Summary
Radiobiology research in rectal cancer has been limited to cell lines, patient-derived organoids (PDOs), or xenografts. Here, we describe a protocol which recapitulates more efficiently the complex contributions of the tumor microenvironment. This approach establishes a preclinical mouse model of rectal cancer by intrarectal transplantation of genetically modified organoids into immunocompetent mice followed by precise image-guided radiotherapy (IGRT) of organoid-induced tumors. This model represents a useful platform to study the cellular and molecular determinants of therapy resistance in rectal cancer.
For complete details on the use and execution of this protocol, please refer to Nicolas et al. (2022).
Subject areas: Cancer, Health sciences, Model organisms
Graphical abstract
Highlights
-
•
Orthotopic transplantation of tumor organoids in immunocompetent mice
-
•
Precise image-guided radiotherapy (IGRT) of rectal tumors
-
•
Study complex contributions of the tumor microenvironment and the immune system
-
•
Study cellular and molecular determinants of therapy resistance in rectal cancer
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Radiobiology research in rectal cancer has been limited to cell lines, patient-derived organoids (PDOs), or xenografts. Here, we describe a protocol which recapitulates more efficiently the complex contributions of the tumor microenvironment. This approach establishes a preclinical mouse model of rectal cancer by intrarectal transplantation of genetically modified organoids into immunocompetent mice followed by precise image-guided radiotherapy (IGRT) of organoid-induced tumors. This model represents a useful platform to study the cellular and molecular determinants of therapy resistance in rectal cancer.
Before you begin
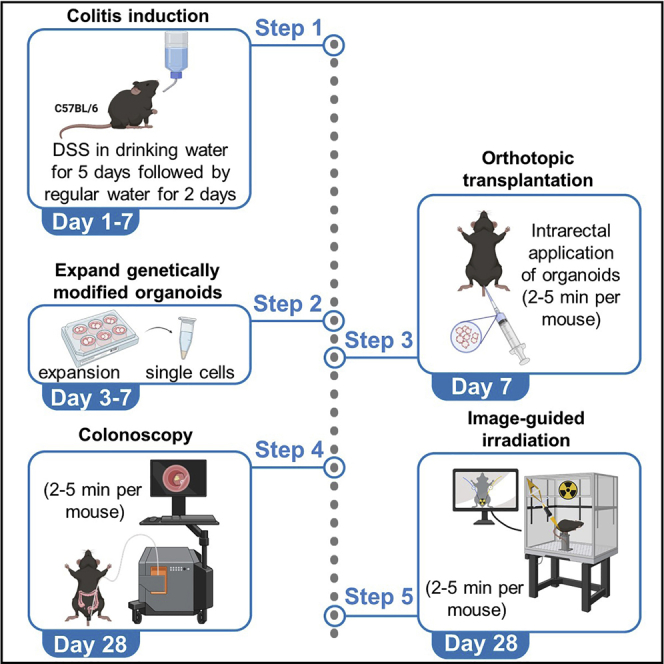
This protocol describes the specific steps to perform orthotopic transplantation of genetically modified organoids into immunocompetent C57BL/6J mice and to perform local fractionated image guided radiotherapy of established distal tumors.
We use male and female C57BL/6J wild-type (Janvier Labs) mice in the following protocol aged 8–10 weeks old. We generated genetically modified organoids mutant for classical colorectal cancer-associated driver mutations including Apc, Trp53, Tgfbr2, K-rasG12D (APTK) and myristoylated AKT (APTKA organoids) as described in (Varga et al., 2020).
The media used for culturing genetically modified tumor organoids differ depending on their mutational phenotypes. Organoids grow in media lacking growth factors present in normal colon organoids medium. APTK and APTKA organoids media are both prepared from a basic medium labeled as Ad-DF+++. The medium composition for each type of organoids is summarized in the section ‘materials and equipment’. APTK and APTKA organoids grow well in basement matrix extract BME (R&D 3533-005-02) whereas, to resuspend dissociated organoids prior to orthotopic injections, we use basement membrane matrix Matrigel (Corning 356231) along PBS as described below in the “step-by-step method details” section.
Preparation of solutions, media and organoids
Timing: 30 min
-
1.
Prepare organoid media in advance and keep it at 4°C for a maximum of 4 weeks.
Note: Growth factors including B27 and N2, N-acetyl cysteine (NAC), puromycin, BME and Matrigel need to be aliquoted and stored at −20°C till further use.
-
2.To culture organoids first thaw and aliquot BME and Matrigel bottles.
-
a.Thaw frozen bottles from −80°C on ice for 4–6 h.
-
b.Aliquot them as 1 mL volume in Eppendorf tubes and store at −20°C.
-
c.Rethaw Matrigel and BME aliquots on ice for 2 h.
-
a.
-
3.
Prior to orthotopic transplantation, expand organoids to allow injection of a volume of approximately 400 μL of BME containing organoids per mouse.
-
4.
Prepare anesthesia solution as described below in the ‘materials and equipment’ section and store it up to 4 weeks at 4°C.
Dextran sulfate sodium-induced colitis
Timing: 5 days
-
5.Subject C57BL/6J female mice purchased from JANVIER LABS, aged 8 weeks old to 4% DSS water for 5 consecutive days (day 0 till day 5).
-
a.Monitor mouse weight daily starting from day 0 till day 11.
-
b.Substitute DSS water by normal water on day 5.
-
a.
CRITICAL: Extent of colonic epithelial injury and ulceration depends on DSS concentration, mouse strain, the amount of DSS consumed by mice and the animal facility (microbiome). DSS toxicity varies largely between C57BL/6 mice ordered from different vendors. Males are more sensitive to DSS treatment and display increased lethality upon treatment with 4% DSS. Therefore, it is recommended to treat male mice using a concentration of 2.8%–3% DSS. Successful DSS-induced colitis is a critical step for tumor transplantation and intraluminal growth (Figure 2A). Successful DSS-induced colitis is assessed by loose stool, diarrhea, intrarectal bleeding and weight loss. Mice that lose > 20% of their initial weight will not recover from colitis and need to be euthanized.
Figure 2.
Tumor imaging
(A) H&E staining representing untreated colon and DSS-induced colitis (day 7) histology.
(B) Colonoscopy images of APTKA orthotopic tumor transplanted in C57BL/6 mouse 2, 3 and 4 weeks post-orthotopic transplantation. White lines mark the tumor area.
(C) Endoscopic probe marked by a ruler and used to determine the distance of the lower tumor margin from the anus.
(D) H&E staining representing APTKA tumors 2 and 3 weeks post-orthotopic transplantation. White lines indicate basal membrane and where invasive tumor area start. Scale bars represent 700 and 200 μm.
Institutional permissions
All animals are housed under standard housing conditions at Georg-Speyer-Haus animal facility and all animal experiments are performed in accordance with the guidelines reviewed and approved by the Regierungspräsidium Darmstadt, Germany.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| APTK murine organoids | This study | NA |
| APTKA murine organoids | This study | NA |
| Chemicals, peptides, and recombinant proteins | ||
| Cultrex Reduced Growth Factor Basement Membrane Extract, Type 2 | R&D Systems | Cat# 3533-010-02 |
| Corning® Matrigel® Growth Factor Reduced (GFR) | Corning | Cat# 356231 |
| GlutaMAX | Thermo Fisher Scientific | Cat# 35050061 |
| HEPES | Thermo Fisher Scientific | Cat# 15630080 |
| Penicillin/ Streptomycin | Thermo Fisher Scientific | Cat# 15070063 |
| B-27 supplement | Thermo Fisher Scientific | Cat# 12587010 |
| N-2 supplement | Thermo Fisher Scientific | Cat# 17502048 |
| N-Acetyl-L-cysteine | Sigma-Aldrich | Cat# A9165 |
| Advanced DMEM/F-12 | Thermo Fisher Scientific | Cat# 12634028 |
| Hygromycin B | Thermo Fisher Scientific | Cat# 10687010 |
| Puromycin dihydrochloride | Merck | Cat# 58-58-2 |
| Dextran Sulfate Sodium (DSS) | MP Biomedicals | Cat# 216011090 |
| Cell recovery solution | Corning | Cat# 354253 |
| Accutase cell dissociation reagent | Thermo Fisher Scientific | Cat# A1110501 |
| Ketamine (10%) | Serumwerk Bernburg (Bernburg) | Cat# 13690.00.00 |
| Xylazine | Serumwerk Bernburg (Bernburg) | Cat# 3100265.00.00 |
| Isoflurane | Zoetis | Cat# TU 061220 |
| Vaseline | Sigma-Aldrich | Cat# 16415 |
| Experimental models: Organisms/strains | ||
| Mouse C57BL/6J (wild type, male and female, 8 weeks-old) | JANVIER LABS | RRID:IMSR_JAX:000664 |
| Software and algorithms | ||
| Built-in Muriplan Software (Small Animal Radiation Research Platform) | Xstrahl, Surrey, UK | NA |
| Other | ||
| Plastic Feeding Tubes | Instech Laboratories, Inc | Cat# FTP-18-38-50 |
Materials and equipment
Ad-DF+++ medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Advanced DMEM/F12 | N/A | 485 mL |
| GlutaMAX (200 mM) | 2 mM | 5 mL |
| HEPES (1 M) | 10 mM | 5 mL |
| Penicillin/ streptomycin (10,000 units/mL) | 100 units/mL | 5 mL |
| Total | N/A | 500 mL |
Once prepared, stored at 4°C for 4 weeks.
APTK organoids culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Ad-DF +++ | N/A | 38. 692 mL |
| B-27 supplement (50×) | 1× | 800 μL |
| N-2 supplement (100×) | 1× | 400 μL |
| N-Acetyl-L-cysteine | 2 mM | 100 μL |
| Puromycin dihydrochloride | 2 μg/mL | 8 μL |
| Total | N/A | 40 mL |
Once prepared, stored at 4°C for 4 weeks.
APTKA organoids culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Ad-DF +++ | N/A | 38.532 mL |
| B-27 supplement (50×) | 1× | 800 μL |
| N-2 supplement (100×) | 1× | 400 μL |
| N-Acetyl-L-cysteine | 2 mM | 100 μL |
| Puromycin dihydrochloride | 2 μg/mL | 8 μL |
| Hygromycin | 200 μg/mL | 160 μL |
| Total | N/A | 40 mL |
Once prepared, stored at 4°C for 4 weeks.
Anesthesia solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Saline solution (0.9% NaCl) | N/A | 8.6 mL |
| Ketamine (10%) | 1% | 1 mL |
| Xylazine (Rompun 20 mg/mL) | 0.8 mg/mL | 400 μL |
| Total | N/A | 10 mL |
Once prepared, stored at 4°C for 4 weeks.
Alternatives: Isoflurane can be administered as an alternative anesthetic agent via a properly calibrated vaporizer.
Step-by-step method details
Collection of APTKA and APTK organoids for transplantation
Timing: 60–90 min
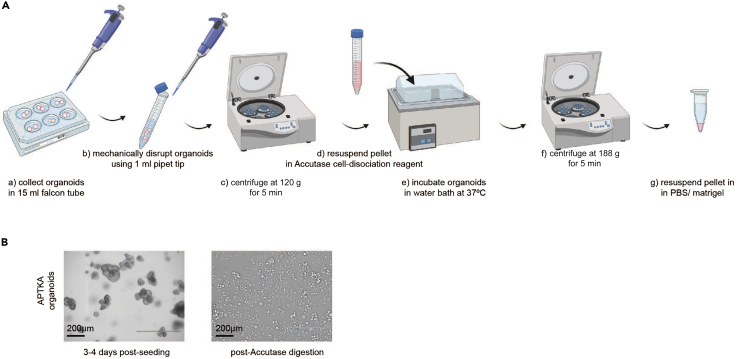
We collect, dissociate and resuspend cultured organoids prior to orthotopic transplantation into the lumen of DSS treated mice. It usually takes 3–4 days post seeding till organoids reach proper size and 70%–80% confluency (Figures 1A and 1B).
-
1.
3–4 days post seeding, carefully aspirate the culture medium of APTKA or APTK organoids.
-
2.
To depolymerize the BME, add 1 mL Cell Recovery Solution (Corning) to each well.
-
3.
With a 1 mL pipet tip, mechanically disrupt the organoids BME drops into smaller clusters of cells by pipetting up and down.
-
4.
In a 15 mL falcon tube, pool 4 wells of organoids embedded in 800 μL BME and pipette up and down 12 times using a 1 mL pipet tip. Incubate at 4°C for 15 min.
-
5.
Add 4 mL PBS and centrifuge at 120 g for 5 min at 4°C.
-
6.
For further dissociation, after centrifugation, remove supernatant and resuspend the pellet in 2 mL pre-warmed Accutase cell dissociation reagent by pipetting up and down with a 1 mL pipet tip for 12 times.
-
7.
Incubate the disrupted organoids in a water bath at 37°C for 10 min.
-
8.
To obtain a homogenous cell suspension, further disrupt the organoids for a final time by pipetting up and down for 12 times using 1 mL pipet tip. Pool the organoids in one 15 mL falcon tube and centrifuge at 188 g for 5 min at 4°C.
-
9.
Aspirate the supernatant. Wash the pellet with 8 mL Ad-DF+++ and centrifuge at 188 g for 5 min at 4°C.
-
10.
Aspirate the supernatant. Wash the pellet with 8 mL PBS and centrifuge at 188 g for 5 min at 4°C.
-
11.
Aspirate the supernatant, resuspend organoids in diluted 20% Matrigel-PBS solution and keep the solution on ice until transplantation.
Note: The final concentration of the cell suspension should correspond to 1–2 × 106 single cells per 100 μL, however, it is not necessary to completely digest organoids into single cells because long digestion times may lead to cell death. The number is an approximation and serves as guidance. We observed that cell clusters engraft as well as single cells.
CRITICAL: Healthy organoids of 70%–80% confluency should be used, typically 3–4 days post seeding and overgrown confluent organoids should be avoided (Figure 1B).
Note: To avoid cells aggregation and clumps, pipette up and down the prepared cells suspension or flick the tube before intralumenal transplantation (Methods video S1). Resuspension of the cells in diluted 20% Matrigel-PBS solution is essential as cells resuspended only in PBS solution will not be successfully transplanted. Post-organoids preparation, directly proceed into intralumenal transplantation to avoid organoids death.
Figure 1.
Collection and digestion of genetically modified organoids
(A) Representative scheme summarizing the multistep process of preparation and digestion of genetically modified organoids prior to transplantation.
(B) Representative microscopic images of ∼70% confluent APTKA organoids prior to collection and Accutase-dissociated organoids resuspended in 20% Matrigel/PBS. Scale bars represent 200 μm.
Orthotopic transplantation of organoids
Timing: 5–10 min per mouse
Taking advantages of the previously published dextran sulfate sodium (DSS) endoluminal protocol (O'Rourke et al., 2017), we perform intrarectal injections of prepared organoids 2 days after termination of DSS administration (experimental day 7) on anesthetized mice.
-
12.
Apply a small amount of eye ointment Bepanthen on the mouse eyes to protect the cornea.
-
13.
Prior to transplantation, anesthetize mice with 2% isoflurane and gently flush the colon with minimum volume of 20°C–25°C PBS (∼ 200 μL) for emptying luminal fecal contents.
-
14.
To proceed into organoids transplantation, anesthetize mice with intraperitoneal injection of Anesthesia solution: 10 μL/g of mouse.
Optional: Intraluminal transplantation can be performed under isoflurane, yet transplantation works better when mice are allowed to sleep longer.
See Methods video S1.
-
15.
Cool down the catheter on ice and keep it cold during the procedure.
-
16.
Keeping the mouse in prone position, gently insert the cold catheter at 2 cm depth into the colon and infuse 100 μL of cells dissolved in 20% Matrigel-PBS.
Note: For a smooth intrarectal injection, the catheter tip can be sparingly coated with Vaseline.
Optional: For intrarectal injections, 200 μL pipet tip can be used instead of the catheter.
-
17.
To prevent leakage of luminal content, immediately seal the anus with Vaseline.
Optional: To seal the anus, tissue adhesive can be used instead of Vaseline, but it must be removed after about 4–6 h post transplantation.
-
18.
Allow the mouse to recover under red lamp or use hot water blanket, warm fluid bag or gloves filled with warm water.
Tumor imaging and monitoring
Timing: 5–10 min per mouse
Determine progression of endoluminal tumor growth and evaluate tumor size weekly starting around 3 weeks post-transplantation using the small animal endoscopy system (Karl Storz Endoscope, El Segundo, CA, USA) on mice under Ketamine/Xylazine anesthesia. Assess tumor volume (mm3) per animal by measuring the longest and shortest diameter of the visible rectal tumor. Successful transplantation will lead to a single distal tumor per mouse at a maximum depth of 2 cm from the anus.
-
19.
Anesthetize mice with Anesthesia solution (10 μL/g of mouse) and apply eye ointment on the mouse eyes to protect the cornea.
See Methods video S2.
-
20.
To remove luminal fecal content, flush the colon with 20°C–25°C PBS (2 mL).
-
21.
Grasp the camera head in a manner that will allow you to cover the port opposite the air infusion intake with a finger to insufflate the colon as the telescope is inserted (Methods video S2).
Note: Alternatively, set an appropriate flow rate for the air pump and close the port that you would cover with your thumb.
CRITICAL: The flow rate should be ∼2 to 3 bubbles per second when the endoscope is inserted into a 50 mL conical tube of H2O.
-
22.
Keep the mouse in prone position, with one hand hold the dorsal fur and with the other hand carefully insert the HOPKINS telescope (1.9 mm outer diameter, length 10 cm; Karl Storz) covered with an endoscopic sheath into the rectum up to 2 cm depth.
-
23.
Keep the colon inflated and start recording as you slowly withdraw the endoscope. Distal and richly vascularized endoscopic lesions, 3–6 weeks post-transplantation, confirm successful engraftment and tumor growth (Figure 2B).
-
24.
Determine the distance of the lower tumor margin from the anus using the endoscopic probe with graduation (Figure 2C).
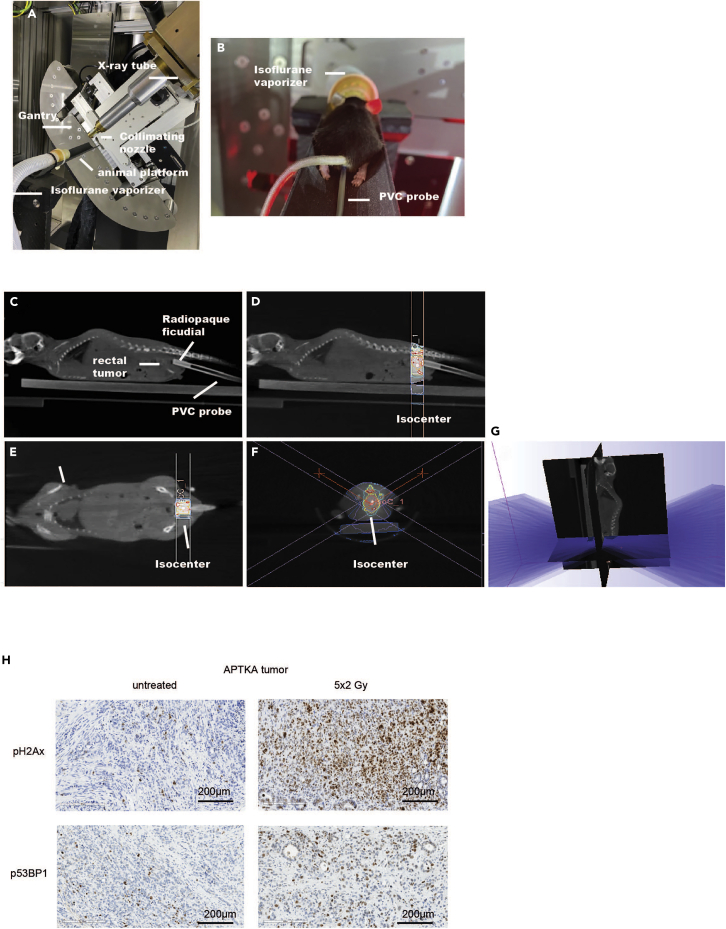
Image-guided radiotherapy using the small animal radiation research platform (SARRP)
Timing: 2–5 min per mouse
The small animal radiation research platform (SARRP) provides a combined use of a precisely aligned defined beam and a volumetric treatment-time imaging system (Figure 3). We irradiate tumors that reached required size (∼2/3 of lumen). If the tumors had not reached the required size, we perform another colonoscopy few days later. All tumors grow to the required size between 3–6 weeks after orthotopic transplantation.
-
25.
Anesthetize mice with 1.5% isoflurane (Figure 3A).
-
26.
Prepare a 2 mm-diameter PVC probe with an endoluminal 1 mm-diameter radiopaque line to insert intrarectally and advance its tip up to the lower margin of the rectal cancer, based on the endoscopy measurements described above (Figure 3B).
-
27.
Acquire onboard cone-beam computer tomography (CBCT) images (Figures 3C–3F) by rotating the mouse between the stationary x-ray source and a 2-dimensional digital flat-panel detector in a horizontal setup applying a tube voltage of 60 kVp, 0.8 mA and 720 projections acquired over 360°. CBCT acquisition is referred to as ‘RT planning CBCT image’.
-
28.
Reconstitute CBCT images using SARRP’s MuriSlice™ software reconstruction algorithm to 0.16 mm isotropic voxels and transfer RT planning CBT images to Muriplan™ treatment planning software (SARRP, Xstrahl, Surrey, UK).
-
29.
Perform segmentation (definition of grey values for each tissue type: air, lung, tissue, fat and bone) and contouring of the target region and organs at risk.
-
30.
Plan irradiation dose and its associated collimator, gantry and couch angles based on the isocenter (defined by the tumor diameter, and distance between lower tumor margin and anus) which is used to define the center of rotation for administration of either a vertical beam or a conical arc, operated at a 60-degree oblique angle.
-
31.
To verify treatment planning compute isodose lines (Figures 3D–3F) and a dose volume histogram (DVH).
-
32.
Execute radiotherapy (RT) planning with fractionated single doses of 2.0 Gy using a 10-mm collimated arc beam operating at 220 kVp, 13 mA applied five times a week to reach a total dose of 10 Gy (Figure 3G).
Figure 3.
Image-guided irradiation
(A) The small animal radiation research platform (SARRP) with illustration showing X-ray tube, collimating nozzle, isoflurane vaporizer, rotating gantry and animal platform.
(B) Mouse with intrarectally inserted PVC probe under isoflurane anesthesia and placed on SARRP rotating animal platform.
(C) CBCT image of the mouse illustrating the radiopaque fiducial inserted endoluminal into the PVC probe and the rectal tumor used to determine the isocenter.
(D–F) Coronal and sagittal view of the radiotherapy planning showing the dose set to the isocenter in the orthotopic tumor model. Fall-off of the radiation dose is visualized by color wash.
(G) Illustration revealing the radiation arcs employed for the RT planning.
(H) Phospho-γH2AX (Ser139) and phospho-53bp1 (Ser1778) immunohistochemistry staining of untreated and 5 × 2 Gy irradiated APTKA orthotopic tumor 7 days post last dose irradiation. Scale bars represent 200 μm.
Expected outcomes
Multiple limitations are attributed to previously published in vivo models in radiobiology research. For instance, PDOs, whose response to therapy has been recently associated with patients outcome, undermine the heterogeneity and complex contribution of the tumor microenvironment to therapy response and resistance (Schmitt and Greten, 2021). Furthermore, in vivo xenografts models in immunodeficient mice lack intact immune compartment and, hence, hamper vigorous assessment of parameters than affect radiotherapy outcomes such as vascularization, hypoxia and cytotoxic T cells infiltration. Additionally, in contrast to clinical manifestations, metastasis is rarely reported in xenografts models. Of note, distant liver and lung metastases occur in up to 30% of rectal cancer patients treated with standard CRT and surgery (Fokas et al., 2014).
Published genetic models based on Cre/lox systems such as villin-cre yield growth of multiple colonic tumors by affecting all intestinal epithelial cells and rarely distal metastasis (Schwitalla et al., 2013). Therefore, we attempted establishment and characterization of a preclinical orthotopic mouse model of rectal cancer. The novelty of this model resides in its close resemblance of the clinical setting with one distal tumor at an identical anatomical location, locally irradiated by fractionated modality in an immunocompetent mouse. The model further allows an in-depth understanding and characterization of tumor microenvironment-derived effects on CRT response. The use of genetically-modified organoids for cancer modeling serve as ideal platform for personalized medicine and individual-tailored therapies by capturing diverse representation of rectal cancer mutational genotypes and clonal diversity.
Histologically, this model resembles the pathology or stepwise progression of human rectal cancer with the following characteristics: abnormal glands infiltrating the submucosa, moderate to poorly differentiated tumors, intraluminal tumor budding and stroma- rich tumors (Figure 2B).
The stepwise tumor evolution model described above largely mimics all stages of human rectal cancer, including metastatic dissemination. For instance, ∼60% of untreated APTKA tumor metastasize to the liver (Varga et al., 2020). Hence, the following model can also be used to study radiotherapy reported abscopal effects with MRI-based assessment of distant metastasis prior and post-CRT.
Collectively, our described model here illustrates a flexible, relatively fast platform to model different stages of rectal cancer by using ex-vivo engineered mouse organoids, also enabling examination of tumor-stroma interactions in genetic chimeras, which is likely to be crucial in CRT response and resistance.
Limitations
We attempted to adapt the protocol on NOD scid gamma mice (NSG) immunodeficient mice employing patient- derived rectal cancer organoids for reconstitution of human epithelium in established tumors. However, inefficient de-epithelization of immunodeficient (NSG) mice was achieved as lack of immune compartment hindered successful colitis induction upon DSS administration. Therefore, for immunodeficient and DSS- sensitive mice such as Trp53 knockout mice of defective tight-junction permeability and decreased epithelial barrier function, an alternative EDTA-based injury orthotopic mouse model can be used (Schwitalla et al., 2013; Sugimoto et al., 2018). Alternatively, orthotopic co-implantation of tumor and stroma cells in cecum and rectum can be used to solve this limitation (Kasashima et al., 2021). Transplantation efficiency is heterogenous between different cohorts and between mice of the same cohort housed together, ranging between 50 to 80%. Low transplantation efficiency and a delayed tumor growth are encountered when organoids with fewer mutations are employed. Therefore, generation of large mice cohorts for multiple treatment groups with synchronous tumor onset and progression is not possible.
Troubleshooting
Problem 1
Variable levels of DSS-induced colitis.
The extent of DSS-induced colitis depends not only on its concentration but also mouse strain, gender, vendors from which they are ordered, prevailing microbiome at animal facility and amount of DSS consumed by mice. DSS variable degrees of induced colitis were even observed among different experimental batches with mice ordered from the same vendor, with comparable age and gender. Successful transplantation and tumor growth highly depend on the DSS- induced colonic injury assessed by loose stool, diarrhea, intrarectal bleeding and weight loss that is detected starting from day 5 post initiation of DSS administration (step 5).
Potential solution
When working with males or genetically modified mice, firstly test lower and different DSS concentrations ranging between ∼2.5 to 3%, monitor mice sensitivity to DSS by daily measurement of body weight before planning further experiment for orthotopic transplantation.
When treating C57BL/6 female mice with 4% DSS water aged 8 weeks old and noticing that on day 5 that there was neither weight loss nor clear sign of diarrhea or intrarectal bleeding, it is recommended to prolong the DSS treatment for one additional day (Day 6) and proceed into the transplantation next day (Day 7).
When treating C57BL/6 female mice with 4% DSS water aged 8 weeks old and noticing that on day 4 weight loss of 12%–15% and clear sign of diarrhea or intrarectal bleeding, it is recommended to shorten the treatment to 4 days and proceed into the organoid transplantation on day 7.
Problem 2
Low transplantation efficiency.
Transplantation efficiency rates vary between one batch and the other. Successful transplantation is reflected by ∼80% tumor formation among transplanted mice detected 3–6 weeks post organoids transplantation. Lower transplantation rate can be encountered with only 50% tumor formation in treated mice (steps 12–18).
Potential solution
Successful DSS-induced colitis and colonic de-epithelization highly impact organoids engraftment and tumor growth, therefore an implementation of the above-mentioned recommendations is required.
The optimum age of DSS-treated and transplanted mice is 8 weeks. Lower transplantation rates are encountered among older mice (∼12 weeks old and more).
Organoid viability prior to transplantation is a key determinant in their in vivo engraftment. Therefore, healthy organoids of 70% confluency shall be used, ideally 3 days post-seeding, and overgrown organoids shall be avoided (Figure 1B).
Problem 3
Lack of tumor growth.
Even if transplantation was successful after DSS induced colitis it can happen that tumors do not grow out in some cases or tumor development time could be too slow (longer as 3 months) (step 23).
Potential solution
Organoids aggressiveness and mutational phenotype are crucial determinants in their in vivo engraftment. Organoids less aggressive then APTK or APTKA, such as APT (Apc, Trp53 and Tgfbr2 knockout) organoids, a higher number of cells is recommended for intrarectal injection (∼2 × 106/100 μL) diluted in 30%–40% Matrigel-PBS. Prior to intraluminal transplantation, it is recommended to pipette the prepared cell suspension in order to avoid cellular aggregates and clumps.
Problem 4
Mouse bearing multiple tumors.
In few cases, a transplanted mouse might develop more than one distal tumor.
Potential solution
Such mouse is not subjected to local radiotherapy (step 23).
Problem 5
Excessive air insufflation during endoscopy.
Excessive insufflation can severely increase the risk of perforation and gastrointestinal track inflation. On the other hand, insufficient air flow leads to poor endoscopic visuality of luminal content (step 23).
Potential solution
Prior to imaging, check the endoscope air pump by immersing the scope into a 50 mL falcon tube filled with distilled water. Set low insufflation speed of 2–3 bubbles per second. Alternatively, switch off the air pump immediately after insufflation while keeping the ports closed. Or grasp the camera head in a manner that will allow you to cover the port opposite the air infusion intake with a finger in order to insufflate the colon as the telescope is inserted.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Florian R. Greten (greten@gsh.uni-frankfurt.de).
Materials availability
Reagents, resources, and materials employed in this study are detailed in the key resources table. Materials and reagents used in this study and generated at our laboratory are available upon request.
Acknowledgments
We thank Esther Engel, Petra Dinse, Kathleen Mohs, Eva Rudolf, Julius Oppermann, Stephanie Hehlgans, Jeannie Peifer, and Christin Danneil for expert technical assistance as well as the staff at the Animal Facility and the Histology Core Facilities at the Georg-Speyer-Haus. We thank Claire Conche and Yasamin Dabiri for video recording.
Graphical Abstract was created with BioRender.com. Work in the lab of F.R.G. is supported by institutional funds from the Georg-Speyer-Haus, by the LOEWE Center Frankfurt Cancer Institute (FCI) funded by the Hessen State Ministry for Higher Education, Research and the Arts [III L 5 - 519/03/03.001 - (0015)], Deutsche Forschungsgemeinschaft (FOR2438: Gr1916/11-1; SFB1292-Project ID: 318346496-TP16; SFB1479-Project ID: 441891347-P02; GRK2336) and the ERC (Advanced Grant PLASTICAN-101021078). The Institute for Tumor Biology and Experimental Therapy, Georg-Speyer-Haus is funded jointly by the German Federal Ministry of Health and the Ministry of Higher Education, Research and the Arts of the State of Hessen (HMWK).
Author contributions
Conceptualization, A.M.N., M.P., F.R., E.F., and F.R.G.; Methodology, A.M.N., M.P., F.R., E.F., and F.R.G.; Visualization, A.M.N., M.P., F.R., and E.F.; Writing – Original draft, A.M.N., M.P., F.R., E.F., and F.R.G.; Supervision, E.F. and F.R.G.; Funding Acquisition, F.R.G.
Declaration of interests
The authors have filed a patent application related to this study. F.R.G. is a consultant for Amazentis not related to this work.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101749.
Data and code availability
This study did not generate datasets nor code.
References
- Fokas E., Liersch T., Fietkau R., Hohenberger W., Beissbarth T., Hess C., Becker H., Ghadimi M., Mrak K., Merkel S., et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J. Clin. Oncol. 2014;32:1554–1562. doi: 10.1200/JCO.2013.54.3769. [DOI] [PubMed] [Google Scholar]
- Kasashima H., Duran A., Cid-Diaz T., Muta Y., Kinoshita H., Batlle E., Diaz-Meco M.T., Moscat J. Mouse model of colorectal cancer: orthotopic co-implantation of tumor and stroma cells in cecum and rectum. STAR Protoc. 2021;2:100297. doi: 10.1016/j.xpro.2021.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas A.M., Pesic M., Engel E., Ziegler P.K., Diefenhardt M., Kennel K.B., Buettner F., Conche C., Petrocelli V., Elwakeel E., et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell. 2022;40:168–184.e13. doi: 10.1016/j.ccell.2022.01.004. [DOI] [PubMed] [Google Scholar]
- O'Rourke K.P., Loizou E., Livshits G., Schatoff E.M., Baslan T., Manchado E., Simon J., Romesser P.B., Leach B., Han T., et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 2017;35:577–582. doi: 10.1038/nbt.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M., Greten F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021;21:653–667. doi: 10.1038/s41577-021-00534-x. [DOI] [PubMed] [Google Scholar]
- Schwitalla S., Ziegler P.K., Horst D., Becker V., Kerle I., Begus-Nahrmann Y., Lechel A., Rudolph K.L., Langer R., Slotta-Huspenina J., et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell. 2013;23:93–106. doi: 10.1016/j.ccr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Sugimoto S., Ohta Y., Fujii M., Matano M., Shimokawa M., Nanki K., Date S., Nishikori S., Nakazato Y., Nakamura T., et al. Reconstruction of the human colon epithelium in vivo. Cell Stem Cell. 2018;22:171–176.e5. doi: 10.1016/j.stem.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Varga J., Nicolas A., Petrocelli V., Pesic M., Mahmoud A., Michels B.E., Etlioglu E., Yepes D., Häupl B., Ziegler P.K., et al. AKT-dependent NOTCH3 activation drives tumor progression in a model of mesenchymal colorectal cancer. J. Exp. Med. 2020;217:e20191515. doi: 10.1084/jem.20191515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets nor code.