental antecedents of young‐onset Parkinson's disease. Neurology 1993;43:1150–1158. [DOI] [PubMed] [Google Scholar]
- 9. Wickremaratchi MM, Perera D, O'Loghlen C, et al. Prevalence and age of onset of Parkinson's disease in Cardiff: a community based cross sectional study and meta‐analysis. J Neurol Neurosurg Psychiatry 2009;80:805–807. [DOI] [PubMed] [Google Scholar]
- 10. Kang GA, Bronstein JM, Masterman DL, Redelings M, Crum JA, Ritz B. Clinical characteristics in early Parkinson's disease in a Central California population‐based study. Mov Disord 2005;20:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schrag A, Schott JM. Epidemiological, clinical, and genetic characteristics of early‐onset parkinsonism. Lancet Neurol 2006;5:355–363. [DOI] [PubMed] [Google Scholar]
- 12. Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry 2000;69:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rana AQ, Siddiqui I, Yousuf MS. Challenges in diagnosis of young onset Parkinson's disease. J Neurol Sci 2012;323:113–116. [DOI] [PubMed] [Google Scholar]
- 14. Mehanna R, Moore S, Hou JG, Sarwar AI, Lai EC. Comparing clinical features of young onset, middle onset and late onset Parkinson's disease. Parkinsonism Relat Disord 2014;20:530–534. [DOI] [PubMed] [Google Scholar]
- 15. Camerucci E, Stang CD, Hajeb M, et al. Early‐onset parkinsonism and early‐onset Parkinson's disease: a population‐based study (2010–2015). J Parkinsons Dis 2021;11:1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morales‐Briceno H, Mohammad SS, Post B, et al. Clinical and neuroimaging phenotypes of genetic parkinsonism from infancy to adolescence. Brain 2020;143:751–770. [DOI] [PubMed] [Google Scholar]
- 17. Mehanna R, Jankovic J. Young‐onset Parkinson's disease: its unique features and their impact on quality of life. Parkinsonism Relat Disord 2019;65:39–48. [DOI] [PubMed] [Google Scholar]
- 18. Post B, van den Heuvel L, van Prooije T, van Ruissen X, van de Warrenburg B, Nonnekes J. Young onset Parkinson's disease: a modern and tailored approach. J Parkinsons Dis 2020;10(s1):S29–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Young‐ versus older‐onset Parkinson's disease: impact of disease and psychosocial consequences. Mov Disord 2003;18:1250–1256. [DOI] [PubMed] [Google Scholar]
- 20. Alves G, Wentzel‐Larsen T, Aarsland D, Larsen JP. Progression of motor impairment and disability in Parkinson disease: a population‐based study. Neurology 2005;65:1436–1441. [DOI] [PubMed] [Google Scholar]
- 21. Ylikotila P, Tiirikka T, Moilanen JS, Kääriäinen H, Marttila R, Majamaa K. Epidemiology of early‐onset Parkinson's disease in Finland. Parkinsonism Relat Disord 2015;21:938–942. [DOI] [PubMed] [Google Scholar]
- 22. Riboldi GM, Frattini E, Monfrini E, Frucht SJ, Fonzo AD. A practical approach to early‐onset parkinsonism. J Parkinsons Dis 2022;12:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2014;29:1583–1590. [DOI] [PubMed] [Google Scholar]
- 24. Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- 25. Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson's disease across North America. Parkinsons Dis 2018;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savica R, Grossardt BR, Rocca WA, Bower JH. Parkinson disease with and without dementia: a prevalence study and future projections. Mov Disord 2018;33:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet 2004;363:1783–1793. [DOI] [PubMed] [Google Scholar]
- 28. Kasamo S, Takeuchi M, Ikuno M, Kawasaki Y, Tanaka S, Takahashi R, Kawakami K. Real‐world pharmacological treatment patterns of patients with young‐onset Parkinson's disease in Japan: a medical claims database analysis. J Neurol 2019;266:1944–1952. [DOI] [PubMed] [Google Scholar]
- 29. Winter Y, Bezdolnyy Y, Katunina E, et al. Incidence of Parkinson's disease and atypical parkinsonism: Russian population‐based study. Mov Disord 2010;25:349–356. [DOI] [PubMed] [Google Scholar]
- 30. Ivashynka A. Parkin (PARK 2) mutations in patients with early‐onset Parkinson's disease [abstract]. Mov Disord 2017;32(suppl 2). [Google Scholar]
- 31. Crosiers D, Picku B, Theuns J, et al. Non‐motor symptoms in a Flanders–Belgian population of 215 Parkinson's disease patients as assessed by the non‐motor symptoms questionnaire. Am J Neurodegener Dis 2012;1:160–167. [PMC free article] [PubMed] [Google Scholar]
- 32. Fiala O, Růžička E. Česká a slovenská neurologie a neurochirurgie: časopis českých a slovenských neurologů a neurochirurgů. Genetika Parkinsonovy nemoci 2009;72:419–428. [Google Scholar]
- 33. https://parkinsoncare.cz/parkinsonova-nemoc/casovy-rozvoj/. Accessed January 15, 2022.
- 34. Svátová J. Parkinson's disease and ability to drive. Int Med Pract 2010;12:205–208. [Google Scholar]
- 35. Heinzel S, Berg D, Binder S, et al. Do we need to rethink the epidemiology and healthcare utilization of Parkinson's disease in Germany? Front Neurol 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Illés A, Csabán D, Grosz Z, et al. The role of genetic testing in the clinical practice and research of early‐onset parkinsonian disorders in a Hungarian cohort: Increasing challenge in genetic counselling, improving chances in stratification for clinical trials. Front Genet 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sveinbjörnsdottir S, Hicks AA, Jonsson T, et al. Familial aggregation of Parkinson's disease in Iceland. N Engl J Med 2000;343:1765–1770. [DOI] [PubMed] [Google Scholar]
- 38. Olszewska DA, McCarthy A, Soto‐Beasley AI, Walton RL, Ross OA, Lynch T. PARKIN, PINK1, and DJ1 analysis in early‐onset Parkinson's disease in Ireland. Ir J Med Sci 2022;191:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sironi F, Primignani P, Ricca S, et al. DJ1 analysis in a large cohort of Italian early onset Parkinson disease patients. Neurosci Lett 2013; Pt B;557:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaiyrzhanov R, Aitkulova A, Vandrovcova J, et al. A glimpse of the genetics of young‐onset Parkinson's disease in Central Asia. Mol Genet Genomic Med 2021;9:e1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hipp G, Vaillant M, Diederich NJ, et al. The Luxembourg Parkinson's study: a comprehensive approach for stratification and early diagnosis. Front Aging Neurosci 2018;10:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gustavsson EK, Trinh J, McKenzie M, Bortnick S, Petersen MS, Farrer MJ, Aasly JO. Genetic identification in early onset parkinsonism among Norwegian patients. Mov Disord Clin Pract 2017;4:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Milanowski ŁM, Ross OA, Friedman A, et al. Genetics of Parkinson's disease in the polish population. Neurol Neurochir Pol 2021;55:241–252. [DOI] [PubMed] [Google Scholar]
- 44. Bras J, Guerreiro R, Ribeiro M, et al. Analysis of Parkinson disease patients from Portugal for mutations in SNCA, PRKN, PINK1 and LRRK2. BMC Neurol 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Atadzhanov M, Zumla A, Mwaba P. Study of familial Parkinson's disease in Russia, Uzbekistan, and Zambia. Postgrad Med J 2005;81:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ilyechova EY, Miliukhina IV, Karpenko MN, Orlov IA, Puchkova LV, Samsonov SA. Case of early‐onset Parkinson's disease in a heterozygous mutation carrier of the ATP7B gene. J Pers Med 2019;9(3):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jankovic MZ, Dobricic V, Kresojevic N, et al. Identification of mutations in the PARK2 gene in Serbian patients with Parkinson's disease. J Neurol Sci 2018;393:27–30. [DOI] [PubMed] [Google Scholar]
- 48. Benetin J. Parkinsonova choroba. Bratislava: Herba; 2009. [Google Scholar]
- 49. Soňa Nevšímalová ER, Tichý J. NEUROLOGIE. Dotisk 1. vydání z r. 2002. KAROLINUM: koedice GALÉN; 2005. [Google Scholar]
- 50. Cristina TP, Pablo M, Teresa PM, et al. A genetic analysis of a Spanish population with early onset Parkinson's disease. PLoS One 2020;15:e0238098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fereshtehnejad SM, Hadizadeh H, Farhadi F, Shahidi GA, Delbari A, Lökk J. Comparison of the psychological symptoms and disease‐specific quality of life between early‐ and typical‐onset Parkinson's disease patients. Parkinsons Dis 2014;2014:819260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gershanik OS. Early onset parkinsonism. Front Biosci 2003;8:s568–s578. [DOI] [PubMed] [Google Scholar]
- 53. Chien HF, Rohé CF, Costa MD, et al. Early‐onset Parkinson's disease caused by a novel parkin mutation in a genetic isolate from North‐Eastern Brazil. Neurogenetics 2006;7:13–19. [DOI] [PubMed] [Google Scholar]
- 54. Camargos ST, Dornas LO, Momeni P, Lees A, Hardy J, Singleton A, Cardoso F. Familial parkinsonism and early onset Parkinson's disease in a Brazilian movement disorders clinic: phenotypic characterization and frequency of SNCA, PRKN, PINK1, and LRRK2 mutations. Mov Disord 2009;24(5):662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. https://www.parkinson.ca/about‐parkinsons/young‐onset‐parkinsons‐disease‐2/. Accessed January 15, 2022.
- 56. Tipton PW, Jaramillo‐Koupermann G, Soto‐Beasley AI, et al. Genetic characterization of Parkinson's disease patients in Ecuador and Colombia. Parkinsonism Relat Disord 2020;75:27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guerrero Camacho JL, Monroy Jaramillo N, Yescas Gómez P, et al. High frequency of Parkin exon rearrangements in Mexican‐mestizo patients with early‐onset Parkinson's disease. Mov Disord 2012;27:1047–1051. [DOI] [PubMed] [Google Scholar]
- 58. Khalil H, Chahine LM, Siddiqui J, et al. Parkinson's disease in the Middle East, North Africa, and South Asia: Consensus from the International Parkinson and Movement Disorder Society Task Force for the Middle East. J Parkinsons Dis 2020;10:729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. https://www.parkinsons.org.au/young-onset-parkinson-s. Accessed January 15, 2022.
- 60. Tsai CH, Lu CS. Early onset parkinsonism in Chinese. J Formos Med Assoc 1991;90:964–969. [PubMed] [Google Scholar]
- 61. Patel SG, Buchanan CM, Mulroy E, et al. Potential PINK1 founder effect in Polynesia causing early‐onset Parkinson's disease. Mov Disord 2021;36:2199–2200. [DOI] [PubMed] [Google Scholar]
- 62. Surathi P, Jhunjhunwala K, Yadav R, Pal PK. Research in Parkinson's disease in India: a review. Ann Indian Acad Neurol 2016;19:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kukkle PL, Goyal V, Geetha TS, et al. Clinical study of 668 Indian subjects with juvenile, young, and early onset Parkinson's disease. Can J Neurol Sci 2022;49:93–101. [DOI] [PubMed] [Google Scholar]
- 64. Muthane UB, Swamy HS, Satishchandra P, Subhash MN, Rao S, Subbakrishna D. Early onset Parkinson's disease: Are juvenile‐ and young‐onset different? Mov Disord 1994;9:539–544. [DOI] [PubMed] [Google Scholar]
- 65. https://www.parkinsons.org.nz/understanding-parkinsons/early-onset-parkinsons. Accessed January 15, 2022.
- 66. Chung EJ, Ki CS, Lee WY, Kim IS, Kim JY. Clinical features and gene analysis in Korean patients with early‐onset Parkinson disease. Arch Neurol 2006;63:1170–1174. [DOI] [PubMed] [Google Scholar]
- 67. Ton ND, Thuan ND, Thuong MTH, et al. Rare and novel variants of PRKN and PINK1 genes in Vietnamese patients with early‐onset Parkinson's disease. Mol Genet Genomic Med 2020;8:e1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Regragui W, Lachhab L, Razine R, et al. Profile of idiopathic parkinson's disease in Moroccan patients. Int Arch Med 2014;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Milanowski LM, Oshinaike O, Broadway BJ, et al. Early‐onset Parkinson disease screening in patients from Nigeria. Front Neurol 2021;11:594927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van der Merwe C, Haylett W, Harvey J, Lombard D, Bardien S, Carr J. Factors influencing the development of early‐ or late‐onset Parkinson's disease in a cohort of south African patients. S Afr Med J 2012;102:848–851. [DOI] [PubMed] [Google Scholar]
- 71. Deng H, Wang P, Jankovic J. The genetics of Parkinson disease. Ageing Res Rev 2018;42:72–85. [DOI] [PubMed] [Google Scholar]
- 72. Payami H, Zareparsi S, James D, Nutt J. Familial aggregation of Parkinson disease: a comparative study of early‐onset and late‐onset disease. Arch Neurol 2002;59:848–850. [DOI] [PubMed] [Google Scholar]
- 73. Schneider SA, Klein C. PINK1 type of young‐onset Parkinson disease. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 2010:1993–2021 [updated 2018 May 24]. [PubMed] [Google Scholar]
- 74. Jiang Y, Yu M, Chen J, et al. Parkin is the most common causative gene in a cohort of mainland Chinese patients with sporadic early‐onset Parkinson's disease. Brain Behav 2020;10:e01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bozi M, Papadimitriou D, Antonellou R, et al. Genetic assessment of familial and early‐onset Parkinson's disease in a Greek population. Eur J Neurol 2014;21:963–968. [DOI] [PubMed] [Google Scholar]
- 76. Kauther KM, Höft C, Rissling I, Oertel WH, Möller JC. The PLA2G6 gene in early‐onset Parkinson's disease. Mov Disord 2011;26:2415–2417. [DOI] [PubMed] [Google Scholar]
- 77. Chen SJ, Ho CH, Lin HY, Lin CH, Wu RM. Lack of PTRHD1 mutation in patients with young‐onset and familial Parkinson's disease in a Taiwanese population. Neurobiol Aging 2021;100:118.e15–118.e16. [DOI] [PubMed] [Google Scholar]
- 78. Youn J, Lee C, Oh E, et al. Genetic variants of PARK genes in Korean patients with early‐onset Parkinson's disease. Neurobiol Aging 2019;75(224):e9–224.e15. [DOI] [PubMed] [Google Scholar]
- 79. Pal GD, Hall D, Ouyang B, et al. Consortium on risk for early onset Parkinson's disease (CORE‐PD) investigators. Genetic and clinical predictors of deep brain stimulation in young‐onset Parkinson's disease. Mov Disord Clin Pract 2016;3:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Milanowski ŁM, Lindemann JA, Hoffman‐Zacharska D, et al. Frequency of mutations in PRKN, PINK1, and DJ1 in patients with early‐onset Parkinson disease from neighboring countries in Central Europe. Parkinsonism Relat Disord 2021;86:48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kanaya Y, Kume K, Morino H, et al. Analysis of genetic risk factors in Japanese patients with Parkinson's disease. J Hum Genet 2021;66:957–964. [DOI] [PubMed] [Google Scholar]
- 82. Malek N, Swallow DM, Grosset KA, et al. Olfaction in Parkin single and compound heterozygotes in a cohort of young onset Parkinson's disease patients. Acta Neurol Scand 2016;134:271–276. [DOI] [PubMed] [Google Scholar]
- 83. Moisan F, Kab S, Mohamed F, et al. Parkinson disease male‐to female ratios increase with age: French nationwide study and meta‐analysis. J Neurol Neurosurg Psychiatry 2016;87:952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Time trends in the incidence of Parkinson disease. JAMA Neurol 2016;73:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry 2004;75:637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson's disease. Mov Disord 2003;18:19–31. [DOI] [PubMed] [Google Scholar]
- 87. Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Currie LJ, Harrison MB, Trugman JM, Bennett JP, Wooten GF. Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol 2004;61:886–888. [DOI] [PubMed] [Google Scholar]
- 89. Ragonese P, D'Amelio M, Callari G, Salemi G, Morgante L, Savettieri G. Age at menopause predicts age at onset of Parkinson's disease. Mov Disord 2006;21:2211–2214. [DOI] [PubMed] [Google Scholar]
- 90. Ragonese P, D'Amelio M, Savettieri G. Implications for estrogens in Parkinson's disease: an epidemiological approach. Ann N Y Acad Sci 2006;1089:373–382. [DOI] [PubMed] [Google Scholar]
- 91. Lucking CB, Durr A, Bonifati V. European consortium on genetic susceptibility in Parkinson's disease, association between early onset Parkinson's disease and mutations in the parkin gene: French Parkinson's disease genetics study group. N Engl J Med 2000;342:1560–1567. [DOI] [PubMed] [Google Scholar]
- 92. Seier M, Hiller A. Parkinson's disease and pregnancy: an updated review. Parkinsonism Relat Disord 2017;40:11–17. [DOI] [PubMed] [Google Scholar]
- 93. Rubin SM. Parkinson's disease in women. Dis Mon 2007;53:206–213. [DOI] [PubMed] [Google Scholar]
- 94. Nageshwaran S, Smith M, Bordelon YM. Movement disorders and pregnancy. In: Klein A, O'Neal MA, Scifres C, Waters J, Waters JH, eds. Neurological Illness in Pregnancy: Principles and Practice. 1st ed. Hoboken: Wiley‐Blackwell; 2016:179–190. [Google Scholar]
- 95. Gatto NM, Deapen D, Stoyanoff S, Pinder R, Narayan S, Bordelon Y, Ritz B. Lifetime exposure to estrogens and Parkinson's disease in California teachers. Parkinsonism Relat Disord 2014;20:1149–1156. [DOI] [PubMed] [Google Scholar]
- 96. Disshon KA, Boja JW, Diuzen DE. Inhibition of the striate dopamine transporter activity by 17beta‐estradiol. Eur J Pharmacol 1998;345:207–211. [DOI] [PubMed] [Google Scholar]
- 97. Benedetti MD, Maraganore DM, Bower JH, et al. Menopause, hysterectomy, and estrogen in Parkinson's disease: an exploratory case–control study. Mov Disord 2001;16:830–837. [DOI] [PubMed] [Google Scholar]
- 98. Lyons KE, Hubble JP, Troster AI, Pahwa R, Koller WC. Gender differences in Parkinson's disease. Clin Neuropharmacol 1998;21:118–121. [PubMed] [Google Scholar]
- 99. Nitkowska M, Czyżyk M, Friedman A. Reproductive life characteristics in females affected with Parkinson's disease and in healthy control subjects—a comparative study on polish population. Neurol Neurochir Pol 2014;48:322–327. [DOI] [PubMed] [Google Scholar]
- 100. Ragonese P, D'Amelio M, Salemi G, et al. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology 2004;62:2010–2014. [DOI] [PubMed] [Google Scholar]
- 101. Yadav R, Shukla G, Goyal V, Singh S, Behari M. A case control study of women with Parkinson's disease and their fertility characteristics. J Neurol Sci 2012;319:135–138. [DOI] [PubMed] [Google Scholar]
- 102. Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson's disease: a review. J Neurol 2017;264:1583–1607. [DOI] [PubMed] [Google Scholar]
- 103. Morissette M, Sweidi SA, Callier S, Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson's disease. Mol Cell Endocrinol 2008;290:60–69. [DOI] [PubMed] [Google Scholar]
- 104. Baraka AM, Korish AA, Soliman GA, Kamal H. The possible role of estrogen and selective estrogen receptor modulators in a rat model of Parkinson's disease. Life Sci 2011;88:879–885. [DOI] [PubMed] [Google Scholar]
- 105. Liu R, Baird D, Park Y, et al. Female reproductive factors, menopausal hormone use, and parkinson's disease. Mov Disord 2014;29:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shulman LM. Is there a connection between estrogen and Parkinson's disease? Parkinsonism Relat Disord 2002;8:289–295. [DOI] [PubMed] [Google Scholar]
- 107. Wang P, Li J, Qiu S, Wen H, Du J. Hormone replacement therapy and Parkinson's disease risk in women: a meta‐analysis of 14 observational studies. Neuropsychiatr Dis Treat 2015;11:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Reproductive factors and clinical features of Parkinson's disease. Parkinsonism Relat Disord 2013;19:1094–1099. [DOI] [PubMed] [Google Scholar]
- 109. Kusters CDJ, Paul KC, Duarte Folle A, et al. Increased menopausal age reduces the risk of Parkinson's disease: a Mendelian randomization approach. Mov Disord 2021;36:2264–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Parkinson Study Group POETRY Investigators . A randomized pilot trial of estrogen replacement therapy in post‐menopausal women with Parkinson's disease. Parkinsonism Relat Disord 2011;17:757–760. [DOI] [PubMed] [Google Scholar]
- 111. Tsang KL, Ho SL, Lo SK. Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology 2000;54:2292–2298. [DOI] [PubMed] [Google Scholar]
- 112. Saunders‐Pullman R, Gordon‐Elliott J, Parides M, Fahn S, Saunders HR, Bressman S. The effect of estrogen replacement on early Parkinson's disease. Neurology 1999;52:1417–1420. [DOI] [PubMed] [Google Scholar]
- 113. Simon KC, Chen H, Gao X, Schwarzschild MA, Ascherio A. Reproductive factors, exogenous estrogen use, and risk of Parkinson's disease. Mov Disord 2009;24:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shi J, Zhang B, Choi JY, et al. Age at menarche and age at natural menopause in east Asian women: a genome‐wide association study. Age (Dordr) 2016;38:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wickremaratchi MM, Knipe MD, Sastry BS, et al. The motor phenotype of Parkinson's disease in relation to age at onset. Mov Disord 2011;26:457–463. [DOI] [PubMed] [Google Scholar]
- 116. Friedman A. Old‐onset Parkinson's disease compared with young‐onset disease: clinical differences and similarities. Acta Neurol Scand 1994;89:258–261. [DOI] [PubMed] [Google Scholar]
- 117. Hely MA, Morris JG, Reid WG, O'Sullivan DJ, Williamson PM, Broe GA, Adena MA. Age at onset: the major determinant of outcome in Parkinson's disease. Acta Neurol Scand 1995;92:455–463. [DOI] [PubMed] [Google Scholar]
- 118. Giovannini P, Piccolo I, Genitrini S, et al. Early‐onset Parkinson's disease. Mov Disord 1991;6:36–42. [DOI] [PubMed] [Google Scholar]
- 119. Nagayama H, Hamamoto M, Nito C, Takagi S, Miyazaki T, Katayama Y. Initial symptoms of Parkinson's disease with elderly onset. Gerontology 2000;46:129–132. [DOI] [PubMed] [Google Scholar]
- 120. Pagano G, Ferrara N, Brooks DJ, Pavese N. Age at onset and Parkinson disease phenotype. Neurology 2016;86:1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol 2014;71:499–504. [DOI] [PubMed] [Google Scholar]
- 122. Kempster PA, O'Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson's disease: a clinico‐pathological study. Brain 2010;133:1755–1762. [DOI] [PubMed] [Google Scholar]
- 123. Jankovic J, Kapadia AS. Functional decline in Parkinson's disease. Arch Neurol 2001;58:1611–1615. [DOI] [PubMed] [Google Scholar]
- 124. van Rooden SM, Heiser WJ, Kok JN, Verbaan D, van Hilten JJ, Marinus J. The identification of Parkinson's disease subtypes using cluster analysis: a systematic review. Mov Disord 2010;25:969–978. [DOI] [PubMed] [Google Scholar]
- 125. Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 2015;72:863–873. [DOI] [PubMed] [Google Scholar]
- 126. Eisinger RS, Hess CW, Martinez‐Ramirez D, Almeida L, Foote KD, Okun MS, Gunduz A. Motor subtype changes in early Parkinson's disease. Parkinsonism Relat Disord 2017;43:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Te Groen M, Bloem BR, Wu SS, Post B. Parkinson's foundation quality improvement initiative investigators. Better quality of life and less caregiver strain in young‐onset Parkinson's disease: a multicentre retrospective cohort study. J Neurol 2021;268:1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kumar N, Van Gerpen JA, Bower JH, Ahlskog JE. Levodopa‐dyskinesia incidence by age of Parkinson's disease onset. Mov Disord 2005;20:342–344. [DOI] [PubMed] [Google Scholar]
- 129. Van Gerpen JA, Kumar N, Bower JH, Weigand S, Ahlskog JE. Levodopa‐associated dyskinesia risk among Parkinson disease patients in Olmsted County, Minnesota, 1976–1990. Arch Neurol 2006;63:205–209. [DOI] [PubMed] [Google Scholar]
- 130. Ku S, Glass GA. Age of Parkinson's disease onset as a predictor for the development of dyskinesia. Mov Disord 2010;25:1177–1182. [DOI] [PubMed] [Google Scholar]
- 131. Knipe MD, Wickremaratchi MM, Wyatt‐Haines E, Morris HR, Ben‐Shlomo Y. Quality of life in young‐ compared with late‐onset Parkinson's disease. Mov Disord 2011;26:2011–2018. [DOI] [PubMed] [Google Scholar]
- 132. Hustad E, Myklebust TÅ, Gulati S, Aasly JO. Increased mortality in young‐onset Parkinson's disease. J Mov Disord 2021;14:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu SY, Wu JJ, Zhao J, et al. Onset‐related subtypes of Parkinson's disease differ in the patterns of striatal dopaminergic dysfunction: a positron emission tomography study. Parkinsonism Relat Disord 2015;21:1448–1453. [DOI] [PubMed] [Google Scholar]
- 134. DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord 2009;15(Suppl. 3):S237–S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Poston KL, Eidelberg D. Functional brain networks and abnormal connectivity in the movement disorders. Neuroimage 2012;62:2261–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, Eidelberg D. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in parkinsonism. Neuroimage 2004;21:1497–1507. [DOI] [PubMed] [Google Scholar]
- 137. Graff‐Radford J, Williams L, Jones DT, Benarroch EE. Caudate nucleus as a component of networks controlling behavior. Neurology 2017;89:2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sossi V, de la Fuente‐Fernandez R, Schulzer M, Adams J, Stoessl AJ. Age related differences in levodopa dynamics in Parkinson's: implications for motor complications. Brain 2006;129:1050–1058. [DOI] [PubMed] [Google Scholar]
- 139. Schirinzi T, Di Lazzaro G, Sancesario GM, et al. Young‐onset and late‐onset Parkinson's disease exhibit a different profile of fluid biomarkers and clinical features. Neurobiol Aging 2020;90:119–124. [DOI] [PubMed] [Google Scholar]
- 140. Schrag A, Banks P. Time of loss of employment in Parkinson's disease. Mov Disord 2006;21:1839–1843. [DOI] [PubMed] [Google Scholar]
- 141. Murphy R, Tubridy N, Kevelighan H, O'Riordan S. Parkinson's disease: How is employment affected? Ir J Med Sci 2013;182:415–419. [DOI] [PubMed] [Google Scholar]
- 142. da Silva AG, Leal VP, da Silva PR, et al. Difficulties in activities of daily living are associated with stigma in patients with Parkinson's disease who are candidates for deep brain stimulation. Braz J Psychiatry 2020;42:190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Calne SM, Kumar A. Young onset Parkinson's disease, practical management of medical issues. Parkinsonism Relat Disord 2008;14:133–142. [DOI] [PubMed] [Google Scholar]
- 144. Bhidayasiri R, Boonmongkol T, Thongchuam Y, et al. Impact of disease stage and age at Parkinson's onset on patients' primary concerns: insights for targeted management. PLoS One 2020;15:e0243051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Maier K, Calne SM. Informal caregiving: a valuable part of the health care team. In: Ebadi M, Pfeiffer RF, eds. Parkinson's Disease. Florida: CRC Press; 2005:999–1008. [Google Scholar]


