Abstract
Background
Since breast cancer is less common in men than in women, data on the use of new therapeutic agents, including cyclin-dependent kinase 4–6 (CDK 4–6) inhibitors, are limited in patients with metastatic hormone receptor positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) male breast cancer. Therefore; we aimed to investigate the treatment responses of metastatic HR+, HER2-male breast cancer patients treated with CDK 4–6 inhibitors in a multicenter real-life cohort.
Methods
Male patients with a diagnosis of HR+ and HER2-metastatic breast cancer, treated with any CDK 4–6 inhibitor, were included in the study. Demographic and clinical characteristics of the patients were recorded. We aimed to determine progression-free survival (PFS) time, response rates and drug related side effects.
Results
A total 25 patients from 14 institutions were recruited. The mean age at diagnosis was 57 years. Median follow-up was 19.53 (95% CI: 14.04–25.02) months. The overall response rate was 60%. While the median PFS was 20.6 months in the whole cohort, it wasn't reached in those using CDK 4–6 inhibitors in first line and 10 months in the subsequent lines (p:0.009). No new adverse events were encountered.
Conclusion
In our study, we found that CDK 4–6 inhibitors are effective and safe options in men with HR+ and HER2-metastatic breast cancer as in women. Our results support the use of CDK 4–6 inhibitor-based combinations in the first-line treatment of HR+ and HER2-metastatic male breast cancer.
Keywords: Male breast cancer, Cyclin-dependent kinase 4–6 inhibitors, Palbociclib, Ribociclib
Highlights
-
•
CDK 4–6 inhibitors are effective and safe agents for male patients.
-
•
They should be used in the first line treatment for the most effective results.
-
•
Palbociclib had numerically better, statistically similar progression-free survival time to ribociclib.
1. Background
Breast cancer is the most common cancer in women in the United States and the second most common cancer causing death. However, male breast cancers accounting for less than 1% of all diagnosed cases [1,2]. According to the estimates of the American Cancer Society, 2710 male patients are expected to be diagnosed and 530 male patients to die with breast cancer in 2022 [1]. Male breast cancer is very rare compared to female breast cancer and there are differences in histological subtypes [3]. Over 80% of the male breast cancers were hormone receptor positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) disease, while the other subtypes consist less than 20% of the cases [3].
Due to the rarity of male breast cancer, phase 3 clinical trials could not be conducted. Treatment management is guided by indirect results from phase 3 clinical trials in female patients. If aromatase inhibitors are to be used in the treatment of advanced male breast cancer, it is recommended to be used together with gonadotropin releasing hormone (GnRH) analogs, and the efficacy of fulvestrant is thought to be similar in male and female patients [4,5]. There are no clinical studies on the use of cyclin-dependent kinase 4–6 (CDK 4–6) inhibitors in male patients, which are the preferred treatment agents in the treatment of metastatic breast cancer. Both palbociclib, abemaciclib, and ribociclib has been demonstrated to improve progression-free survival (PFS) in studies conducted in the large-scale clinical trials in female patients with metastatic HR+, HER2-metastatic breast cancer and became the standard of care [[6], [7], [8]].
The CDK4/6 inhibitors block the transition of the cell cycle from the G1 phase to the S phase and efficacy and side effects are not expected to differ in male and female [9]. It was also used in male patients in two phase-1 studies in solid tumors and non-Hodgkin Lymphoma, and no difference was observed in safety profile [10,11].
Case-reports and a retrospective study of palbociclib are available in the literature on the use of CDK 4–6 inhibitors in male breast cancer patients [12,13]. There are no studies on the use of other CDK 4–6 inhibitors. Therefore, our study aimed to investigate the efficacy and safety of CDK 4–6 inhibitors in male breast cancers in a multicenter cohort including patients treated with several CDK 4–6 inhibitors.
2. Materials and methods
In our study, the data of 25 male patients with HR+ and HER2-breast cancer who were treated with any CDK 4–6 inhibitor in 14 tertiary centers from Turkey between 2019 and 2022 were retrospectively evaluated. Demographic characteristics of the patients, metastatic sites at the time of diagnosis, treatments before CDK 4–6 inhibitors, response to treatment with CDK 4–6 inhibitors, drug-related side effects, and PFS data were recorded.
The primary endpoint was PFS and defined as the time between the onset of CDK 4–6 inhibitors and the date of progression or death. The secondary endpoints were response rates and safety.
Response rates were assessed according to “Response evaluation criteria in solid tumors” (RECIST) 1.1. “Common Terminology Criteria for Adverse Events” (CTCAE version 4.03) was used to evaluate AEs.
IBM SPSS Statistics Version 22 package program was used for statistical analysis. The association of clinical factors with PFS was examined with Kaplan-Meier curves. The survival times were reported with medians and 95% confidence intervals (CI). Cox's regression analysis was not performed due to insufficient number of patients. A p value of <0.05 was considered statistically significant.
The ethical approval obtained from the Local Research Ethics Committee of Hacettepe University School of Medicine, with decision number of GO/22732. All procedures and stages in this multicenter and retrospective study were carried out in line with the World Medical Association Declaration of Helsinki, “Ethical Principles for Medical Research Involving Human Subjects”.
3. Results
3.1. Baseline characteristics
A total of 25 male patients were included in this study. The mean age at starting CDK 4–6 inhibitors was 57.25 ± 12.55 years. All of the patients had an Eastern Cooperative Oncology Group (ECOG) 0 or 1 performance status. All patients used gonadotropin releasing hormone (GnRH) and fulvestrant (n = 9) or aromatase inhibitor (n = 16) together with CDK 4–6 inhibitor. While 16 of the patients used palbociclib, 9 patients used ribociclib. Twelve patients used CDK 4–6 inhibitors in the first line, 10 patients used in the second line and 3 patients used in the subsequent line. Of the patients, 12 had de novo metastatic disease and 13 had recurrent disease. At the time of initiation of CDK 4–6 inhibitor therapy, 17 patients had bone metastases, 4 patients had liver metastases, and 15 patients had lung metastases. Demographic and clinical characteristics of the patients are shown in Table 1. The BRCA analysis results were not available.
Table 1.
Baseline characteristics.
| Characteristics | n | % |
|---|---|---|
| Age (mean ± standard deviation) | 57.25 ± 12.55 | |
|
ECOG score 0 1 |
18 7 |
72 28 |
|
Treatment agent Palbociclib Ribociclib |
16 9 |
64 36 |
| Bone metastasis | 17 | 68 |
| Liver metastasis | 4 | 16 |
| Lung Metastasis | 15 | 60 |
|
Recurrent disease De novo metastatic disease |
13 12 |
52 48 |
|
Treatment line (for metastatic disease) 1 2 3 |
12 10 3 |
48 40 12 |
|
Combination (CDK 4–6 inhibitor plus) Aromatase inhibitors + GnRH analogs Fulvestrant + GnRH analogs |
16 9 |
64 36 |
3.2. Survival outcomes and response rates
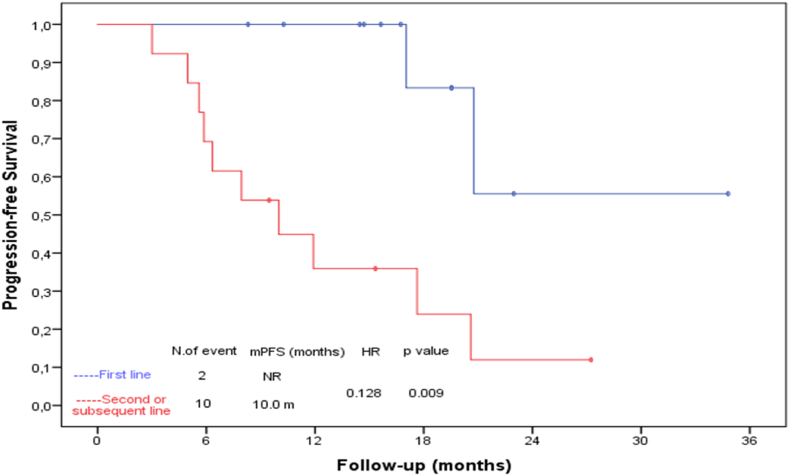
At the median 19.53 (95% CI: 14.04–25.02) months follow-up 6 (24%) patients passed away. The disease progressed in 12 patients and the median PFS was 20.6 months (95% CI: 15.37–25.83) in the whole group. No statistically significant difference was found between palbociclib and ribociclib in terms of PFS (mPFS: 20.6 months (95% CI: 13.73–27.46) vs 11.9 months (95% CI: 3.85–19.95) respectively, p:0.085). Patients using the first line; had a better PFS than the patients who used it on the second and later lines (mPFS: NA vs 10 months (95% CI: 3.90–16.09) respectively, p:0.009). Kaplan-Meier estimates for PFS is shown in Fig. 1. PFS of 9 patients using palbociclib in first-line therapy was not achieved, while PFS of 3 patients using ribociclib was 20.76 months (95% CI: NA-NA p: 0.617). Four patients with liver metastases had a significantly shorter PFS than the others (mPFS: 20.6 months (95% CI: 16.72–24.47) vs 4.96 months (95% CI: 2.15–7.77) respectively, p:0.003). There was no difference in PFS between de novo metastatic disease and recurrent disease (mPFS: 20.6 months (95% CI: 8.13–33.06) vs 17.6 months (95% CI: 6.62–28.64), p:0.348). PFS was 20.6 months when used with an aromatase inhibitor, and 17 months (95% CI:4.50–29.56) with fulvestrant (p:0.119).
Fig. 1.
The relationship between progression-free survival and treatment line.
In Kaplan Meier analysis, only the line of treatment and the presence of liver metastasis were found to be associated with PFS. COX regression analysis was not performed as there were only 4 patients with liver metastases.
The median overall survival (OS) time could not be reached. Survival at 24 months was 68.8% in first line treatments, and 63.9% in later lines. There were 2 deaths in the first-line treatment group and 4 deaths in the next-line groups. All deaths were considered as disease-related. The ages of the two patients who died in first line group were 50 (at 6 months of treatment and there was bone and lung metastases at the time of diagnosis) and 68 (at 21 months of treatment).
Complete and partial responses were obtained in 3 and 12 patients, respectively. While stable disease was observed in 7 and progressive disease was observed in 3 patients. The overall response rate (ORR) was 60% and the disease control rate was 88%. The best responses with CDK 4–6 inhibitors are shown in Table 2.
Table 2.
Best Response with CDK 4–6 inhibitors.
| Response | n | % |
|---|---|---|
| Complete Remission (CR) | 3 | 12 |
| Partial Remission (PR) | 12 | 48 |
| Stable Disease (SD) | 7 | 28 |
| Progressive Disease | 3 | 12 |
| Objective Response Rate (CR + PR) | 15 | 60 |
| Disease Control Rate (CR + PR + SD) | 22 | 88 |
3.3. Safety
No new adverse events (AEs) were observed in all patients. The most common side effect was neutropenia (88%), others include fatigue (40%), anemia (28%), thrombocytopenia (20%), increased alanine aminotransferase (ALT) (12%), asthenia (15%), and elevated creatinine (12%) level. Grade 3 and above AEs were neutropenia (28%), anemia (4%), and increased ALT (4%). All AEs are shown in Table 3. Neutropenic fever was observed in 1 patient and did not cause death. Dose reductions and treatment interruptions were recorded in 9 patients. There was no difference in PFS between groups with and without dose reductions and treatment interruptions (95% CI: 17 months 9.93–24.13) vs 20.76 months (95% CI: 10.25–31.27) respectively, p:0.45). One patient could not tolerate the drug due to fatigue. No drug-related death was encountered.
Table 3.
Adverse events according to “Common Terminology Criteria for Adverse Events”.
| Adverse events | Grade 1–2, n (%) | Grade 3–4, n (%) |
|---|---|---|
| Any adverse events | 24 (96) | 8 (32) |
| Neutropenia | 15 (60) | 7 (28) |
| Anemia | 6 (24) | 1 (4) |
| Thrombocytopenia | 5 (20) | 0 (0) |
| ALT elevation | 2 (8) | 1 (4) |
| Asthenia | 3 (15) | 0 (0) |
| Creatinin elevation | 3 (12) | 0 (0) |
| QTc | 1 (4) | 0 (0) |
| Fatigue | 9 (36) | 1 (4) |
4. Discussion
To the best of our knowledge, this was the first study that assessed the effect of CDK 4–6 inhibitors (palbociclib and ribociclib) on the PFS and safety outcomes in male patients with metastatic breast cancer. In this study, we showed that CDK 4–6 inhibitors are effective and safe agents in the treatment of metastatic breast cancer in male patients as well as in females.
Although CDK 4–6 inhibitors revolutionized PFS and OS in female patients with HR+ and HER2-metastatic breast cancer, there are no phase 3 studies in male patients. In a retrospective analysis, which evaluated only palbociclib, it was shown that 26 patients using a combination of palbociclib and letrozole had a longer median duration of treatment than patients using letrozole [12]. However, only 12 patients had response evaluation in this study and the median duration of response was 9.4 months. Data on PFS were not available in this study [12]. There is a case report of male breast cancer using CDK 4–6; partial response was obtained in the 6th month of the treatment, but no information is available about the next follow-up [13]. In another case report, a complete response was obtained with the use of abemaciclib, fulvestrant and leuprolide in the second line treatment, and the treatment is still continuing at the 18th month of treatment [14]. In a case report published in 2021, partial response was achieved with palbociclib, letrozole, and leuprolide and PFS for more than 1 year was achieved as of the date of publication [15]. As seen in these case reports, there are promising results of CDK 4–6 inhibitors in metastatic male breast cancer.
In our study, PFS was 20 months in patients using palbociclib and 11 months in patients using ribociclib. While the PFS of 3 patients using ribociclib in first line treatment was 20.76 months, it could not be reached at 34 months in 9 patients using palbociclib. Due to the small number of patients using ribociclib in first line treatment in our study, it is difficult to say that palbociclib is a more effective agent in male breast cancer. It is clear that, the prospective studies with larger numbers of patients are needed.
In the MONALEESA-3 study, second-line treatment in postmenopausal female patients; 9 months of PFS is achieved with fulvestrant, while the PFS increases to 14 months with the addition of ribociclib to the treatment [16]. In the PALOMA 3 study, 12-month PFS was achieved with the combination of palbociclib and fulvestrant [17]. In our study; similar to these two phase-3 studies conducted in postmenopausal women, a 10 months PFS was achieved with the use of CDK 4–6 in second-line therapy. In the treatment of HR+, HER2-metastatic breast cancer in female patients, the most effective treatment choice in the first line is considered to be combination therapies containing CDK 4–6. In MONALEESA-3, PALOMA 2 and MONARCH 3 studies; with the combination of CDK 4–6 inhibitors with fulvestrant or aromatase inhibitor in first line, progression-free survival of around 24 months is provided [6,16,18]. In our study, a progression-free survival time of over 20 months was found with use as the first-line therapy. Considering the scarcity of treatment options in male breast cancer, the use of CDK 4–6 inhibitors in first-line therapy should be recommended as in female patients.
The ORR in MONALEESA 3, PALOMA 2 and MONARCH 3 trial were 40%, 55%, 59% and disease control rates (DCR) in PALOMA 2 and MONARCH 3 trial were 84%, 78% respectively [6,16,18]. In our trial, ORR and DCR were 60% and 88%. We have shown that palbociclib and ribociclib provide an effective treatment response in male patients as well as in female patients.
In our study, no new adverse events of CDK 4–6 inhibitors were found, except for the known ones. Our safety data in male patients were comparable to phase 3 studies in female patients [6,16,18].
Since the reimbursement of CDK 4–6 inhibitors is approved later in our country than in the USA and European countries, the number of our patients is limited to 25.
Our study has several limitations, mainly based on its retrospective nature. However, considering the low incidence of male breast cancer, it should be kept in mind that it will be difficult to conduct a prospective study. The number of our patients was not sufficient to examine the relationship between PFS and several clinical features due to limited patient numbers in most subgroups. It was not possible to compare the two treatments because the patients included in the study used palbociclib and ribociclib at different treatment lines. Our study had a 19.53 months follow-up period, and longer follow-up periods are needed for a more accurate evaluation of efficacy and safety data.
5. Conclusion
This is the first study to evaluate the progression-free survival data of ribociclib and palbociclib in male patients with metastatic breast cancer, and we showed that these agents can be used effectively and safely both in first-line therapy and in later lines.
Funding
The authors received no financial support for this article.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of Hacettepe University.
Declaration of competing interest
The authors have declared no conflicts of interest.
References
- 1.Xia C., et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miao H., et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29(33):4381–4386. doi: 10.1200/JCO.2011.36.8902. [DOI] [PubMed] [Google Scholar]
- 3.Chavez‐MacGregor M., et al. Male breast cancer according to tumor subtype and race: a population‐based study. Cancer. 2013;119(9):1611–1617. doi: 10.1002/cncr.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y., et al. Breast cancer screening in high-risk men: a 12-year longitudinal observational study of male breast imaging utilization and outcomes. Radiology. 2019;293(2):282–291. doi: 10.1148/radiol.2019190971. [DOI] [PubMed] [Google Scholar]
- 5.Zagouri F., et al. Fulvestrant and male breast cancer: a case series. Ann Oncol. 2013;24(1):265–266. doi: 10.1093/annonc/mds597. [DOI] [PubMed] [Google Scholar]
- 6.Finn R.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 7.Johnston S., et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5(1):1–8. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hortobagyi G.N., et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 9.VanArsdale T., et al. Molecular pathways: targeting the cyclin D–CDK4/6 axis for cancer treatment. Clin Cancer Res. 2015;21(13):2905–2910. doi: 10.1158/1078-0432.CCR-14-0816. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz G., et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1) Br J Cancer. 2011;104(12):1862–1868. doi: 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty K.T., et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced CancerPhase I, dose-escalation trial of PD 0332991, 4-week cycle. Clin Cancer Res. 2012;18(2):568–576. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- 12.Kraus A.L., et al. Real‐world data of palbociclib in combination with endocrine therapy for the treatment of metastatic breast cancer in men. Clin Pharmacol Therapeut. 2022;111(1):302–309. doi: 10.1002/cpt.2454. [DOI] [PubMed] [Google Scholar]
- 13.Sorscher S. A first case of male breast cancer responding to combined aromatase inhibitor/palbociclib therapy. Int J Cancer Clin Res. 2016;3(5):69. [Google Scholar]
- 14.Hansra D., et al. Male patient with metastatic stage IV breast cancer achieves complete remission on second line Abemaciclib, Fulvestrant and Leuprolide: a case report. Molecul Clin Oncol. 2020;12(2):120–125. doi: 10.3892/mco.2019.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zattarin E., et al. Prolonged benefit from palbociclib plus letrozole in heavily pretreated advanced male breast cancer: case report. Tumori J. 2021;107(6):NP15–NP19. doi: 10.1177/0300891620976981. [DOI] [PubMed] [Google Scholar]
- 16.Slamon D.J., et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 17.Turner N.C., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 18.Goetz M.P., et al. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]