Abstract
The KdpB polypeptides in the cyanobacterium Anabaena torulosa were shown to be two membrane-bound proteins of about 78 kDa, expressed strictly under K+ deficiency and repressed or degraded upon readdition of K+. In both Anabaena and Escherichia coli strain MC4100, osmotic and ionic stresses caused no significant induction of steady-state KdpB levels during extreme potassium starvation.
Potassium is an important nutritional requirement for all bacteria. The major roles attributed to this alkali metal cation in bacterial cells relate to maintenance of turgor (13) and intracellular pH (10), enzyme activation (35), gene expression (19, 37), and regulation of stress responses (12, 27). In a heterocystous, nitrogen-fixing cyanobacterium, Anabaena torulosa, we earlier showed that deprivation of K+ caused pleiotropic effects, resulting especially in the impairment of the vital metabolic processes of photosynthesis and nitrogen fixation in addition to the expected loss of turgor (2).
In bacterial cells, K+ levels range from 0.2 to 0.6 M (15) and are maintained by multiple K+ transport systems whose variety increases further during stress conditions (12). For example, Escherichia coli possesses at least two constitutive, low-affinity K+ transport systems, the Trk and Kup systems (34), and an inducible high-affinity K+-specific transport system, the Kdp system (3, 23). The Kdp system is an emergency or backup system expressed and used to scavenge K+ from very low (less than 1 mM)-K+ environs (3) when the other transport systems are unable to meet the cell's need for potassium (13).
The E. coli Kdp system is an ion-motive P-type ATPase comprising the KdpA, KdpB, KdpC, and KdpF proteins (3, 18) encoded by a single kdpFABC operon whose expression is regulated by an adjoining kdpDE operon. The kdpDE operon encodes a membrane-spanning sensor kinase, KdpD, and a cytosolic transcriptional activator, KdpE (28, 32). The KdpD protein in E. coli consists of (i) a cytosolic N-terminal domain of about 400 amino acids, (ii) four hydrophobic transmembrane domains (TMDs) of nearly 100 amino acid residues, and (iii) a hydrophilic cytosolic C-terminal domain (CTD) of about 400 amino acids (4). Most of the N-terminal domain is dispensable (29), except for amino acid residues 12 to 128, whose deletion deregulates the phosphatase activity of the KdpD protein (20). Modification of TMDs inactivates KdpD and desensitizes E. coli cells to K+ levels (36). The CTD closely resembles the transmitter domain of other bacterial sensor kinases. In response to an appropriate signal(s), it transphosphorylates the KdpE protein (4), which in turn switches on the transcription of the kdpFABC operon (26). KdpD is believed to sense at least the following two types of signals: (i) a change in membrane stretch (36), possibly caused by an alteration in turgor pressure (14, 25), and (ii) the external and internal potassium levels (33, 36). The turgor model of regulation has been questioned at times (8, 17).
The kdp genes are widely distributed among bacteria (38). DNA sequencing has also revealed the presence of kdp homologs in the genomes of two filamentous heterocystous cyanobacteria, Anabaena sp. strain L-31 (GenBank accession no. AF213466) and Anabaena sp. strain PCC7120 (www.kazusa.or.jp/cyano/anabaena), and in the genome of a unicellular cyanobacterium, Synechocystis sp. strain PCC6803 (21). In all three genomes the kdpD gene is truncated and completely lacks the coding sequences for the TMDs and the CTD of E. coli. Also, the kdpE gene appears to be absent. This raises interesting questions about the expression and regulation of the Kdp system in cyanobacteria. We have earlier reported the presence of cross-reactive KdpB-like proteins of very similar molecular mass in three different Anabaena spp. (5). The present work examines the regulation of KdpB expression in A. torulosa, a brackish water, salt-tolerant (7), but relatively osmo-sensitive (16) cyanobacterial strain. Our results show that Anabaena Kdp-ATPase expression displays physiological regulation by environmental K+ levels but is not significantly influenced by osmotic stresses.
Axenic cultures of the filamentous, heterocystous, nitrogen-fixing cyanobacterium A. torulosa (2, 6, 7) were grown in BG-11 medium (11) without combined nitrogen. The medium was modified to obtain either BG-11/K0 (wherein K2HPO4 was replaced by equimolar Na2HPO4) or BG-11/K5 (BG-11/K0 containing 5 mM KCl) medium (2). When required, media were supplemented with nitrogen by the addition of 5 mM NH4Cl and 5 mM MOPS. The initial pH of all media was adjusted to 7.0. Cultures were grown under continuous aeration (2 liters min−1) and illumination (2.5 mW cm−2) from white fluorescent lamps at 25 ± 2°C. Protein content was determined by the Folin phenol method (24). Cellular extracts containing proteins (150 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto Boehringer Mannheim positively charged nylon membranes, as described earlier (2, 5). The two antisera (anti-KdpB and anti-KdpABC) raised in rabbits against the corresponding purified proteins from E. coli were kindly provided by K. Altendorf (University of Osnabrück, Osnabrück, Germany) and were used at dilutions of 1:5,000 and 1:2,000, respectively. Immunodetection of KdpB was carried out as described earlier (2).
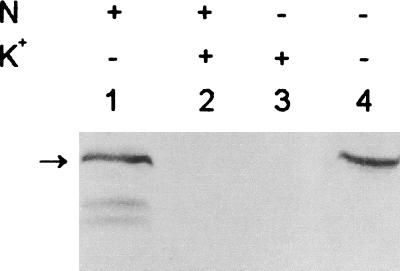
For K+ starvation, 3-day-old BG-11/K5-grown Anabaena cells were harvested by centrifugation (5,000 × g for 5 min), washed three times with 5 volumes of BG-11/K0 medium each, inoculated in BG-11/K0 or BG-11/K5 medium at a density of approximately 1 μg of chlorophyll a ml−1, and grown. Using an anti-KdpB antiserum, the KdpB was immunodetected in A. torulosa as a protein of about 78 kDa (Fig. 1 and 2A) which resolved into two protein bands (Fig. 2B; see Fig. 4) if a longer duration of electrophoresis was employed. K+ deprivation caused KdpB expression in cells grown in both N-supplemented and N-deficient media. KdpB was never detected in cells grown in K+-supplemented media. Identical results were obtained when an anti-KdpABC antiserum was used, i.e., no bands corresponding to KdpA or KdpC were observed (data not shown), as has been the case in Alicyclobacillus acidocaldarius and Rhodobacter sphaeroides (1, 9), in which only the most conserved KdpB was detected.
FIG. 1.
Identification of Anabaena KdpB homolog. Proteins were extracted from A. torulosa cultures grown in N-supplemented (lanes 1 and 2) or N-deficient (lanes 3 and 4) conditions in BG-11/K5 (lanes 2 and 3) or BG-11/K0 (lanes 1 and 4) medium for 3 days. Equal amounts of protein (150 μg) were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (100 V for 1 h followed by 200 V for 3.5 h) and then electroblotted. The blots were allowed to cross-react with a primary anti-E. coli KdpB antiserum followed by a secondary anti-rabbit immunoglobulin G coupled to alkaline phosphatase.
FIG. 2.
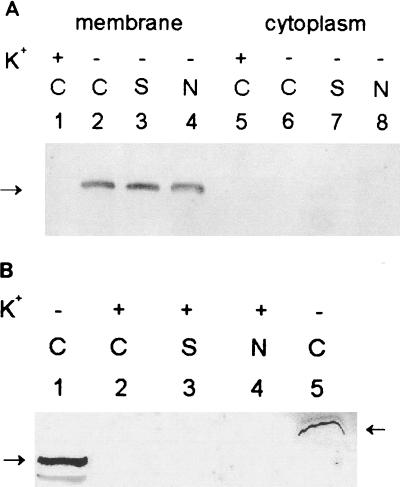
Cellular location of and effect of osmotic stresses on KdpB levels in Anabaena. (A) Nitrogen-fixing cultures were grown in BG-11/K5 (lanes 1 and 5) or BG-11/K0 (lanes 2 and 6) medium. On day 2, parts of the BG-11/K0 culture were stressed with either 0.2 M sucrose (lanes 3 and 7) or 0.1 M NaCl (lanes 4 and 8) for 24 h. Cellular extracts were separated into membrane (lanes 1 to 4) and cytosolic (lanes 5 to 8) fractions and the proteins were resolved by electrophoresis, as described for Fig. 1. C, medium controls; S, addition of sucrose; N, addition of NaCl. (B) Nitrogen-fixing cultures grown in BG-11/K5 (lane 2) medium for 2 days were subjected to 0.2 M sucrose (lane 3) or 0.1 M NaCl (lane 4) for 24 h. Membrane fractions were isolated and electrophoretically resolved for a longer duration (100 V for 1 h followed by 200 V for 4.5 h). Protein samples from E. coli MC4100 grown in BG-11/K0 medium for 6 h (lane 1) and from A. torulosa grown in BG-11/K0 for 24 h (lane 5) were included for comparison. Other details were as given for Fig. 1.
FIG. 4.
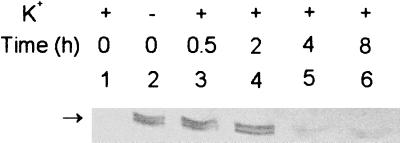
The negative effect of exogenous K+ addition on KdpB levels in Anabaena. Cultures were grown in BG-11/K5 (lane 1) or BG-11/K0 (lane 2) medium for 2 days. On day 2, 5 mM KCl was added to part of the BG-11/K0 culture at time zero. Samples were removed 0.5 (lane 3), 2 (lane 4), 4 (lane 5), or 8 (lane 6) h after K+ addition, electrophoretically resolved for a longer duration, and processed for KdpB immunodetection as for Fig. 2B.
Under all conditions tested, in A. torulosa KdpB was seen exclusively in the crude membrane fraction and none was detected in the cytosol (Fig. 2A). When osmotic stress (0.1 M NaCl or 0.2 M sucrose) adequate to induce an osmotic stress response in this strain (6, 16) was imposed on K+-deficient A. torulosa cultures, the steady-state levels of KdpB did not change significantly (Fig. 2A, lanes 3 and 4). Similarly, exposure of cells grown in 5 mM K+ to such osmotic stresses also failed to cause any detectable KdpB expression (Fig. 2B). Clearly, therefore, the turgor perturbations caused by osmotic stresses did not significantly enhance the KdpB expression in Anabaena, irrespective of the K+ status of cells.
In E. coli strains carrying mutations in either the kdp, trk, or kup genes and grown in media containing 1 to 5 mM K+, the addition of NaCl enhances the steady-state levels of β-galactosidase expressed from the kdpFABC promoter (8, 20, 23, 26, 33, 36). This contrasts with the situation in Anabaena. However, the effects of osmotic stresses over and above that of severe K+ deficiency (BG-11/K0) on the actual synthesis of Kdp polypeptides were not reported for wild-type E. coli strains. To examine whether the results for Anabaena (Fig. 2A) were really at variance with the situation in E. coli, an experiment was performed with E. coli strain MC4100, which possesses all the K+ transport systems (Fig. 3). Cells grown in K115 medium (23) were washed with and inoculated in either K115 medium or BG-11/K0 medium, wherein K2HPO4 and KH2PO4 were replaced with equimolar concentrations of corresponding sodium salts. Figure 3 shows that in BG-11/K0 medium, the KdpB levels were not noticeably enhanced by short (6 h) or prolonged (24 h) exposure to osmotic stress (0.25 M NaCl or 0.4 M sucrose) in this E. coli strain.
FIG. 3.
Effect of osmotic and salinity stresses on KdpB levels in E. coli strain MC4100. Cells were grown either in K115 (lanes 1 and 5) or in BG-11/K0 (lanes 2 and 6) medium. Part of the cells grown in BG-11/K0 medium were stressed with either 0.4 M sucrose (lanes 3 and 7) or 0.25 M NaCl (lanes 4 and 8). Samples were collected at 6 (lanes 1 to 4) or 24 (lanes 5 to 8) h after the initiation of K+ deprivation. Other details were as given for Fig. 2B.
A characteristic feature of Kdp-ATPase is its negative regulation by high levels of external K+. To test this, Anabaena cells were grown in BG-11/K0 medium for 2 days to express KdpB optimally and then supplemented with 5 mM KCl. Extended electrophoretic resolution of protein extracts from such cultures clearly revealed the presence of two KdpB bands of nearly equal intensity in K+-starved cells (Fig. 4). In the K+-replete cells, the presynthesized KdpB bands were not affected for at least 2 h but were rapidly degraded thereafter and were barely detectable after 4 h (Fig. 4). Thus, restoration of K+ not only represses further Kdp synthesis as in E. coli (3, 13, 14) but also triggers its degradation in Anabaena.
DNA sequencing has revealed the existence of two independent kdpABC operons each in Anabaena sp. strain PCC7120 and Anabaena sp. strain L-31, while only one such operon is found in Synechocystis sp. strain PCC6803 (www.kazusa.or.jp/cyano/; our unpublished results). The two KdpB bands observed in A. torulosa (Fig. 4) may well be the products of two kdpB genes, apparently typical of Anabaena genomes. The KdpB polypeptides of A. torulosa have been clearly and unambiguously shown to be (i) absent in the presence of 5 mM K+ in the medium; (ii) present solely under conditions of K+ limitation; (iii) insensitive to osmoinduction; (iv) exclusively localized in the membranes; (v) of a molecular mass of about 78 kDa, in agreement with the expected molecular mass of the Anabaena KdpB protein (74.6 kDa), which is larger than the E. coli KdpB protein (72 kDa); (vi) strongly immunologically cross-reactive to both the anti-KdpB and anti-KdpABC antisera; and (vii) repressed and degraded upon the readdition of K+.
The apparent osmoinsensitivity of kdp expression (Fig. 2) appears to be a regulatory feature unique to Anabaena. In E. coli strains carrying most K+ transporters, the osmoinduction is maximal at 1 to 5 mM K+ (20) and decreases both at higher (26, 36) and even at lower (33) K+ concentrations. Similar results have been reported for Salmonella enterica serovar Typhimurium in which under severe K+ deficiency (Kdp− Trk− strain grown at 1 mM K+), NaCl represses Kdp expression (see Table 2 in reference 17). Our results (Fig. 2A and 3) clearly show that under extreme K+ starvation (BG-11/K0), the Kdp expression becomes independent of the osmotic signal both in Anabaena and in E. coli. Thus, among the two signals sensed by KdpD, the K+ signal is a dominating one compared to turgor perturbations or membrane stretch caused by osmotic upshock. Indeed, the osmoinduced β-galactosidase activities (from kdpFABC::lacZ) in any strain never exceed those observed at very low K+ levels (0.1 to 1 mM).
A novel irreversible inactivation of presynthesized Kdp by 20 mM K+ was recently shown in E. coli (33). It was suggested that when a high K+ level is encountered the Kdp complex may be dissociated and degraded, although a previous study of E. coli showed that Kdp proteins were stable for at least two generations (2 h) following the readdition of excess K+ (22). The Kdp-ATPase thus appears to be a liability when cells have enough K+ available to them. In Anabaena, the Kdp system appears to be tightly regulated in that it is (i) neither expressed nor osmoinduced at 5 mM K+ and (ii) repressed and rapidly degraded (presynthesized Kdp) when exposed to 5 mM K+ (Fig. 4).
As stated earlier, the cyanobacterial KdpD has no membrane-spanning domains and it is not clear if it can anchor in the cytoplasmic membrane and/or sense membrane stretch or turgor drop. This, along with the apparent absence of KdpE, had raised doubts about how kdp expression is regulated in these microbes. The osmoinsensitivity of kdp expression in Anabaena reported here may well be related to its unique truncated KdpD, but that remains to be established. The present study clearly shows that expression of the membrane-bound Kdp-ATPase in Anabaena is primarily regulated by K+ levels in the environment. This study also provides a molecular basis for earlier reports on the presence of high-affinity turgor-responsive K+ transport systems in Anabaena (30, 31).
REFERENCES
- 1.Abee T, Knol J, Hellingwerf K L, Bakker E P, Siebers A, Konings W L. A Kdp-like, high affinity, K+-translocating ATPase is expressed during growth of Rhodobacter sphaeroides in low potassium media. Arch Microbiol. 1992;158:374–380. [Google Scholar]
- 2.Alahari A, Apte S K. Pleiotropic effects of potassium deficiency in a heterocystous, nitrogen-fixing cyanobacterium, Anabaena torulosa. Microbiology. 1998;144:1557–1563. doi: 10.1099/00221287-144-6-1557. [DOI] [PubMed] [Google Scholar]
- 3.Altendorf K, Epstein W. Kdp-ATPase of Escherichia coli. Cell Physiol Biochem. 1993;4:160–168. [Google Scholar]
- 4.Altendorf K, Voelkner P, Puppe W. The sensor kinase KdpD and the response regulator KdpE control expression of the kdpFABC operon in Escherichia coli. Res Microbiol. 1994;145:374–381. doi: 10.1016/0923-2508(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 5.Apte S K, Alahari A. Role of alkali cations (K+ and Na+) in cyanobacterial nitrogen fixation and adaptation to salinity and osmotic stress. Indian J Biochem Biophys. 1994;31:267–279. [PubMed] [Google Scholar]
- 6.Apte S K, Bhagwat A A. Salinity stress-induced proteins in two nitrogen-fixing Anabaena strains differentially tolerant to salt. J Bacteriol. 1989;171:909–915. doi: 10.1128/jb.171.2.909-915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apte S K, Reddy B R, Thomas J. Relationship between Na+ influx and salt tolerance of nitrogen-fixing cyanobacteria. Appl Environ Microbiol. 1987;53:1934–1939. doi: 10.1128/aem.53.8.1934-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asha H, Gowrishankar J. Regulation of kdp operon expression in Escherichia coli: evidence against turgor as signal for transcriptional control. J Bacteriol. 1993;175:4528–4537. doi: 10.1128/jb.175.14.4528-4537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakker E P, Borchard A, Michels M, Altendorf K, Siebers A. High-affinity potassium uptake system in Bacillus acidocaldarius showing immunological cross-reactivity with the Kdp system from Escherichia coli. J Bacteriol. 1987;169:4342–4348. doi: 10.1128/jb.169.9.4342-4348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth I R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castenholz R W. Culturing of cyanobacteria. Methods Enzymol. 1988;167:68–93. [Google Scholar]
- 12.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 13.Epstein W. Osmoregulation by potassium transport in Escherichia coli. FEMS Microbiol Rev. 1986;39:73–78. [Google Scholar]
- 14.Epstein W. Kdp, a bacterial P-type ATPase whose expression and activity are regulated by turgor pressure. Acta Physiol Scand. 1992;146:193–199. [PubMed] [Google Scholar]
- 15.Epstein W, Schultz S G. Cation transport in Escherichia coli. V. Regulation of cation content. J Gen Physiol. 1965;49:221–234. doi: 10.1085/jgp.49.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes T, Iyer V, Apte S K. Differential responses of nitrogen-fixing cyanobacteria to salinity and osmotic stresses. Appl Environ Microbiol. 1993;59:899–904. doi: 10.1128/aem.59.3.899-904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frymier J S, Reed T D, Fletcher S A, Csonka L N. Characterization of transcriptional regulation of the kdp operon of Salmonella enterica serovar Typhimurium. J Bacteriol. 1997;179:3061–3063. doi: 10.1128/jb.179.9.3061-3063.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaßel M, Möllenkamp T, Puppe W, Altendorf K. The KdpF subunit is part of the K+-translocating Kdp complex of Escherichia coli and is responsible for stabilization of the complex in vitro. J Biol Chem. 1999;274:37901–37907. doi: 10.1074/jbc.274.53.37901. [DOI] [PubMed] [Google Scholar]
- 19.Giaever H M, Styrvold O B, Kaasen I, Strom A R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988;170:2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung K, Altendorf K. Truncation of amino acids 12–128 causes deregulation of the phosphatase activity of the sensor kinase KdpD of Escherichia coli. J Biol Chem. 1998;273:17406–17410. doi: 10.1074/jbc.273.28.17406. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuna A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 22.Laimins L A, Rhoads D B, Altendorf K, Epstein W. Identification of the structural proteins of an ATP-driven potassium transport system of Escherichia coli. Proc Natl Acad Sci USA. 1978;75:3216–3219. doi: 10.1073/pnas.75.7.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laimins L A, Rhoads D B, Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosenbrough N J, Farr A L, Randall R J. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Malli R, Epstein W. Expression of the Kdp ATPase is consistent with regulation by turgor pressure. J Bacteriol. 1998;180:5102–5108. doi: 10.1128/jb.180.19.5102-5108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima K, Sugiura A, Kanamaru K, Mizuno T. Signal transduction between two regulatory components involved in the regulation of the kdpABC operon in Escherichia coli: phosphorylation-dependent functioning of the positive regulator, KdpE. Mol Microbiol. 1993;7:109–116. doi: 10.1111/j.1365-2958.1993.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 27.Palleros D R, Reid K L, Shi L, Welch W J, Fink A L. ATP-induced protein-Hsp70 dissociation requires K+ but not ATP hydrolysis. Nature. 1993;365:664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- 28.Polarek J W, Williams G, Epstein W. The products of the kdpDE operon are required for expression of the Kdp ATPase of Escherichia coli. J Bacteriol. 1992;174:2145–2151. doi: 10.1128/jb.174.7.2145-2151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puppe W, Zimann P, Jung K, Lucassen M, Altendorf K. Characterisation of truncated forms of the KdpD protein, the sensor kinase of the K+-translocating Kdp system of Escherichia coli. J Biol Chem. 1996;271:25027–25034. doi: 10.1074/jbc.271.40.25027. [DOI] [PubMed] [Google Scholar]
- 30.Reed R H, Stewart W D P. Characterisation of the transport of potassium ions in the cyanobacterium Anabaena variabilis Kurtz. Eur J Biochem. 1981;116:323–330. doi: 10.1111/j.1432-1033.1981.tb05337.x. [DOI] [PubMed] [Google Scholar]
- 31.Reed R H, Stewart W D P. Evidence for a turgor-sensitive K+ influx in the cyanobacterium Anabaena variabilis ATCC29413 and Synechococcus PCC6714. Biochim Biophys Acta. 1985;812:155–162. [Google Scholar]
- 32.Rhoads D B, Laimins L, Epstein W. Functional organization of the kdp genes of Escherichia coli K-12. J Bacteriol. 1978;135:445–452. doi: 10.1128/jb.135.2.445-452.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roe A J, McLaggan D, O'Byrne C P, Booth I R. Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol Microbiol. 2000;35:1235–1243. doi: 10.1046/j.1365-2958.2000.01793.x. [DOI] [PubMed] [Google Scholar]
- 34.Stumpe S, Schlosser A, Schleyer M, Bakker E P. K+ circulation across the prokaryotic cell membrane: K+ uptake systems. In: Konings W N, Kaback H R, Lolkema J S, editors. Handbook of biological physics. 2. Transport processes in eukaryotic and prokaryotic organisms. Amsterdam, The Netherlands: Elsevier; 1996. pp. 473–499. [Google Scholar]
- 35.Suelter C H. Enzymes activated by monovalent cations: patterns and predictions for the enzyme-catalysed reactions are explored. Science. 1970;168:789–795. doi: 10.1126/science.168.3933.789. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura A, Hirokawa K, Nakashima K, Mizuno T. Signal sensing mechanisms of the putative osmosensor KdpD in Escherichia coli. Mol Microbiol. 1994;14:929–938. doi: 10.1111/j.1365-2958.1994.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland L, Cairney J, Elmore M J, Booth I R, Higgins L F. Osmotic regulation of transcription of the proU betaine transport gene is dependent on accumulation of intracellular potassium. J Bacteriol. 1986;168:805–814. doi: 10.1128/jb.168.2.805-814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walderhaug M O, Litwack D E, Epstein W. Wide distribution of homologs of Escherichia coli Kdp K+-ATPase among gram-negative bacteria. J Bacteriol. 1989;171:1192–1195. doi: 10.1128/jb.171.2.1192-1195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]