Abstract
Objectives
Rapid plasma reagin (RPR) and Treponema pallidum (TP) antibody test kits are often used to diagnose syphilis, although the relationship between their measured values is unclear. We aimed to reveal the relevance of these kits’ results.
Design and methods
In all, 143 sera from 110 patients were tested using 12 TP kits and 5 RPR kits and the results compared.
Results
The specificity and sensitivity of RPR kits were 81–96% and 95–100%, respectively. The correlation coefficients (0.849–0.934) considerably differed between the manual RPR card test and latex agglutination (LA) assay kits. The following sensitivities were obtained: 82–91% for TP fluorescent treponemal antibody absorption assay (FTA-ABS), TP hemagglutination assay (HA), and TP particle agglutination assay (PA); 94–95% for TP LAs; and 92–100% for chemiluminescent immunoassay (CLIA), chemiluminescent enzyme immunoassay (CLEIA), and immunochromatography assay (IC). Correlation coefficients between TP kits were 0.753–0.974, and the measured values varied. Changes in RPR and quantifiable TP kits were the same for patients with reinfected syphilis and with syphilis under treatment.
Conclusions
RPR tests had lower specificity than TP antibody tests. RPR card test and RPR LAs had similar specificity and sensitivity, but their measured values were different. RPR should be measured using automatic RPR LA without setting the upper limit of the reported value. RPR LA should also be standardized. The sensitivity of TP antibody was better in CLIA, CLEIA, and IC than in FTA-ABS, HA, PA, and LA. Therefore, TP antibody kits should be standardized and quantified.
Keywords: Syphilis, Nontreponemal antibody test, Treponemal antibody test
Highlights
-
•
RPR CT and four RPR LAs have similar specificity and sensitivity.
-
•
RPR CT and four RPR LAs have different measured values.
-
•
CLIA, CLEIA, and IC have more sensitive TP antibodies than other tests.
-
•
The measured values of 12 TP antibody kits vary.
-
•
Quantitative TP antibodies are similar to RPR in the clinical course of syphilis.
Abbreviations
- CLEIA
chemiluminescent enzyme immunoassay
- CLIA
chemiluminescent immunoassay
- CT
card test assay
- FTA-ABS
fluorescent treponemal antibody absorption assay
- HA
hemagglutination assay
- IC
immunochromatography assay
- LA
latex agglutination assay
- PA
particle agglutination assay
- RPR
rapid plasma reagin
- TP
Treponema pallidum subspecies pallidum
- VDRL
venereal disease research laboratory
1. Introduction
Syphilis is a multisystem infection caused by Treponema pallidum subspecies pallidum (TP). It is commonly sexually transmitted but can also be vertically transmitted during pregnancy, causing congenital syphilis [1]. It is a global public health problem.
45 million people were infected with syphilis in 2015 [2]. Each year, six million new cases of syphilis occur in low- and middle-income countries [3]. Furthermore, 300,000 fetal and neonatal deaths are attributed to syphilis, and an additional 215,000 infants are at increased risk of early death [4]. In Japan, the number of syphilis diagnoses increased from 500 to 900 from 2000 to 2012; it increased more than five times from 1,228 in 2013 to 7,002 in 2018. This increase was especially prominent in large cities, such as Tokyo [5].
Syphilis diagnosis depends on a combination of clinical and laboratory criteria [2]. Therefore, an accurate diagnostic test is required. However, direct TP detection tests, such as PCR tests, are not commercially available. The presumptive laboratory diagnosis of syphilis is based on the results of two serological tests: nontreponemal (venereal disease research laboratory [VDRL] or rapid plasma reagin [RPR] tests) and treponemal tests. Usually, nontreponemal test antibody titers correlate with disease activity and are used to monitor treatment responses; reactive results are then confirmed with a treponemal test.
RPR tests can be carried out by automated quantification latex agglutination (LA) or manual card test (CT) assays. The Center for Disease Control and Prevention recommends that nontreponemal tests should be reported quantitatively. A four-fold change in titer, equivalent to a change of two dilutions (e.g., from 1:16 to 1:4), is necessary to demonstrate a clinically significant difference between two nontreponemal test results obtained using the same serologic test, preferably by the same laboratory [2]. However, the same nontreponemal test is sometimes unavailable for patients referred from different facilities during syphilis treatment.
Treponemal tests may be performed using many methods, such as fluorescent treponemal antibody absorption assay (FTA-ABS), hemagglutination assay (HA), particle agglutination assay (PA), immunochromatography assay (IC), LA, chemiluminescent immunoassay (CLIA), and chemiluminescent enzyme immunoassay (CLEIA). TP FTA-ABS has been considered the reference method for treponemal antibody tests [6].
Many nontreponemal and treponemal tests are used to diagnose syphilis, although they yield different measured values, and the relationship between these values is unclear [6,7]. However, studies have yet to compare these values under routine clinical conditions.
Therefore, in this study, to reveal the relevance of treponemal and nontreponemal kit results, we compared the measured values of 12 commercial treponemal antibody kits and 5 commercial nontreponemal antibody kits often used in Japan by examining samples that were ordered to the clinical laboratory of Kobe University Hospital.
2. Materials and methods
2.1. Samples
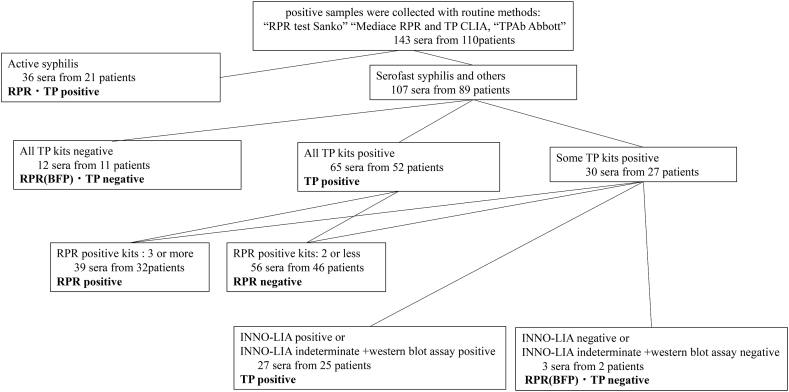
A total of 143 sera from 110 patients were collected from patients with suspected syphilis at Kobe University Hospital from July to December 2018. Nontreponemal or treponemal positive samples were collected by routine methods: RPR CT, RPR test Sanko (Sekisui Medical, Tokyo, Japan); RPR LA, Mediace RPR (Kyokuto Pharmaceutical, Tokyo, Japan); and TP CLIA, TPAb Abbott (Abbott, Chicago, IL, USA). Clinical diagnoses of active syphilis, serofast syphilis, and others were performed in accordance with clinical and laboratory criteria through a review of electronic medical records by a board-certified ID physician. Serofast syphilis was defined by prior treatment history in the documented chart and known history of RPR titer. The sera of RPR biological false positives were defined as negative for TP. RPR was defined as negative once three or more negative RPR assays were performed after receiving RPR biological false-positive results. TP negatives were defined as negative for all TP kits. TP positive results were confirmed using the INNO-LIA syphilis score assay (INNO, Fujirebio Europe, Gent, Belgium); samples were selected through confirmatory [8] and western blot assays on samples that were not positive in all TP kits. TP positive samples that were INNO-LIA positive or INNO-LIA indeterminate were confirmed with a target band detected via western blot assay (Fig. 1).
Fig. 1.
Classification of sera as negative or positive after Rapid Plasma Reagin test and Treponema pallidum antibody test. Sera were measured using 12 TP kits and 5 RPR kits. BFP: biological false-positive; RPR: rapid plasma reagin test; TP: Treponema pallidum antibody.
This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Ethics Committee at the Kobe University Graduate School of Medicine [approval number 180295].
2.2. Detections of RPR and TP antibodies
Five RPR kits, namely, the RPR test Sanko (Sekisui Medical), Mediace RPR (Kyokuto), RAPIDIA Auto RPR (Fujirebio), Accuras Auto RPR (Shino-Test, Tokyo, Japan), and LASAY (Denka Seiken, Tokyo, Japan), were used. Twelve TP antibody kits, namely, FTA-ABS test-SG kit (KW; Japan BCG Laboratory, Tokyo, Japan), SERODIA-TP (Fujirebio), SERODIA-TP PA (Fujirebio), Treponema pallidum latex agglutination (Kyokuto), RAPIDIA Auto TP (Fujirebio), LASAY auto TP Ab (Denka Seiken), Accuras Auto TP (Syphilis)-A (Shino-Test), TPAb Abbott (Abbott), Lumipulse Presto TP (Fujirebio), HISCL TPAb (Sysmex, Kobe, Japan), ESPLINE TP (Fujirebio), and DAINA SCREEN TPAb (Abbott Diagnostics Medical, Tokyo, Japan), were used in this study. RPR test Sanko and Mediace RPR were obtained from different companies, but the manufacturing company was the same. All tests were performed in accordance with the manufacturer's instructions. RPR LA kits were quantitative tests with a reported upper limit of 20 R U. In the TP antibody tests, three TP LA kits, namely, Treponema pallidum latex agglutination, RAPIDIA Auto TP, and LASAY auto TP Ab were quantitative tests, and only Treponema pallidum latex agglutination did not require an upper limit. The details are shown in Table 1, Table 2.
Table 1.
Characteristics of five commercial kits of rapid plasma reagin test.
| Reagent | RPR test "SANKO" | Mediace RPR | RAPIDIA Auto RPR | Accuras Auto RPR | LASAY |
|---|---|---|---|---|---|
| Principle | CT | LA | LA | LA | LA |
| Automation/manual | Manual | Automation | Automation | Automation | Automation |
| Semi-quantitative | Quantitative | Quantitative | Quantitative | Quantitative | |
| Cut-off value | 1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Unit | titer | RU | RU | RU | RU |
| Upper limit of reported value | none | 20 | 20 | 20 | 20 |
CT: Card test, LA: latex agglutination.
Table 2.
Characteristics of 12 commercial kits of Treponema pallidum antibody test.
| Reagent | FTA-ABS test-SG KIT (KW) | SERODIA-TP | SERODIA-TP・PA | Treponema pallidum latex agglutination | RAPIDIA Auto TP | LASAY auto TP Ab | Accuras Auto TP (Syphilis)-A | TPAb Abbott | Lumipulse Presto TP | HISCL TPAb | ESPLINE TP | DAINA SCREEN・TPAb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Principle | FTA-ABS | HA | PA | LA | LA | LA | LA | CLIA | CLEIA | CLEIA | IC | IC |
| Antigen | Native | Native | Native | Native | Recombinant | Recombinant | Recombinant | Recombinant | Recombinant | Recombinant | Recombinant | Native |
| Automation/manual | Manual | Manual | Manual | Automation | Automation | Automation | Automation | Automation | Automation | Automation | Manual | Manual |
| Semi-quantitative | Semi-quantitative | Semi-quantitative | Quantitative | Quantitative | Quantitative | Qualitative | Qualitative | Qualitative | Qualitative | Qualitative | Qualitative | |
| Cut-off value | 20 | 80 | 80 | 10 | 10 | 20 | 1.0 | 1.0 | 1.0 | 1.0 | – | – |
| Unit | titer | titer | titer | TU | U/mL | U/mL | COI | S/CO | COI | COI | – | – |
| Upper limit of reported value | None | 20480 | 20480 | None | 500 | 500 | 20 | 20 | 100 | 200 | – | – |
FTA-ABS: Fluorescent treponemal antibody absorption assay; HA: hemagglutination; PA: particle agglutination; LA: latex agglutination; CLIA: chemiluminescent immunoassay; CLEIA: chemiluminescent enzyme immunoassay; and IC: immunochromatography.
2.3. Correlation with RPR and TP kits
A total of 143 samples from 110 patients were tested with RPR and TP kits, and their measured values were analyzed. Correlation coefficients were calculated via Spearman's rank test.
3. Results
3.1. RPR kits
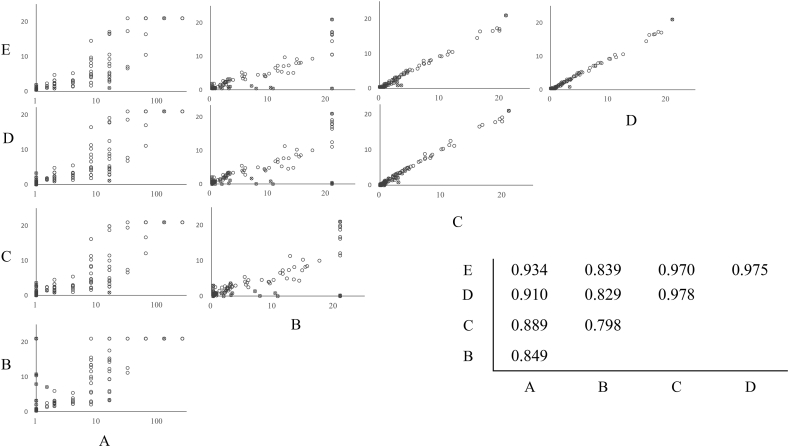
In active syphilis, most samples tested positive when RPR kits were used. Only one sample, which was obtained during medical treatment, was negative when tested with RPR test Sanko (CT) and Mediace RPR (LA). The other RPR LA kits yielded low positive values. The specificities, sensitivities, and agreements of these kits were 81–96%, 95–100%, and 88–98%, respectively. The agreements of RAPIDIA Auto RPR (LA), Accuras Auto RPR (LA), and LASAY (LA) were over 95% (Table 3). The correlation among RAPIDIA Auto RPR, Accuras Auto RPR, and LASAY was good, with a correlation coefficient ≥0.97. The correlation coefficient between Mediace RPR and other LA kits ranged from 0.798 to 0.839, and measured values differed. The correlation coefficient between the RPR test Sanko (manual CT) and LA kits ranged from 0.849 to 0.934, and their measured values largely differed. CT did not have an upper limit for the reported values. However, the upper limit for the reported values of LA kits was 20; therefore, it could not reflect the pathological condition at high values (Fig. 2). Furthermore, 14 (9.8%) samples from 12 (11.0%) patients were biological false positives. The measured values of biological false positives varied from 1 to 128 depending on the kit. Biological false-positive samples were observed in cases such as patients with immune disorders and pregnant women (Table 4).
Table 3.
Specificity, sensitivity, and agreement of Rapid Plasma Reagin Tests for the detection of syphilis.
| Positive | Negative | Sensitivity | Specificity | % Agreement | ||
|---|---|---|---|---|---|---|
| RPR test "SANKO" (CT) | ||||||
| Reactive | 71 | 4 | 95 | 94 | 94 | |
| Non reactive | 4 | 64 | ||||
| Mediace RPR (LA) | ||||||
| Reactive | 71 | 13 | 95 | 81 | 88 | |
| Non reactive | 4 | 55 | ||||
| RAPIDIA Auto RPR (LA) | ||||||
| Reactive | 74 | 5 | 99 | 93 | 96 | |
| Non reactive | 1 | 63 | ||||
| Accuras Auto RPR (LA) | ||||||
| Reactive | 75 | 6 | 100 | 91 | 96 | |
| Non reactive | 0 | 62 | ||||
| LASAY (LA) | ||||||
| Reactive | 75 | 3 | 100 | 96 | 98 | |
| Non reactive | 0 | 65 | ||||
CT: card test; LA: latex agglutination.
Fig. 2.
Correlations between Rapid Plasma Reagin tests. Spearman's correlation coefficients are shown in the insert on the graph. A: RPR test "SANKO" (card test), B: Mediace RPR (latex agglutination (LA)), C: RAPIDIA Auto RPR (LA), D: Accuras Auto RPR (LA), E: LASAY (LA) o: Positive; x: Negative.
Table 4.
Biological false positive by rapid plasma reagin.
| Sample number | CT |
LA |
LA |
LA |
LA |
||
|---|---|---|---|---|---|---|---|
| RPR test "SANKO" |
Mediace RPR |
RAPIDIA Auto RPR |
Accuras Auto RPR |
LASAY |
|||
| Cut-off value | 1 titer | 1.0 RU | 1.0 RU | 1.0 RU | 1.0 RU | ||
| 6 | 16 + | 3.4 + | 0.8 - | 1.1 + | 1.0 + | Myasthenia gravis | |
| 39 | 1 + | 7.1 + | 1.4 + | 1.7 + | 1.2 + | Systemic lupus erythematosus | |
| 54 | (−) | >20 + | 0.0 - | 0.0 - | <0.5 - | Antiphospholipid antibody syndrome | |
| 60 | (−) | 3.2 + | 0.3 - | 0.2 - | <0.5 - | Ulcerative colitis | |
| 61 | (−) | 2 + | 0.0 - | 0.0 - | <0.5 - | Aortic dissection | |
| #85 | (−) | <0.4 - | 2.9 + | 2.1 + | 1.9 + | Pregnancy | |
| 89 | (−) | 7.9 + | 0.1 - | 0.1 - | <0.5 - | Thyroid-associated ophthalmopathy | |
| ##90 | (−) | >20 + | 0.1 - | 0.2 - | <0.5 - | Systemic lupus erythematosus | |
| 93 | 128 + | >20 + | >20 + | >20 + | >20 + | Cataract | |
| 96 | (−) | 3.1 + | 3.6 + | 3.3 + | 0.9 - | Uveitis | |
| ##103 | (−) | >20 + | 0.2 - | 0.4 - | <0.5 - | Systemic lupus erythematosus | |
| #144 | (−) | <0.4 - | 2.0 + | 1.4 + | 1.4 + | Pregnancy | |
| 145 | (−) | 10.8 + | 0.0 - | 0.0 - | <0.5 - | Fatty liver | |
| 155 | (−) | 10.4 + | 0.8 - | 0.8 - | 0.7 - | Diabetes |
CT: Card test; LA: latex agglutination; #: Same patient.
3.2. TP kits
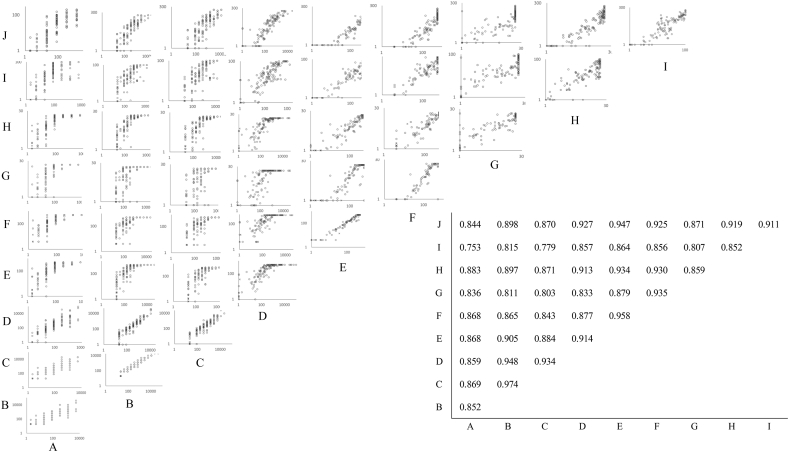
In active syphilis, all samples were positive when tested with TP kits. FTA-ABS, HA, and PA had 100% specificity, 82–91% sensitivity, and <92% agreements. However, these were lower than those of LAs, which had specificity, sensitivity, and agreement of 93–100%, 94–95%, and 94–96%, respectively. CLIA and CLEIA had specificity, sensitivity, and agreement of 80–100%, 92–100%, and 93–99%, respectively. ICs had specificity, sensitivity, and agreement of 87–100%, 98–100%, and 98–99%, respectively (Table 5). Almost all correlation coefficients between TP kits were low, ranging from 0.753 to 0.974. Measured values varied among kits. The correlation between SERODIA-TP (HA) and SERODIA-TP PA (PA), both produced by Fujirebio, was good (r = 0.974) (Fig. 3). The samples of 3 sera from 2 patients were false-positive, and their INNO-LIA scores were indeterminate (Table 6).
Table 5.
Specificity, sensitivity, and agreement of Treponema pallidum antibody tests for the detection of syphilis.
| Positive | Negative | Sensitivity | Specificity | % Agreement | ||
|---|---|---|---|---|---|---|
| FTA-ABS test-SG KIT (FTA-ABS) | ||||||
| Reactive | 113 | 0 | 88 | 100 | 90 | |
| Non reactive | 15 | 15 | ||||
| SERODIA-TP (HA) | ||||||
| Reactive | 105 | 0 | 82 | 100 | 84 | |
| Non reactive | 23 | 15 | ||||
| SERODIA-TP・PA (PA) | ||||||
| Reactive | 116 | 0 | 91 | 100 | 92 | |
| Non reactive | 12 | 15 | ||||
| Treponema pallidum latex agglutination (LA) | ||||||
| Reactive | 122 | 0 | 95 | 100 | 96 | |
| Non reactive | 6 | 15 | ||||
| RAPIDIA Auto TP (LA) | ||||||
| Reactive | 122 | 1 | 95 | 93 | 95 | |
| Non reactive | 6 | 14 | ||||
| LASAY auto TP Ab (LA) | ||||||
| Reactive | 120 | 0 | 94 | 100 | 94 | |
| Non reactive | 8 | 15 | ||||
| Accuras Auto TP (Syphilis)-A (LA) | ||||||
| Reactive | 121 | 0 | 95 | 100 | 95 | |
| Non reactive | 7 | 15 | ||||
| TPAb・Abbott (CLIA) | ||||||
| Reactive | 128 | 3 | 100 | 80 | 98 | |
| Non reactive | 0 | 12 | ||||
| Lumipulse Presto TP (CLEIA) | ||||||
| Reactive | 126 | 0 | 98 | 100 | 99 | |
| Non reactive | 2 | 15 | ||||
| HISCL TPAb (CLEIA) | ||||||
| Reactive | 118 | 0 | 92 | 100 | 93 | |
| Non reactive | 10 | 15 | ||||
| ESPLINE TP (IC) | ||||||
| Reactive | 128 | 2 | 100 | 87 | 99 | |
| Non reactive | 0 | 13 | ||||
| DAINA SCREEN・TPAb (IC) | ||||||
| Reactive | 125 | 0 | 98 | 100 | 98 | |
| Non reactive | 3 | 15 |
CLEIA: chemiluminescent enzyme immunoassay; CLIA: chemiluminescent immunoassay; FTA-ABS: fluorescent treponemal antibody absorption assay; HA: hemagglutination; IC: immunochromatography; LA: latex agglutination; PA: particle agglutination.
Fig. 3.
Correlation between Treponema pallidum antibody tests. Spearman's correlation coefficients are shown in the insert on the graph. A: FTA-ABS test-SG KIT (FTA-ABS test-SG KIT), B: SERODIA-TP (hemagglutination), C:SERODIA-TP-PA(particle agglutination), D: Treponema pallidum latex agglutination(LA), E: LASAY (latex agglutination (LA)), E: RAPIDIA Auto TP (LA), F: LASAY auto TP Ab (LA), G: Accuras Auto TP (Syphilis)-A (LA), H: TPAb-Abbott(chemiluminescent immunoassay (CLIA)), I: Lumipulse Presto TP (chemiluminescent enzyme immunoassay (CLEIA)), J: HISCL TPAb (CLEIA). o: Positive; x: Negative.
Table 6.
False positives reported by Treponema pallidum antibody test.
| Sample number | FTA-ABS |
HA |
PA |
LA |
LA |
LA |
LA |
CLIA |
CLEIA |
CLEIA |
IC |
IC |
Line immunoassay |
Western blot |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FTA-ABS test-SG KIT (KW) |
SERODIA-TP |
SERODIA-TP・PA |
Treponema pallidum latex agglutination |
RAPIDIA Auto TP |
LASAY auto TP Ab |
Accuras Auto TP (Syphilis)-A |
TPAb・Abbott |
Lumipulse Presto TP |
HISCL TPAb |
ESPLINE TP |
DAINA SCREEN・TPAb |
INNO-LIA Score |
||||||||||
| Cut-off value | 20 titer | 80 titer | 80 titer | 10 TU | 10 U/mL | 20 U/mL | 1.0 COI | 1.0 S/CO | 1.0 COI | 1.0 COI | – | – | TpN17 | TpN47 | TpN15 | TmpA | Interpretation | TpN17 | TpN47 | TpN15 | ||
| #85 | (−) | (−) | (−) | 0.3 - | 0 - | 4 - | 0.0 - | 1.63 + | 0.2 - | 0.1 - | + | (−) | (−) | 1+ | (−) | (−) | Indeterminate | (−) | (−) | (−) | Pregnancy | |
| #144 | (−) | (−) | (−) | 1.2 - | 35 + | 14 - | 0.0 - | 8.02 + | 0.6 - | 0.8 - | + | (−) | (−) | 2+ | (−) | (−) | Indeterminate | (−) | (±) | (−) | Pregnancy | |
| 153 | (−) | (−) | (−) | 0.0 - | 0 - | 4 - | 0.0 - | 1.28 + | 0.1 - | 0.0 - | (−) | (−) | (−) | (−) | 1+ | (−) | Indeterminate | (−) | (−) | (±) | Brain tumor | |
FTA-ABS: fluorescent treponemal antibody absorption assay, HA: hemagglutination, PA: particle agglutination, LA: latex agglutination, CLIA: chemiluminescent immunoassay, CLEIA: chemiluminescent enzyme immunoassay, IC: immunochromatography, #: same patient.
3.3. Clinical course
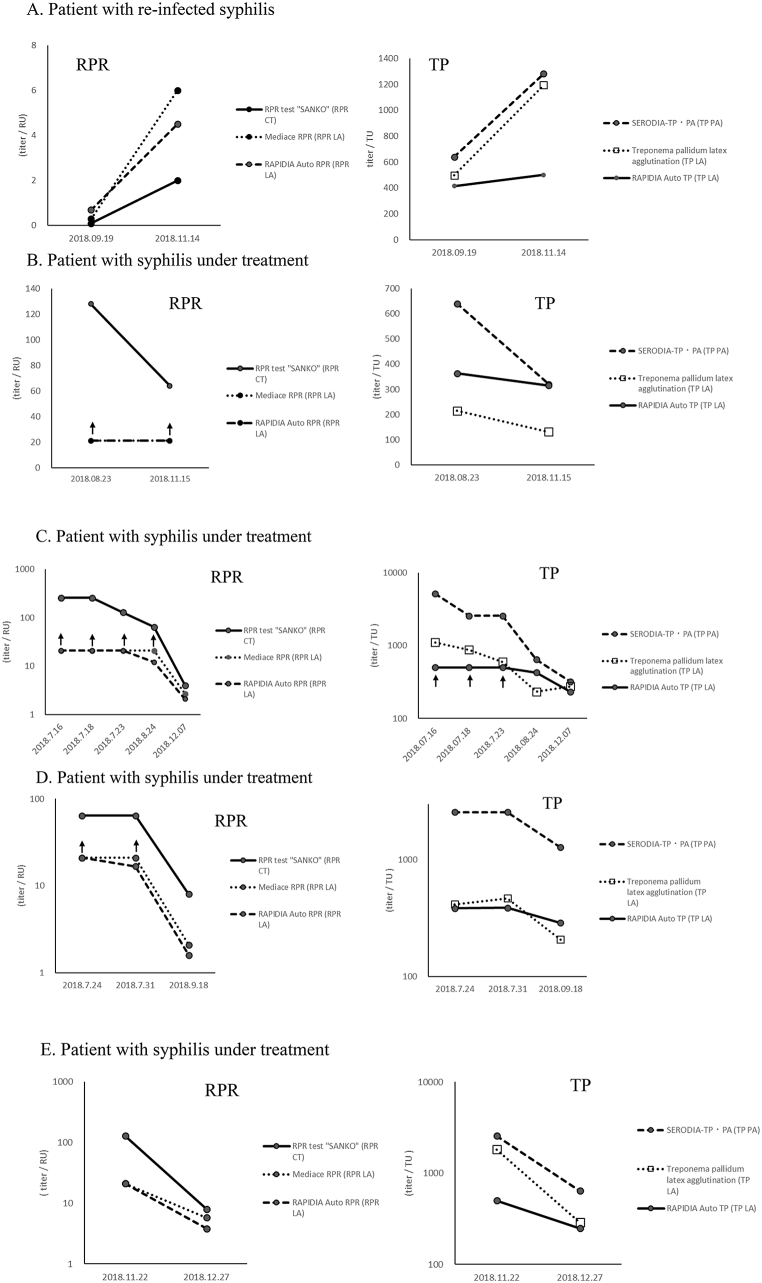
The responses of RPR kits, namely, RPR test Sanko (CT), Mediace RPR (LA), and RAPIDIA Auto RPR (LA), and quantifiable TP kits, namely, SERODIA-TP PA (PA), Treponema pallidum latex agglutination (LA), and RAPIDIA Auto TP (LA), in the clinical course of patients with syphilis were examined. Changes in RPR and TP kits were the same in patients with reinfected syphilis and those with syphilis under treatment (Fig. 4).
Fig. 4.
Clinical course of a patient with reinfected syphilis and patients with syphilis under treatment. RPR: Rapid plasma reagin, TP: Treponema pallidum antibody, CT: card test, LA: latex agglutination, PA: particle agglutination. ↑: Upper limit of reported value.
4. Discussion
Nontreponemal and treponemal antibody tests are important for the diagnosis and treatment of syphilis. In Japan, many kinds of RPR and TP kits are available. Therefore, we compared the measured values of 12 TP and 5 RPR commercial kits commonly used in Japan under routine clinical conditions. We used samples, including those from patients under treatment, ordered to the clinical laboratory of Kobe University Hospital.
RPR tests yield biological false-positives in some cases, such as in patients with systemic lupus erythematosus, pregnant women, and the general population [9]. In this study, biological false-positive samples were detected in patients with immune disorders, pregnant women, and others. The measured values of biological false positives varied from low to high depending on the kit. RPR kits had 81–96% specificity. Mediace RPR (LA) had the lowest specificity, whereas RPR test Sanko (CT) had the highest specificity. Positive samples were collected using Mediace RPR (LA), which is likely one of the reasons why Mediace RPR (LA) had low specificity. Nontreponemal antibody tests have lower sensitivity (72.6%) than do TP PA, but they yield 85.9% sensitivity in primary syphilis [10]. In this study, RPR kits had good sensitivity (97–100%). This study targeted active syphilis and serofast syphilis and did not include primary syphilis. The correlation coefficients among RAPIDIA Auto RPR (LA), Accuras Auto RPR (LA), and LASAY (LA) were ≥0.97. The manufacturers of these may have included the same antibody in each kit. However, the correlation coefficients with Mediace RPR (LA), and its correlation coefficient with RPR test Sanko (CT) in particular, were low, and measured values were very dissimilar to those from other kits. LA kits set an upper limit of the reported value; therefore, it could not reflect pathological conditions at higher values. Onoe et al. [7] reported that significant correlations were observed between most of the automated testing. This discrepancy can be explained as follows: in Japan, automated RPR tests usually require an upper limit of 20, but Onoe et al. [7] did not set an upper limit. Many RPR automated kits are commercially available, although many laboratories use the same automated RPR kit.
In Japan, some laboratories use automated RPR kits for screening; then, they confirm and semi-quantify their results using manual CT kits. In this study, the measured values of Sanko (CT) and LA kits did not match. Manual RPR CT has been regarded as the reference standard for nontreponemal tests [11]. An automated RPR LA is a good alternative to manual RPR CT for syphilis diagnosis and treatment response evaluation [12]. Measuring RPR LA titers via automated methods is more sensitive and effective than via manual RPR CT [7]. Therefore, we considered that manual CT kits were unnecessary, but automated RPR LA kits should be standardized, and the upper limit of the reported values of RPR LA kits should be eliminated.
TP antibody tests are performed using various methods. Their measured values differ depending on the kit despite the application of the same measurement principle. In this study, the number of negative samples was less; therefore, specificity could not be fully evaluated. However, the specificities were relatively good. TPAb Abbott (CLIA) had low specificity because it involves collecting positive samples. In this study, INNO-LIA and western blot were used with serofast sera to study sensitivity. Traditional FTA-ABS, HA, and PA had lower specificities than LA, CLIA, CLEIA, and IC except HISCL TPAb. FTA-ABS [6] and PA [13] are considered the gold standards of TP antibody. TP FTA-ABS showed poor sensitivity in primary syphilis, and TP PA is preferred to TP FTA-ABS to adjudicate discordant results with a reverse sequence algorithm [14]. CLIA, CLEIA, and IC are more sensitive and specific than PA. The positive rate of CLIA is higher than that of PA even at the first onset of syphilis [15]. In light of our results, we consider CLIA and CLEIA as the gold standards of TP antibody tests. In measurement principle, CLIA and CLEIA are more sensitive than INNO-LIA and western blot. Therefore, false positives obtained using TP kits should be further examined, and the clinical course of TP antibodies should be investigated with ultra-sensitive methods, such as CLIA and CLEIA.
Many qualitative TP kits and few quantitative TP kits are available. RPR is used to evaluate therapeutic effects, and TP antibody is used to diagnose syphilis. However, in this study, changes detected by quantitative TP kits were similar to those observed using RPR kits in both patients with reinfection and those with syphilis under treatment. Therefore, quantitative TP kits without an upper limit may help monitor therapeutic effects. The correlation coefficients were low (0.753–0.974) among TP kits. Measured values also varied among kits. Therefore, TP kits should be quantified and standardized for use in monitoring therapeutic effects.
RPR tests sometimes present biological false positives; in this study, 9.8% of all RPR results were biological false positives. TP antibody tests had fewer false positives than RPR tests (2%). RPR detects active syphilis but misses very early syphilis more than TP antibody does [16]. Nontreponemal antibody tests have lower sensitivity than TP PA in primary syphilis [10]. Therefore, TP antibody tests may be better than RPR tests as screening methods for primary syphilis.
5. Conclusions
The specificity of RPR tests was lower than that of TP antibody tests. The specificity and sensitivity of RPR CTs were like those of LAs, but the measured values of RPR CTs and LAs were different. Therefore, RPR should be measured using an automatic RPR LA without setting the upper limit of the reported value. In addition, RPR LA should be standardized. The sensitivity of TP antibody was better in CLIA, CLEIA, and IC excluding HISCL than in FTA-ABS, HA, PA, and LA. Quantitative TP antibodies gave similar results to RPR in clinical course during reinfection and treatment; furthermore, they may contribute to the follow-up of treatment responses, and TP antibody tests may potentially be better than RPR tests as screening methods for primary syphilis. Therefore, TP antibody kits should be standardized and quantified.
Funding
This work was supported by the Japan Society for the Promotion of Science, Japan [grant number for “Grant-in-Aid for Encouragement of Scientists”: 19H00433].
CRediT authorship contribution statement
Itsuko Sato: Data acquisition and analysis. Yuji Nakamachi: Formal analysis, manuscript preparation. Goh Ohji: Study protocol development, data acquisition and analysis, and manuscript review. Yoshihiko Yano: Manuscript review. Jun Saegusa: Manuscript review.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all companies for their collaboration in this study. We would like to thank Editage (www.editage.com) for English language editing.
Contributor Information
Itsuko Sato, Email: itsuko@med.kobe-u.ac.jp.
Yuji Nakamachi, Email: nakamati@med.kobe-u.ac.jp.
Goh Ohji, Email: ohji@med.kobe-u.ac.jp.
Yoshihiko Yano, Email: yanoyo@med.kobe-u.ac.jp.
Jun Saegusa, Email: jsaegusa@med.kobe-u.ac.jp.
Data availability
No data was used for the research described in the article.
References
- 1.Peeling R.W., Mabey D., Kamb M.L., Chen X.S., Radolf J.D., Benzaken A.S. Syphilis, Nat. Rev. Dis. Primers. 2017;3 doi: 10.1038/nrdp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman L., Rowley J., Vander Hoorn S., et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor M., Alonso-González M., Gómez B., Korenromp E., Broutet N. World Health Organization global health sector strategy on sexually transmitted infections: an evidence-to-action summary for Colombia. Rev. Colomb. Obstet. Ginecolog. 2017;68:193–201. doi: 10.18597/rcog.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute of Infectious Diseases National epidemiological surveillance of infectious diseases. https://www.niid.go.jp/niid/en/survaillance-data-table-english.html
- 6.Park B.G., Yoon J.G., Rim J.H., Lee A., Kim H.S. Comparison of six automated Treponema-specific antibody assays. J. Clin. Microbiol. 2016;54:163–167. doi: 10.1128/JCM.02593-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onoe T., Honda M., Matsuo K., et al. Examination of the correlation between the manual and automated serological testing methods for syphilis. J. Dermatol. 2012;39:355–361. doi: 10.1111/j.1346-8138.2011.01341.x. [DOI] [PubMed] [Google Scholar]
- 8.Ebel A., Vanneste L., Cardinaels M., et al. Validation of the INNO-LIA syphilis kit as a confirmatory assay for Treponema pallidum antibodies. J. Clin. Microbiol. 2000;38:215–219. doi: 10.1128/JCM.38.1.215-219.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smikle M.F., James O.B., Prabhakar P. Biological false positive serological tests for syphilis in the Jamaican population. Genitourin. Med. 1990;66:76–78. doi: 10.1136/sti.66.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creegan L., Bauer H.M., Samuel M.C., Klausner J., Liska S., Bolan G. An evaluation of the relative sensitivities of the Venereal Disease Research Laboratory test and the Treponema pallidum particle agglutination test among patients diagnosed with primary syphilis. Sex. Transm. Dis. 2007;34:1016–1018. doi: 10.1097/OLQ.0b013e3181124473. [DOI] [PubMed] [Google Scholar]
- 11.Workowski K.A., Bolan G.A. Centers for disease Control and prevention, sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuboi M., Nishijima T., Aoki T., et al. Usefulness of automated latex turbidimetric rapid plasma reagin test for diagnosis and evaluation of treatment response in syphilis in comparison with manual card test: a prospective cohort study. J. Clin. Microbiol. 2018;56:e01003–e01018. doi: 10.1128/JCM.01003-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morshed M.G., Singh A.E. Recent trends in the serologic diagnosis of syphilis. Clin. Vaccine Immunol. 2015;22:137–147. doi: 10.1128/CVI.00681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park I.U., Fakile Y.F., Chow J.M., et al. Performance of treponemal tests for the diagnosis of syphilis. Clin. Infect. Dis. 2019;68:913–918. doi: 10.1093/cid/ciy558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park I.U., Chow J.M., Bolan G., Stanley M., Shieh J., Schapiro J.M. Screening for syphilis with the treponemal immunoassay: analysis of discordant serology results and implications for clinical management. J. Infect. Dis. 2011;204:1297–1304. doi: 10.1093/infdis/jir524. [DOI] [PubMed] [Google Scholar]
- 16.Janier M., Unemo M., Dupin N., et al. European guideline on the management of syphilis. J. Eur. Acad. Dermatol. Venereol. 2020;35(2021):574–588. doi: 10.1111/jdv.16946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.