Abstract
Background and Aims
Acute-on-chronic liver failure (ACLF) tends to progress rapidly with high short-term mortality. We aimed to create a widely applicable, simple prognostic (WASP) score for ACLF patients.
Methods
A retrospective cohort of ACLF cases recruited from three centers in China were divided into training and validation sets to develop the new score. A prospective longitudinal cohort was recruited for further validation.
Results
A total of 541 cases were included in the training set, and seven independent ACLF prognostic factors were screened to construct a new quantitative WASP-ACLF table. In the validation set of 671 cases, WASP-ACLF showed better predictive ability for 28-day and 90-day mortality than the currently used prognostic scores at baseline, day 3, week 1, and week 2. The predictive efficacy and clinical validity of the model improved over time. Patients were assigned to low-, intermediate-, and high-risk groups by their WASP-ACLF scores. Compared with the other two groups, intermediate-risk patients had a more uncertain prognosis, with a 90-day mortality of 44.4–50.6%. Sequential assessments at weeks 1 and 2 found the 90-day mortality of intermediate-risk groups was <20% for patients with a ≥2 point decrease in WASP-ACLF and was up to 56% for patients with a ≥2 points increase. Similar results were observed in prospective data.
Conclusions
The new ACLF prognostic score was simple, widely applicable, and had good predictive efficacy. Continuous assessments and trend of change in WASP-ACLF need to be considered, especially for intermediate-risk patients.
Keywords: Acute-on-chronic liver failure, Scoring model, Trends, Prognosis
Graphical abstract
Introduction
Acute-on-chronic liver failure (ACLF) is a complex clinical syndrome characterized by acute deterioration of liver function and multiple organ failure. It is typically triggered by a precipitating event in the backdrop of chronic liver disease. ACLF has a rapid onset and progression, and is associated with high short-term 30–50% 28 day and 50–80% 90 day mortality.1,2 Liver transplantation is the main treatment for ACLF and can significantly improve the prognosis.3 However, given the shortage of liver sources, the risks associated with liver transplantation, and the high medical costs, risk stratification of patients with ACLF is a key imperative. Many evaluation methods have been developed to assess the risk of ACLF progression.4 This approach can facilitate timely individualized intervention and support rational allocation of medical resources.
Owing to the complex nature of liver failure, accurate assessment of ACLF progression and the extent of hepatic impairment is inherently challenging. Several comprehensive multifactor models have been developed to predict ACLF progression. The Child-Turcotte-Pugh score (CTP)5 and the model for end-stage liver disease (MELD)6 are commonly used prognostic methods for liver disease. However, these models have significant limitations for predicting mortality in patients with ACLF. The Asian Pacific Association for the Study of the Liver proposed a new prognostic scoring model for ACLF that includes hepatic encephalopathy (HE), total bilirubin (TB), international normalized ratio (INR), creatinine (Cr), and lactate.7 However, in some countries, lactate is not routinely assessed in patients with ACLF, limiting its application. The chronic liver failure-sequential organ failure assessment score (CLIF-SOFA)8 and the chronic liver failure consortium organ function (CLIF-OF)9 proposed by the European Association for the Study of Liver Diseases have high prognostic evaluation value. However, data for calculation of some parameters rely on the assessment of the respiratory and circulatory systems, which are difficult to obtain from most general wards. Therefore, a widely applicable and simple ACLF prognostic score is required for wider clinical application.
In this study, we identified independent prognostic factors for ACLF patients, and developed a new quantitative nomogram for prognostic prediction based on each factor’s regression coefficients and cutoff values. In addition, we assessed the predictive efficacy of our prognostic model by comparing it with other known prognostic scores.
Methods
Study design, participants, and data collection
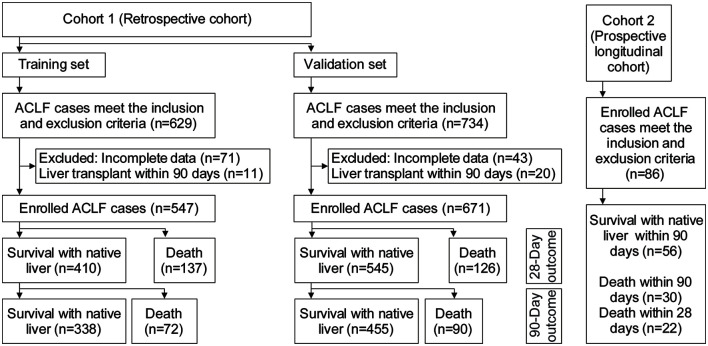
Two cohorts of ACLF patients were enrolled in two separate investigations (Fig. 1). In the first investigation, a retrospective cohort was recruited at multiple centers in China. This retrospective cohort comprised of a training set used for model construction and a validation set. Cases in the training set were sourced from among patients hospitalized in the Tianjin Third Central Hospital between January 1, 2008 and December 31, 2014. Cases in the validation set were sourced from among patients hospitalized in the Tianjin Third Central Hospital, Beijing You’an Hospital, and The Fifth Medical Center of Chinese PLA General Hospital between January 1, 2015 and June 30, 2019. In the second investigation, a prospective longitudinal cohort of 86 patients hospitalized in the Tianjin Third Central Hospital was enrolled from January 1, 2021 to June 30, 2021. The inclusion criteria were occurrence of liver failure manifesting as jaundice with a TB ≥5 mg/dL) and coagulation dysfunction with an INR ≥1.5, or prothrombin activity (PTA) <40%) within 4 weeks in the backdrop of chronic liver disease, including chronic hepatitis, compensated cirrhosis, and decompensated cirrhosis. The exclusion criteria were presence of concomitant human immunodeficiency virus infection, severe extrahepatic chronic disease, liver cancer, or other malignancies, or pregnancy. Clinical data were retrieved from manual and electronic medical records. All study procedures complied with the principles of the Declaration of Helsinki. The study was approved by the Ethics Committees of all the participating institutions.
Fig. 1. Flow chart of ACLF cases in the respective and prospective cohorts.
ACLF, acute-on-chronic liver failure.
Scoring models
The CTP score5 was calculated using TB, serum albumin (ALB), prothrombin time (PT), ascites, and West Haven stage of HE. The MELD6 and MELD-Na10 scores were calculated as MELD = 3.78 × ln (TB mg/dL) + 11.2 × ln (INR) + 9.57 × ln (Cr mg/dL) and MELD-Na = MELD + 1.59 × (135-Na mmol/L). The CLIF-SOFA score8 and CLIF-OF score9 were determined by the sum of the organ failure severity grades. Details can be found in the cited references.
Statistical analysis
Continuous data were reported as means ± standard deviation and between-group differences were assessed using the t-test. Categorical variables were reported as frequency (%), and between-group differences were assessed using chi-squared test or Fisher’s exact tests. Univariate and multivariate Cox regression analyses were used to identify independent prognostic predictors of ACLF. A binary logistic regression equation was constructed according to the forward likelihood ratio test method. The area under the receiver operating characteristic (ROC) curve (AUC) for the various prognostic scores were compared using the z-test with the Delong method. Decision curve analysis (DCA) was used to determine the clinical validity of the model. Two-tailed p-values <0.05 were considered statistically significant for all tests. Statistical analysis and mapping were performed with SPSS Statistics 22.0 (IBM Corp., Armonk, New York, USA), and R (version 3.6.3),11 with rms and survival packages (Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org).
Results
Clinical characteristics of patients in the retrospective cohort
For the training set, 547 of the 629 eligible ACLF cases were enrolled after excluding 71 cases with incomplete data and 11 who received liver transplants within 90 days. Of the 547 cases, 209 died within 90 days and 338 cases survived. For the validation set, 671 of the 734 eligible cases were enrolled after excluding 43 with incomplete data and 20 with liver transplantation within 90 days. In the validation set, 356 cases were from the Tianjin Third Central Hospital, 185 cases from the Fifth Medical Center of Chinese PLA General Hospital, and 130 cases from Beijing You’an Hospital; 216 cases died within 90 days, and 455 cases survived.
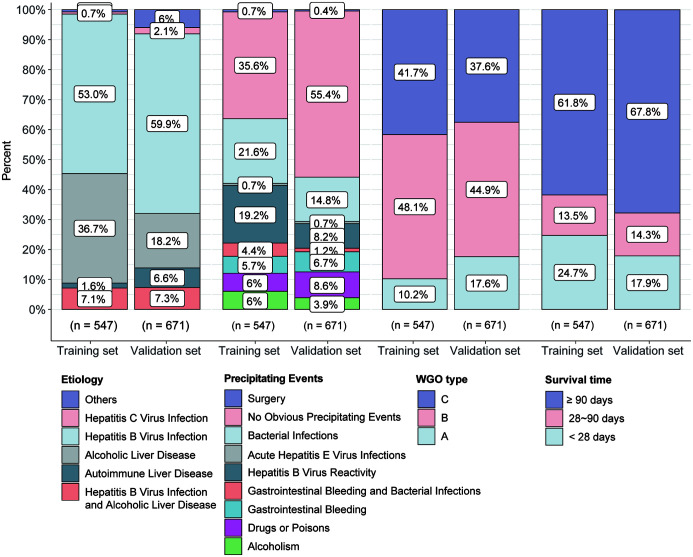
There were no significant differences between the training and validation sets with respect to etiology, precipitating events, or short-term mortality (Fig. 2). The most common etiologies were hepatitis B virus (HBV) infection (53.0% in the training set, 59.9% in the validation set), alcoholic liver disease (ALD; 36.7% in the training set, 18.2% in the validation set), and HBV infection combined with ALD (7.1% in the training set, 7.3% in the validation set). The largest proportion of cases in both the training set (35.6%) and validation set (55.4%) had no obvious precipitating event. Other precipitating events were bacterial infection (21.6% in the training set and 14.8% in the validation set) and HBV reactivation (19.2% in the training set and 8.2% in the validation set). The cases were classified by the ACLF criteria proposed by the World Gastroenterology Organization (WGO)12 as type A/B/C. WGO types in the training set were 10.2% for type A, 48.1% for type B, and 41.7% for type C. The 90 day mortality was 38.2%. The WGO types in the validation set were 17.6% for type A, 44.9% for type B, and 37.6% for type C. The 90 day mortality was 32.2% (Fig. 2).
Fig. 2. Etiology, precipitating event, WGO type, and survival rate of ACLF cases in the training and validation sets of the retrospective cohort.
WGO, World Gastroenterology Organization; ACLF, acute-on-chronic liver failure.
Prognostic factor analysis
The training set of 547 cases included 338 survivors and 209 nonsurvivors. The ACLF prognostic factors identified by univariate Cox regression analysis (Table 1) were age, WGO type, HE, gastrointestinal bleeding (GIB), ascites, coinfection, hemoglobin (HB), hematocrit (HCT), white blood cell (WBC) count, neutrophil ratio (N), C-reactive protein (CRP), platelets (PLTs), total cholesterol (TC), ALB, TB, Cr, blood urea nitrogen (BUN), serum potassium (K), serum sodium (Na), PT, PTA, INR, and alpha-fetoprotein (AFP). In addition, nonsurvivors had significant higher levels of CTP, MELD, MELD-Na, CLIF-SOFA, and CLIF-OF scores than survivors. All of the scores were closely related to the prognosis of ACLF (Table 1). On multivariate Cox analysis, age, WGO type, HE, and levels of WBC, TB, Cr, and INR were identified as independent prognostic factors of ACLF (Table 1). Logistic regression analysis was performed to assess the standard regression coefficients and the cutoff values of each independent prognostic factor. The factors with high influence weights were TB (0.441, 18.1), Cr (0.519, 1.3), INR (0.655, 2.5), and HE (0.535, 1). Factors with low influence weights (Supplementary Table 1) were age (0.169, 57), WGO type (0.216, 3), and WBC (0.186, 10.0).
Table 1. Prognostic factors in ACLF cases.
| Survivors (n=338) | Nonsurvivors (n=209) | Univariate Cox regression |
Multivariate Cox regression |
||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||||
| Age, years | 49.314±10.814 | 53.034±10.725 | 1.027 (1.014–1.041) | <0.001 | 1.034 (1.027–1.087) | <0.001 | |
| Male | 277 (82.0) | 174 (83.3) | 1.091 (0.759–1.568) | 0.64 | |||
| Etiology | HBV | 178 (52.7) | 112 (53.6) | 0.991 (0.755–1.301) | 0.949 | ||
| Alcoholic | 115 (34.0) | 86 (41.1) | 1.274 (0.967–1.679) | 0.086 | |||
| HBV and Alcoholic | 30 (8.8) | 9 (4.3) | 0.534 (0.274–1.042) | 0.066 | |||
| WGO type | A | 50 (14.8) | 6 (2.9) | 1.838 (1.463–2.309) | <0.001 | 1.383 (1.057–1.810) | 0.018 |
| B | 172 (50.9) | 91 (43.5) | |||||
| C | 116 (34.3) | 112 (53.6) | |||||
| HE, West Haven Stage | 0 | 277 (82.0) | 70 (33.5) | 1.77 (1.624–1.928) | <0.001 | 1.422 (1.288–1.571) | <0.001 |
| 1 | 6 (1.8) | 7 (3.3) | |||||
| 2 | 26 (7.7) | 20 (9.6) | |||||
| 3 | 22 (6.5) | 67 (32.1) | |||||
| 4 | 7 (2.1) | 45 (21.5) | |||||
| GIB | 37 (10.9) | 64 (30.6) | 2.551 (1.899–3.427) | <0.001 | – | – | |
| Ascites | 246 (72.3) | 180 (86.1) | 1.650 (1.154–2.361) | 0.006 | – | – | |
| Coinfection | 217 (64.2) | 151 (72.2) | 1.364 (1.004–1.853) | 0.047 | – | – | |
| HB, g/L | 114.072±27.271 | 109.402±27.234 | 0.995 (0.99–1) | 0.035 | – | – | |
| HCT | 37.020±23.783 | 34.068±11.896 | 0.981 (0.964–0.997) | 0.022 | – | – | |
| RDW | 16.186±5.139 | 16.794±3.088 | 1.015 (0.996–1.033) | 0.121 | |||
| WBC, ×109/L | 10.229±5.951 | 15.461±8.942 | 1.073 (1.057–1.088) | <0.001 | 1.057 (1.028–1.088) | <0.001 | |
| N, ×106/L | 72.230±11.195 | 78.266±11.830 | 1.047 (1.032–1.062) | <0.001 | – | – | |
| CRP, mg/dL | 27.305±25.184 | 27.932±24.809 | 1.015 (1.011–1.02) | <0.001 | – | – | |
| PLT, ×109/L | 90.027±52.565 | 79.395±53.585 | 0.997 (0.994–1) | 0.027 | – | – | |
| ALT, U/L | 357.154±594.490 | 371.101±764.236 | 1 (1–1) | 0.652 | |||
| AST, U/L | 318.502±496.134 | 399.364±782.904 | 1 (1–1) | 0.058 | |||
| ALP, U/L | 133.225±56.531 | 140.981±80.287 | 1.002 (1–1.004) | 0.093 | |||
| GGT, U/L | 150.412±172.012 | 138.412±167.238 | 1 (0.999–1.001) | 0.51 | |||
| CHE, IU/L | 2,780.144±1,385.580 | 2,732.775±1,558.847 | 1 (1–1) | 0.854 | |||
| TC, mmol/L | 2.776±0.833 | 2.631±0.867 | 0.834 (0.707–0.984) | 0.032 | – | – | |
| ALB, g/L | 28.502±6.086 | 27.443±5.252 | 0.964 (0.937–0.991) | 0.009 | – | – | |
| PA, g/L | 4.878±3.508 | 4.302±3.158 | 0.959 (0.919–1.001) | 0.053 | |||
| GLO, g/L | 33.645±8.058 | 32.439±9.390 | 0.984 (0.967–1) | 0.051 | |||
| TG, mmol/L | 1.350±1.275 | 1.190±1.122 | 0.902 (0.774–1.053) | 0.191 | |||
| TB, mg/dL | 15.029±7.275 | 20.881±9.782 | 1.055 (1.040–1.07) | <0.001 | 1.028 (1.011–1.046) | 0.001 | |
| Cr, mg/dL | 1.021±0.782 | 2.304±1.992 | 1.285 (1.227–1.331) | <0.001 | 1.187 (1.066–1.322) | 0.001 | |
| BUN, mmol/L | 6.968±5.315 | 11.318±8.007 | 1.066 (1.05–1.083) | <0.001 | – | – | |
| K, mmol/L | 3.979±0.719 | 4.083±0.977 | 1.542 (1.315–1.808) | <0.001 | – | – | |
| Na, mmol/L | 133.921±6.331 | 131.371±6.833 | 0.959 (0.942–0.975) | <0.001 | – | – | |
| PT, s | 23.883±5.115 | 28.294±9.066 | 1.063 (1.049–1.078) | <0.001 | – | – | |
| PTA, s | 38.861±9.595 | 32.962±11.089 | 0.952 (0.938–0.965) | <0.001 | – | – | |
| INR | 2.471±0.959 | 4.002±2.186 | 1.278 (1.227–1.331) | <0.001 | 1.113 (1.028–1.205) | 0.001 | |
| Abnormal AFP | 164 (48.5) | 92 (44.0) | 0.841 (0.640–1.105) | 0.213 | |||
| CTP | 11.029±1.590 | 11.588±1.323 | 1.236 (1.124–1.359) | <0.001 | |||
| MELD | 6.681±8.529 | 11.038±12.590 | 1.017 (1.007–1.027) | <0.001 | |||
| MELD-Na | 25.707±9.843 | 41.014±13.256 | 1.063 (1.054–1.071) | <0.001 | |||
| CLIF-SOFA | 8.506±2.391 | 13.330±3.521 | 1.266 (1.23–1.302) | <0.001 | |||
| CLIF-OF | 9.459±1.738 | 12.684±2.20 | 1.443 (1.378–1.511) | <0.001 | |||
Data are number (%) or mean±standard deviation, as indicated. Assignment of indexes in Cox regression analysis was: WGO type A, B, and C of ACLF were 1, 2, and 3 respectively; normal and abnormal AFP were 0 and 1; positive complications including coinfection, gastrointestinal bleeding, ascites were 1, and 0 for no complications; hepatic encephalopathy was 0, 1, 2, 3, and 4 by the West Haven stages 0, I, II, III, and IV, respectively. ACLF, acute-on-chronic liver failure; AFP, alpha-fetoprotein; ALB, serum albumin; ALD, alcoholic liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CHE, cholinesterase; CLIF-OF, chronic liver failure consortium organ function; CLIF-SOFA, chronic liver failure-sequential organ failure assessment; Cr, creatinine; CRP, C-reactive protein; CTP, Child-Turcotte-Pugh; GGT, γ-glutamyl transpeptidase; GIB, gastrointestinal bleeding; GLO, globulins; HB, hemoglobin; HBV, hepatitis B virus; HCT, hematocrit; HE, hepatic encephalopathy; INR, international normalized ratio; K, serum potassium; MELD, model for end-stage liver disease; N, neutrophil ratio; Na, serum sodium; PA, prealbumin; PLT, platelet; PT, prothrombin time; PTA, prothrombin activity; RDW, red cell volume distribution width; TB, total bilirubin; TC, total cholesterol; TG, triglyceride; WBC, white blood cell; WGO, World Gastroenterology Organization.
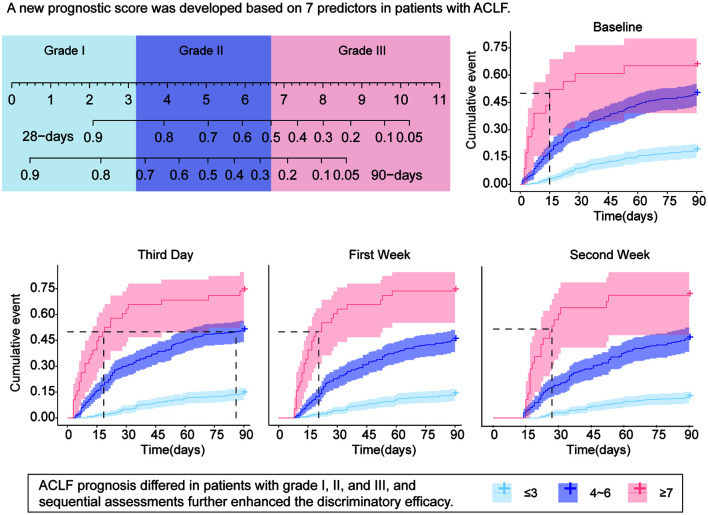
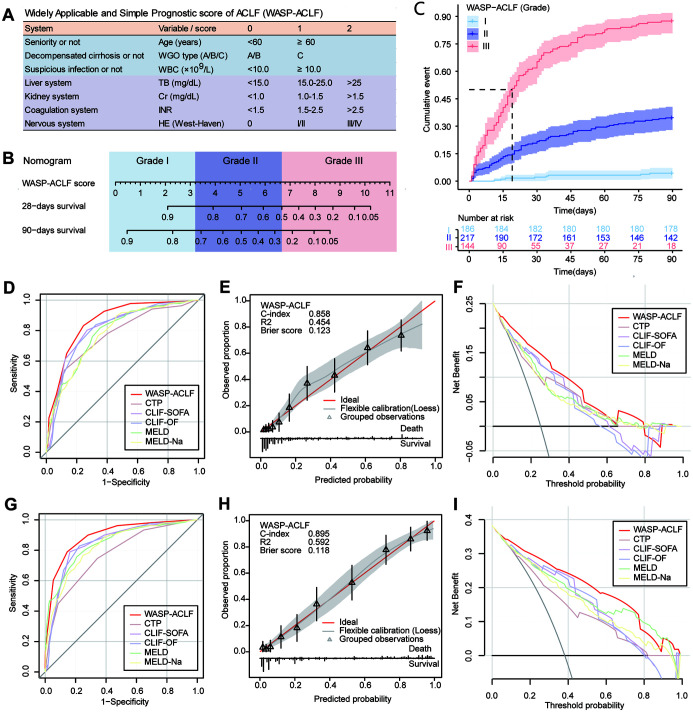
A widely applicable and simple prognostic score of ACLF (WASP-ACLF) was constructed by the standard coefficients and the cutoff values of the above parameters. A score of 1 each was assigned to advanced age (≥60 years), type-C ACLF, and suspected coinfection (WBC ≥10.0 × 109/L); and four factors representing the liver, kidney, coagulation, and the nervous system, TB, Cr, INR, and HE, were assigned scores of 0, 1, and 2 depending on the different level (Fig. 3A).
Fig. 3. (A) Parameter composition and quantitative assignment table of WASP-ACLF. (B) Nomogram showing the 28-and 90-day mortality corresponding to the WASP-ACLF scores, as well as the classification of patients into grade I (0–3), grade II (4–6), and grade III (7–11). (C) Cumulative mortality rate from 0 to 90 days for grades I, II, and III patients in the training set. (D–F) ROC curves, calibration curve, and DCA for the 28-day prognosis of patients in the training set. (G–I) ROC curves, calibration curve and DCA curves for the 90-day prognosis of patients in the training set.
ACLF, acute-on-chronic liver failure; CLIF-OF, chronic liver failure consortium organ function; CLIF-SOFA, chronic liver failure-sequential organ failure assessment; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; ROC, receiver operating characteristic; DCA, decision curve analysis; Cr, creatinine; HE, hepatic encephalopathy; INR, international normalized ratio; TB, total bilirubin; WBC, white blood cell; WGO, World Gastroenterology Organization; WASP-ACLF, widely applicable and simple prognostic score of ACLF.
Predictive performance of WASP-ACLF in the training set
The WASP-ACLF scores of patients ranged from 0 to 11; the corresponding 28-day and 90-day mortality rates are shown in the nomogram. Based on the WASP-ACLF score and cutoff values, ACLF cases were classified into three grades, grade I (0–3) was the low-risk group, grade II (4–6) was the intermediate-risk group, and grade III (7–11) was the high-risk group (Fig. 3B). The 28-day and 90-day mortalities for grade III cases in the training set were 61.8% and 87.5%, respectively, which were much higher than those for grade I (1.6% and 4.3%,) and grade II (20.7% and 34.6%) cases (p<0.001, Fig. 3C). On ROC curve analysis (Fig. 3D, Supplementary Table 2), the AUC for the WASP-ACLF 28-day prognosis (0.858) was significantly greater than that of other scores, such as CTP (0.770), CLIF-SOFA (0.816), CLIF-OF (0.807), MELD (0.787), and MELD-Na (0.779). The calibration curve showed good consistency between the WASP-ACLF and 28-day prognosis, with a Brier score of 0.123 (Fig. 3E), and DCA curve analysis showed the best clinical validity of WASP-ACLF compared with other scores (Fig. 3F). For 90-day prognosis (Fig. 3G, Supplementary Table 2), the AUC of WASP-ACLF (0.895) was also significantly higher than that of other scores, i.e., CTP (0.773), CLIF-SOFA (0.849), CLIF-OF (0.847), MELD (0.847), and MELD-Na (0.832). Similarly, calibration curves showed good consistency between the WASP-ACLF and 90-day prognosis, with a Brier score of 0.118 (Fig. 3H), and DCA curve analysis showed the best clinical validity of WASP-ACLF (Fig. 3I).
External validation of WASP-ACLF
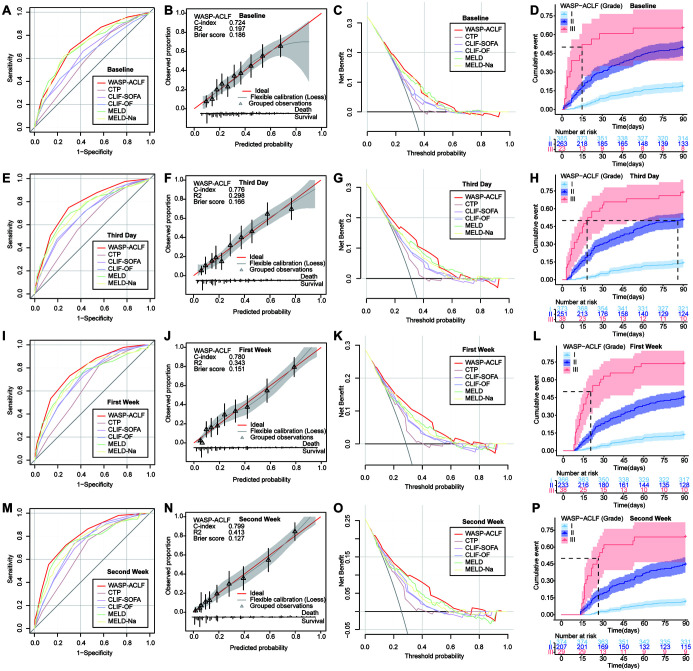
In the validation set, the predictive efficacy of WASP-ACLF, based on data obtained at baseline, the third day, the first week, and the second week after admission, was compared with that of other prognostic scores. On ROC curve analysis of 90-day prognosis, the AUC of WASP-ACLF at the admission baseline was 0.724, and the Brier calibration curve consistency score was 0.186. The AUC value and the clinical validity of WASP-ACLF were significantly greater than those of CTP, CLIF-SOFA, and CLIF-OF, but there were no significant differences compared with MELD and MELD-Na (Fig. 4A–C, Table 2). The 90-day mortality rates of grade I (18.4%), II (49.4%), and III (65.2%) patients were significantly different (p<0.001; Fig. 4D and Supplementary Table 3). On performing ROC curve analysis of 90-day prognosis on the third day, the AUC value of the WASP-ACLF score was 0.776 and the Brier consistency score was 0.166. The AUC value and the clinical validity of WASP-ACLF were significantly greater than those of other scores (Fig. 4E–G, Table 2), with significant differences in the 90-day mortality rates of grade I (13.9%), II (50.6%), and III (73.7%) patients (p<0.001, Fig. 4H and Supplementary Table 3). On performing ROC curve analysis of 90-day prognosis at the first week, the AUC value of WASP-ACLF was 0.780 and the Brier consistency score was 0.151. The AUC value and the clinical validity of WASP-ACLF were significantly higher than those of other scores (Fig. 4I–K, and Table 2), with significant differences in the 90-day mortality rates of grade I (13.4%), II (45.1%), and grade III (73.7%) patients (p<0.001; Fig. 4L and Supplementary Table 3). On performing ROC curve analysis of 90-day prognosis at the second week, the AUC value of WASP-ACLF was 0.799, and the Brier consistency score was 0.127. The AUC value and the clinical validity of WASP-ACLF were significantly higher than those of other scores (Fig. 4M–O, Table 2), with significantly different 90-day mortalities of grade I (11.5%), II (44.4%), and III (69.0%) patients (p<0.001, Fig. 4P, Supplementary Table 3). Similarly, the AUC values and clinical validity of WASP-ACLF for 28-day prognosis were significantly higher than those of other prognostic scores (Supplementary Fig. 1 and Supplementary Table 3 and 4). In addition, the results showed a gradual increase of the AUC values and a gradual decrease of the Brier scores of the WASP-ACLF over time, suggesting that sequential assessment may improve the predictive accuracy and consistency of the model.
Fig. 4. ROC curves, calibration curve and DCA curves for 90-day prognosis, and cumulative 0-90 days mortality of grade I, II, and III patients in the validation set at (A–D) baseline, (E–H) the third day after admission, (I–L) the first week, and (M–P) the second week.
ACLF, acute-on-chronic liver failure; CLIF-OF, chronic liver failure consortium organ function; CLIF-SOFA, chronic liver failure-sequential organ failure assessment; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; WASP-ACLF, widely applicable and simple prognostic score of ACLF; ROC, receiver operating characteristic; DCA, decision curve analysis.
Table 2. Predictive efficacy of each score for 90-day prognosis by receiver operating characteristic curve analysis in the validation set.
| Time (cases) | Variable | AUC (95% CI) | St | Cutoff value | Sensitivity | Specificity | z statistic (p-value)* |
|---|---|---|---|---|---|---|---|
| Baseline (n=671) | CTP | 0.600 (0.561–0.637) | 0.022 | 11 | 0.824 | 0.341 | 5.435 (p<0.001) |
| CLIF-SOFA | 0.649 (0.611–0.685) | 0.022 | 8 | 0.662 | 0.578 | 4.170 (p<0.001) | |
| CLIF-OF | 0.625 (0.587–0.662) | 0.023 | 10 | 0.426 | 0.776 | 4.643 (p<0.001) | |
| MELD | 0.709 (0.673–0.743) | 0.022 | 25 | 0.620 | 0.721 | 0.779 (p=0.436) | |
| MELD-Na | 0.713 (0.677–0.747) | 0.021 | 28 | 0.620 | 0.743 | 0.585 (p=0.559) | |
| WASP-ACLF | 0.724 (0.689–0.758) | 0.020 | 4 | 0.689 | 0.758 | NA | |
| Third day (n=662) | CTP | 0.626 (0.588–0.663) | 0.022 | 11 | 0.826 | 0.369 | 6.521 (p<0.001) |
| CLIF-SOFA | 0.695 (0.658–0.730) | 0.021 | 8 | 0.710 | 0.571 | 4.874 (p<0.001) | |
| CLIF-OF | 0.694 (0.657–0.729) | 0.021 | 10 | 0.473 | 0.807 | 4.316 (p<0.001) | |
| MELD | 0.739 (0.704–0.772) | 0.022 | 25 | 0.652 | 0.741 | 2.036 (p=0.042) | |
| MELD-Na | 0.727 (0.691–0.761) | 0.022 | 24 | 0.797 | 0.560 | 2.760 (p=0.006) | |
| WASP-ACLF | 0.776 (0.743–0.808) | 0.019 | 4 | 0.749 | 0.706 | NA | |
| First week (n=637) | CTP | 0.643 (0.604–0.680) | 0.022 | 11 | 0.824 | 0.440 | 6.124 (p<0.001) |
| CLIF-SOFA | 0.713 (0.676–0.748) | 0.021 | 8 | 0.731 | 0.576 | 4.142 (p<0.001) | |
| CLIF-OF | 0.711 (0.674–0.746) | 0.021 | 9 | 0.753 | 0.582 | 3.518 (p<0.001) | |
| MELD | 0.725 (0.688–0.759) | 0.023 | 23 | 0.692 | 0.679 | 2.564 (p=0.010) | |
| MELD-Na | 0.733 (0.697–0.767) | 0.022 | 24 | 0.802 | 0.578 | 2.317 (p=0.021) | |
| WASP-ACLF | 0.780 (0.746–0.812) | 0.019 | 4 | 0.731 | 0.697 | NA | |
| Second week (n=610) | CTP | 0.672 (0.634–0.710) | 0.023 | 11 | 0.768 | 0.534 | 5.112 (p<0.001) |
| CLIF-SOFA | 0.730 (0.693–0.765) | 0.021 | 8 | 0.690 | 0.655 | 4.003 (p<0.001) | |
| CLIF-OF | 0.732 (0.695–0.766) | 0.022 | 9 | 0.743 | 0.620 | 3.613 (p<0.001) | |
| MELD | 0.754 (0.717–0.787) | 0.024 | 22 | 0.742 | 0.695 | 2.057 (p=0.040) | |
| MELD-Na | 0.746 (0.710–0.780) | 0.023 | 23 | 0.794 | 0.600 | 2.447 (p=0.014) | |
| WASP-ACLF | 0.799 (0.765–0.830) | 0.020 | 4 | 0.723 | 0.728 | NA |
ACLF, acute-on-chronic liver failure; CLIF-OF, chronic liver failure consortium organ function; CLIF-SOFA, chronic liver failure-sequential organ failure assessment; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; WASP-ACLF, widely applicable and simple prognostic score of ACLF.
Trends in WASP-ACLF prognostic benefit assessment
Classification of cases by WASP-ACLF revealed that patients in the low-risk group had a better prognosis than those in the high-risk group. The 90-day mortality rates of patients who were grade I at baseline, the third day, the first week, and the second week were 18.4%, 13.9%, 13.4%, and 11.5%, respectively. The corresponding rates were 49.4%, 50.6%, 45.1 and 44.4% for grade II patients, and 65.2%, 73.7%, 73.7%, and 69.0%, for grade III patients. As the results had considerable uncertainty about the short-term prognosis of grade II patients, trends in WASP-ACLF scores were calculated to further analyze their prognosis. On the third day after admission, grade II patients had a 90-day mortality of 50.6%. Differences of the 90-day mortality of patients with decreases ≥2 points (71.4%), fluctuations within 2 points (50.0%), and increases ≥2 points (50.0%) in the WASP-ACLF score from baseline values were not significant (Table 3). In contrast, after the first week, the 90-day morality rates of patients with a decrease of ≥2 points, fluctuation within 2 points, and an increase of ≥2 points in WASP-ACLF from baseline were 20.0%, 44.3%, and 56.8%, respectively. Similarly, the 90-day morality rates were 18.8%, 43.1%, and 55.6% at the second week, and all differences were statistically significant (p<0.05, Table 3). The 28-day morality rates of grade II patients also differed significantly with different trends of WASP-ACLF at the first and second week (p<0.05, Table 3).
Table 3. Short-term mortality of grade II cases according to the trend of change in the WASP-ACLF score.
| Time | Updated WASP-ACLF |
Trend ≥2 points vs. baseline | 28-day mortality, % (n) | χ2-value, p-value | 90-day mortality, % (n) | χ2-value, p-value |
|---|---|---|---|---|---|---|
| 28-day mortality, % (n) | ||||||
| 90-day mortality, % (n) | ||||||
| Third day | Grade II | Decrease | 57.1 (4) | χ2=3.261, p=0.200 | 71.4 (5) | χ2=1.200, p=0.622 |
| 29.9 (75) | No obvious change | 28.2 (61) | 50.0 (108) | |||
| 50.6 (127) | Increase | 35.7 (10) | 50.0 (14) | |||
| First week | Grade II | Decrease | 6.7 (1) | χ2=6.768, p=0.032 | 20.0 (3) | χ2=6.308, p=0.043 |
| 22.7 (53) | No obvious change | 20.7 (36) | 44.3 (77) | |||
| 45.1 (105) | Increase | 36.4 (16) | 56.8 (25) | |||
| Second week | Grade II | Decrease | 0.0 (0) | χ2=7.107, p=0.023 | 18.8 (3) | χ2=7.084, p=0.029 |
| 18.4 (38) | No obvious change | 16.8 (23) | 43.1 (59) | |||
| 44.4 (92) | Increase | 27.8 (15) | 55.6 (30) |
WASP-ACLF, widely applicable and simple prognostic score of acute-on-chronic liver failure.
Validation of WASP-ACLF in the prospective longitudinal cohort
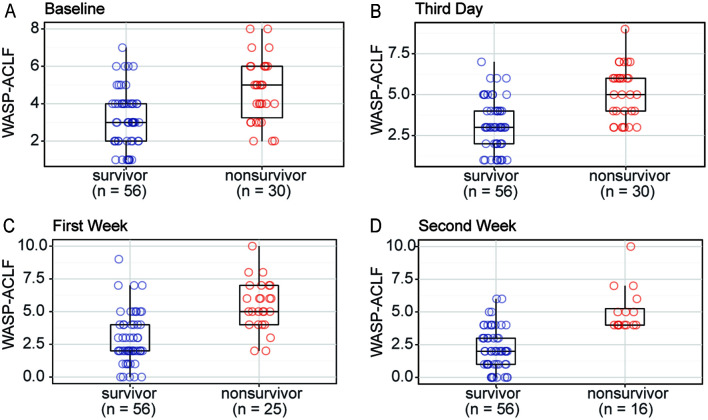
A total of 86 cases that met the inclusion and exclusion criteria were enrolled in the prospective study. All had a follow-up duration of >90 days except for those who died. There were 51 men and 35 women cases in the cohort and the mean age was 57.919±12.391 years. The etiologies included 45 cases (52.3%) with HBV, 15 (17.4%) with ALD, three (3.5%) with HBV combined with ALD, and 17 (19.8%) with autoimmune liver disease. Fifty-six cases survived for more than 90 days and 30 died. The WASP-ACLF scores of nonsurvivors at baseline, day 3, week 1, and week 2 were 4.700±1.684, 5.100±1.626, 5.480±1.874, and 5.063±1.692, respectively. The corresponding survivor scores were 3.179±1.515, 3.179±1.539, 2.857±1.939, and 2.196±1.589. The nonsurvivor scores were significantly higher than those of the survivors (p<0.001), and the between-group difference tended to increase gradually over time, indicating that sequential assessment did improve the differentiation ability of WASP-ACLF (Fig. 5). Because of the limited number of prospective cases, further validation of the trends in WASP-ACLF was performed only at week 2, and at that time the 90-day mortality rates of intermediate-risk patients with a decrease of ≥2 points, fluctuation within 2 points, and an increase of ≥2 points in WASP-ACLF from baseline were 33.3%, 46.7%, and 66.7%, respectively. These results were in general agreement with the retrospective statistics, but no significant differences were observed because the small number of cases (p>0.05).
Fig. 5. Comparison of the WASP-ACLF scores of the survivors and nonsurvivors in the prospective longitudinal cohort at.
(A) baseline, (B) the third day, (C) the first week (C), and (D) the second week after admission. WASP-ACLF, widely applicable and simple prognostic score of acute-on-chronic liver failure.
Discussion
There is considerable worldwide variability in the definition and diagnostic criteria for ACLF, partly because of differences in the etiology of in Eastern and Western countries. The main cause of ACLF in Asian countries is HBV infection. ALD is the main cause in America and European countries.12 Therefore, the precipitating events for ACLF tend to vary. For example, in the CANONIC study8 the most common precipitating events for ACLF were infections, followed by alcohol abuse. In Asia, HBV reactivity remains the most common cause, but the proportion of alcohol abuse has shown an increase over successive years.7 To overcome this problem, the WGO has proposed a globally harmonized consensus definition of ACLF that states that ACLF can occur at all stages of the natural history of chronic liver disease, including in the absence of cirrhosis as well as in the backdrop of compensatory cirrhosis and uncompensated cirrhosis. They further defined ACLF at each of these stages as types A, B, and C.12 The ACLF definition and classification have been adopted in the latest Chinese guidelines for liver failure.13 The American Association for the Study of Liver Diseases also supports a consensus definition of ALCF that recommends narrowing the differences and simplifying the criteria.1 Therefore, the inclusion criteria of this study included cases with chronic hepatitis, and both compensated and decompensated cirrhosis.
Consistent with previous studies, the main etiologies of the ACLF cases included in our study were HBV infection and ALD, with no significant precipitating events in most cases. In our study, WGO type was an independent prognostic factor for patients with ACLF. In recent years, the WGO type has been applied in several ACLF studies. For example, Mu et al.14 categorized 1,159 HBV-infected ACLF patients with 90 day follow-up data as types A, B, and C with significant between-group differences in 90 day mortality of 31.1% for type A; 40.9% for type B, and 61.4% for type C. ACLF type was identified as an independent risk factor for 90 day mortality in patients with HBV-ACLF.14 A study by Tang et al.15 found that HBV reactivity was the main precipitating event in patients with type A HBV-ACLF, bacterial infection predominated in type B cases and type C HBV-ACLF; liver and coagulation failures were most common in patients with type A, and renal failure was mainly observed in type C subjects. Tri-typing of HBV-related ACLF following the WGO definition can help distinguish the clinical characteristics, including precipitating events, organ failure, and short-term prognosis.15
In our study, age, WBC, HE, TB, Cr, and INR levels were also independent prognostic factors for ACLF. In many studies, age was negatively associated with ACLF prognosis.4,16 This may be partly attributable to aging and age-related complications, such as diabetes, skeletal muscle loss, and coronary artery disease, which adversely affect the outcomes of ACLF. Consistent with our study, Verma et al.17 found that ammonia level was a significant predictor of HE occurrence and HE classification; in addition to the above-mentioned factors, they also identified ammonia, lactate, and systemic inflammatory response syndrome as independent predictors of 30-day mortality in patients with ACLF. Elevated WBC counts suggest potential infection, and the CLIF C-ACLF model incorporates WBC in the calculation equation, with high levels of WBC associated with increased risk of ACLF mortality.9 In addition, the latest simplified version of the HBV-ACLF prognostic score proposed by the Chinese Group on the Study of Severe Hepatitis B also incorporates six indicators, including neutrophils and age, and has achieved highly predictive assessments.18
The regression equation and formula were too complex for clinical application. For example, a previous study conducted by our group proposed a prognostic model for ACLF based on the trends in clinical indicators and baseline patient characteristics.19 However, the complex formula and calculations make it less convenient and intuitive to use. On the other hand, a quantitative table is easier to apply in clinical settings. The WASP-ACLF developed in this study consists of seven parameters with broad applicability and easy availability. At day 1 and week 2 of sequential assessment, WASP-ACLF had better predictive efficacy than the existing prognostic scores, such as CTP, CLIF-SOFA, CLIF-OF, MELD, and MELD-Na. In addition, we observed an improvement in the AUC and DCA values over time, which reflect the predictive efficacy and clinical validity. Sequential assessment at multiple time-points may accurately reflect the clinical course and the responsiveness to medical treatment, and theoretically improve the prognostic ability compared with assessment at a single time point.20 Therefore, sequential WASP-ACLF assessment was more helpful in determining the prognosis of ACLF than other methods.
In this study, patients with ACLF were classified as low-risk, intermediate-risk, and high-risk by their WASP-ACLF scores. From the admission baseline to the second week, grade I patients had 28-day mortality rates of no more than 10% and 90-day mortality rates of no more than 20%. In contrast, grade III patients a 28-day mortality of approximately 60% and 90-day mortality of up to 70%. Therefore, more attention should be paid to intermediate-risk patients, in whom the 28-day mortality was 18.4–29.9%, and the 90-day mortality was 44.4–50.6%, suggesting significant uncertainty in the short-term prognosis. Our results illustrated that the trend of change in WASP-ACLF allowed for better prognostic assessment of grade II patients. On sequential assessment at the first or second week, the 90-day mortality of intermediate-risk groups was <20% for patients with a decrease in WASP-ACLF of ≥2 points, while it was up to 56% for patients with a ≥2 point increase. Similar results were also found in the prospective longitudinal cohort. Consistent with our results, in a previous study, the change in MELD score at the second week was found to be an ideal time point to determine the prognosis of ACLF patients; the predicted survival rate in the next 60 days was 93.8% in patients who showed no increase in MELD score at this time point.21 These findings strongly suggest that along with continuous assessment, trends in WASP-ACLF need to be considered, especially for intermediate-risk patients. Comprehensive data allows for more accurate assessment of patient condition and prognosis, facilitating personalized treatment and liver transplantation strategies.
Conclusion
In conclusion, the WASP-ACLF proposed in this study is a simple and widely applicable tool with good predictive efficacy. Sequential assessments and evaluation of the trend of change in WASP-ACLF facilitates accurate risk stratification of ACLF patients.
Supporting information
ACLF, acute-on-chronic liver failure; CLIF-OF, chronic liver failure consortium organ function; CLIF-SOFA, chronic liver failure-sequential organ failure assessment; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; WASP-ACLF, widely applicable and simple prognostic score of ACLF; ROC, receiver operating characteristic; DCA, decision curve analysis.
Acknowledgments
The authors wish to thank all China Network for Severe Liver Diseases members for their continued support with data collection.
Abbreviations
- ACLF
acute-on-chronic liver failure
- AFP
alpha-fetoprotein
- ALB
serum albumin
- ALD
alcoholic liver disease
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under ROC curve
- BUN
blood urea nitrogen
- CHE
cholinesterase
- CLIF-OF
chronic liver failure consortium organ function
- CLIF-SOFA
chronic liver failure-sequential organ failure assessment
- Cr
creatinine
- CRP
C-reactive protein
- CTP
Child-Turcotte-Pugh
- DCA
decision curve analysis
- GGT
γ-glutamyl transpeptidase
- GIB
gastrointestinal bleeding
- GLO
globulins
- HB
hemoglobin
- HBV
hepatitis B virus
- HCT
hematocrit
- HE
hepatic encephalopathy
- INR
international normalized ratio
- K
serum potassium
- MELD
model for end-stage liver disease
- N
neutrophil ratio
- Na
serum sodium
- PA
prealbumin
- PLT
platelet
- PT
prothrombin time
- PTA
prothrombin activity
- RDW
red cell volume distribution width
- ROC
receiver operating characteristic
- TB
total bilirubin
- TC
total cholesterol
- TG
triglyceride
- WASP-ACLF
widely applicable and simple prognostic score of ACLF
- WBC
white blood cell
- WGO
World Gastroenterology Organization
Data sharing statement
All data generated or analyzed in this study are available from the corresponding author for the reasonable request.
References
- 1.Bajaj JS, Moreau R, Kamath PS, Vargas HE, Arroyo V, Reddy KR, et al. Acute-on-chronic liver failure: getting ready for prime time? Hepatology. 2018;68(4):1621–1632. doi: 10.1002/hep.30056. [DOI] [PubMed] [Google Scholar]
- 2.Wu D, Sun Z, Liu X, Rao Q, Chen W, Wang J, et al. HINT: a novel prognostic model for patients with hepatitis B virus-related acute-on-chronic liver failure. Aliment Pharmacol Ther. 2018;48(7):750–760. doi: 10.1111/apt.14927. [DOI] [PubMed] [Google Scholar]
- 3.Sundaram V. Editorial: Transplantation in the cirrhotic patient with multi-organ failure: adding more pieces to an incomplete puzzle. Am J Transplant. 2020;20(9):2297–2298. doi: 10.1111/ajt.15927. [DOI] [PubMed] [Google Scholar]
- 4.Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67(12):2181–2191. doi: 10.1136/gutjnl-2017-314641. [DOI] [PubMed] [Google Scholar]
- 5.Pugh R, Murray-Lyon I, Dawson J, Pietroni M, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 6.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 7.Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(4):353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130(6):1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11. R Development Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, 2020. Available from: https://www.R-project.org/
- 12.Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin HC, et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147(1):4–10. doi: 10.1053/j.gastro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association Guideline for diagnosis and treatment of liver failure. Zhonghua Gan Zang Bing Za Zhi. 2019;27(1):18–26. doi: 10.3760/cma.j.issn.1007-3418.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Mu X, Tong J, Xu X, Chen J, Su H, Liu X, et al. World Gastroenterology Organisation classification and a new type-based prognostic model for hepatitis B virus-related acute-on-chronic liver failure. Clin Res Hepatol Gastroenterol. 2021;45(3):101548. doi: 10.1016/j.clinre.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Qi T, Li B, Li H, Huang Z, Zhu Z, et al. Tri-typing of Hepatitis B-related acute-on-chronic liver failure defined by the World Gastroenterology Organization. J Gastroenterol Hepatol. 2021;36(1):208–216. doi: 10.1111/jgh.15113. [DOI] [PubMed] [Google Scholar]
- 16.Szabo G. More than meets the eye: Severe alcoholic hepatitis can present as acute-on-chronic liver failure. J Hepatol. 2018;69(2):269–271. doi: 10.1016/j.jhep.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Verma N, Dhiman RK, Choudhury A, Taneja S, Duseja A, Singh V, et al. Dynamic assessments of hepatic encephalopathy and ammonia levels predict mortality in acute-on-chronic liver failure. Hepatol Int. 2021;15:970–982. doi: 10.1007/s12072-021-10221-7. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Liang X, You S, Feng T, Zhou X, Zhu B, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75(5):1104–1115. doi: 10.1016/j.jhep.2021.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Yu Z, Zhang Y, Cao Y, Xu M, You S, Chen Y, et al. A dynamic prediction model for prognosis of acute-on-chronic liver failure based on the trend of clinical indicators. Sci Rep. 2021;11(1):1810. doi: 10.1038/s41598-021-81431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira G, Baldin C, Piedade J, Reis V, Valdeolivas T, Victor L, et al. Combination and sequential evaluation of acute-on-chronic liver failure (ACLF) and hyponatremia and prognosis in cirrhotic patients. Dig Liver Dis. 2020;52(1):91–97. doi: 10.1016/j.dld.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R, Krishnamoorthy TL, Tan HK, Lui HF, Chow WC. Change in model for end-stage liver disease score at two weeks, as an indicator of mortality or liver transplantation at 60 days in acute-on-chronic liver failure. Gastroenterol Rep. 2015;3(2):122–127. doi: 10.1093/gastro/gou075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ACLF, acute-on-chronic liver failure; CLIF-OF, chronic liver failure consortium organ function; CLIF-SOFA, chronic liver failure-sequential organ failure assessment; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; WASP-ACLF, widely applicable and simple prognostic score of ACLF; ROC, receiver operating characteristic; DCA, decision curve analysis.