Abstract
Disruption of pstS encoding the Pi-binding protein in Escherichia coli generally leads to the constitutive expression of the pho regulon. We demonstrate that Pi-controlled expression is restored when the activity of the Pi transporter PitA or PitB is increased. Apparently, PstS is not an essential component of the signal transduction pathway.
Growth of Escherichia coli under Pi limitation results in the induction of the pho regulon, which includes phoA encoding alkaline phosphatase (18) and the pst operon encoding a Pi transporter. This Pi transporter consists of a periplasmic Pi-binding protein (PstS), two integral membrane proteins (PstA and PstC), and an ATP-binding protein (PstB) (4, 16). Central to the regulation of the pho regulon is a two-component regulatory system encoded by the phoBR operon (21). In addition, the Pst system plays a role in Pi regulation, since mutations in any of the genes of the pst operon generally lead to constitutive expression of the pho regulon (21–23). This constitutive expression is not due to decreased intracellular phosphate levels, which were reported to be maintained at a high level under high-Pi conditions by a secondary Pi transporter, PitA (11, 24). Furthermore, the regulatory and transport roles of the Pst system could be uncoupled by specific amino acid substitutions in PstC or PstA (5, 6). Since periplasmic PstS binds Pi with high affinity, it could potentially act as the primary sensor of external Pi (20). The interaction of Pi-loaded PstS with the membrane components of the Pst system might lead to a conformational change, which is sensed by the product of the fifth gene of the pst operon, PhoU. PhoU is not involved in Pi transport (15), but probably forms the regulatory link between the Pi transporter and the PhoBR system (20).
To study the role of PstS in signal perception, we wished to isolate missense mutations in pstA or pstC that allow the Pst system to transport Pi in the absence of PstS. In a previous study, such mutants were not obtained, but a third Pi transporter, PitB, was discovered (9). In this study, we demonstrate that PitA or PitB activity can restore Pi regulation of the pho regulon in the absence of PstS.
Pseudorevertants in pitA restore Pi regulation in a pstS mutant.
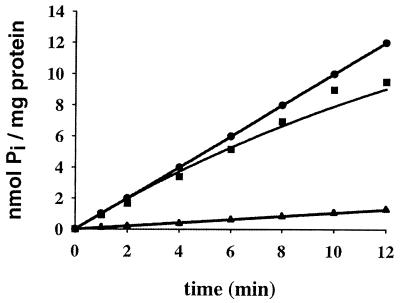
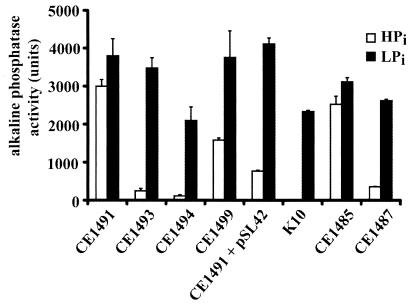
To study the role of PstS in Pi regulation of the pho regulon, we attempted to isolate mutants in pstA or pstC, allowing the Pst system to transport Pi in the absence of PstS. Therefore, strain CE1491, which lacks all three known Pi transporters (Table 1), was mutagenized with ethylmethane sulfonic acid, and mutants that could grow on minimal medium plates (9) with 660 μM Pi as the sole source of phosphate were selected. Emanating from the idea that restored Pi transport via the Pst system might also restore Pi control of the pho regulon, the mutants obtained were tested for expression of alkaline phosphatase on L broth plates containing the chromogenic substrate 5-bromo-4-chloro-3-indolyl phosphate (XP) (3). Six of 300 mutants tested showed drastically reduced alkaline phosphatase activity, and two mutants, designated CE1493 and CE1494, were characterized in detail. Quantitative analysis showed that alkaline phosphatase activity was reduced after growth in complex medium almost to the level found in pitA Pst+ strain K10 (Table 1). Furthermore, uptake of 33Pi was considerably improved compared to that in the parental strain, CE1491 (Fig. 1). However, after P1 transductions with the revertants as donors and strain K10 as acceptor, all Kanr transductants tested (50 in each case) failed to grow on Pi as a phosphate source, and alkaline phosphatase was highly expressed (data not shown), showing that the reversion is not closely linked to the pstS::kan mutation. Furthermore, since CE1493 and CE1494 were resistant to gentamicin, the pitB::gm mutation had not reverted. Hence, we considered the possibility that the pitA mutation had reverted. First, the pitB::gm allele was replaced with a wild-type pitB gene by P1 transduction with the metC162::Tn10 strain CAG18475 (13) as the donor, resulting in strains CE1495 and CE1496 (note that a wild-type pitB gene gives a PitB− phenotype) (9). Subsequently, a pitA::gm mutation was introduced. The resulting strains, CE1497 and CE1498, respectively, had lost the ability to grow on Pi (results not shown), showing that the reversion in strains CE1493 and CE1494 is linked to the pitA locus. Furthermore, they produced high levels of alkaline phosphatase (Table 1), demonstrating that the reduced alkaline phosphatase activity in strains CE1493 and CE1494 results from the same mutation and is not due to a secondary mutation (for example, in the phoBR genes). After PCR amplification and cloning in pCRII− TOPO (Invitrogen), the pitA alleles of the revertants were sequenced. A point mutation resulting in the substitution of Thr41 (which is highly conserved in a large superfamily of Pi transporters) (12) by Ile was found in both strains. The original pitA mutation of strain K10, which resulted in the Gly220Asp substitution (9), was retained, demonstrating that the revertants CE1493 and CE1494 carry a compensatory mutation in pitA, rather than a true reversion. To investigate whether the pho regulon can be induced in strains CE1493 and CE1494, the cells were grown in high-phosphate (HPi) and low-phosphate (LPi) media, and alkaline phosphatase activity was determined. Indeed, high activity was measured after growth of these strains in LPi medium (Fig. 2). These results demonstrate that the requirement for PstS in Pi regulation of the pho regulon can be substituted by PitA activity.
TABLE 1.
Alkaline phosphatase activities of various E. coli strainsa
| Strainb | Relevant characteristics | Plasmidc | PhoA activityd |
|---|---|---|---|
| K10 | pitA10 relA1 spoT1 | 16 ± 3 | |
| CE1485 | K10 pstS::kan | 2,180 ± 45 | |
| CE1491 | CE1485 pitB::gm | 2,070 ± 300 | |
| CE1493 | CE1491 PitA+ | 40 ± 4 | |
| CE1494 | CE1491 PitA+ | 25 ± 3 | |
| CE1495 | CE1493 pitB+metC::Tn10 | 40 ± 3 | |
| CE1496 | CE1494 pitB+metC::Tn10 | 23 ± 2 | |
| CE1497 | CE1495 pitA::gm | 1,570 ± 140 | |
| CE1498 | CE1496 pitA::gm | 1,300 ± 125 | |
| CE1491 | CE1485 pitB::gm | pJF118EH | 1,885 ± 280 |
| CE1491 | CE1485 pitB::gm | pSL41 (pitB) | 42 ± 15 |
| CE1491 | CE1485 pitB::gm | pSL42 (pitA) | 100 ± 10 |
| CE1487 | CE1485 PitB+ | 80 ± 10 | |
| CE1492 | CE1487 Δ(pstSCAB-phoU)::cam | 2,940 ± 170 | |
| MG1655 | 15 ± 2 | ||
| CE1500 | MG1655 pitA::gm pstS::kan | pJF118EH | 1,690 ± 110 |
| CE1501 | MG1655 pitA::gm Δ(pstSCAB- phoU)::cam | pJF118EH | 2,080 ± 150 |
| CE1500 | MG1655 pitA::gm pstS::kan | pSL41 (pitB) | 48 ± 5 |
| CE1501 | MG1655 pitA::gm Δ(pstSCAB- phoU)::cam | pSL41 (pitB) | 1,645 ± 230 |
Strains were grown overnight at 37°C in L broth supplemented with 1 mM glucose-3-phosphate. Addition of glucose-3-phosphate resulted in equal growth of all strains and did not influence alkaline phosphatase activities (data not shown).
Strains K10 and MG1655 were obtained from the E. coli Genetic Stock Center, Department of Biology, Yale University, Conn. Strains CE1485, CE1491, and CE1487 were described previously (9), and all other strains were constructed in the present work. The pstS::kan mutation is an insertion of a kanamycin resistance cassette in the PvuI site approximately in the middle of the pstS gene. Strains carrying this mutation do not produce the Pi-binding protein, as was verified by Western blotting (9).
The alkaline phosphatase activities were determined with para-nitrophenyl phosphate as a substrate (17). Alkaline phosphatase activity is expressed in units, which are defined as nanomoles of para-nitrophenol released per minute per optical density at 660 nm of cell culture. Values represent the averaged results of three or four independent experiments, and standard deviations are given.
FIG. 1.
Uptake of 33Pi by cells of strains CE1491 (▴), CE1493 (●), and CE1494 (▪). Growth of cells and uptake experiments were performed essentially as described previously (9). The experiments were repeated twice with essentially the same results, and the data from one of these experiments are shown.
FIG. 2.
Alkaline phosphatase activities in K10 and its derivatives grown in HPi or LPi medium. Cells were grown overnight in the peptone-based HPi and LPi media (10) as described previously (9). The alkaline phosphatase activities were determined and are expressed as in Table 1. The data represent averaged results of three or four independent experiments, and standard deviations are given.
Wild-type pitA can restore Pi regulation in a pstS mutant.
To investigate whether expression of a wild-type pitA gene can restore Pi regulation in a pstS mutant, the mutant pitA allele in CE1491 was replaced with the wild-type gene by P1 transduction with zhf-5::Tn10 strain CAG18450 (13) as the donor. A Tetr transductant that could grow on Pi as a phosphate source was designated CE1499. Even though colonies of this strain were blue on XP-containing L broth plates, quantative analysis showed that the alkaline phosphatase activity was reduced compared to that of the parental strain CE1491, although not to the same extent as in the pseudorevertants CE1493 and CE1494 (Fig. 2). Alkaline phosphatase activity was induced again in strain CE1499 after growth in LPi medium (Fig. 2). The relatively weak repression of alkaline phosphatase activity in strain CE1499 after growth under Pi-replete conditions may explain why pseudorevertants with a compensatory mutation in pitA rather than true revertants were picked in the original mutant selection described in the previous paragraph. Probably true revertants were among the strains that could grow on the minimal medium plates with Pi as the sole source of phosphate, but were blue on XP-containing L broth plates. However, introduction of plasmid pSL42, carrying pitA, into CE1491 resulted in severe repression of alkaline phosphatase synthesis in HPi and complex medium (Fig. 2 and Table 1, respectively), and phoA expression could be induced when cells were grown in LPi medium (Fig. 2). These results demonstrate that the activity of the wild-type PitA transporter can substitute for PstS in Pi regulation.
PitB expression also restores Pi regulation in a pstS mutant.
Because pitA pstS strain CE1487 had recovered the ability to grow on Pi due to the expression by gene amplification of pitB (9), it was of interest to determine whether normal regulation of the pho regulon was regained in this strain as well. Indeed, the expression of alkaline phosphatase in strain CE1487 was significantly reduced compared to that in its parental strain CE1485 after growth in HPi medium, and it could be induced by growth in LPi medium (Fig. 2). Furthermore, alkaline phosphatase was constitutively expressed in strain CE1491, a pitB::gm derivative of CE1487, directly demonstrating that the regained Pi control on phoA expression in the pstS mutant CE1487 is due to the expression of pitB and not to a secondary mutation. In addition, introduction of plasmid pSL41, carrying the pitB gene, into this strain resulted in a drastic reduction of alkaline phosphatase expression under HPi conditions, whereas cells carrying the control plasmid pJF118EH still showed high enzyme activity (Table 1). Thus, like PitA activity, PitB activity can compensate for the absence of pstS in the Pi regulation of the pho regulon.
Other pst genes are still required for Pi regulation in the absence of PstS.
To investigate whether the other genes of the pst operon are still required for Pi control of the pho regulon in the absence of PstS, a strain was constructed in which all genes of the entire pstSCAB-phoU locus were deleted. For this purpose, plasmid pSN507 (2) was digested with MunI and SnaBI, thereby removing a DNA segment encompassing approximately the 3′ half of pstS, the entire pstC, pstA, and pstB genes, and approximately two-thirds from the 5′ end of phoU, and ligated with a Camr cassette (1). The resulting plasmid was digested with EcoRI, and the 6.4-kb DNA fragment with the ΔpstSCAB-phoU mutation was used to transform recBC sbc strain AM1095 (8). One Camr transformant, which expressed alkaline phosphatase constitutively and did not produce PstS and PhoU as verified by Western blotting, was designated CE1489. The deletion was then transferred by P1 transduction to PitB+ strain CE1487. High levels of alkaline phosphatase activity were detected in the resulting strain, CE1492, after growth in complex medium (Table 1). This result demonstrates that PstCAB and/or PhoU is still required for Pi control of the pho regulon even when PitB is active and that the Pi signaling proceeds (at least in part) via the same pathway as in the wild-type strain.
Influence of the genetic background.
All experiments described so far were carried out in the genetic background of strain K10. Although this strain is the classical strain for studies of the pho regulon, it carries relA and spoT mutations, which were recently shown to affect Pi regulation in other strains (14). To exclude the possibility that our results were influenced by this background, several mutations were transferred by P1 transduction into strains MG1655 and W3110, which do not carry spoT or relA mutations. As expected, alkaline phosphatase was produced constitutively in the pstS::kan pitA::gm and Δ(pstSCAB-phoU)::cam pitA::gm derivatives of MG1655, designated CE1500 and CE1501, respectively (Table 1). The subsequent introduction of plasmid pSL41 containing pitB did not affect alkaline phosphatase activity in strain CE1501, but severely reduced this activity in strain CE1500 (Table 1). Similar results were obtained for the derivatives of strain W3110 (data not shown). Hence, also in different genetic backgrounds, PitB activity can compensate for the loss of Pi control of the pho regulon in the absence of PstS, but the products of other genes of the pst operon remain required for this control.
Our observation that the Pi regulation of the pho regulon can be restored in the absence of the Pi-binding protein by increased PitA or PitB activity is very unexpected in the light of previous publications (11, 21, 24). The results demonstrate that Pi signaling via the Pst system can occur in the absence of PstS and challenge the view that extracellular rather than intracellular Pi concentrations control the expression of the pho regulon. It is conceivable that PhoU associated with the Pst system has direct access to Pi that enters the cell via this system. This Pi might modify PhoU, or, alternatively, PhoU may convert a proportion of the Pi into a metabolic compound (21), and either action may repress the autokinase activity of PhoR. Pi that enters the cell via PitA or PitB may be channeled into other metabolic routes or may not be available to PhoU, because it remains associated with divalent cations (19). However, when intracellular Pi increases due to overproduction of PitA or PitB, it may become accessible to PhoU.
Acknowledgments
We thank Paul Schoondermark for constructing the Δ(pstSCAB-phoU)::cam strain CE1489. We are indebted to The Netherlands Culture Collection of Bacteria (NCCB) for providing us with plasmids and strains.
This research was supported by the Life Sciences Foundation (ALW), which is subsidized by The Netherlands Organization for Scientific Research (NWO).
REFERENCES
- 1.Alexeyev M F, Shokolenko I N, Croughan T P. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene. 1995;160:63–67. doi: 10.1016/0378-1119(95)00108-i. [DOI] [PubMed] [Google Scholar]
- 2.Amemura M, Shinagawa H, Makino K, Otsuji N, Nakata A. Cloning of and complementation tests with alkaline phosphatase regulatory genes (phoS and phoT) of Escherichia coli. J Bacteriol. 1982;152:692–701. doi: 10.1128/jb.152.2.692-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and ϕ80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 4.Chan F-Y, Torriani A. PstB protein of the phosphate-specific transport system of Escherichia coli is an ATPase. J Bacteriol. 1996;178:3974–3977. doi: 10.1128/jb.178.13.3974-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox G B, Webb D, Godovac-Zimmermann J, Rosenberg H. Arg-220 of the PstA protein is required for phosphate transport through the phosphate-specific transport system in Escherichia coli but not for alkaline phosphatase repression. J Bacteriol. 1988;170:2283–2286. doi: 10.1128/jb.170.5.2283-2286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox G B, Webb D, Rosenberg H. Specific amino acid residues in both the PstB and PstC proteins are required for phosphate transport by the Escherichia coli Pst system. J Bacteriol. 1989;171:1531–1534. doi: 10.1128/jb.171.3.1531-1534.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 8.Hoekstra W P M, de Haan P G, Bergmans J E N, Zuidweg E M. Transformation in E. coli K12: relation of linkage to distance between markers. Mol Gen Genet. 1976;145:109–110. doi: 10.1007/BF00331565. [DOI] [PubMed] [Google Scholar]
- 9.Hoffer S M, Schoondermark P, van Veen H W, Tommassen J. Activation by gene amplification of pitB, encoding a third phosphate transporter of Escherichia coli K-12. J Bacteriol. 2001;183:4659–4663. doi: 10.1128/JB.183.15.4659-4663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levinthal A, Signer E R, Fetherolf K. Reactivation and hybridisation of reduced alkaline phosphatase. Proc Natl Acad Sci USA. 1962;48:1230–1237. doi: 10.1073/pnas.48.7.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg H, Gerdes R G, Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977;131:505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saier M H, Jr, Eng B H, Fard S, Garg J, Haggerty D A, Hutchinson W J, et al. Phylogenetic characterization of novel transport families revealed by genome analysis. Biochim Biophys Acta. 1999;1422:1–56. doi: 10.1016/s0304-4157(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 13.Singer M, Baker T A, Schnitzler G, Deischel S M, Goel M, Dove W, Jaacks K J, Grossman A D, Erickson J W, Gross C A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spira B, Yagil E. The relation between ppGpp and the pho regulon in Escherichia coli. Mol Gen Genet. 1998;257:469–477. doi: 10.1007/s004380050671. [DOI] [PubMed] [Google Scholar]
- 15.Steed P M, Wanner B L. Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J Bacteriol. 1993;175:6797–6809. doi: 10.1128/jb.175.21.6797-6809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surin B P, Rosenberg H, Cox G B. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J Bacteriol. 1985;161:189–198. doi: 10.1128/jb.161.1.189-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tommassen J, Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980;143:151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torriani A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960;38:460–470. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- 19.Van Veen H W, Abee T, Kortstee G J J, Konings W N, Zehnder A J B. Translocation of metal phosphates via the phosphate inorganic transport system of Escherichia coli. Biochemistry. 1994;33:1766–1770. doi: 10.1021/bi00173a020. [DOI] [PubMed] [Google Scholar]
- 20.Wanner B L. Gene regulation by phosphate in enteric bacteria. J Cell Biochem. 1993;51:47–54. doi: 10.1002/jcb.240510110. [DOI] [PubMed] [Google Scholar]
- 21.Wanner B L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella. Cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1357–1381. [Google Scholar]
- 22.Willsky G R, Bennett R L, Malamy M H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973;113:529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willsky G R, Malamy M H. Control of the synthesis of alkaline phosphatase and the phosphate-binding protein in Escherichia coli. J Bacteriol. 1976;127:595–609. doi: 10.1128/jb.127.1.595-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willsky G R, Malamy M H. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol. 1980;144:356–365. doi: 10.1128/jb.144.1.356-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]