Abstract
We sequenced severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genomes from nasal and throat swabs of a hospitalized patient during the fifth wave of coronavirus disease 2019 (COVID-19) pandemic in Hong Kong. Genomic characteristics and viral load dynamics of an Omicron BA.2.2 variant before and after molnupiravir treatment were presented.
Keywords: BA.2.2, Molnupiravir, Mutation, Omicron, SARS-CoV-2, Viral load
Molnupiravir has been included as a conditional recommendation in World Health Organization's coronavirus disease 2019 (COVID-19) therapeutics guidelines and introduced in Hong Kong in March 2022 (World Health Organization, 2022; South China Morning Post, 2022). Data on safety and efficacy of molnupiravir has been revealed from clinical trials and hamster models (Fischer 2nd et al., 2022; Jayk Bernal et al., 2022; Rosenke et al., 2021; Abdelnabi et al., 2021), but its impact on predominant Omicron variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) within human host remains obscure. In our hospital, we have recently started treating COVID-19 patients with molnupiravir. Here we report a set of temporal data on genomic characteristics and viral load dynamics of an Omicron BA.2.2 variant from a hospitalized patient treated with molnupiravir.
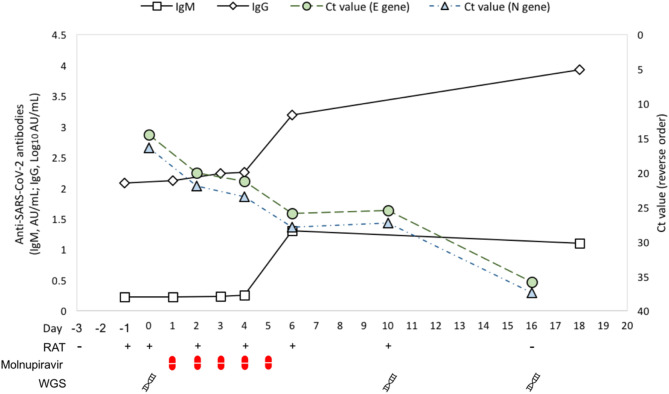
Patient A was a 74-year-old gentleman presented with impaired cognitive function recently. Magnetic resonance imaging of brain showed a 4.9 × 2.8 × 2.4 cm enhancing left temporal mass and confirmed by positron emission tomography scan that it was likely a primary brain tumor. The patient was vaccinated with two doses of CoronaVac (52 and 24 days ago respectively) and admitted for craniotomy with a negative COVID-19 rapid antigen test (RAT) (day −3). He was visited by his son after the operation (day −2) but the son was later found to be RAT-positive on the next day. The patient was then screened by RAT (day −1) and real-time reverse transcription polymerase chain reaction (rRT-PCR) (day 0) and was found to be positive by both methods. Cycle threshold (Ct) values of E and N genes were 14.5 and 16.4, respectively. Blood tests on day 0 showed lymphopenia (0.49 × 109/L), severe thrombocytopenia (34 × 109/L) and drop in hemoglobin from 16.3 to 8.3 g/dL. Liver function was normal but creatinine was mildly elevated at 107 μmol/L. C-reactive protein was elevated at 85 mg/L and procalcitonin was normal at 0.1 ng/mL. There was a hematoma at the scalp wound and the patient was found to have high fever. Molnupiravir (800 mg twice daily orally) was started on day 1 for five days. Intermittent surveillance of viral load and serological response was done on combined nasal and throat swabs (NS-TS) for rRT-PCR and serum for quantitative IgM and IgG against SARS-CoV-2 spike protein every two to four days until discharge. E- and N-gene Ct values rose to 35.8 and 37.4 respectively on day 16, with IgM at 1.1 AU/mL and IgG at 8572.3 AU/mL (or 3.93 Log10 AU/mL) on day 18. Details are summarized in Fig. 1 .
Fig. 1.
Timeline of clinical management for Patient A. The figure presents serum anti-SARS-CoV-2 spike protein antibody levels (IgM in AU/mL and IgG in Log10 AU/mL, primary vertical axis), viral load (represented by cycle threshold (Ct) value in reverse order, secondary vertical axis), COVID-19 rapid antigen test (RAT), duration of molnupiravir regimen and time points designated for SARS-CoV-2 whole genome sequencing (WGS).
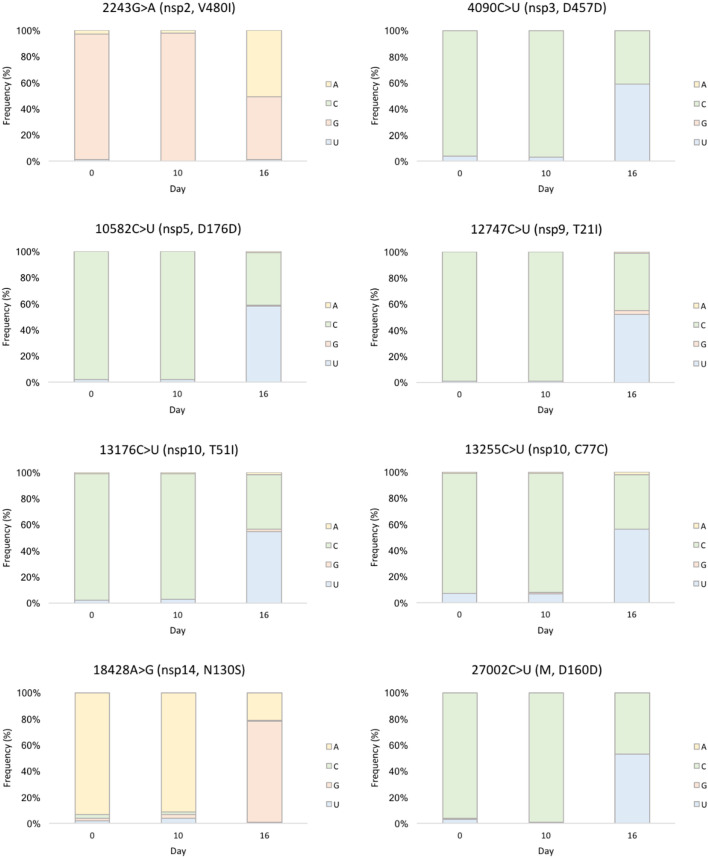
To study the effect of molnupiravir at genomic level, total nucleic acid was extracted from pre- (day 0) and post-treatment (day 10 and 16) NS-TS for direct sequencing of SARS-CoV-2 genomes, as described previously (Au et al., 2020). All three genomes were assigned to clade 21 L (Omicron, Pango lineage BA.2.2) by Nextclade (Nextclade, 2022) (Fig. S1). Variant calling by Unipro UGENE (Okonechnikov et al., 2012) revealed that day-0 and 10 genomes shared identical mutation pattern, whereas eight additional nucleotide substitutions were found on day 16, including 2243G > A and 18428A > G transitions, as well as six C-to-U transitions (nucleotide positions 4090, 10,582, 12,747, 13,176, 13,255 and 27,002), with variant allele frequency (VAF) of 51 to 77% (Fig. 2 ). Half of these substitutions were missense mutations in non-structural protein (nsp) genes, including 2243G > A in nsp2 (V480I), 12747C > U in nsp9 (T21I), 13176C > U in nsp10 (T51I) and 18428A > G in nsp14 (N130S).
Fig. 2.
Eight additional nucleotide substitutions harboured by day-16 SARS-CoV-2 sequence from Patient A. Variant allele frequencies (VAF) ranged from 51 to 77%, supported by sequencing depth of 378 to 765 × .
To estimate the extent of spontaneous mutation, two patients without molnupiravir treatment (Patient B and C) were randomly selected for comparison (Table 1 ). All the SARS-CoV-2 strains were BA.2.2 variants, and no additional nucleotide substitution was found on day 22 and 14, respectively. From the linear regression and scatter plot of Ct values versus day of rRT-PCR (Fig. S2), the slope of Patient A's line of best fit was the steepest, yet without statistically significant difference from that of Patient B and C (p values were 0.26 and 0.62, respectively). Quantification of genomic (gRNA) and subgenomic RNA (sgRNA) by periscope (Parker et al., 2021) did not reveal any appreciable pattern distinguishing between treated and untreated patients (Fig. S3).
Table 1.
Summary of Ct values and whole genome sequencing results at designated time points.
| Patient A |
Patient B |
Patient C |
|||||
|---|---|---|---|---|---|---|---|
| Day | 0 | 10 | 16 | 0 | 22 | 0 | 14 |
| Molnupiravir treatment | Yes, day 1 to 5 | No | No | ||||
| E gene Ct values | 14.5 | 25.5 | 35.8 | 16.6 | 33.3 | 14.1 | 30.9 |
| N gene Ct values | 16.4 | 27.3 | 37.4 | 19.2 | 35.8 | 16.2 | 33.1 |
| Reference coverage | 97% | 95% | 92% | 90% | 76% | 94% | 88% |
| Average depth | 403× | 404× | 384× | 311× | 155× | 356× | 304× |
| WHO label | Omicron | Omicron | Omicron | ||||
| Pango lineage | BA.2.2 | BA.2.2 | BA.2.2 | ||||
| Nextstrain clade | 21 L | 21 L | 21 L | ||||
| Additional nucleotide changes | N/A | 0 | 8 | N/A | 0 | N/A | 0 |
To the best of our knowledge, this is one of the earliest reports on temporal genomic changes and viral load dynamics of an Omicron variant in a patient treated with molnupiravir, as recent clinical trials were initiated before emergence of Omicron variants in November 2021 (Fischer 2nd et al., 2022; Jayk Bernal et al., 2022). To conclude, our data might shed light on some features of molnupiravir's mechanism of action in vivo. First, the rate of viral RNA clearance in treated patient was similar to that of untreated patients, which coincided with the observation from Phase 3 MOVe-OUT trial (MERCK, 2022). Second, the SARS-CoV-2 was under selection pressure from both elevated serum level of host anti-spike protein antibodies (approximately from day 6) and action of the nucleoside analog molnupiravir (regimen lasted from day 1 to 5). From whole-genome sequencing data, the additional nucleotide changes were primarily found in first half of SARS-CoV-2 genome (6/8, 75%) but not in mutation hotspots like spike and nucleocapsid protein genes (Rochman et al., 2021). In contrast to the findings by Fischer and colleagues (Fischer 2nd et al., 2022), we did not observe any additional nucleotide changes in RdRp gene, albeit the sequencing technique we used might be less favourable for detecting low-allele-fraction variants. C > U transitions were most common (6/8, 75%), which was in agreement with the findings on various coronaviruses (Agostini et al., 2019; Sheahan et al., 2020). The four missense mutations were found at genomic locations associated with 3′-to-5′ exonuclease activity (nsp14), replication and transcription (nsp2, 9 and 10) (Ma et al., 2021; de Araújo et al., 2021; Saramago et al., 2021). Nevertheless, the significance of these mutations is not well understood and awaits further investigation. Third, from Phase 3 MOVe-OUT trial, infectious virus was detected in 0% of the patients on day 3 of molnupiravir treatment. We did not have culture data to determine viral viability at different time points, but from whole-genome sequencing data, quasispecies harbouring the nucleotide substitutions was not observed on day 10 (5 days after completion of regimen), and was finally detected on day 16. As NS-TS were not available between these two time-points, we could not track the change in VAF very closely, but as the majority of canonical subgenomic RNA types were detected on day 10, it is an educated guess that viral transcription has taken place at least on day 10 and accumulation of quasispecies continued thereafter. This delayed predominance of variant population, together with molnupiravir's hotspots of action in SARS-CoV-2 genome, could be interesting questions to be addressed by larger collection of whole-genome and clinical data for further assessing the efficacy and molecular mechanism of molnupiravir on SARS-CoV-2.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material
The datasets are available from the corresponding author on reasonable request.
Ethics declarations
Informed consent was obtained from Patient A. Patient-identifying information was removed throughout the whole study. This study was approved by HKSH Medical Group Research Committee (reference number: RC-2022-06).
CRediT authorship contribution statement
Wai Sing Chan: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing. Janet Hei Yin Law: Investigation. Matthew Kam Shing Ho: Writing – review & editing. Tsun Leung Chan: Writing – review & editing. Edmond Shiu Kwan Ma: Writing – review & editing. Bone Siu Fai Tang: Conceptualization, Data curation, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
We thank the colleagues of Department of Pathology, Hong Kong Sanatorium & Hospital for their dedicated and professional work on routine laboratory diagnostics.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.meegid.2022.105376.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Abdelnabi R., Foo C.S., De Jonghe S., Maes P., Weynand B., Neyts J. Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a Hamster infection model. J. Infect. Dis. 2021;224(5):749–753. doi: 10.1093/infdis/jiab361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M.L., Pruijssers A.J., Chappell J.D., Gribble J., Lu X., Andres E.L., Bluemling G.R., Lockwood M.A., Sheahan T.P., Sims A.C., Natchus M.G., Saindane M., Kolykhalov A.A., Painter G.R., Baric R.S., Denison M.R. Small-molecule antiviral β-d-N4-Hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 2019;93(24) doi: 10.1128/JVI.01348-19. (e01348–19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au C.H., Chan W.S., Lam H.Y., Ho D.N., Lam S., Zee J., Chan T.L., Ma E. Genome sequences of SARS-CoV-2 strains detected in Hong Kong. Microbiology Resource Announcements. 2020;9(31) doi: 10.1128/MRA.00697-20. (e00697–20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo J., Pinheiro S., Zamora W.J., Alves C.N., Lameira J., Lima A.H. Structural, energetic and lipophilic analysis of SARS-CoV-2 non-structural protein 9 (NSP9) Sci. Rep. 2021;11(1):23003. doi: 10.1038/s41598-021-02366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W.A., 2nd, Eron J.J., Jr., Holman W., Cohen M.S., Fang L., Szewczyk L.J., Sheahan T.P., Baric R., Mollan K.R., Wolfe C.R., Duke E.R., Azizad M.M., Borroto-Esoda K., Wohl D.A., Coombs R.W., James Loftis A., Alabanza P., Lipansky F., Painter W.P. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 2022;14(628):eabl7430. doi: 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L., Du J., Pedley A., Assaid C., Strizki J., Grobler J.A., Shamsuddin H.H., Tipping R., Wan H., Paschke A., Butterton J.R., et al. Molnupiravir for Oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Chen Y., Wu W., Chen Z. Structure and function of N-terminal zinc finger domain of SARS-CoV-2 NSP2. Virol. Sin. 2021;36(5):1104–1112. doi: 10.1007/s12250-021-00431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERCK . 2022. Merck and Ridgeback to present data demonstrating that treatment with LAGEVRIOTM (molnupiravir) was associated with more rapid elimination of infectious SARS-CoV-2 than placebo.https://www.merck.com/news/merck-and-ridgeback-to-present-data-demonstrating-that-treatment-with-lagevrio-molnupiravir-was-associated-with-more-rapid-elimination-of-infectious-sars-cov-2-than-placebo/ Accessed on April 10, 2022 from. [Google Scholar]
- Nextclade Clade Assignment, Mutation Calling, and Sequence Quality Checks. 2022. https://clades.nextstrain.org
- Okonechnikov K., Golosova O., Fursov M., UGENE team Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics (Oxford, England) 2012;28(8):1166–1167. doi: 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- Parker M.D., Lindsey B.B., Leary S., Gaudieri S., Chopra A., Wyles M., Angyal A., Green L.R., Parsons P., Tucker R.M., Brown R., Groves D., Johnson K., Carrilero L., Heffer J., Partridge D.G., Evans C., Raza M., Keeley A.J., Smith N., et al. Subgenomic RNA identification in SARS-CoV-2 genomic sequencing data. Genome Res. 2021;31(4):645–658. doi: 10.1101/gr.268110.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman N.D., Wolf Y.I., Faure G., Mutz P., Zhang F., Koonin E.V. Ongoing global and regional adaptive evolution of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2021;118(29) doi: 10.1073/pnas.2104241118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenke K., Hansen F., Schwarz B., Feldmann F., Haddock E., Rosenke R., Barbian K., Meade-White K., Okumura A., Leventhal S., Hawman D.W., Ricotta E., Bosio C.M., Martens C., Saturday G., Feldmann H., Jarvis M.A. Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model. Nat. Commun. 2021;12(1):2295. doi: 10.1038/s41467-021-22580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saramago M., Bárria C., Costa V.G., Souza C.S., Viegas S.C., Domingues S., Lousa D., Soares C.M., Arraiano C.M., Matos R.G. New targets for drug design: importance of nsp14/nsp10 complex formation for the 3′-5′ exoribonucleolytic activity on SARS-CoV-2. FEBS J. 2021;288(17):5130–5147. doi: 10.1111/febs.15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., 3rd, Stevens L.J., Chappell J.D., Lu X., Hughes T.M., George A.S., Hill C.S., Montgomery S.A., Brown A.J., Bluemling G.R., Natchus M.G., Saindane M., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Science Translational Medicine. 2020;12(541):eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South China Morning Post . 2022. Coronavirus: 16,000 Hong Kong patients receive new oral anti-Covid drugs with favourable outcomes, few side effects.https://www.scmp.com/news/hong-kong/health-environment/article/3171980/coronavirus-16000-hong-kong-patients-receive-new Accessed on April 7, 2022 from. [Google Scholar]
- World Health Organization WHO Updates its Treatment Guidelines to Include Molnupiravir. 2022. https://www.who.int/news/item/03-03-2022-molnupiravir Accessed on April 7, 2022 from.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.
Data will be made available on request.