Abstract
Background
Type 2 Diabetes Mellitus (T2DM) is one of the health issues causing untoward low-grade systemic inflammation. Aerobic Training (AT) and Vitamin D (Vit D) supplementation are among the approaches that improve lipid profile and liver enzymes in T2DM. However, the mechanisms responsible for these improvements are not fully elucidated.
Objectives
This study aimed to evaluate the effects of AT and Vit D supplementation on lipid profile, liver enzymes, Interleukin-6 (IL-6), Interleukin-10 (IL-10), Cluster of differentiation 27 (CD27), Chemokine (C-X-C motif) Ligand 13 (CXCL13), Interferon-Gamma (IFN-γ) and Transforming Growth Factor-Beta 1 (TGF-β1) gene expressions in patients with T2DM.
Methods
In this study, 40 male T2DM patients aged 35–50 years were randomly selected and assigned into four groups (n = 10 for each); AT+vitamin D supplementation (AT+Vit D), AT+placebo (AT), Vit D supplementation (Vit D), and control+placebo (C). The intervention consisted of 8 weeks of 20–40 minutes AT protocol at 60–75% HRmax 3 sessions/week and taking 50,000 IU of Vit D supplement once a week. Serum levels of lipid profile and liver enzymes and gene expression of IL-6, IL-10, CD27, CXCL13, IFN-γ, and TGF-β1 in Peripheral Blood Mononuclear Cells (PBMCs) were measured. One-way analysis of variance (ANOVA), Tukey’s post hoc, and paired sample t-test at P-values less than 0.05 were used to analyze the data using SPSS software.
Results
AT+Vit D, AT, and Vit D significantly decreased TC, TG, LDL, AST, ALT, and GGT while increased HDL after 8 weeks in favor of AT+Vit D. Also, gene expressions of IL-6, IL-10, CD27, CXCL13, IFN-γ, and TGF-β1 were downregulated significantly in AT+Vit D, AT, and Vit D, while upregulated in C. Furthermore, compared to individual AT or Vit D, AT+Vit D significantly downregulated IL-6 (P = 0.013; P = 0.025), IL-10 (P = 0.012; P = 0.026), CD27 (P = 0.023; P = 0.041), CXCL13 (P = 0.014; P = 0.025), IFN-γ (P = 0.017; P = 0.026), and TGF-β1 (P = 0.001; P = 0.028).
Conclusion
In comparison to individual AT or Vit D, AT+Vit D may enhance lipid profile, and liver enzymes and drive the balance to favor inhibition of inflammation by downregulating gene expression of inflammation-related factors. As a result, AT+Vit D may be considered appropriate therapy for managing T2DM.
Keywords: Exercise, Inflammation, Diabetes, Vitamin D
Introduction
Diabetes, particularly Type 2 Diabetes Mellitus (T2DM), is a health issue with many cardiovascular and neuromuscular diseases complications. Chronic hyperglycemia in diabetes gradually induces insulin resistance in many tissues, causing untoward low-grade systemic inflammation and creating insulin resistance-inflammation dual in diabetic patients. Several clinical trials have focused on non-pharmacological complementary approaches to prevent and control T2DM and its complication [1].
Accumulating evidence suggests that regular Aerobic Training (AT) helps facilitate skeletal muscle glucose uptake via insulin-dependent and noninsulin-dependent pathways, increasing GLUT4 abundance, boosting weight loss, and improving the liver enzyme and lipid profile [2, 3]. Thus, apart from these beneficial effects, AT may improve T2DM complications by targeting the possible mechanisms that drive the inflammatory pathways [4]. The mechanisms underlying AT-induced improvements are unknown. It is thought that AT, as a mechanical stimulus, may play a key role in modifying endothelial activity and the expression of a variety of proteins, particularly those involved in local inflammation (e.g., Interleukin-6 (IL-6), Interleukin-10 (IL-10), Cluster of Differentiation 27 (CD27), Chemokine (C-X-C motif) Ligand 13 (CXCL13). Studies investigating the effects of AT on inflammation in T2DM models are inconsistent [5, 6] and limited to serum level analyses [7, 8] or animal studies [9, 10].
Recent studies have focused on vitamin supplements (i.e., Vitamin D (Vit D)) in improving and controlling chronic disease. Additionally, studies reported the high prevalence of hypovitaminosis D in T2DM patients [11–13]. Beyond its captious role in the calcium-related insulin secretion [14], glucose transporter type 4 (GLUT-4) translocation [15], and glycemic status [12, 16], Vit D supplementation has also been found to play a critical role in the pathogenesis of the inflammatory disease via downregulating the production of the inflammatory cytokines [17].
Hoseini et al. [18] found that combined exercise and high dosages of Vit D supplementation lowered insulin, blood glucose, and homeostatic model evaluation for insulin resistance and increased peroxisome proliferator-activated receptor (PPAR) gene expression in ovariectomized rats. Also, Hoseini et al. [19] reported the combination of AT+ Vit D supplementation significantly reduced liver enzymes, anthropometric and glycemic indices, and improved lipid profile in older women with Vit D deficiency and NAFLD.
The literature supports the improvement of liver enzymes and lipid profiles after combined AT + Vit D supplementation and serum levels of inflammatory biomarkers after AT or Vit D supplementation in animal models of diabetes or other inflammation-related chronic diseases; however, few studies have investigated the effects of separate or combined AT and Vit D supplementation on gene expression of inflammatory biomarkers. Thus, we aimed at investigating the effects of AT and Vit D supplementation on IL-6, IL-10, CD27, CXCL13, IFN-, and TGF-1 gene expressions in patients with T2DM.
Methodology
Study design and participants
This is a randomized, single-blinded clinical trial with a placebo control conducted on 40 T2DM patients (over 3 years of diagnosed diabetes history) aged 35–50 years. This study was registered on the Iranian website for clinical trial registration IRCT20210811052151N1 on 01/09/2021, performed following the Declaration of Helsinki, and approved by the Research Ethics Committees of Kermanshah Razi University (IR.RAZI.REC.1400.044). All participants completed and signed written informed consent.
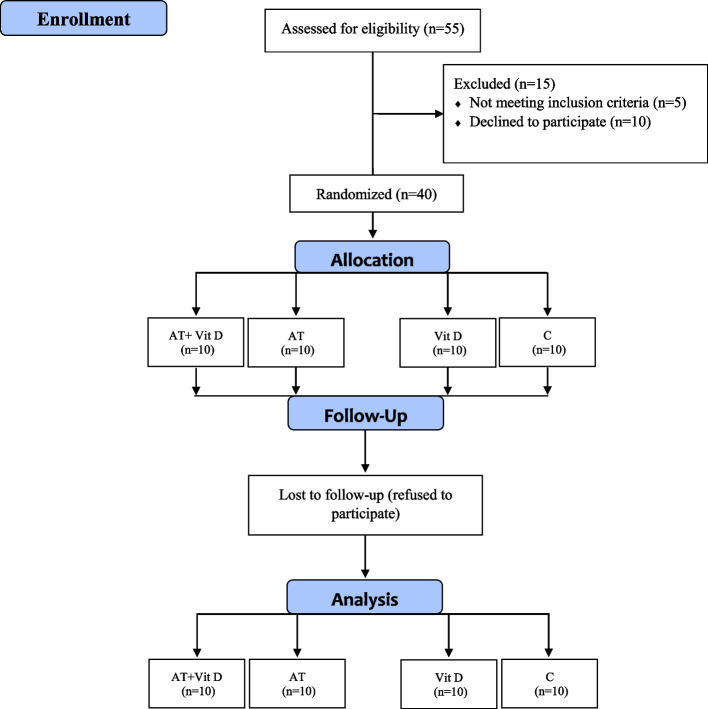
The main exclusion criteria included: more than 6 months of regular exercise schedule, history of orthopedic disorders, smoking, consumption of immunosuppressive drugs, muscle injuries, inability to perform the exercises, COVID-19 infection during the study period, taking anti-inflammatory and antioxidant supplements such as Vit D, E, and C, and omega-3 fatty acids within past 6 months. The sample size was evaluated at 36 subjects using G. POWER 3.1 software (alpha error of 0.05, power of 0.99, and effect size of 0.85). Due to the probability of refusal to continue the study, 40 male T2DM patients aged 35–50 years were randomly selected from the Diabetes Medical & Health Center. Then, they were randomly located in four groups: AT+ Vit D supplementation (AT+Vit D; n = 10), AT+placebo (AT; n = 10), Vit D supplementation (Vit D; n = 10), and control+placebo (C; n = 10) using the Random Number Generator method; all subjects had the same chance of being selected (Fig. 1).
Fig. 1.
Flow chart of the study population
Aerobic training
The AT and AT+Vit D groups participants were required to exercise 3 days per week for 8 weeks. The exercise training protocol started at 20 minutes of 60% HRmax. It progressed to 40 minutes of 75% HRmax per session at the end of the eighth week, following the American Diabetes Association (ADA)'s guidelines [20]. Each session also included a 10-minute warmup and a 10-minute cooldown. A combination of the HRmax formula [HRmax = 220 − age] [21] and the 6–20 Rating of Perceived Exertion (RPE) scale were used in the outsets to control the intensity of the training sessions and ensure that the target heart rate (exercise intensity) was obtained and sustained during the study [22]. All participants learned the pulse palpation method to count their heart rates. Furthermore, the training sessions were conducted under the supervision of exercise physiologists (Table 1).
Table 1.
The aerobic training
| Variables | Week | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Intensity (HRMax) | 60–65% | 60–65% | 60–65% | 65–70% | 65–70% | 65–70% | 70–75% | 70–75% |
| Time (min) | 20 | 25 | 30 | 30 | 35 | 35 | 40 | 40 |
| Borg scale | 10 | 10 | 11 | 11 | 12 | 12 | 13 | 13 |
Diet and supplementation
In this study, Vit D and AT+Vit D groups consumed 50,000 IU of Vit D supplement (Zahravi Pharmaceutical Company, Tabriz, Iran) once a week, while AT and C consumed a placebo with the same taste, color, and shape (Barij Essence Pharmaceutical Company, Kashan, Iran) [19, 23]. Additionally, a self-reported three-day food record [analyzed by nutritionist IV software (First Databank, San Bruno, CA)] was used at the baseline in weeks 2, 4, 6, and 8 of the intervention to describe the daily diet precisely.
Measurements
Anthropometric and body composition
The initial measurements were taken 3 days before and after the intervention. Height and Waist Circumference (WC) was measured to the nearest 0.5 cm using a stadiometer (DETECTO, Model 3PHTROD-WM, USA) and a non-elastic tape measure. The Bioelectrical impedance analysis (BIA; Zeus 9.9 PLUS; Jawon Medical Co., Ltd., Kungsang Bukdo, South Korea) was used to measure Bodyweight (BW), Body Mass Index (BMI), Waist–Hip Ratio (WHR), and Body Fat Percentage (BFP) after at least 12-h of fasting between 8 and 9 in the morning. The participants were asked not to participate in intensive physical activities 72 h before the measurements.
Blood sampling
Forty-eight hours before and after the intervention, 15 ml of fasting blood was obtained from the median cubital vein. Serum levels of lipid profile (Triglyceride (TG), Total Cholesterol (TC), High-Density Lipoprotein (HDL), and Low-Density Lipoprotein (LDL); using Hitachi kits, Tokyo, Japan). Additionally, liver enzymes (Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Gamma-Glutamyl Transferase (GGT); with the ELISA method (Greiner Bio-One Kit, Germany)) were measured.
Isolation of PBMCs
The Peripheral Blood Mononuclear Cells (PBMCs) isolated from blood samples were used to examine the gene expression levels associated with inflammation [24]. The gene expression of IL-6, IL-10, CD27, CXCL13, IFN-γ, and TGF-β1 were assessed as the primary outcomes due to their role in the signaling pathway of diabetic low-grade inflammation and secondary outcomes [25–27]. Blood samples were mixed through 3-part diluted blood to 2-part Ficoll-Hypaque and centrifuged for 30-min at 500×g for PBMCs isolation. Then, using a sterile Pasteur pipet, PBMCs were carefully aspirated, and 10 mL of phosphate-buffered saline (PBS) was added and centrifuged for 10 minutes at 400 g, followed by Hanks balanced salt solution and Percoll. The cloudy layer in the top 5 mm was transferred into a separate tube for further analysis after centrifugation for 25 minutes at 370 g, 25 °C.
RNA extraction and real-time PCR
Total RNAs were extracted from one aliquot of 5 × 106 unactivated PBMCs with the trizol reagent (Invitrogen, USA) using an RNX-plus kit (Cinnacolon, Tehran, Iran) based on the manufacturer’s instructions. The RNA was quantified using a UV spectrophotometer, which revealed no contamination with protein or DNA (OD 260/280 ratio between 1.7 and 2.1), followed by reverse transcription to the cDNA library (via Moloney murine leukemia virus reverse transcriptase). Then, glyceraldehyde-3-phosphate dehydrogenase primers as housekeeping gene and quantitative RT-PCR method (LightCycler technology, Roche Diagnostics, Rotkreuz, Switzerland; SYBR green detection and Amplicon Kit), the gene expression of IL-6, IL-10, CD27, CXCL13, IFN-γ, and TGF-β1 were evaluated. Additionally, the Primer Express (Applied Biosystems, Foster City) and Beacon designer (Takaposizt, Tehran, Iran) software were used to design the primers and Pffafi or 2 − 11CT method to calculate relative transcription levels (Table 2).
Table 2.
Forward and Reverse Primers Used for Real-Time Quantitative PCR
| Gene | Primer | Product size (bp) | Annealing temperature (C) |
|---|---|---|---|
| IL-6 |
F: GGTACATCCTCGACGGCATCT R: GT GCCTCTTTGCTGCTTTCAC |
250 | 60 |
| IL-10 |
F: AAGGCAGTGGAGCAGGTGAA R: CCAGCAGACTCAATACACAC |
250 | 58 |
| CD27 |
F: TGTCGGCACTGTAACTCTGGTCT R: CCTGCACTGCCAGCCAT |
250 | 59 |
| CXCL13 |
F: GCTTGAGGTGTAGATGTGTCC R: CCCACGGGGCAAGATTTGAA |
150 | 59 |
| IFN-γ |
F: GGCATTTTGAAGAATTGGAAAG R: TTTGGATGCTCTGGTCATCTT |
286 | 60 |
| TGF-β1 |
F: CCCAGCATCTGCAAAGCTC R: GTCAATGTACAGCTGCCGCA |
286 | 60 |
Statistical analysis
The SPSS software (IBM Corp., Armonk, NY, USA) was used to perform the statistical analyses. Mean ± standard deviation (SD) was used to present the data. The Shapiro–Wilk test was used to check the normal distribution of the variables. One-way analysis of variance (ANOVA) and Tukey’s post hoc were used to analyze the between-group differences. The paired sample t-test analyzed within-group differences at P-values less than 0.05.
Results
Table 3 presents the Mean ± SD of baseline levels of anthropometric and physiologic variables and their between-group comparison. The results of the one-way ANOVA show no significant differences in the mean of WC, BW, BMI, BFP, WHR, FBG, and insulin in the pre-test between groups.
Table 3.
Mean ± SD of anthropometric and physiologic variables before the intervention in patients with T2DM
| Variables | AT + Vit D | AT | Vit D | C | P-Value |
|---|---|---|---|---|---|
| Age (years) | 48.32 ± 2.23 | 47.13 ± 3.12 | 49.10 ± 1.23 | 48.27 ± 2.17 | 0.540 |
| Height (cm) | 159.14 ± 2.13 | 161.17 ± 2.21 | 160.21 ± 2.28 | 162.12 ± 3.18 | 0.304 |
| WC (cm) | 10.13 ± 3.23 | 9.10 ± 2.13 | 11.15 ± 1.89 | 10.25 ± 3.26 | 0.215 |
| BW (Kg) | 75.11 ± 2.12 | 76.12 ± 3.14 | 74.18 ± 3.09 | 75.09 ± 2.17 | 0.451 |
| BMI (kg/m2) | 29.66 ± 1.25 | 29.30 ± 1.43 | 28.90 ± 1.23 | 28.57 ± 1.27 | 0.356 |
| BFP (%) | 32.16 ± 1.56 | 31.11 ± 2.14 | 30.23 ± 1.56 | 29.76 ± 2.08 | 0.084 |
| WHR (cm) | 0.94 ± 0.01 | 0.93 ± 0.03 | 0.92 ± 0.04 | 0.92 ± 0.02 | 0.078 |
| Insulin (μU/mL) | 7.87 ± 0.29 | 7.59 ± 0.56 | 6.89 ± 0.23 | 6.73 ± 0.33 | 0.117 |
| FBG (mg/dl) | 169.76 ± 2.05 | 158.23 ± 2.41 | 150.18 ± 1.44 | 153.33 ± 2.54 | 0.063 |
Data analysis was done by the analysis of one-way analysis of variance and least significant difference post-hoc Tukey’s test after adjustment for baseline values
AT Aerobic Training, Vit D Vitamin D Supplementation, C Control, WC Waist Circumferences, BW Bodyweight, BMI Body Mass Index, BFP Body Fat Percentage, WHR Waist To Hip Ratio, FBG Fasting Blood Glucose
Table 4 shows the between-group comparisons of the lipid profile (TC, TG, LDL, and HDL), liver enzymes (AST, ALT, and GGT), and serum 25-OH-Vit D. The results of the t-test indicate significant differences in the post-test compared to the pre-test. After 8 weeks, TC, TG, LDL, AST, ALT, and GGT decreased significantly in AT+Vit D, AT, and Vit D but not significantly in C (Table 3). Based on the results, there was a significant increase in the HDL and 25-OH-Vit D after 8 weeks in AT+Vit D, AT, and Vit D groups. Comparing the pre-test to the post-test, no significant differences were found in HDL levels in the C group. The results of one-way ANOVA indicate significant between-group differences in the Δ (post-pre) of variables mentioned previously.
Table 4.
Mean ± SD of lipid profile and liver enzymes before and after the 8-week intervention in patients with T2DM
| Variables | AT + Vit D | AT | Vit D | C | P-Value |
|---|---|---|---|---|---|
| AST (U/L) | |||||
| Before | 35.17 ± 2.29 | 34.59 ± 2.56 | 36.89 ± 2.23 | 35.73 ± 1.30 | |
| After | 25.13 ± 1.11 | 27.73 ± 1.24 | 30.20 ± 1.22 | 36.97 ± 2.23 | |
| P† | 0.001* | 0.022* | 0.039* | 0.398 | |
| Δ | −10.04 ± 1.18μ€β | −6.86 ± 1.32€β | −5.69 ± 1.01β | 1.24 ± 0.93 | 0.012¥ |
| ALT (U/L) | |||||
| Before | 33.36 ± 1.65 | 32.53 ± 2.28 | 34.18 ± 2.64 | 35.43 ± 1.50 | |
| After | 24.04 ± 2.07 | 26.10 ± 1.37 | 30.12 ± 1.73 | 35.89 ± 1.34 | |
| P† | 0.011* | 0.024* | 0.043* | 0.624 | |
| Δ | −9.32 ± 0.42μ€β | −6.43 ± 0.91 €β | −4.06 ± 0.91β | 0.46 ± 0.16 | 0.002¥ |
| GGT (U/L) | |||||
| Before | 38.34 ± 1.29 | 36.97 ± 2.26 | 36.23 ± 1.19 | 37.35 ± 1.26 | |
| After | 26.24 ± 2.23 | 28.07 ± 1.17 | 30.14 ± 2.31 | 38.71 ± 2.15 | |
| P† | 0.001* | 0.014* | 0.029* | 0.287 | |
| Δ | −12.10 ± 0.94 μ€β | −8.90 ± 1.09 €β | −6.09 ± 1.12 β | 1.36 ± 0.89 | 0.003 ¥ |
| TC (mg/dl) | |||||
| Before | 187.19 ± 3.87 | 175.27 ± 5.11 | 179.04 ± 2.94 | 186.14 ± 3.26 | |
| After | 164.17 ± 1.21 | 162.14 ± 3.54 | 169.36 ± 4.13 β | 188.21 ± 1.49 | |
| P† | 0.001* | 0.001* | 0.012* | 0.282 | |
| Δ | −23.02 ± 2.66μ€β | −13.13 ± 1.57β | −9.68 ± 1.19β | 2.07 ± 1.77 | 0.001 ¥ |
| TG (mg/dl) | |||||
| Before | 163.42 ± 5.07 | 160.15 ± 3.07 | 155.43 ± 4.64 | 153.08 ± 3.26 | |
| After | 146.13 ± 2.92μ€β | 150.25 ± 2.23 | 147.32 ± 2.76β | 156.35 ± 2.38 | |
| P† | 0.001* | 0.003* | 0.018* | 0.216 | |
| Δ | −17.29 ± 2.15μ€β | −9.90 ± 0.84€β | −8.11 ± 1.88β | 3.27 ± 0.88 | 0.003 ¥ |
| LDL (mg/dl) | |||||
| Before | 143.12 ± 4.34 | 140.26 ± 3.56 | 147.12 ± 6.47 | 142.14 ± 3.23 | |
| After | 123.07 ± 2.33 | 128.27 ± 2.06 | 139.02 ± 2.02 | 144.17 ± 2.66 | |
| P† | 0.001* | 0.001* | 0.017* | 0.294 | |
| Δ | −20.05 ± 2.01μ€β | −11.99 ± 1.50€β | −8.10 ± 4.45β | 2.03 ± 0.57 | 0.002 ¥ |
| HDL (mg/dl) | |||||
| Before | 31.14 ± 2.63 | 32.29 ± 1.38 | 33.18 ± 1.27 | 32.54 ± 1.29 | |
| After | 42.07 ± 1.82μ€β | 38.06 ± 2.11β | 37.92 ± 1.42β | 30.36 ± 2.15 | |
| P† | 0.001* | 0.031* | 0.042* | 0.092 | |
| Δ | 10.93 ± 0.81μ€β | 5.77 ± 0.73β | 4.74 ± 0.15β | −2.28 ± 0.86 | 0.026 ¥ |
| serum 25-OH-Vit D (ng/mL) | |||||
| Before | 22.22 ± 1.29 | 21.45 ± 2.57 | 23.17 ± 1.02 | 23.20 ± 3.41 | |
| After | 39.67 ± 0.84 | 28.26 ± 1.62 | 34.18 ± 1.24 | 22.70 ± 1.64 | |
| P† | 0.001* | 0.002* | 0.001* | 0.111 | |
| Δ | 17.45 ± 0.45μ€β | 6.81 ± 0.95€β | 11.01 ± 0.22β | −0.50 ± 1.77 | 0.001 ¥ |
Data analysis was done by one-way analysis of variance and least significant difference post-hoc Tukey’s test after adjustment for baseline values
AT Aerobic Training, Vit D Vitamin D Supplementation, C Control Group, Δ pos-pre
P†Statistical analysis was done by paired sample t-test
*Significantly different in comparison pre and post within the groups
¥Significantly different comparing Δ between groups
μSignificantly different compared with AT
€Significantly different compared with Vit D
βSignificantly different compared with C
Compared to the C group, AT+Vit D, AT, and Vit D significantly decreased TC, TG, LDL, AST, and ALT, GGT while increased HDL and 25-OH-Vit D. Additionally, the AT+Vit D group significantly decreased TC, TG, LDL, AST, ALT, and GGT levels and increased HDL, and 25-OH-Vit D levels compared to the individual AT and Vit D groups. The results also indicate decreased TC, TG, LDL, AST, and ALT and increased HDL, and 25-OH-Vit D in AT compared to Vit D, although they were not significant.
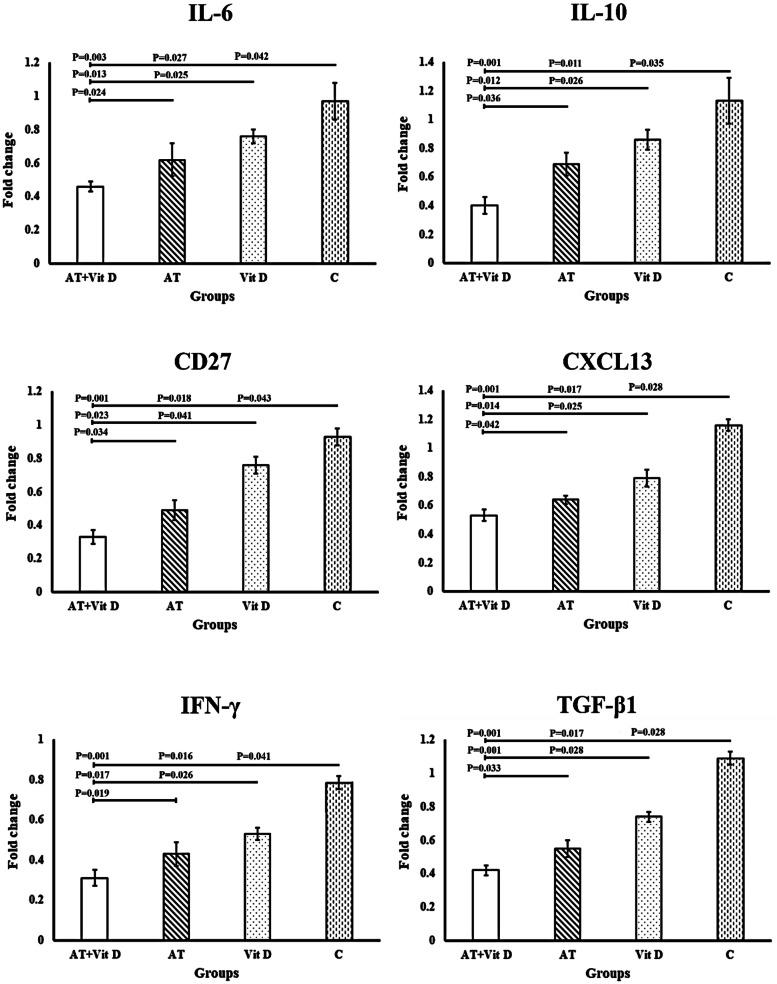
Based on the results of the t-test, gene expressions of IL-6, IL-10, CD27, CXCL13, IFN-γ, and TGF-β1 were downregulated significantly after 8 weeks of AT+Vit D, AT, and Vit D while upregulated significantly in C group (Fig. 2). Furthermore, compared to C group AT+Vit D, AT, and Vit D significantly downregulated IL-6 (P = 0.003; P = 0.027; P = 0.042), IL-10 (P = 0.001; P = 0.011; P = 0.035), CD27 (P = 0.001; P = 0.018; P = 0.043), CXCL13 (P = 0.001; P = 0.0017; P = 0.028), IFN-γ (P = 0.001; P = 0.016; P = 0.041), and TGF-β1 (P = 0.001; P = 0.019; P = 0.037) gene expressions. Also, significant differences were observed in the gene expressions of IL-6 (P = 0.013; P = 0.025), IL-10 (P = 0.012; P = 0.026), CD27 (P = 0.023; P = 0.041), CXCL13 (P = 0.014; P = 0.025), IFN-γ (P = 0.017; P = 0.026), and TGF-β1 (P = 0.001; P = 0.028) in the AT+Vit D compared to individual AT or Vit D, respectively. Moreover, AT significantly downregulated the gene expressions of IL-6 (P = 0.024), IL-10 (P = 0.036), CD27 (P = 0.034), CXCL13 (P = 0.042), IFN-γ (P = 0.019), and TGF-β1 (P = 0.033) than Vit D (Fig. 2).
Fig. 2.
Effect of aerobic training and vitamin d supplementation on IL-6, IL-10, CD27, CXCL13, IFN-γ, and TGF-β1 in patients with T2DM. IL-6: Interleukin-6; IL-10: Interleukin-10; CD27: Cluster of Differentiation 27; CXCL13: Chemokine (C-X-C motif) Ligand 13; IFN-γ: Interferon-Gamma a; TGF-β1: Transforming Growth Factor-Beta 1. P-value was obtained from One-way ANOVA and Tukey’s post hoc test. Data are means ± standard deviation
Discussion
The present study investigated whether separate or combined AT and Vit D induce more significant changes in the liver enzymes, lipid profile, and inflammation-related gene expression in individuals with T2DM. We showed that 8-week AT and Vit D significantly improved lipid profile accompanied by a significant decrease in liver enzymes (ALT and AST). In line with our findings, previous research has shown that AT is an essential component of T2DM management (31) and is associated with improved hepatic risk factors (i.e., ALT, AST, and GGT) [19]. Interestingly, combined AT+ Vit D appears to produce more beneficial changes in lipid profile and liver enzymes than single interventions. Consistent with our findings, Vit D and AT have significantly reduced BW, BMI, visceral fat, liver enzymes, and improved lipid profile in the rat [28] and human [19] models. Improved liver enzymes following exercise might result from weight loss pathophysiological changes that lead to improved insulin sensitivity, reduced free fatty acid transfusion into the liver, and decreased inflammatory mechanisms [29, 30]. The improvement of lipid profile could be due to the use of fat as an energy substrate both in exercising and recovery states. Also, exercise appears to increase lipoprotein A, lipoprotein lipase (LPL) enzyme levels, and catabolism of the lipid as a consequence [31–33]. Additionally, Carmeliet et al. [34] reported that Vit D reduces the liver secretion of triglyceride via increasing intracellular calcium, fecal excretion of bile acids, and creating calcium-fatty acid soap [34, 35]. Hypovitaminosis D may generally interfere with the proper function of insulin receptors and GLUT-4 in target tissues, probably via increasing parathyroid hormone levels [36, 37]. Therefore, combined AT and Vit D might be beneficial for inducing liver enzyme and lipid profile improvements in T2DM patients through the mechanisms mentioned above.
This study showed downregulated IL-6, IL-10, CD27, CXCL13, IFN-γ, and TGF-β1 gene expression after 8 weeks in all three experimental groups compared to the control group. Although AT and Vit D groups induced significant anti-inflammatory effects in human-derived PBMCs in the present study, these alterations were more significant in the AT+ Vit D group. Thus, combined AT+ Vit D was significantly more beneficial in downregulating IL-6, IL-10, CD27, CXCL13, IFN-γ, and TGF-β1 gene expression than AT and Vit D alone.
There is mounting evidence that those who are physically active or become physically active have a reduction in biomarkers associated with chronic inflammation [38–40]. The primary mechanisms through which exercise training promotes anti-inflammatory effects are still unknown. However, some intriguing possibilities might include: reduced visceral adiposity [41, 42], increased heat shock proteins releasement [43, 44], decreased local hypoxia and ischemia [44], reduced adipocytes infiltration via macrophages [41], altered immune cell phenotype, and lower monocyte toll-like receptor 4 (TLR4) expression [38]. In the absence of regular exercise training, these reciprocal adjustments tend to produce a vicious cycle leading to increased fat mass and inflammation because exercise-induced fat loss and reduced adiposity mediate the expression of inflammatory genes.
Regardless of the contradictory results in the literature, Vit D is also believed to modulate innate immune response and inflammation [45–47]. Considering the existence of Vit D Receptor (VDR) in the pancreas [48], vascular endothelial cells and PBMC [49], skeletal muscle cells, and other tissues [50] modulating a wide range of physiological functions are expected. The downstream signaling cascades of VDR in PBMCs remain unclear; however, recent studies indicate the alteration of inflammation pathways following Vit D supplementation. The activated VDR then might act as a transcription factor [51, 52] or modulate other unidentified molecules inducing the post-translational modifications or inhibitory effects [53] that regulate target gene expression [47] and reduce the level of inflammatory factors, in turn.
Finally, it seems that AT and Vit D suppress target gene expressions through different mechanisms that might converge in the downstream elements, resulting synergically in reduced inflammation.
Limitation of the study
This study was a placebo-controlled, single-blind, randomized trial with a low dropout rate evaluating the alterations of gene expression in human subjects. The small sample size is one of the limitations of this study thus concluding a conclusive response is premature. Therefore, we suggest investigating the same intervention in larger sample sizes and other tissues (e.g skeletal muscle cells). Also, it is suggested to investigate the level of inflammatory biomarkers in the diabetes population without Vit D deficiency or in animal models to better control Vit D intake as well as, exposure to UV.
Conclusion
Finally, any event that could modulate the vicious cycle of inflammation could play a key role in improving low-grade systemic inflammation in T2DM patients. As a result, AT+ Vit D could drive the balance to favor inhibition of inflammation and break this vicious cycle by improving lipid profile and liver enzymes while downregulating gene expression of inflammation-related factors. As a result, AT+ Vit D could be considered an ideal therapy for managing T2DM.
Acknowledgments
The authors would like to thank the participants for their cooperation.
Abbreviations
- AT
Aerobic Training
- Vit D
Vitamin D
- T2DM
Type 2 Diabetes Mellitus
- FBG
Fasting Blood Glucose
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- CD27
Cluster of differentiation 27
- CXCL13
Chemokine (C-X-C motif) Ligand 13
- IFN-γ
Interferon-Gamma
- TGF-β1
Transforming Growth Factor-Beta 1
Authors’ contributions
RH: designed the study. HA.R: experimented. RH: analyzed the data and wrote the manuscript. JK.A: was involved in the interpretation of data, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
The authors declared that the research did not receive any financial grants.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to ongoing data analysis but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Razi University of Kermanshah (IR.RAZI.REC.1400.044) and was registered in the Iranian Clinical Trial Registration Center (code: IRCT20210811052151N1 on 01/09/2021).
All procedures followed were following the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients.
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khademi Z, Imani E, Heidary Khormizi M, Poordad Khodaei A, Sarneyzadeh M, Nikparvar M. A Study on The Variation of Medicinal Plants Used for Controlling Blood Sugar and Causes of Self–Medication by Patients Referred to Bandarabbas Diabetic Center. J Diabetes Nurs. 2013;1(1):12–20. [Google Scholar]
- 2.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghalavand A, Shakeriyan S, Monazamnezhad A, Dadvar N, Heidarnezhad M, Delaramnasab M. The effects of aerobic training on blood glycemic control and plasma lipid profile in men with type 2 diabetes. Sylwan. 2014;158(6):1–10. [Google Scholar]
- 4.Abd El-Kader SM, Al-Shreef FM. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr Health Sci. 2018;18(1):120–131. doi: 10.4314/ahs.v18i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikniaz L, Ghojazadeh M, Nateghian H, Nikniaz Z, Farhangi MA, Pourmanaf H. The interaction effect of aerobic exercise and vitamin D supplementation on inflammatory factors, anti-inflammatory proteins, and lung function in male smokers: a randomized controlled trial. BMC Sports Sci Med Rehabil. 2021;13(1):1–8. doi: 10.1186/s13102-021-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeno FP, Ramis TR, Munhoz SV, Farinha JB, Moritz CE, Leal-Menezes R, Ribeiro JL, Christou DD, Reischak-Oliveira A. Effect of aerobic and resistance exercise training on inflammation, endothelial function and ambulatory blood pressure in middle-aged hypertensive patients. J Hypertens. 2020;38(12):2501–2509. doi: 10.1097/HJH.0000000000002581. [DOI] [PubMed] [Google Scholar]
- 7.Krause M, Rodrigues-Krause J, O’Hagan C, Medlow P, Davison G, Susta D, Boreham C, Newsholme P, O’Donnell M, Murphy C. The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur J Appl Physiol. 2014;114(2):251–260. doi: 10.1007/s00421-013-2769-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Yoo JH, Kang S, Woo JH, Shin KO, Kim KB, Cho SY, Roh HT, Kim YI. The effects of 12 weeks regular aerobic exercise on brain-derived neurotrophic factor and inflammatory factors in juvenile obesity and type 2 diabetes mellitus. J Phys Ther Sci. 2014;26(8):1199–1204. doi: 10.1589/jpts.26.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry JC, Simtchouk S, Durrer C, Jung ME, Mui AL, Little JP. Short-term exercise training reduces anti-inflammatory action of interleukin-10 in adults with obesity. Cytokine. 2018;111:460–469. doi: 10.1016/j.cyto.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Wang S-Q, Li D, Yuan Y. Long-term moderate intensity exercise alleviates myocardial fibrosis in type 2 diabetic rats via inhibitions of oxidative stress and TGF-β1/Smad pathway. J Physiol Sci. 2019;69(6):861–873. doi: 10.1007/s12576-019-00696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik R, Farooq R, Mehta P, Ishaq S, Din I, Shah P, Majid S. Association of Vitamin D receptor gene polymorphism in adults with type 2 diabetes in the Kashmir Valley. Can J Diabetes. 2018;42(3):251–256. doi: 10.1016/j.jcjd.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Wang SY, Shen TT, Xi BL, Shen Z, Zhang X. Vitamin D affects the neutrophil-to-lymphocyte ratio in patients with type 2 diabetes mellitus. J Diabetes Investig. 2021;12(2):254–265. doi: 10.1111/jdi.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H-J, Kang C-K, Park H, Lee M-G. Effects of vitamin D supplementation and circuit training on indices of obesity and insulin resistance in T2D and vitamin D deficient elderly women. J Exerc Nutr Biochem. 2014;18(3):249. doi: 10.5717/jenb.2014.18.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grammatiki M, Karras S, Kotsa K. The role of vitamin D in the pathogenesis and treatment of diabetes mellitus: a narrative review. Hormones. 2019;18(1):37–48. doi: 10.1007/s42000-018-0063-z. [DOI] [PubMed] [Google Scholar]
- 15.Nachankar A, Kotwal N, Upreti V, Verma V, Kumar KH. Association of vitamin D and parathyroid hormone with insulin sensitivity, beta cell function and gestational diabetes in pregnancy: a cross-sectional, observational study. Diabetes Ther. 2018;9(5):2081–2090. doi: 10.1007/s13300-018-0508-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhat MH, Mohd M, Dar IH, Bhat JA. Role of vitamin D deficiency in type 2 diabetes: association or coincidence? Clin Diabetol. 2021;10(2):188–194. [Google Scholar]
- 17.Dadrass A, Mohamadzadeh Salamat K, Hamidi K, Azizbeigi K. Anti-inflammatory effects of vitamin D and resistance training in men with type 2 diabetes mellitus and vitamin D deficiency: a randomized, double-blinded, placebo-controlled clinical trial. J Diabetes Metab Disord. 2019;18(2):323–331. doi: 10.1007/s40200-019-00416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoseini R, Damirchi A, Babaei P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. 2017;36:54–59. doi: 10.1016/j.nut.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Hoseini Z, Behpour N, Hoseini R. Co-treatment with vitamin D supplementation and aerobic training in elderly women with Vit D deficiency and NAFLD: a single-blind controlled trial. Hepat Mon. 2020;20(2):1–11.
- 20.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branco BHM, de Oliveira MF, Ladeia GF, Bertolini SMMG, Badilla PV, Andreato LV. Maximum heart rate predicted by formulas versus values obtained in graded exercise tests in Brazilian jiu-jitsu athletes. Sport Sci Health. 2020;16(1):39–45. doi: 10.1007/s11332-019-00570-0. [DOI] [Google Scholar]
- 22.Rahimi E. Physical activity and type 2 diabetes: a narrative review. J Phys Act Hormones. 2019;2(4):51–62. [Google Scholar]
- 23.Imanparast F, Javaheri J, Kamankesh F, Rafiei F, Salehi A, Mollaaliakbari Z, Rezaei F, Rahimi A, Abbasi E. The effects of chromium and vitamin D3 co-supplementation on insulin resistance and tumor necrosis factor-alpha in type 2 diabetes: a randomized placebo-controlled trial. Appl Physiol Nutr Metab. 2020;45(5):471–477. doi: 10.1139/apnm-2019-0113. [DOI] [PubMed] [Google Scholar]
- 24.Mizuarai S, Irie H, Kotani H. Gene expression-based pharmacodynamic biomarkers: the beginning of a new era in biomarker-driven anti-tumor drug development. Curr Mol Med. 2010;10(6):596–607. doi: 10.2174/1566524011009060596. [DOI] [PubMed] [Google Scholar]
- 25.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung YT, Aziz F, Guerrero-Castilla A, Arguelles S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr Pharm Des. 2018;24(14):1449–1484. doi: 10.2174/1381612824666180327165604. [DOI] [PubMed] [Google Scholar]
- 28.Hoseini R, Damirchi A, Babaei P. The interaction effect of aerobic training and different doses of intramuscular vitamin D on body weight, visceral fat and food intake in female Wistar rats. J Arak Univ Med Sci. 2015;18(7):24–33. [Google Scholar]
- 29.Slentz CA, Bateman LA, Willis LH, Shields AT, Tanner CJ, Piner LW, Hawk VH, Muehlbauer MJ, Samsa GP, Nelson RC. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301(5):E1033–E1039. doi: 10.1152/ajpendo.00291.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo R, Liong EC, So KF, Fung M-L, Tipoe GL. Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2015;14(2):139–144. doi: 10.1016/S1499-3872(15)60355-1. [DOI] [PubMed] [Google Scholar]
- 31.Greene NP, Martin SE, Crouse SF. Acute exercise and training alter blood lipid and lipoprotein profiles differently in overweight and obese men and women. Obesity. 2012;20(8):1618–1627. doi: 10.1038/oby.2012.65. [DOI] [PubMed] [Google Scholar]
- 32.Kostrzewa-Nowak D, Nowak R, Jastrzębski Z, Zarębska A, Bichowska M, Drobnik-Kozakiewicz I, Radzimińsk Ł, Leońska-Duniec A, Ficek K, Cięszczyk P. Effect of 12-week-long aerobic training programme on body composition, aerobic capacity, complete blood count and blood lipid profile among young women. Biochem Med (Zagreb) 2015;25(1):103–113. doi: 10.11613/BM.2015.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansour-Ghanaei F, Joukar F, Mobaraki SN, Mavaddati S, Hassanipour S, Sepehrimanesh M. Prevalence of non-alcoholic fatty liver disease in patients with diabetes mellitus, hyperlipidemia, obesity and polycystic ovary syndrome: a cross-sectional study in north of Iran. Diabetes Metab Syndr. 2019;13(2):1591–1596. doi: 10.1016/j.dsx.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab. 2015;29(4):621–631. doi: 10.1016/j.beem.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Babaei P, Damirchi A, Hoseini R. The interaction effects of aerobic exercise training and vitamin D supplementation on plasma lipid profiles and insulin resistance in ovariectomized rats. J Exerc Nutr Biochem. 2015;19(3):173. doi: 10.5717/jenb.2015.15070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asbaghi O, Khosroshahi MZ, Kashkooli S, Abbasnezhad A. Effect of calcium-vitamin d co-supplementation on insulin, insulin sensitivity, and glycemia: a systematic review and meta-analysis of randomized clinical trials. Horm Metab Res. 2019;51(05):288–295. doi: 10.1055/a-0887-0205. [DOI] [PubMed] [Google Scholar]
- 37.Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc. 2018;2(7):687–709. doi: 10.1210/js.2017-00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collao N, Rada I, Francaux M, Deldicque L, Zbinden-Foncea H. Anti-inflammatory effect of exercise mediated by toll-like receptor regulation in innate immune cells–a review: anti-inflammatory effect of exercise mediated by toll-like receptor regulation in innate immune cells. Int Rev Immunol. 2020;39(2):39–52. doi: 10.1080/08830185.2019.1682569. [DOI] [PubMed] [Google Scholar]
- 39.Estébanez B, Rodriguez AL, Visavadiya NP, Whitehurst M, Cuevas MJ, González-Gallego J, Huang C-J. Aerobic training Down-regulates Pentraxin 3 and Pentraxin 3/toll-like receptor 4 ratio, irrespective of oxidative stress response, in elderly subjects. Antioxidants. 2020;9(2):110. doi: 10.3390/antiox9020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.e Silva LLS, de Sousa Fernandes MS, Kubrusly MS, Muller CR, Américo ALV, Stefano JT, Evangelista FSA, Oliveira CP, Jukemura J. Effects of aerobic exercise protocol on genes related to insulin resistance and inflammation in the pancreas of Ob/Ob mice with NAFLD. Clin Exp Gastroenterol. 2020;13:223. doi: 10.2147/CEG.S242393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ara I, Auerbach P, Larsen S, Mata E, Stallknecht B, Ploug T, Prats C, Helge JW. Low-grade inflammation is not present in former obese males but adipose tissue macrophage infiltration persists. Biomedicines. 2020;8(5):123. doi: 10.3390/biomedicines8050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Della Guardia L, Codella R. Exercise tolls the bell for key mediators of low-grade inflammation in dysmetabolic conditions. Cytokine Growth Factor Rev. 2021;62:83–93. doi: 10.1016/j.cytogfr.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 43.El-Zayat SR, Sibaii H, Mannaa FA. Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl Res Centre. 2019;43(1):1–12. doi: 10.1186/s42269-019-0227-2. [DOI] [Google Scholar]
- 44.Archer AE, Von Schulze AT, Geiger PC. Exercise, heat shock proteins and insulin resistance. Philos Trans R Soc Lond Ser B Biol Sci. 2018;373(1738):20160529. doi: 10.1098/rstb.2016.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wamberg L, Cullberg K, Rejnmark L, Richelsen B, Pedersen S. Investigations of the anti-inflammatory effects of vitamin D in adipose tissue: results from an in vitro study and a randomized controlled trial. Horm Metab Res. 2013;45(06):456–462. doi: 10.1055/s-0032-1331746. [DOI] [PubMed] [Google Scholar]
- 46.Sokol SI, Srinivas V, Crandall JP, Kim M, Tellides G, Lebastchi A, Yu Y, Gupta AK, Alderman MH. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med. 2012;17(6):394–404. doi: 10.1177/1358863X12466709. [DOI] [PubMed] [Google Scholar]
- 47.Laird E, Rhodes J, Kenny RA. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J. 2020;113(5):81. [PubMed] [Google Scholar]
- 48.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523(1):123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Sun J, Liu C, Zhang S, Yi B, Gui M, Zhang W, Li YC, Zhang H. Vitamin D receptor expression in peripheral blood mononuclear cells is inversely associated with disease activity and inflammation in lupus patients. Clin Rheumatol. 2019;38(9):2509–2518. doi: 10.1007/s10067-019-04594-2. [DOI] [PubMed] [Google Scholar]
- 50.Maestro B, Dávila N, Carranza MC, Calle C. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84(2–3):223–230. doi: 10.1016/S0960-0760(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 51.Contreras-Bolívar V, García-Fontana B, García-Fontana C, Muñoz-Torres M. Mechanisms involved in the relationship between vitamin D and insulin resistance: impact on clinical practice. Nutrients. 2021;13(10):3491. doi: 10.3390/nu13103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rafiq S, Jeppesen PB. Insulin resistance is inversely associated with the status of vitamin D in both diabetic and non-diabetic populations. Nutrients. 2021;13(6):1742. doi: 10.3390/nu13061742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salehpour A, Hedayati M, Shidfar F, Neshatbini Tehrani A, Farshad AA, Mohammadi S. 1, 25-Dihydroxyvitamin D3 modulates adipogenesis of human adipose-derived mesenchymal stem cells dose-dependently. Nutr Metab. 2021;18(1):1–9. doi: 10.1186/s12986-021-00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to ongoing data analysis but are available from the corresponding author on reasonable request.