Abstract
HSCT recipients are at increased risk for COVID-19-associated morbidity and mortality. Early treatment of symptomatic SARS-CoV-2 infection is an important means to decreasing risk for severe disease and death. While some HSCT recipients, particularly those who are early post-transplant and severely immunosuppressed, may have diminished response to COVID-19 vaccines, the benefits of vaccination are uncontested. Public health, healthcare facility and individual level approaches are all necessary to mitigate risk for infection in this vulnerable population.
Keywords: COVID-19, SARS-CoV-2, Hematopoietic stem cell transplant, HSCT, Prevention, Treatment
1. Introduction
In late 2019, a novel coronavirus resulted in a cluster of pneumonia cases in Wuhan, China. With rapid spread and ultimately declaration of a global pandemic in February 2020, the World Health Organization designated the disease caused by severe acute respiratory coronavirus 2 (SARS-CoV-2) to be COVID-19 (coronavirus disease 2019). Since that time, no corner of the globe and no sector of healthcare has been left untouched by COVID-19, its impact felt disproportionally by those persons who are medically and/or socioeconomically most vulnerable. While the pace of response to the pandemic from medical research and development has been unprecedented, with availability of highly effective vaccines and other therapeutics in record time, the evolution of the SARS-CoV-2 virus has directed the epidemiology of the pandemic in ways that could not have been predicted at the outset. Going forward, there are still many uncertainties, even as we surpass 6.5 million COVID-19-associated deaths worldwide [1]. In this chapter, we will focus attention on the impact and management of COVID-19 on HSCT recipients, acknowledging their special vulnerabilities and risks.
2. Impact of COVID-19 on transplant centers and clinical practices
Early in the pandemic, several groups and societies advised that nonurgent transplants be delayed and that conditioning regimens or stem cell source be modified in some instances [[2], [3], [4]] to decrease risk for severe COVID-19 disease in patients receiving intensive immunosuppression for hematopoietic stem cell transplantation (HSCT) and in donors, and to preserve hospital capacity. In this context, centers reported a decrease in HSCT volume, both for non-malignant and malignant disorders, along with a shift in stem cell source distribution and in patient-level protocols [5,6]. A study from the World Marrow Donor Association examining global trends during the COVID-19 pandemic found that donations from unrelated donors decreased by 3.5% from 2019 to 2020, compared to an average annual growth rate of 3.9% from 2015 to 2019 [7]. The reasons for this decline are likely multifactorial in nature, including disruption in courier and other services as a consequence of travel restrictions or strained health care systems, and prioritization of patients with acute disease, among other potential factors. Over time, however, rapid adaptations allowed for a safe supply of donor products. With potential barriers to accessing allogeneic product from international or otherwise remote donors, or in considering that a donor might become infected with SARS-CoV-2 after recipient conditioning is begun, the EBMT and NMDP issued guidelines recommendations in 2020 that centers consider use of frozen stem cell products to ensure availability when conditioning is started [8,9]. This guidance, in turn, resulted in a shift from bone marrow to peripheral blood stem cells (PBSC) in some instances, given the complexity of cryopreserving the former. A 2021 CIBMTR study suggested that cryopreservation during the COVID-19 pandemic was associated with slower engraftment of PBSC grafts, but no statistically significant effect on non-relapse mortality, progression-free survival or overall survival [10]. Encouragingly, there are early data to suggest the shift to cryopreserved products among other adaptations, allowed lifesaving transplant operations to continue [11]; and, over time, cryopreserved products were increasingly acceptable to transplant centers [12].
In this same era, broad recommendations and policies for SARS-CoV-2 screening of recipients and donors with deferral periods for individuals testing positive were codified, in an effort to mitigate risk both to individual patients and more broadly risk for transmission in the healthcare setting [13,14]. Admittedly, the screening protocols and management algorithms with regard to deferral timeline in the case of a positive test, in particular in a potential donor, are not “data driven.” While there is evidence of donor-derived COVID-19 transmitted by lung transplantation [15,16], there is to date no confirmed instance of transmission via cellular transplant [17]. In fact, there are several reports describing of use of stem cells harvested from donors who tested positive for SARS-CoV-2 by PCR on nasopharyngeal swab at the time of cell collection, without documented transmission to the recipients [12,18,19]. However, in the absence of data and while SARS-CoV-2 continues to circulate in the community, it will fraught to step back or pivot from donor and recipient screening protocols.
3. Clinical presentation of COVID-19 in HSCT recipients
The clinical presentation of COVID-19 is variable, with a range of severity, from asymptomatic to severe illness, with extrapulmonary manifestations and late sequelae (“long COVID”). As with the population at large, the risk for COVID-19 and the severity of the clinical presentation in HSCT recipients is highly dependent on a multitude of patient characteristics, including the degree of immunosuppression, age, comorbidities, vaccination status as well as immune response to vaccination, and by the virulence of the circulating virus, a variable that continues to evolve with the emergence of successive variants. Acknowledging these variables, apart from a higher risk for severe COVID-19, the clinical presentation is not distinctly different in the HSCT population; that said, there are several important considerations that warrant mention.
Prolonged SARS-CoV-2 shedding is well described in the HSCT population [20,21]. Chemotherapeutic agents employed in the treatment of malignancy in the time leading up to transplant and in the conditioning regimen along with immunosuppressive medications for prevention and treatment of GVHD contribute to the immune suppressed state that allow for prolonged shedding. Among other agents, the CD-20 monoclonal antibodies (eg., rituximab) have been associated with prolonged, asymptomatic shedding [22]. At the same time, other infections (eg., cytomegalovirus, community respiratory viruses, Pneumocystis carinii, etc.) and noninfectious pulmonary complications (eg., engraftment syndrome with diffuse alveolar damage, idiopathic pneumonia syndrome, diffuse alveolar damage) are common in the HSCT population, particularly in allogeneic recipients [23]. Furthermore, while prolonged shedding often represents fragment RNA from non-viable virus, there are reports in HSCT recipients of shedding of viable SARS-CoV-2 for several months after initial infection [24], and even reports of clinical relapse with the same or evolved strain types in other similarly immunocompromised patient populations [25,26]. With this background in mind, the finding of positive SARS-CoV-2 PCR in a patient with prior COVID-19 and presenting either for planned HSCT or post-HSCT with diffuse ground glass consolidation on chest imaging can present significant diagnostic and management challenges.
4. Morbidity and mortality in HSCT recipients
The literature on morbidity and mortality of COVID-19 in HSCT recipients consists largely of single center and consortium studies, with significant heterogeneity in inclusion by testing criteria, and with variability in reported patient characteristics, follow-up periods, endpoints and outcomes (Table 1 ) [20,[27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. Furthermore, there is a significant bias toward reporting earlier in the pandemic, at which time testing was less available, management less sophisticated, and vaccination not or less available, and when disease associated with SARS-CoV-2 was more severe than with later variants and subvariants. That said, it is uncontested that COVID-19 disease severity and morbidity are increased in the HSCT population, relative to the population at large. A systematic review and meta-analysis that included 19 studies published from the outset of the pandemic through December 31, 2021 reported on outcomes from 2031 HCT recipients who had a median age of 57 years, a median time from transplant of 23 months for autologous recipients and 16 months for allogeneic recipients, and a median time from COVID-19 diagnosis of 28 days (range 0–262); a pooled analysis revealed an intensive care unit admission rate of 29% and a mortality rate of 19% [37]. There was a slightly higher, though not statistically significant, mortality rate among allogeneic HCT recipients compared with autologous recipients, 21% vs 17%, respectively. Age was associated with mortality in most but not all of the studies included in this analysis. Likewise, many but not all studies have demonstrated increased mortality in association with shorter time from transplant [27,31,[33], [34], [35], [36],38]. Not surprisingly, several studies have demonstrated active GVHD [27,36] and active malignancy [29] to be associated with higher mortality.
Table 1.
Morbidity and mortality of COVID-19 in HSCT recipients. Auto = autologous, Allo = allogeneic, IQR = interquartile range. ǂvariable definition of severe disease, *variable follow-up time.
| Author, site(s) | Diagnosis period | # HSCT recipients: auto/allo/CAR-T | Age, years – median (range) | Time since HSCT or CAR-T – median (range) | % asymptomatic | % hospitalized | % required supplemental O2 | % required ICU care | % require mechanical ventilation | % severe COVIDǂ | % COVID-specific mortality* | % overall mortality* | % mortality (by time from diagnosis) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altuntas, Republic of Turkey (Ministry of Health database) [31] | 3/11/20–5/29/20 | 32: 20/12/0 | 56.5 (19-74) | 100 | 22 | 16 | 22 | 16 | |||||
| Coll, Spain (national)*not all lab confirmed [32] | on or before 7/13/20 | 113: 42/71/0 | Auto: 60 (55-64) | 18 months (4-53) | 97 | 17 | 15 | 24 | |||||
| Allo: 48 (34-61) | 15 months (7-37) | 80 | 7 | 8 | 20 | ||||||||
| Camargo, US (University of Miami) [33] | March 2020–December 2020 | 28: 12/15/1 | 57 (50-67) | 656 days (IQR 33–1274) | 57 | 80 (12 of 15 assessable) | 25 | 21 | 36 | Auto:25 | 14 (30 days) | ||
| Allo:27 | |||||||||||||
| El Fakih, Middle East (10 HCT tertiary care centers, adult and pediatric) [34] | 3/15/20–12/1/20 | 91: 39/52/0 | 35 | 14.9 months (IQR 16.3–38.9) | 14 | 53 | 18 | Total: 16 | Total: 10 | 5 | 4 | ||
| Admitted:31 | Admitted:19 | ||||||||||||
| Xhaard, France, Belgium and Switzerland (SFGM-TC centers, adult and pediatric) [35] | March 2020–May 2020 | 54: 0/54/0 | 57.4 (5.5–80.4) | 1.3 years (11 days −19 years) | 52 | 24 | ? | 39 | 24 | 26 | |||
| Ljungman, 22 European countries (ESBMT or GETH, adult and pediatric) [20] | on or before 7/31/20 | 382: 146/236/0 | Auto:60.6 (7.7–81.6) | Auto:24.6 months (0.9–350.3) | 8.9 | 35 | 22 | 25 | Auto:28 | Auto: 28 (6 weeks) | |||
| Allo:54.1 (1–80.3) | Allo:15.8 months (0.2–292.7) | Allo:29 | Allo: 22 (6 weeks) | ||||||||||
| Mushtaq, US (University of Kansas) [27] | March 2020–May 2021 | 58: 23/32/3 | 58 (24-77) | 17.7 months (0.2–201.9) | 11 | 6 | Overall:16 | ||||||
| Allo:28% | |||||||||||||
| Pinana, 41 hospitals in Spain (GETH, adult and pediatric) [28] | 3/1/20–5/15/20 | 123: 58/65/0 | Auto: 61 (34-75) | Auto:8 | Auto:41 | Auto: 14 | Auto: 17 | Auto:21 | |||||
| Allo: 48 (1-70) | Allo:10 | Allo:49 | Allo: 11 | Allo:18 | Allo:20 | ||||||||
| Shah, US (MSKCC) [29] | 3/15/20–5/7/20 | 77: 37/35/5 | Overall: 62 (IQR, 52-68)] | 782 days (IQR 354–1611) | 44 | 43 | 12 | 22 | 18 | Overall: 22 (30 days) | |||
| Auto: 64 (IQR, 52-69) | Allo: 27 (30 days) | ||||||||||||
| Allo: 60 (IQR, 51-65) | Auto: 13 (30 days) | ||||||||||||
| CAR-T: 63 (IQR, 58-74) | CAR-T: 40 (30 days) | ||||||||||||
| Sharma, US (CIBMTR) [30] | 3/27/20–8/12/20 | 318: 134/184/0 | Auto: 23 (IQR 8–51) | Auto:13 | Auto:13 | Auto:19 | |||||||
| Allo: 17 (IQR 8–46) | Allo:15 | Allo:15 | Allo:22 | ||||||||||
| Varma, US (Rush, University of Chicago, Mt. Sinai, Northwestern) [36] | 34: 20/14/0 | Overall: 57 | Overall: 17.4 months | Overall: 74 | 32 | 8 | 41 | 21 (7 of 25 admitted patients) | |||||
| Auto: 59 | Auto: 13.2 months | ||||||||||||
| Allo: 54 | Allo: 18.9 months |
5. Treatment of COVID-19 in HSCT recipients
Management of SARS-CoV-2 infection continues to evolve as therapeutic options expand. Targeted antiviral therapies available at this writing include remdesivir, nirmaterelvir/ritonavir (Paxlovid) and molnupiravir. The efficacy data for remdesivir has been variable [39,40], and with intravenous administration required over several days this agent has largely been restricted to use in hospitalized patients. Of the antivirals, nirmatrelvir/ritonavir, has demonstrated the greatest risk reduction in hospitalization or death when begun within 5 days of symptom onset [41]. Very relevant to the allogeneic HCT population, however, is the potent drug-drug interaction between the protease inhibitor ritonavir and calcineurin inhibitors (CNIs) and mammalian target of rapamycin (mTOR) inhibitors [42]. For this reason, either use of a SARS-CoV-2 monoclonal antibody (see below), if available, or careful coadministration of ritonavir-boosted nirmatrelvir while withholding certain immunosuppressive agents (eg., tacrolimus, sirolimus) or reducing the dosage of certain immunosuppressive agents (eg., cyclosporine), monitoring closely for toxicities, and performing therapeutic drug monitoring during and following treatment is advised [[43], [44], [45], [46]]. A series of monoclonal antibodies targeting the spike protein of SARS-CoV-2, the specifics or which have evolved over time in response to virus-mediated immune escape, have received EUA by the FDA for treatment of non-hospitalized patients with COVID-19 who are at high risk of progression to severe disease. With significant supply/demand mismatch and other barriers to use of targeted antivirals and monoclonal antibodies at various times in the course of the pandemic, the NIH has issued prioritization guidelines for the treatment and prevention of COVID-19 when there are logistical or supply constraints [47].
As much of the severe disease related to COVID-19 is a consequence of immune-mediated injury, adjuvant therapy with systemic immunosuppression has been an area of intense study. For hospitalized patients with moderate to severe COVID-19 requiring supplemental oxygen or mechanical ventilation, dexamethasone (6 mg once daily for up to 10 days) has been demonstrated to improve survival [48]. In addition to dexamethasone, in hospitalized patients requiring supplemental oxygen with systemic inflammation as demonstrated by elevation of C-reactive protein, the adjuvant use of tocilizumab, an interleukin-6 (IL-6) inhibitor, or baricitinib, a Janus kinase (JAK) inhibitor, have been demonstrated to additively decrease the composite endpoint of need for mechanical ventilation or death, or mortality, respectively [49,50]. It is important to acknowledge that HSCT recipients were specifically excluded from many of these studies. The risk of secondary infection as a consequence of immunomodulation in the HSCT population is likely to be magnified, and as such these therapies should be used with caution in this population.
6. Investigative therapies
Mesenchymal stem cells (MSCs) have been studied in various applications for their immunomodulatory, anti-inflammatory and regenerative properties [51]. Based on safety and the suggestion of efficacy in preclinical and early-stage clinical studies, there are numerous trials in progress to evaluate the effectiveness of MSCs in management of COVID-19 [52]. However, at this writing MSCs are not FDA-approved for the treatment of COVID-19 and should only be used in the context of a clinical trial. Likewise, based on the premise that T cells are critical in immune response to SARS-CoV-2 infection, there are several studies underway to evaluate the potential therapeutic role of adoptive T-cell therapy for COVID-19 [53].
7. Prevention of COVID-19 in HSCT recipients
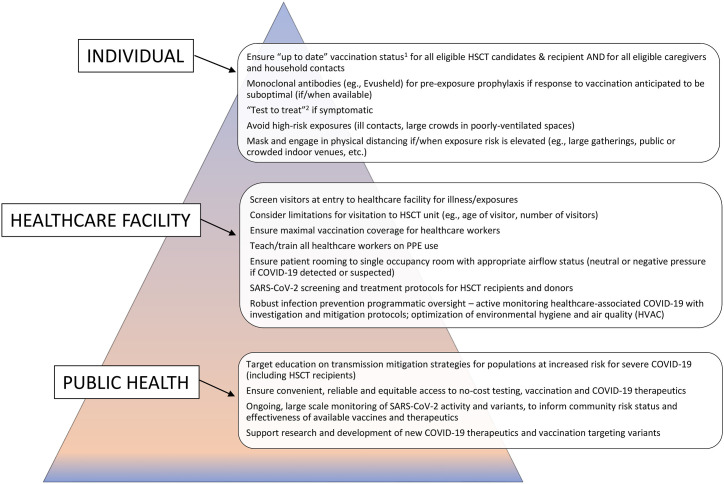
With increased risk for COVID-19-associated morbidity and mortality in HSCT recipients, preventing SARS-CoV-2 infection is critically important in this vulnerable population. There is a hierarchy of controls with regard to prevention, a layering of protection measures that rely on public health, the healthcare facility, and the individual (Fig. 1 ).
Fig. 1.
COVID-19 “prevention pyramid” – hierarchy of controls: PPE = personal protective equipment; HVAC = heating, ventilation, and air conditioning. 1Use of COVID-19 Vaccines in the United States [Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html]. 2US Department of Health & Human Services ASPR COVID-19 Test to Treat [Available from: https://aspr.hhs.gov/TestToTreat/Pages/default.aspx?ACSTrackingID=USCDC_1052-DM76108&ACSTrackingLabel=COCA%20Now%3A%20New%20COVID-19%20Test%20to%20Treat%20Initiative%20and%20Locator%20Tool&deliveryName=USCDC_1052-DM76108].
8. Vaccination in HSCT recipients
The clinical trials that evaluated safety and efficacy of the COVID-19 vaccines and led to EUA and ultimate FDA approval specifically excluded HSCT recipients and other severely immunocompromised individuals [[54], [55], [56]]. That said, given that all of the currently authorized or approved COVID-19 vaccines licensed in the United States (US) are not live virus vaccines, according to the Advisory Committee on Immunization Practices (ACIP) they can be safely administered to immunocompromised individuals [57]. In December 2021 the ACIP expressed preference for the mRNA vaccines (BNT162b2 or mRNA-1273) for primary and booster vaccination, based on concerns for thrombotic events associated with the adenovirus-vector vaccine (Ad26.COV2.S) currently available in the US under EUA.
Vaccine safety in HSCT recipients: Not unexpectedly, given the sheer number of vaccine administrations to a population with a high baseline risk for events, there have been several case reports describing the onset of immune-mediated complications (eg., GVHD, acute interstitial lung disease, immune thrombotic thrombocytopenic purpura) occurring in temporal association with COVID-19 vaccination [[58], [59], [60], [61]]. Whether or not these events are causally related to vaccination is difficult to untangle on an individual, case by case basis. Ultimately, large scale prospective vaccine studies in HSCT recipients will provide clarity on safety in this population. That said, the scarcity of such reports and the absence of a safety signal in the numerous retrospective studies of vaccine administration in HSCT recipients on balance with the enormous number of persons vaccinated provides a significant degree of reassurance.
Vaccine efficacy in HSCT recipients: While highly immunogenic in the populations included in the large-scale studies that led to vaccine licensing, at least against the original outbreak strain of SARS-CoV-2, numerous, mostly single-center immunogenicity studies have demonstrated decreased, but highly variable, in vitro immune response in HSCT recipients to a 2- or 3-dose mRNA vaccine series as measured by neutralizing antibody and/or T-cell response [12,[62], [63], [64], [65], [66], [67], [68], [69], [70], [71]]. Not surprisingly, recency of HSCT, immunosuppressive therapy, lymphopenia, active malignancy and GVHD have all been demonstrated to be associated with dampened immune response. And while an attenuated in vitro immune response has been consistently reported in the HSCT population as a whole, it is important to note that only a small minority had complete absence of an in vitro immune response. Furthermore, In vitro correlates are an imperfect surrogate for in vivo vaccine efficacy. While there is no large-scale prospective efficacy data for COVID-19 vaccination in the HSCT population at the current time, the benefits of COVID-19 vaccination for this highly vulnerable population are indisputable. Ongoing [72] and future prospective studies focusing on the type, timing and cadence of COVID-19 vaccination in HSCT recipients will undoubtedly inform optimal vaccine strategies.
9. COVID-19 vaccination recommendations for HSCT recipients
The current US vaccination schedule for individuals with moderate or severe immunocompromising conditions, under which HSCT recipients who are within 2 years of transplant, receiving active treatment with high-dose steroids or other immunocompromising drugs, and/or receiving active cancer treatment fall, includes a 3-dose primary series of an mRNA vaccine (or a single-dose adenovirus-vector vaccine followed by a single-dose mRNA vaccine), followed by 2 booster doses for those 12 years and older (or by 1 booster dose for those 5–11 years) [73]. It is anticipated that the vaccine guidelines and the definition of “up to date” with COVID-19 vaccination will be iteratively updated as the science evolves and as the virus evolves. While relied upon in immunologic studies of vaccine efficacy and despite accumulating evidence that suggests the presence of antibodies following infection offers some degree of protection from reinfection, SARS-CoV-2 antibody testing is not currently recommended to assess for immunity following COVID-19 vaccination or to assess the need for vaccination or quarantine in a previously infected person [74].
There are several considerations regarding vaccination that are unique to the HSCT setting that warrant specific mention. There is limited data to suggest adoptive transfer of immune memory, as assessed by anti-spike glycoprotein-specific IgG, from vaccinated stem cell donor to recipient [18]. While it is generally advised that all eligible persons, including stem cell donors, remain up to date with vaccination, there is not yet sufficient rationale to include donor SARS-CoV-2 serological status as a determinant of donor choice. Time from transplant to vaccination and immune recovery after transplant have been demonstrated to correlate with humoral immune response to vaccination [75]. At this writing, ASTCT and EBMT guidelines suggest COVID-19 vaccination start at least 3 months after allogeneic or autologous HSCT [76,77]. The optimal timing of vaccination after HSCT, with regard to immunogenicity and durability, is an area of active investigation (CIBMTR SC21-07/BMT CTN 2101) [78]. For persons who received 1 or more doses of COVID-19 vaccine prior to HSCT, the ACIP in alignment with ASTCT, CIBMTR and NMDP, recommends revaccination at least 3 months following transplant [73,79]. Routine post-transplant vaccines can be given concomitantly with COVID-19 vaccines.
Acknowledging the suboptimal immune response to vaccination in certain HSCT recipients, “cocoon vaccination” with SARS-CoV-2 vaccination of all eligible caregivers and household contacts is absolutely critical as another strategy to decrease risk for COVID-19 in this population [80].
10. Other prevention strategies in HSCT recipients
When considering risk mitigation apart from vaccination, there are a number of other pharmacologic, structural, individual and public health interventions that warrant consideration.
Evusheld (tixagevimab-cilgavimab) has an EUA for use as pre-exposure prophylaxis of COVID-19 in individuals 12 years and older with moderate to severe immune compromise and who may not mount an adequate response to vaccination, including HSCT recipients [81]. Data supporting authorization date to the pre-Omicron era [82], and there has been demonstration of diminished neutralization capacity against Omicron and its sublineages [83,84]. Moving forward, it is absolutely critical to monitoring emerging SARS-CoV-2 variant strains for antibody neutralization potency and in vivo protection.
In the face of risk for severe COVID-19 and with suboptimal immune response to vaccination in HSCT recipients, and as the virus evolves with variants leading to immune escape and future waves of infection, the timeline for individual, systems and public health level precautions is uncertain. Persons at increased risk for severe COVID-19 are strongly encouraged to layer prevention strategies, with masking and avoiding high risk settings (eg., large crowds, poorly ventilated indoor venues) when warranted by local or community infection activity. Healthcare systems, in particular, have a responsibility to optimize protection of vulnerable HSCT recipients while SARS-CoV-2 continues to circulate. With prolonged hospital stays, often on dedicated units and in rooms with positive pressure ventilation, situational awareness of engineering controls and airflow is critical. Persons with active COVID-19 should not be placed in a positive pressure room, as this poses risk for transmission to other patients and staff on the unit.
11. Lessons learned
The COVID-19 pandemic has led to devastating loss of life on a worldwide scale and has posed unprecedented challenge to the healthcare infrastructure. This has prompted rapid restructuring of standard work protocols, with several collateral improvements in care delivery and outcomes for the HSCT population. Several sites have reported on successes in shifting transplant to home-based rather than hospital-based care [85,86]. Widespread routine masking has resulted in marked reduction in the spread of community respiratory virus infections in HSCT recipients, formerly a relatively frequent cause of morbidity and mortality in this population, with some calling to “maintain mask momentum” in this population in the post-pandemic era [87,88]. Undoubtedly there is much more we will learn from the pandemic, both with regard to how best to manage and mitigate COVID-19 in HSCT recipients, but also how best to approach post-transplant vaccinations and prevention of infection more broadly. We must “be nimble” and ready for the next challenge.
12. Practice points
-
1.
SARS-CoV-2 infection is associated with increased disease severity and increased mortality in HSCT recipients, with risk largely related to degree of immune suppression.
-
2.
Prolonged viral shedding is common in HSCT recipients, introducing complexity when sorting through disease attribution, management and venue of care with regard to duration of transmission-based isolation precautions.
-
3.
Early treatment of symptomatic infection in HSCT recipients is critical to decrease risk for severe disease and death. Ritonavir-boosted nirmatrelvir (Paxlovid) is a highly efficacious SARS-CoV-2 antiviral, with important drug-drug interaction with calcineurin inhibitors and mTOR inhibitors.
-
4.
While some HSCT recipients may have a diminished response to COVID-19 vaccination when compared with the immunocompetent population, it is strongly advised that all HSCT recipients as well as their caregivers and close contacts be up to date with COVID-19 vaccination.
-
5.
Prevention of COVID-19 in HSCT recipients includes a layered strategy of vaccination, masking in certain situations, and system-level controls to minimize risk for SARS-CoV-2 exposure.
13. Research agenda
-
1.
Prospective studies of COVID-19 vaccination, both with respect to time post-transplant, cadence and type of vaccine, are needed to clarify an optimal strategy for HSCT recipients.
-
2.
The qualitative study of public health, healthcare facility and individual level approaches to mitigate risk for acquisition of SARS-CoV-2 will be important to inform durable prevention strategies for HSCT recipients, both with regard to COVID-19 and as generalizable to other community respiratory viruses.
Declaration of competing interest
None.
References
- 1.COVID-19 dashboard by the center for systems science and engineering. https://coronavirus.jhu.edu/map.html (CSSE) at Johns Hopkins University (JHU) [Available from: [DOI] [PMC free article] [PubMed]
- 2.Waghmare A., Abidi M.Z., Boeckh M., Chemaly R.F., Dadwal S., El Boghdadly Z., et al. Guidelines for COVID-19 management in hematopoietic cell transplantation and cellular therapy recipients. Biol Blood Marrow Transplant. 2020;26(11):1983–1994. doi: 10.1016/j.bbmt.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algwaiz G., Aljurf M., Koh M., Horowitz M.M., Ljungman P., Weisdorf D., et al. Real-World issues and potential solutions in hematopoietic cell transplantation during the COVID-19 pandemic: perspectives from the worldwide network for blood and marrow transplantation and center for international blood and marrow transplant research health services and international studies committee. Biol Blood Marrow Transplant. 2020;26(12):2181–2189. doi: 10.1016/j.bbmt.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungman P., Mikulska M., de la Camara R., Basak G.W., Chabannon C., Corbacioglu S., et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;55(11):2071–2076. doi: 10.1038/s41409-020-0919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passweg J.R., Baldomero H., Chabannon C., Corbacioglu S., de la Camara R., Dolstra H., et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. 2022;57(2):742–752. doi: 10.1038/s41409-022-01604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo D., Polverelli N., Malagola M., Farina M., Leoni A., Bernardi S., et al. Changes in stem cell transplant activity and procedures during SARS-CoV2 pandemic in Italy: an Italian bone marrow transplant group (GITMO) nationwide analysis (TransCOVID-19 survey) Bone Marrow Transplant. 2021;56(9):2272–2275. doi: 10.1038/s41409-021-01287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jöris M.M., Schmidt A.H., Bernas S.N., Feinberg J., Sacchi N., Elmoazzen H., et al. Impact of COVID-19 pandemic on global unrelated stem cell donations in 2020—report from World Marrow Donor Association. Bone Marrow Transplant. 2022;57(6):1021–1024. doi: 10.1038/s41409-022-01667-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronavirus disease COVID-19: EBMT recommendations. https://www.ebmt.org/sites/default/files/2022-02/EBMT%20COVID-19%20guidelines%20v.17.2.pdf version 17 - January 26, 2022 [Available from:
- 9.Updates for Transplant Centers and Cooperative Registries Up-to-date information on NMDP/Be the Match response to COVID-19. https://network.bethematchclinical.org/news/nmdp/be-the-match-response-to-covid-19/updates-for-transplant-centers-and-cooperative-registries/#TemporaryCryopreservation Updated Jan. 11, 2022 [Available from:
- 10.Hsu J.W., Farhadfar N., Murthy H., Logan B.R., Bo-Subait S., Frey N., et al. The effect of donor graft cryopreservation on allogeneic hematopoietic cell transplantation outcomes: a center for international blood and marrow transplant research analysis. Implications during the COVID-19 pandemic. Transplant Cell Ther. 2021;27(6):507–516. doi: 10.1016/j.jtct.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auletta J.J., Novakovich J.L., Stritesky G.L., Newman J., Fridy-Chesser S.T., Hailperin K., et al. Meeting the demand for unrelated donors in the midst of the COVID-19 pandemic: rapid adaptations by the national marrow donor program and its network partners ensured a safe supply of donor products. Transplant Cell Ther. 2021;27(2):133–141. doi: 10.1016/j.jtct.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaekal O.K., Gomez-Arteaga A., Chen Z., Soave R., Shore T., Mayer S., et al. Predictors of covid-19 vaccination response after in-vivo T-cell-depleted stem cell transplantation. Transplant Cell Ther. 2022;28(9):618 e1–e10. doi: 10.1016/j.jtct.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riches M. The new world: hematopoietic stem cell transplant during a pandemic. Curr Opin Hematol. 2021;28(6):389–393. doi: 10.1097/MOH.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 14.Worel N., Shaw B.E., Aljurf M., Koh M., Seber A., Weisdorf D., et al. Changes in hematopoietic cell transplantation Practices in response to COVID-19: a survey from the worldwide network for blood & marrow transplantation. Transplant Cell Ther. 2021;27(3):270 e1–e6. doi: 10.1016/j.jtct.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaul D.R., Valesano A.L., Petrie J.G., Sagana R., Lyu D., Lin J., et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;21(8):2885–2889. doi: 10.1111/ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar D., Humar A., Keshavjee S., Cypel M. A call to routinely test lower respiratory tract samples for SARS-CoV-2 in lung donors. Am J Transplant. 2021;21(7):2623–2624. doi: 10.1111/ajt.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaussen A., Hornby L., Rockl G., O'Brien S., Delage G., Sapir-Pichhadze R., et al. Evidence of SARS-CoV-2 infection in cells, tissues, and organs and the risk of transmission through transplantation. Transplantation. 2021;105(7):1405–1422. doi: 10.1097/TP.0000000000003744. [DOI] [PubMed] [Google Scholar]
- 18.Leclerc M., Fourati S., Menouche D., Challine D., Maury S. Allogeneic haematopoietic stem cell transplantation from SARS-CoV-2 positive donors. Lancet Haematol. 2021;8(3):e167–e169. doi: 10.1016/S2352-3026(21)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anurathapan U., Apiwattanakul N., Pakakasama S., Pongphitcha P., Thitithanyanont A., Pasomsub E., et al. Hematopoietic stem cell transplantation from an infected SARS-CoV2 donor sibling. Bone Marrow Transplant. 2020;55(12):2359–2360. doi: 10.1038/s41409-020-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljungman P., de la Camara R., Mikulska M., Tridello G., Aguado B., Zahrani M.A., et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35(10):2885–2894. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Ruiz M., Aguado J.M. Severe acute respiratory syndrome coronavirus 2 infection in the stem cell transplant recipient - clinical spectrum and outcome. Curr Opin Infect Dis. 2021;34(6):654–662. doi: 10.1097/QCO.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183(7):1901–19012 e9. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucena C.M., Torres A., Rovira M., Marcos M.A., de la Bellacasa J.P., Sanchez M., et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant. 2014;49(10):1293–1299. doi: 10.1038/bmt.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aydillo T., Gonzalez-Reiche A.S., Aslam S., van de Guchte A., Khan Z., Obla A., et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton C.S., Huntley K., Berenger B.M., Bristow M., Evans D.H., Fonseca K., et al. Prolonged SARS-CoV-2 infection following rituximab treatment: clinical course and response to therapeutic interventions correlated with quantitative viral cultures and cycle threshold values. Antimicrob Resist Infect Control. 2022;11(1):28. doi: 10.1186/s13756-022-01067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morel A., Imbeaud S., Scemla A., Pere H., Fourgeaud J., Amrouche L., et al. Severe relapse of SARS-CoV-2 infection in a kidney transplant recipient with negative nasopharyngeal SARS-CoV-2 RT-PCR after rituximab. Am J Transplant. 2022;22(8):2099–2103. doi: 10.1111/ajt.17000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mushtaq M.U., Shahzad M., Chaudhary S.G., Luder M., Ahmed N., Abdelhakim H., et al. Impact of SARS-CoV-2 in hematopoietic stem cell transplantation and chimeric antigen receptor T cell therapy recipients. Transplant Cell Ther. 2021;27(9):796 e1–e7. doi: 10.1016/j.jtct.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinana J.L., Martino R., Garcia-Garcia I., Parody R., Morales M.D., Benzo G., et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah G.L., DeWolf S., Lee Y.J., Tamari R., Dahi P.B., Lavery J.A., et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest. 2020;130(12):6656–6667. doi: 10.1172/JCI141777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma A., Bhatt N.S., St Martin A., Abid M.B., Bloomquist J., Chemaly R.F., et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altuntas F., Ata N., Yigenoglu T.N., Basci S., Dal M.S., Korkmaz S., et al. COVID-19 in hematopoietic cell transplant recipients. Bone Marrow Transplant. 2021;56(4):952–955. doi: 10.1038/s41409-020-01084-x. [DOI] [PubMed] [Google Scholar]
- 32.Coll E., Fernandez-Ruiz M., Sanchez-Alvarez J.E., Martinez-Fernandez J.R., Crespo M., Gayoso J., et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21(5):1825–1837. doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camargo J.F., Mendoza M.A., Lin R., Moroz I.V., Anderson A.D., Morris M.I., et al. Clinical presentation and outcomes of COVID-19 following hematopoietic cell transplantation and cellular therapy. Transpl Infect Dis. 2021;23(4) doi: 10.1111/tid.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Fakih R., Haroon A., Alfraih F., Al-Khabori M.K., Alzahrani M., Alhuraiji A., et al. Clinical course and outcomes of COVID-19 in hematopoietic cell transplant patients, a regional report from the Middle East. Bone Marrow Transplant. 2021;56(9):2144–2151. doi: 10.1038/s41409-021-01312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xhaard A., Xhaard C., D'Aveni M., Salvator H., Chabi M.L., Berceanu A., et al. Risk factors for a severe form of COVID-19 after allogeneic haematopoietic stem cell transplantation: a Societe Francophone de Greffe de Moelle et de Therapie cellulaire (SFGM-TC) multicentre cohort study. Br J Haematol. 2021;192(5):e121–e124. doi: 10.1111/bjh.17260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varma A., Kosuri S., Ustun C., Ibrahim U., Moreira J., Bishop M.R., et al. COVID-19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34(10):2809–2812. doi: 10.1038/s41375-020-01019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahzad M., Chaudhary S.G., Zafar M.U., Hassan M.A., Hussain A., Ali F., et al. Impact of COVID-19 in hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis. 2022;24(2) doi: 10.1111/tid.13792. [DOI] [PubMed] [Google Scholar]
- 38.Sharma A., Bhatt N.S., Hijano D.R. Clinical experience of coronavirus disease 2019 in hematopoietic cell transplant and chimeric antigen receptor T-cell recipients. Curr Opin Hematol. 2021;28(6):394–400. doi: 10.1097/MOH.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 39.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Consortium W.H.O.S.T., Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fact Sheet For Healthcare Providers Emergency use authorization for paxlovid. https://www.fda.gov/media/155050/download [Available from:
- 43.Lange N.W., Salerno D.M., Jennings D.L., Choe J., Hedvat J., Kovac D.B., et al. Nirmatrelvir/ritonavir use: managing clinically significant drug-drug interactions with transplant immunosuppressants. Am J Transplant. 2022;22(7):1925–1926. doi: 10.1111/ajt.16955. [DOI] [PubMed] [Google Scholar]
- 44.Salerno D.M., Jennings D.L., Lange N.W., Kovac D.B., Shertel T., Chen J.K., et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022;22(8):2083–2088. doi: 10.1111/ajt.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NIH COVID-19 treatment guidelines - special considerations in solid organ transplant, hematopoietic stem cell transplant, and cellular immunotherapy candidates, donors, and recipients. https://www.covid19treatmentguidelines.nih.gov/special-populations/transplant/?utm_source=site&utm_medium=home&utm_campaign=highlights [Available from:
- 46.AST statement on oral antiviral therapy for COVID-19 for organ transplant recipients. https://www.myast.org/sites/default/files/AST%20Statement%20on%20Oral%20Antiviral%20Therapy%20for%20COVID%20Jan%204%20%282%29.pdf [Available from:
- 47.NIH coronavirus disease. 2019. https://www.covid19treatmentguidelines.nih.gov/ COVID-19) Treatment Guidelines [Available from:
- 48.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marconi V.C., Ramanan A.V., de Bono S., Kartman C.E., Krishnan V., Liao R., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li N., Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74(13):2345–2360. doi: 10.1007/s00018-017-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu R., Feng Z., Wang F.S. Mesenchymal stem cell treatment for COVID-19. EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toor S.M., Saleh R., Sasidharan Nair V., Taha R.Z., Elkord E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology. 2021;162(1):30–43. doi: 10.1111/imm.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadoff J., Gray G., Vandebosch A., Cardenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.COVID-19 ACIP vaccine recommendations. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html [Available from:
- 58.Trunk A.D., Shewan S.K., Lee C.J., Parker C.J., Couriel D.R. Chronic graft-versus-host disease exacerbation after SARS-CoV-2 vaccination. Bone Marrow Transplant. 2022;57(3):502–503. doi: 10.1038/s41409-021-01543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pabst C., Benning L., Liebers N., Janssen M., Caille L., Speer C., et al. Humoral responses and chronic GVHD exacerbation after COVID-19 vaccination post allogeneic stem cell transplantation. Vaccines (Basel). 2022;10(2) doi: 10.3390/vaccines10020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueno T., Ohta T., Sugio Y., Ohno Y., Uehara Y. Severe acute interstitial lung disease after BNT162b2 mRNA COVID-19 vaccination in a patient post HLA-haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022;57(5):840–842. doi: 10.1038/s41409-022-01633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Innao V., Urso S., Insalaco M., Borraccino A., Consoli U. Immune Thrombotic Thrombocytopenic Purpura following Pfizer-BioNTech anti-COVID-19 vaccination in a patient healed from lymphoma after allogeneic hematopoietic stem cell transplantation. Thromb Res. 2022;210:91–93. doi: 10.1016/j.thromres.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ram R., Hagin D., Kikozashvilli N., Freund T., Amit O., Bar-On Y., et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-A single-center prospective cohort study. Transplant Cell Ther. 2021;27(9):788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clemenceau B., Guillaume T., Coste-Burel M., Peterlin P., Garnier A., Le Bourgeois A., et al. SARS-CoV-2 T-Cell responses in allogeneic hematopoietic stem cell recipients following two doses of BNT162b2 mRNA vaccine. Vaccines (Basel). 2022;10(3) doi: 10.3390/vaccines10030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balcells M.E., Le Corre N., Duran J., Ceballos M.E., Vizcaya C., Mondaca S., et al. Reduced immune response to inactivated SARS-CoV-2 vaccine in a cohort of immunocompromised patients in Chile. Clin Infect Dis. 2022;75(1):e594–e602. doi: 10.1093/cid/ciac167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jarisch A., Wiercinska E., Daqiq-Mirdad S., Hellstern H., Ajib S., Cremer A., et al. SARS-CoV-2-specific T cells are generated in less than half of allogeneic HSCT recipients failing to seroconvert after COVID-19 vaccination. Eur J Immunol. 2022;52(7):1194–1197. doi: 10.1002/eji.202149771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang A., Cicin-Sain C., Pasin C., Epp S., Audige A., Muller N.J., et al. Antibody response to SARS-CoV-2 vaccination in patients following allogeneic hematopoietic cell transplantation. Transplant Cell Ther. 2022;28(4):214 e1–e11. doi: 10.1016/j.jtct.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinana J.L., Lopez-Corral L., Martino R., Montoro J., Vazquez L., Perez A., et al. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am J Hematol. 2022;97(1):30–42. doi: 10.1002/ajh.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shem-Tov N., Yerushalmi R., Danylesko I., Litachevsky V., Levy I., Olmer L., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in haematopoietic stem cell transplantation recipients. Br J Haematol. 2022;196(4):884–891. doi: 10.1111/bjh.17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsushima T., Terao T., Narita K., Fukumoto A., Ikeda D., Kamura Y., et al. Antibody response to COVID-19 vaccine in 130 recipients of hematopoietic stem cell transplantation. Int J Hematol. 2022;115(5):611–615. doi: 10.1007/s12185-022-03325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beerlage A., Leuzinger K., Valore L., Mathew R., Junker T., Drexler B., et al. Antibody response to mRNA SARS-CoV-2 vaccination in 182 patients after allogeneic hematopoietic cell transplantation. Transpl Infect Dis. 2022;24(3) doi: 10.1111/tid.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maillard A., Redjoul R., Klemencie M., Labussiere Wallet H., Le Bourgeois A., D'Aveni M., et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139(1):134–137. doi: 10.1182/blood.2021014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.https://www.clinicaltrials.gov/ct2/results?cond=covid+vaccine+AND+%22Hematologic+Neoplasms%22 ClinicalTrials.gov ClinicalTrials.gov [Available from:
- 73.https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html Use of COVID-19 Vaccines in the United States [Available from:
- 74.Interim guidelines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html Antibody Testing [Available from:
- 75.Tamari R., Politikos I., Knorr D.A., Vardhana S.A., Young J.C., Marcello L.T., et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T-cell therapy. Blood Cancer Discov. 2021;2(6):577–585. doi: 10.1158/2643-3230.BCD-21-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.ASH-ASTCT COVID-19 Vaccination for HCT and CAR T Cell Recipients Frequently asked questions. https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients Version 5.0; last updated March 22, 2022) [Available from:
- 77.COVID-19 and BMT - EBMT: COVID-19 vaccines. Version 7, October 3, 2021 [Available from: https://www.ebmt.org/sites/default/files/2021-10/COVID%20vaccines%20version%207.22%20-%202021-10-03.pdf.
- 78.CIBMTR SC21-07/BMT CTN 2101 COVID observational study materials. https://web.emmes.com/study/bmt2/protocol/2101_protocol/2101_protocol.html [Available from:
- 79.SARS-CoV-2 Revaccination for persons who have received a hematopoietic cell transplant or cellular therapy. https://bethematch.org/workarea/downloadasset.aspx?id=15032387963 [updated September 20, 2021. Available from:
- 80.Woodfield M.C., Pergam S.A., Shah P.D. Cocooning against COVID-19: the argument for vaccinating caregivers of patients with cancer. Cancer. 2021;127(16):2861–2863. doi: 10.1002/cncr.33598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fact Sheet For Healthcare Providers EMERGENCY USE AUTHORIZATION FOR EVUSHELD™ (tixagevimab co-packaged with cilgavimab. https://www.fda.gov/media/154701/download [Available from:
- 82.Levin M.J., Ustianowski A., De Wit S., Launay O., Avila M., Templeton A., et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of covid-19. N Engl J Med. 2022;386(23):2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boschi C., Colson P., Bancod A., Moal V., La Scola B. Omicron variant escapes therapeutic mAbs including recently released Evusheld (R), contrary to eight prior main VOC. Clin Infect Dis. 2022;75(1):e534–e535. doi: 10.1093/cid/ciac143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stuver R., Shah G.L., Korde N.S., Roeker L.E., Mato A.R., Batlevi C.L., et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell. 2022;40(6):590–591. doi: 10.1016/j.ccell.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez-Roca A., Rodriguez-Lobato L.G., Ballestar N., Gallego C., Fernandez-Aviles F. Personalized at-home autologous hematopoietic stem cell transplantation during the SARS-CoV-2 outbreak. Leuk Res. 2021;106 doi: 10.1016/j.leukres.2021.106589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sung A.D., Nichols K.R., Chao N.J. House calls for stem cell transplant patients during the COVID-19 pandemic. Blood. 2020;136(3):370–371. doi: 10.1182/blood.2020006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puius Y.A., Bartash R.M., Zingman B.S. Maintaining mask momentum in transplant recipients. Transpl Infect Dis. 2021;23(4) doi: 10.1111/tid.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De la Puerta R., Montoro J., Aznar C., Lorenzo I., Gonzalez-Barbera E.M., Balaguer-Rosello A., et al. Common seasonal respiratory virus infections in allogeneic stem cell transplant recipients during the SARS-COV-2 pandemic. Bone Marrow Transplant. 2021;56(9):2212–2220. doi: 10.1038/s41409-021-01319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]