Abstract
The World Health Organization (WHO) has set the goal of eliminating hepatitis as a threat to public health by 2030. Blocking mother-to-child transmission (MTCT) of hepatitis B virus (HBV) is not only the key to eliminating viral hepatitis, but also a hot issue in the field of hepatitis B prevention and treatment. To standardize the clinical management of preventing MTCT of HBV and achieve zero HBV infection among infants, the Chinese Foundation for Hepatitis Prevention and Control organized experts to compile a management algorithm for prevention of MTCT of HBV based on the latest research progress and guidelines, including 10 steps of pregnancy management and postpartum follow-up, among which screening, antiviral treatment, and infant immunization are its core components.
Keywords: Hepatitis B, Mother-to-child transmission, Algorithm
Introduction
On May 2016, the World Health Assembly endorsed a Global Health Sector Viral Hepatitis Strategy (2016–2021), which put forth a goal of eliminating the public health threat of viral hepatitis by 2030, including reducing the new infection rate of viral hepatitis by 90% and the mortality rate by 65% in 2030 based on the 2015 data.1 Also, reducing the hepatitis B virus (HBV) infection rate of 5-year-old children to 0.1% was required. Mother-to-child transmission (MTCT) is a major route of transmitting HBV. Preventing MTCT of HBV is the key to eliminating HBV and strengthening standardized management of pregnant women with chronic HBV infections and their babies is an effective measure in eliminating MTCT of HBV. In recent years, strategies to eliminate viral hepatitis implemented in China and abroad have provided a favorable opportunity to eliminate MTCT of HBV. In order to further standardize clinical management for preventing MTCT of HBV in China, the Chinese Foundation for Hepatitis Prevention and Control organized experts to compile and publish a management algorithm for prevention of MTCT of HBV in 2017,2,3 which has been widely used in clinical practice and has had a positive role in preventing MTCT of HBV. Blocking MTCT of HBV has become a hot topic in the field of public health and remarkable progress has been made in related research. Based on consensus guidelines and latest published research results of preventing MTCT of HBV in China and abroad,4–15 we updated the first edition of the management algorithm for prevention of MTCT of HBV in order to help clinicians provide standard clinical management for preventing MTCT of HBV.
Management algorithm
Screening of pregnant women with HBV infection
As a medium-high endemic area of HBV, the prevalence of hepatitis B surface antigen (HBsAg) in general population is approximately 6.1%,16 and is 6.3% in pregnant women in China.17 The general screening of serological markers of HBV infection in pregnant women is the first step in preventing MTCT of HBV. In China, a national integrated prevention of MTCT of human immunodeficiency virus (HIV), syphilis, and hepatitis B (iPMTCT) program was started in 2011 and expanded to all counties nationwide by 2015.18 All the healthcare facilities providing midwifery and antenatal care services were required to participated in the program, which requires that all pregnant women in their first trimester be screened for HIV, syphilis, and HBV. HBsAg was required to be tested in pregnant women during antenatal care following standardized national procedures. The program provided an essential prerequisite for preventing MTCT. To screen HBV infection, and HBV serological markers, at least HBsAg, should be tested. If the pregnant woman is HBsAg negative, it usually indicates that there is no HBV infection, and regular pregnancy care services should be provided. Additionally, serological markers of HBV infections of their husbands should be tested. If the pregnant woman is HBsAg positive, it indicates the presence of HBV infection; therefore, her detailed medical and family history should be collected, after which the situation related to HBV infection needs to be evaluated following the management algorithm. Because of the family clustering of hepatitis B, it is recommended that family members be screened for hepatitis B.
Assessment of HBV infection
HBsAg-positive pregnant women need to be tested for hepatitis B e antigen (HBeAg), anti-HBe, HBV DNA level, and biochemical markers of liver function, and then evaluated by upper abdominal ultrasound to determine whether they have hepatitis activity and to stage fibrosis. Special attention should be paid to the presence of cirrhosis.
When pregnant women have been diagnosed as HBV DNA-positive and have significant hepatic activity, alanine aminotransferase (ALT) levels ≥ 5 times the upper limit of normal (ULN), excluding other factors leading to ALT elevation, such as drug induced liver injury, fatty liver, and others, or found to have liver cirrhosis or advanced fibrosis, an evaluation by a specialist in infectious disease or hepatology should be authorized. Noninvasive tests, such as the aspartate aminotransferase to platelet ratio index (APRI),19 fibrosis 4 score (FIB-4),19 or upper abdominal ultrasonic examination could be used to diagnose advanced fibrosis or liver cirrhosis. Thereafter, an antiviral treatment with tenofovir disoproxil fumarate (TDF) is often a primary recommendation; TDF should be administered after an informed consent by the patient. However, if ALT flare resolves spontaneously overtime, there is no need to initiate antiviral therapy immediately and antiviral therapy could be discontinued after birth.
In pregnant women who are HBV DNA-positive, ALT levels fluctuate between ≥1 and ≤5× the ULN, and total bilirubin (TBIL) is <2× the ULN, antiviral treatment can be delayed. If ALT levels increase to ≥5× the ULN or if TBIL is ≥2× the ULN, then treatment will be the same as in (1) above. If ALT levels decrease to <1× the ULN, then treatment will be the same as (3) below. If ALT levels remain within the range of ≥1 to ≤ 5× ULN at 24 weeks of gestation, TDF should be given as antiviral therapy with informed consent of the patient.
In pregnant women who are HBV DNA-positive with normal ALT levels and no manifestation of liver cirrhosis, antiviral therapy can be postponed and follow-up of liver functions should be continued. During the follow-up period, if there is a persistent elevation of ALT ≥1× the ULN, treatment decisions should follow (1) or (2) depending on the ALT level. In addition, TBIL and PTA should be tested to evaluate the severity of liver injury.
In pregnant women with HBV DNA levels below the lower limit of detection, indicating that the patient may be in an inactive stage, it is recommended to retest HBV DNA at 24 weeks of gestation. If they are still below the detection limit, no intervention is required. For pregnant women who have hepatitis activity and need antiviral treatment, TDF is the first choice. However, tenofovir alafenamide fumarate (TAF) can be used if patients have osteoporosis, kidney damage, or have other risk factors of kidney damage.
Antiviral prophylaxis for MTCT of HBV
For pregnant women with high HBV loads, antiviral therapy in the third trimester combined with neonatal hepatitis B vaccine and hepatitis B immunoglobulin (HBIG) vaccination can further reduce the incidence of MTCT of HBV without increasing adverse pregnancy outcomes of the fetus.9–11 Therefore, antiviral therapy during pregnancy to block MTCT of HBV has been widely accepted and applied in clinical practice and has played a positive role in eliminating MTCT of HBV.15 In pregnant women who have normal liver functions and do not take antiviral drugs, HBV DNA levels should be tested with highly sensitive reagents in the second trimester (12–24 weeks). HBV DNA levels should be used to determine whether antiviral treatment is needed to prevent MTCT of HBV. When quantitative HBV DNA testing is unavailable, HBeAg can be used as an alternative marker, and those who are HBeAg positive should be given antiviral therapy.6
If HBV DNA is >2×105 IU/mL, TDF should be administered after informed consent of the patient at 28 weeks of gestation. TAF or telbivudine could be used as an option for pregnant women who have osteoporosis, kidney damage, or high-risk factors leading to kidney damage or severe gastrointestinal symptoms. TAF has a better renal and bone safety profile than TDF, similar antiviral efficacy in a phase III study,20 and has been used in China to prevent MTCT (see below). HBV DNA tests should be repeated before delivery to evaluate the effects of antiviral treatment and risks of MTCT of HBV. After TAF was approved for marketing in China, it was initially used in clinical research and clinical practice for preventing MTCT. Existing data13,14 showed that TAF was effective and safe for preventing MTCT of HBV during pregnancy and expected to be administered as a new option.
If HBV DNA levels are <2×105 IU/mL, the risk of MTCT of HBV is quite low and can be prevented by HBV vaccination plus HBIG immunization in pregnant women’s newborns and antiviral therapy is not recommended.
For pregnant women who first visit clinics after 28 weeks of gestation with HBV DNA ≥2×105 IU/mL, it is recommended to give antiviral treatment as soon as possible.
Delivery and newborn care
Mode of delivery
Regarding the relationship between mode of delivery and MTCT of HBV, existing research results are inconsistent. Although studies have shown that cesarean sections can reduce the incidence of HBV infection in babies born to women with high viral loads,21,22 a meta-analysis23 found that there was no significant relationship between the type of delivery and risk of MTCT of HBV, and cesarean sections did not reduce incidences of MTCT of HBV. Therefore, it is not recommended to choose a method of delivery according to HBV DNA level or HBeAg status. That should be determined by the obstetric indications.
Care of the newborn
Move the newborn to the resuscitation station immediately after birth and leave the environment contaminated by maternal blood. Completely remove the blood, mucus, and amniotic fluid from body’s surface. Before disposing of umbilical cord, blood, and other contaminants on the surface of the umbilical cord should be cleansed and wiped again; the umbilical cord should be cut off safely according to operating procedures.
Discontinuation of antivirals
The appropriate time to discontinue antivirals for mothers taking antiviral drugs during pregnancy depends on the purpose of antiviral treatment during pregnancy. (1) Pregnant women who take antiviral drugs to prevent MTCT of HBV should be quantitatively tested for HBsAg and HBeAg after delivery. If HBsAg and/or HBeAg levels are significantly reduced, then the antiviral treatment was effective and can be continued. If the decrease in HBsAg and/or HBeAg levels was not significant, then discontinue the drug immediately after delivery.
For the purpose of treating mother’s hepatitis B, antiviral drugs should not be discontinued after delivery. Long-term antiviral treatment is necessary for mothers with hepatitis.
Neonatal immunoprophylaxis
Neonatal immunization is the most important measure to prevent MTCT of HBV. For newborns whose mothers test HBsAg positive, timing of the first dose of hepatitis B vaccine and HBIG is very critical and the combined immunization of hepatitis B vaccine and HBIG should be completed as soon as possible within 12 hours after birth.2,24 The first dose of hepatitis B vaccine should be administered as soon as possible within the 12 h after birth for newborns whose mothers are HBsAg negative. The risk of MTCT was significantly reduced by up to 95% by timely neonatal HBV vaccination and the administration of HBIG after birth in infants born to HBV-infected mothers in China.25
Routine vaccination
Administer a recombinant yeast hepatitis B vaccine (10 µg/0.5 mL) into the anterior lateral muscle of the thigh or the deltoid muscle of the upper arm. Then inject 100 IU of HBIG into the corresponding muscle on the contralateral side. In the 0-1-6 schedule, the second and third injections of hepatitis B vaccine, with the same dose, are given at 1 month and 6 months of age.
Immunization of normal newborns
For newborns of HBsAg-positive mothers, recombinant yeast hepatitis B vaccine 10 µg/0.5 mL + HBIG 100 IU should be administered within 12 h of birth, and two additional 10µg/0.5 mL doses of recombinant yeast hepatitis B vaccine are given 1 and 6 months of age. Newborns, whose mother’s HBsAg status is unknown should be treated as HBsAg positive, receiving recombinant yeast hepatitis B vaccine 10 µg/0.5 mL + HBIG 100 IU within 12 h of birth. HBsAg should be tested as soon as possible to determine the mother’s HBsAg status, and hepatitis B vaccine should be administered to infants in a timely manner following the guidelines. For newborns of HBsAg-negative mothers, recombinant yeast hepatitis B vaccine 10 µg/0.5 mL should be given within 12 h of birth, with an additional two doses of recombinant yeast hepatitis B vaccine 10 µg/0.5 mL at 1 and 6 months of age.
Immunization of low birth weight infants weighing <2,000 g or premature infants <37 weeks of gestation should be completed as soon as possible within 12 h of birth. Recombinant yeast hepatitis B vaccine 10 µg/0.5 mL + HBIG 100 IU, and the additional three doses of hepatitis B vaccine should be given at 1, 2, and 7 months of age. For low birth weight infants or premature infants with HBsAg-unknown mothers, combined immunization of recombinant yeast hepatitis B vaccine 10 µg/0.5 mL + HBIG 100 IU should be completed as soon as possible within 12 h after birth. The mother’s HBsAg should be ascertained as soon as possible. On confirmation of mother’s HBsAg status, infants should be given the hepatitis B vaccine at the times specified in the guidelines. For low birth weight infants or premature infants of HBsAg-negative mothers, it is better to administer the first dose of recombinant yeast hepatitis B vaccine 10 µg/0.5 mL within 12 h after birth and an additional three doses of recombinant yeast hepatitis B vaccine 10 µg/0.5 mL at 1, 2, and 7 months of age. The recombinant yeast hepatitis B vaccine can also be given at discharge or at 1 month of age, and an additional two doses given at 2 and 7 months of age.
Immunization of newborns with critical conditions
Infants with very low birth weights (<1,500 g), severe birth defects, severe asphyxia, respiratory distress syndrome, etc., should be vaccinated as soon as possible after their vital signs are stable (see the immunization recommendations for low birth weight infants of <2,000 g) or premature infants of <37 weeks of gestation.
Treatment of delayed vaccination
To ensure the effectiveness of infant hepatitis B immunization, it is recommended to strictly follow the 0-1-6 immunization schedule, especially for newborns of HBsAg-positive mothers, and as much as possible to not postpone or delay the vaccinations. If the baby has special circumstances and cannot receive the second dose of hepatitis B vaccine as scheduled, the second dose of hepatitis B vaccine can be delayed; however, the maximum delay should not be more than 3 months, after which the third dose of vaccine can still be given at 6 months of age.
Breastfeeding
Breastfeeding does not increase the rate of HBV infection in infants,26,27 and mothers infected with HBV can breastfeed after delivery. It is not necessary to assay HBsAg or HBV DNA in breast milk. As breastfeeding does not increase the incidence of MTCT of HBV, newborns whose mothers are not taking antiviral drugs can breastfeed after receiving standardized combined immunization.2–8 If the mother has hepatitis activity during breastfeeding, antiviral treatment can be given according to treatment indications for chronic hepatitis B (CHB) patients in the 2019 edition of the Guidelines for Prevention and Treatment of CHB.4 Breastfeeding is appropriate for mothers treated with TDF. Antiviral drugs should be discontinued after delivery if pregnant women received antiviral drugs for the purpose of preventing MTCT of HBV and their infants can be breastfed after the combined immunizations. Pregnant women who take antiviral drugs for the purpose of treating hepatitis B should continue antiviral treatment after delivery. If TDF is taken, breastfeeding is not contraindicated because the content of TDF in the milk is infinitesimal.28
Follow-up of mothers after delivery
ALT elevation may occur in mothers with HBV infections postpartum. Studies have shown that approximately 28% of mothers with HBV infections have abnormal liver functions within 24 weeks postpartum, and a high load of HBV DNA in pregnant women is a risk factor for abnormal liver function.29 Those who continue antiviral treatment after childbirth should be followed up according to follow-up schedules for CHB patients. Liver functions and HBV DNA should be tested every 3 months within 1 year after delivery, and hepatitis B serological markers, alpha-fetoprotein, upper abdominal ultrasound, and liver transient elastography should be repeated every 6 months. For those who stop antiviral treatments after delivery and those who did not take antiviral drugs, biochemical parameters of liver functions and HBV DNA should be checked 6 to 8 weeks postpartum. If liver function is normal, liver function and HBV DNA should be checked every 3 months after delivery. If liver function is abnormal and meets the indications of antiviral treatment, antiviral treatment should be initiated.
Post-vaccination serological testing of infants
For infants of HBsAg-positive mothers, 1 to 2 months after completing the full course of hepatitis B immunization, venous blood should be drawn to detect HBV serological markers, including at least HBsAg and anti-HBs. Quantitative detection methods are recommended. If HBsAg is positive, further testing of HBV DNA levels and biochemical parameters of liver functions are required, and the biochemical and virological parameters should be retested every 6 months afterward.
Evaluating efficacy of infant’s hepatitis B immunization
Successful immunization
Post-vaccination serologic testing (PVST) should be performed 1 to 2 months after completion of hepatitis B vaccination. If HBsAg is negative and anti-HBs is positive (anti-HBs ≥10 mIU/mL), it indicates that the immunization was successful and no additional treatment is required. If anti-HBs is <100 mIU/mL, it is a low response, and if anti-HBs is ≥100 mIU/mL, it is a medium-strong response. Regardless of a low or moderate response, there is no need to revaccinate.
Nonresponse to immunization
If the serological test results show that HBsAg is negative and anti-HBs is <10 mIU/mL after the completion of the whole course of hepatitis B immunization, regardless of whether anti-HBe and anti-HBc are positive, repeated immunization is required following the 0-1-6 schedule, and the recombinant yeast hepatitis B vaccine (10 µg/0.5 mL) should be used. One month after completion of repeated vaccination, testing of HBsAg and anti-HBs is required to evaluate the immune response and HBV infection.
Immunization failure and MTCT of HBV
If 1–2 months after infant completes the full course of immunization with hepatitis B vaccine, PVST shows that HBsAg is positive, with or without HBeAg positivity, infants with MTCT of HBV should be tested for HBV DNA and liver functions, and followed up as general patients with chronic HBV infection. If hepatitis activity occurs, antiviral treatment should be carried out in the appropriate time. Interferon alpha, entecavir, or TDF can be used to treat children with hepatitis B.
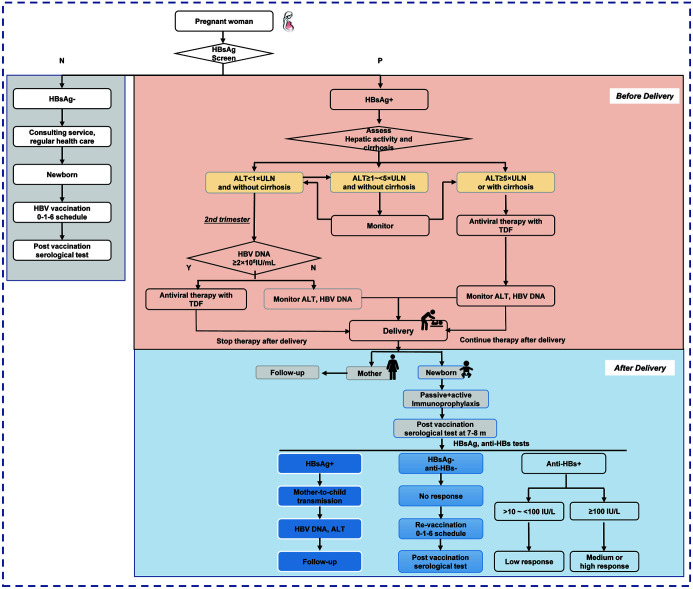
The main points of the above 10 steps are summarized as a management flowchart in Figure 1, which is convenient for reference in clinical practice.
Fig. 1. Management algorithm for preventing mother-to-child transmission of hepatitis B virus.
HBsAg, hepatitis B surface antigen; ALT, alanine aminotransferase; ULN, the upper limit of normal; TDF, tenofovir disoproxil fumarate; HBV, hepatitis B virus; anti-HBs, hepatitis B surface antibody.
Problems to be solved
Whether TAF, an anti-HBV drug, can be used to prevent MTCT of HBV
China approved TAF for the treatment of CHB in 2018. TAF is an upgraded version of TDF, with a reduced incidence of adverse events such as renal function impairment and bone mineral density reduction. Currently, TAF is recommended as the first-line anti-HBV drug in the hepatitis B guidelines of the American Association for the study Liver Diseases (AASLD), European Association for the study of the Liver (EASL), Chinese Medical Association (CMA). As TAF is safer than TDF, can it be used to block MTCT of HBV during pregnancy? Preliminary results of clinical research indicate that TAF is effective and safe in preventing MTCT of HBV and is expected to become a new option for blocking MTCT of HBV.13,14 In the future, additional rigorous randomized controlled studies are needed to provide higher-grade evidence for TAF to prevent MTCT of HBV.
MTCT prevention regimen without HBIG
Inoculation of hepatitis B vaccine and HBIG within 12 h after birth for newborns of mothers with HBV infection is a standard procedure to prevent MTCT of HBV. It has been widely used worldwide and is highly effective for preventing MTCT. However, in developing countries and regions, accessibility of HBIG is limited. In the absence of HBIG, how effective is the antiviral intervention during pregnancy combined with the infant hepatitis B vaccination? This is a new scientific question with practical significance. Multicenter clinical trials have been carried out in China to study this problem.
Safety of antiviral treatment during pregnancy
Published studies have shown that antiviral treatment during pregnancy has no adverse effects on the growth and development of the fetus,11,12,30,31 does not increase the incidence of birth defects, and has no significant effect on bone metabolism, growth, and development of the baby after birth. However, for preventing MTCT of HBV, antiviral treatment is initiated in the third trimester; therefore, current safety data are mostly obtained from situational antiviral treatment in the third trimester. There are few data on the safety of antiviral treatment before 24 weeks of pregnancy, and especially before 12 weeks. and there is a lack of comparative studies on the safety of antiviral therapy at different stages of pregnancy.
The antiretroviral pregnancy registry (APR) was established in the USA in 1989 to detect infant abnormalities associated with exposure to antiretroviral drugs during pregnancy. The APR is an international, voluntary, prospective cohort study with data from 24, 258 pregnant women who had been exposed to antiviral drugs during pregnancy.30 The HBV infection rate among pregnant women in China is about 6.3%17 and there are about 1 million pregnant women infected with HBV every year. The number of pregnant women taking antiviral drugs during pregnancy is increasing. Therefore, it is necessary to establish a registration system for pregnant women with antiviral therapy during pregnancy in China. This will provide the foundation for researching the safety of antiviral drugs during pregnancy based on big data.
Hepatitis flare during pregnancy and postpartum
Pregnant women are considered to be a unique population in terms of immunological state. During pregnancy, a series of changes in hormones and components of the immune system occur, due to tolerance of fetal semi-allogeneic antigens and fetus development.32 The immune status of pregnant women is dynamic, with enhanced immune tolerance during pregnancy and Th1 predominance postpartum.33 In the special period of pregnancy, the dynamic immunity of mothers may have influence on HBV infection. However, the effects of changes in immunological state during pregnancy on HBV infection of mothers are still unknown. On the other hand, chronic HBV infection may have effect on pregnancy outcomes. A large population-based cohort study showed that maternal prepregnancy HBV infection was independently associated with higher risk of preterm birth and early preterm birth.34 In addition, another study35 showed that HBV-infected pregnant women with higher HBV DNA level tended to have higher rates of adverse perinatal/neonatal outcomes, such as preterm birth, fetal growth restriction, oligohydramnios, etc. Based on the finding, it is interesting to know whether antiviral therapy could reduce adverse perinatal/neonatal outcomes by decreasing HBV DNA level in pregnant women with HBV infection. Published consensus guidelines related to MTCT prevention recommend that mothers who take antiviral drugs to prevent the MTCT of HBV can stop antiviral treatment after delivery. However, it has been found in clinical practice that the HBV DNA of some mothers who started antiviral therapy in their third trimesters was lower than the detection limit after postpartum reexamination, and HBsAg and/or HBeAg levels were significantly decreased, suggesting that they may not be immunotolerant at that stage of HBV infection. Therefore, continued antiviral treatment is expected to achieve a favorable outcome. At present, there is a lack of research on long-term follow-up after delivery and withdrawal of HBV-infected mothers. Establishing a follow-up cohort of mothers with postpartum drug withdrawals, and observing changes in virological, serological, and biochemical parameters can provide a basis for optimizing postpartum management.
The status quo of MTCT of HBV
Neonatal immunization is the cornerstone for interrupting MTCT of HBV, reducing up to 95% of perinatal transmissions in China.25 National seroprevalence surveys in China indicated that the prevalence of HBsAg among children under 5 years of age declined from 9.7% in 1992 to 0.32% in 2014.36 Eliminating MTCT of HBV is one of the core tasks to achieve elimination of viral hepatitis as public threat by 2030. The World Health Organization (WHO) suggested 2% as the target for eliminating MTCT of HBV. However, recent studies on MTCT of HBV in China showed that a lower MTCT incidence can be expected. A prospective, multicenter, large sample size study showed that after a standard immunization schedule, only 13 of the 955 infants (1.4%) were positive for HBsAg at 12 months.37 In addition, in Shield Project stage 1,15 a real-world study was carried out to investigate MTCT of HBV after comprehensive management including immunoprophylaxis of infants and antiviral prophylaxis of mothers was adopted. We observed that the overall rate of MTCT was 0.9% (8 of 924 infants enrolled). In Shield Project stage 2 and 3(data not published), with more HBV-infected mothers taking antiviral drugs during pregnancy, the rate of MTCT was below 0.3%, much lower than WHO target for elimination of MTCT. Therefore, based on updated evidence, the 2030 WHO target for elimination of MTCT might be adjusted to below 0.3%, in the context of comprehensive preventive strategy for MTCT has been implemented in China and worldwide.
Abbreviations
- ALT
alanine aminotransferase
- CHB
chronic hepatitis B
- HBsAg
Hepatitis B surface antigen
- HBeAg
hepatitis B e antigen
- HBIG
hepatitis B immunoglobulin
- HBV
hepatitis B virus
- MTCT
mother-to-child transmission
- PVST
post-vaccination serologic testing
- TAF
tenofovir alafenamide fumarate
- TDF
tenofovir disoproxil fumarate
- ULN
upper limit of normal
- WHO
World Health Organization
References
- 1. WHO global health sector strategy on viral hepatitis 2016-2021: towards ending viral hepatitis. Geneva: World Health Organization; 2016 [EB/OL] Available from: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1.
- 2.Chinese Foundation for Hepatitis Prevention and Control, Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association [Management algorithm for interrupting mother-to-child transmission of hepatitis B] Zhonghua Gan Zang Bing Za Zhi. 2017;25(4):254–256. doi: 10.3760/cma.j.issn.1007-3418.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Hou J, Cui F, Ding Y, Dou X, Duan Z, Han G, et al. Management Algorithm for Interrupting Mother-to-Child Transmission of Hepatitis B Virus. Clin Gastroenterol Hepatol. 2019;17(10):1929–1936.e1. doi: 10.1016/j.cgh.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association Guidelines for the prevention and treatment of chronic hepatitis B (version 2019) J Clin Hepatol. 2019;35(12):2648–2669. doi: 10.3969/j.issn.1001-5256.2019.12.007. [DOI] [Google Scholar]
- 5.Obstetrics Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association, Chinese Society of Perinatal Medicine, Chinese Medical Association 2020 clinical guidelines on prevention of mother-to-child transmission of hepatitis B virus. J Clin Hepatol. 2020;36(7):1474–1481. doi: 10.3760/cma.j.cn112141-20200213-00101. [DOI] [Google Scholar]
- 6. World Health Organization. Prevention of mother-to-child transmission of hepatitis B virus: guidelines on antiviral prophylaxis in pregnancy. Available from: https://www.who.int/publications/i/item/978-92-4-000270-8. [PubMed]
- 7.Chinese Society of Hepatology, Chinese Medical Association Consensus on clinical management of hepatitis B virus- infected women of childbearing age. Zhonghua Gan Zang Bing Za Zhi. 2018;26(3):204–208. doi: 10.3760/cma.j.issn.1007-3418.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Chinese Society of Hepatology, Chinese Medical Association, China Grade Center 2019 Chinese practice guideline for prevention and treatment of hepatitis B virus mother-to-child transmission. Chin J Infect Dis. 2019;37(7):388–396. [Google Scholar]
- 9.Zhang H, Pan CQ, Pang Q, Tian R, Yan M, Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014 doi: 10.1002/hep.27034. [DOI] [PubMed] [Google Scholar]
- 10.Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62(2):375–386. doi: 10.1002/hep.27837. [DOI] [PubMed] [Google Scholar]
- 11.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N Engl J Med. 2016;374(24):2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 12.Jourdain G, Ngo-Giang-Huong N, Harrison L, Decker L, Khamduang W, Tierney C, et al. Tenofovir versus Placebo to Prevent Perinatal Transmission of Hepatitis B. N Engl J Med. 2018;378(10):911–923. doi: 10.1056/NEJMoa1708131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng QL, Yu ZJ, Ji F, Li GM, Zhang GF, Xu JH, et al. Tenofovir Alafenamide to Prevent Perinatal Hepatitis B Transmission: A Multicenter, Prospective, Observational Study. Clin Infect Dis. 2021;73(9):e3324–e3332. doi: 10.1093/cid/ciaa1939. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y, Cao L, Zhu L, Huang Y, Lin C, Wang Y, et al. Efficacy and safety of tenofovir alafenamide fumarate for preventing mother-to-child transmission of hepatitis B virus: a national cohort study. Aliment Pharmacol Ther. 2020;52(8):1377–1386. doi: 10.1111/apt.16043. [DOI] [PubMed] [Google Scholar]
- 15.Yin X, Han G, Zhang H, Wang M, Zhang W, Gao Y, et al. A Real-world Prospective Study of Mother-to-child Transmission of HBV in China Using a Mobile Health Application (Shield 01) J Clin Transl Hepatol. 2020;8(1):1–8. doi: 10.14218/JCTH.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polaris Observatory Collaborators Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 17.Cui F, Woodring J, Chan P, Xu F. Considerations of antiviral treatment to interrupt mother-to-child transmission of hepatitis B virus in China. Int J Epidemiol. 2018;47(5):1529–1537. doi: 10.1093/ije/dyy077. [DOI] [PubMed] [Google Scholar]
- 18.Wang AL, Qiao YP, Wang LH, Fang LW, Wang F, Jin X, et al. Integrated prevention of mother-to-child transmission for human immunodeficiency virus, syphilis and hepatitis B virus in China. Bull World Health Organ. 2015;93(1):52–56. doi: 10.2471/BLT.14.139626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver; Clinical Practice Guideline Panel; Chair; EASL Governing Board representative; Panel members EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75(3):659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68(4):672–681. doi: 10.1016/j.jhep.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 21.Pan CQ, Zou HB, Chen Y, Zhang X, Zhang H, Li J, et al. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen-positive women to their infants. Clin Gastroenterol Hepatol. 2013;11(10):1349–1355. doi: 10.1016/j.cgh.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Peng S, Wan Z, Liu T, Li X, Du Y. Cesarean section reduces the risk of early mother-to-child transmission of hepatitis B virus. Dig Liver Dis. 2018;50(10):1076–1080. doi: 10.1016/j.dld.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Chen HL, Cai JY, Song YP, Zha ML, Qin G. Vaginal delivery and HBV mother to child transmission risk after immunoprophylaxis: A systematic review and a meta-analysis. Midwifery. 2019;74:116–125. doi: 10.1016/j.midw.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67(1):1–31. doi: 10.15585/mmwr.rr6701a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Zhang G, Zheng H, Miao N, Shen L, Wang F, et al. Post-vaccination serologic testing of infants born to hepatitis B surface antigen positive mothers in 4 provinces of China. Vaccine. 2017;35(33):4229–4235. doi: 10.1016/j.vaccine.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Chen J, Wen J, Xu C, Zhang S, Zhou YH, et al. Breastfeeding is not a risk factor for mother-to-child transmission of hepatitis B virus. PLoS One. 2013;8(1):e55303. doi: 10.1371/journal.pone.0055303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Z, Yang Y, Wang H, Ma L, Schreiber A, Li X, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165(9):837–846. doi: 10.1001/archpediatrics.2011.72. [DOI] [PubMed] [Google Scholar]
- 28.Ehrhardt S, Xie C, Guo N, Nelson K, Thio CL. Breastfeeding while taking lamivudine or tenofovir disoproxil fumarate: a review of the evidence. Clin Infect Dis. 2015;60(2):275–278. doi: 10.1093/cid/ciu798. [DOI] [PubMed] [Google Scholar]
- 29.Yi W, Pan CQ, Li MH, Wan G, Lv YW, Liu M, et al. The characteristics and predictors of postpartum hepatitis flares in women with chronic hepatitis B. Am J Gastroenterol. 2018;113(5):686–693. doi: 10.1038/s41395-018-0010-2. [DOI] [PubMed] [Google Scholar]
- 30. Antiretroviral Pregnancy Registry Interim Report for 1 January 1989-31 January 2020. Available from: http://www.apregistry.com/forms/exec-summary.pdf.
- 31.Wen WH, Chen HL, Shih TT, Wu JF, Ni YH, Lee CN, et al. Long-term growth and bone development in children of HBV-infected mothers with and without fetal exposure to tenofovir disoproxil fumarate. J Hepatol. 2020;72(6):1082–1087. doi: 10.1016/j.jhep.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Sifnaios E, Mastorakos G, Psarra K, Panagopoulos ND, Panoulis K, Vitoratos N, et al. Gestational Diabetes and T-cell (Th1/Th2/Th17/Treg) Immune Profile. In Vivo. 2019;33(1):31–40. doi: 10.21873/invivo.11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apps R, Kotliarov Y, Cheung F, Han KL, Chen J, Biancotto A, et al. Multimodal immune phenotyping of maternal peripheral blood in normal human pregnancy. JCI Insight. 2020;5(7):134838. doi: 10.1172/jci.insight.134838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Zhang S, Liu M, Wang Q, Shen H, Zhang Y. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: a population-based cohort study. Lancet Glob Health. 2017;5(6):e624–e632. doi: 10.1016/S2214-109X(17)30142-0. [DOI] [PubMed] [Google Scholar]
- 35.Unal C, Tanacan A, Ziyadova G, Fadiloglu E, Beksac MS. Effect of viral load on pregnancy outcomes in chronic hepatitis B infection. J Obstet Gynaecol Res. 2019;45(9):1837–1842. doi: 10.1111/jog.14065. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Liang XF, Wang FZ, Yan L, Li RC, Li YP, et al. Hepatitis B vaccine alone may be enough for preventing hepatitis B virus transmission in neonates of HBsAg (+)/HBeAg (-) mothers. Vaccine. 2017;35(1):40–45. doi: 10.1016/j.vaccine.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Zou H, Chen Y, Zhang H, Tian R, Meng J, et al. The effects of increased dose of hepatitis B vaccine on mother-to-child transmission and immune response for infants born to mothers with chronic hepatitis B infection: a prospective, multicenter, large-sample cohort study. BMC Med. 2021;19(1):148. doi: 10.1186/s12916-021-02025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]