Abstract
Objectives
Human monkeypox (MPX) cases are escalating worldwide. Smallpox vaccination, which was compulsory in Austria until 1981, was reported to confer 85% cross-protection against MPX.
Methods
To assess the impact of smallpox vaccine-induced protection, the age-dependent vaccine-induced immunity against human MPX and the probability of infection according to age in the general population of Vienna, Austria, were determined using a modified susceptible-infected-removed model.
Results
Within the population born before 1981, the average vaccine-induced protective effect was calculated at 50.4%, whereas in the population born thereafter, protection was lacking. The overall probability of infection after exposure to an infected patient was calculated at 73.8%, which exceeds the threshold value of 46.9% for an index patient to infect at least one other person (R ≥1.0).
Conclusion
Our model shows that if no additional interventions are taken, the collective immunization status of the population alone will not suffice to contain human MPX. Although the majority of cases have occurred in a subpopulation, given the steadily increasing incidence, dissemination into the general population remains possible, as observed before with HIV. Our model emphasizes the need for adequate containment measures and may aid in specific risk assessment because it can easily be adapted to other populations and cohorts worldwide.
Keywords: Human monkeypox, Monkeypox virus, Smallpox vaccination, Risk assessment
Introduction
As of September 2, 2022, a total of 53,027 confirmed cases of human monkeypox (MPX) affecting 93 nonendemic countries and seven countries in central and western Africa have been reported (Centers for Disease Control and Prevention, 2022). Since the identification of the first case of this international outbreak on May 7, the number of cases has escalated steadily worldwide (Mathieu et al., 2022). In this regard, the World Health Organization declared the outbreak a public health emergency of international concern on July 23, 2022.
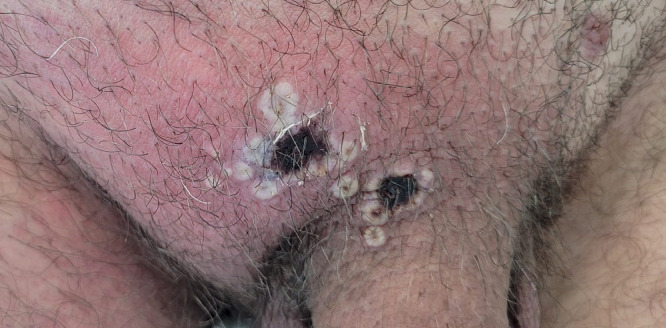
Human MPX is a zoonotic disease caused by the MPX virus (MPXV), which is an enveloped, double-stranded DNA virus belonging to the Orthopoxvirus genus (Petersen et al., 2019). Recently, a new nomenclature has been proposed for the MPXV, terming the more virulent “Congo Basin” lineage as clade 1 and the “West African” strain as clades 2 and 3, the latter constituting the 2022 outbreak virus (Happi et al., 2022; Petersen et al., 2022). After an average incubation period of 7-14 days, vesiculopustular, occasionally ulcerous lesions (Figure 1 ) develop, which can generalize and are frequently accompanied by fever and lymphadenopathy (Bragazzi et al., 2022; Reynolds et al., 2006; Thornhill et al., 2022). The disease is generally self-limited, however, the clinical course may be more severe in children, pregnant women, and immunocompromised individuals. Complications can include secondary bacterial infections, sepsis, encephalitis, and corneal scarring. Transmission occurs after close contact with infected animals or humans and viral DNA is detected in infective skin and mucosal lesions, body fluids including blood, saliva, semen, urine, and feces, or on contaminated fomites (Adler et al., 2022; Fine et al., 1988; Nolen et al., 2015; Nörz et al., 2022; Peiró-Mestres et al., 2022). However, a hallmark of the present 2022 outbreak is the unprecedented predominance of sexually transmitted human MPX cases, which primarily affects the subpopulation of gay, bisexual, and other men-who-have-sex-with-men (GBMSM) in Europe and the Americas (Bragazzi et al., 2022; Philpott et al., 2022; Tarín-Vicente et al., 2022; Thornhill et al., 2022). This is contrary to observations from previous surveillance programs conducted in certain African countries, where human MPX is endemic, and from individual national outbreaks (Beer and Rao, 2019; Fine et al., 1988; Jezek et al., 1988).
Figure 1.
Human monkeypox lesions and regional lymphadenopathy.
Smallpox vaccination was reported to prevent smallpox in 95% of the vaccinees (Amanna et al., 2007; Metzger and Mordmueller, 2007) and global eradication was achieved by the Intensified Smallpox Eradication Program in 1980, allowing the cessation of compulsory mass vaccination. The efficacy of the first-generation smallpox vaccines against human MPX among household contacts was estimated in the order of 85% (Fine et al., 1988). Previous estimations based on data obtained from Zaire reported an average annual primary attack rate of human MPX of 0.04 per 10,000 in smallpox-vaccinated contact persons, whereas in nonvaccinated contacts, the rates were higher at 1.7 per 10,000 (Jezek et al., 1988). Among nonvaccinated household contacts of confirmed cases, a recent pooled estimate indicated a secondary attack rate of approximately 8%, ranging from 0-11% (Beer and Rao, 2019).
In this study, we applied population data obtained from the database of the Federal Statistical Office of Austria (“Statistics Austria”) into a modified stochastic spatiotemporal susceptible-infectious-removed (SIR) epidemic model. Under the assumption that no additional concurrent measures to contain MPXV infections are introduced, this model allowed the assessment of the segregated age-dependent protection against human MPX by previous compulsory smallpox vaccination and the probability of infection according to age in the population of a major European capital.
Methods
Study population
Publicly available data regarding the absolute numbers of the population of the capital of Austria, Vienna, according to the year of birth were extracted from the database of “Statistics Austria,” dated January 1, 2022 (Statistik, 2022). The total population was divided into two cohorts according to the year of birth, with the year 1981 being the threshold when compulsory smallpox vaccination in Austria had ended.
Model system
The classical SIR model (Kermack and McKendrick, 1927), an epidemic compartmental model for the prediction of the spread of an infectious disease with long-term immunity formation, was modified to assess the protective effect of smallpox vaccination against human MPX. Here, translated as a recursive exponential growth function (extrapolation), the weights S (susceptible) and I (infected) are described at a certain time point t, and R (recovered/removed) is represented as a probability distribution for infection. Therefore, I represents the number of initial index cases in the reference system (S), which is the entire cohort, to be extrapolated with a growth rate under the influence of R. Supplementary Figure S1 illustrates the calculation process, and Supplementary Table S1 shows the entire dataset.
Calculation of the susceptible and protected population according to age
The decay rate λ for vaccine-induced protection against smallpox was calculated from the reported half-life of 92 years (Amanna et al., 2007). The proportion at risk for infection among individuals born in the year A at time t, SA,t, was determined under the assumption of a 100% vaccination rate for individuals born before 1981, at the time of compulsory vaccination, and 0% afterward. The variable t represents the year of interest (e.g., 2022). The value 0.85 refers to the smallpox vaccination-induced reported cross-protection against human MPX (Fine et al., 1988).
Next, the fraction of the individuals born in the year A in the total population, fA, was calculated by dividing the absolute number of individuals according to the year of birth by the absolute number of the total cohort. The total proportion of susceptible individuals at time t, St, was calculated by summing over the birth years A under consideration of the fraction of cohort A in the total population fA.
As the fraction of infected individuals, It, is negligibly small as long as there is no major outbreak, the overall proportion of protected individuals is given by Rt = 1 - St.
Determination of epidemic growth
According to the SIR model, the growth of the proportion of infected individuals is proportional to the product of . Assuming an infectious cycle of length Δ, a simplified approach using the information on the basic reproduction number R0, gives the following recursion equation for the first few cycles of a potential epidemic:
The infection cycle corresponds to the time period between two consecutive cases in an infection chain. The length of the infectious cycle remains undefined in the present scenario and can be adapted as new information becomes available.
Results
Population characteristics
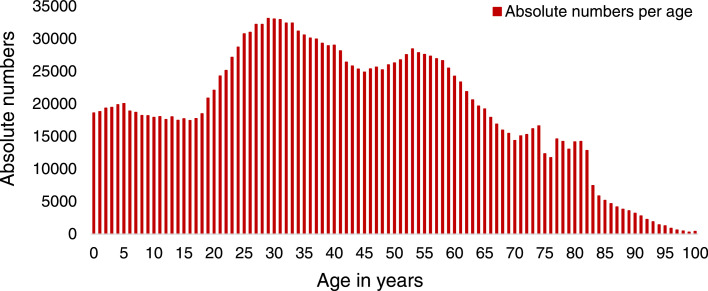
The overall population of Vienna consisted of 1,931,830 inhabitants with an age range from 0 to ≥100 years (Figure 2 , Supplementary Table S1) as of January 1, 2022. The 406 individuals aged ≥100 years represented 0.02% of the total population and were subsumed into one group due to the lack of stratification by year of birth and documented maximum age in the database. The first cohort, encompassing 998,047 (51.7%) individuals born before 1981, was assumed to be immunized with the smallpox vaccine, and the second cohort of 933,783 (48.3%) individuals born during or later than 1981 was presumed not to be immunized.
Figure 2.
Absolute numbers of inhabitants in Vienna by age, as of January 1, 2022.
Protection against human MPX and smallpox by previous compulsory smallpox vaccination
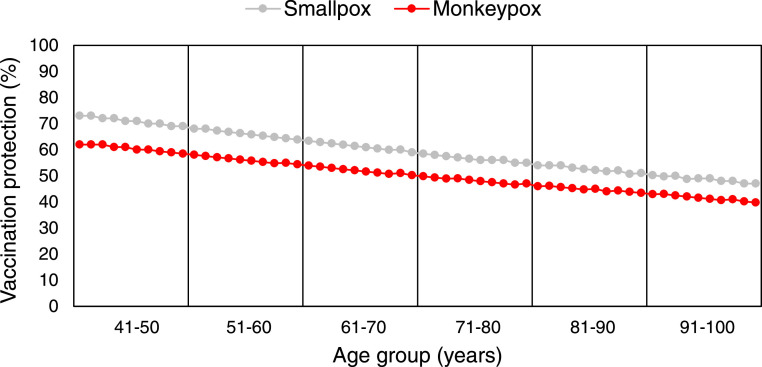
On the basis of the annual decay rate for smallpox vaccination determined at 0.007534208, protection in the cohort born before 1981 was calculated with an overall average protection of 59.3%, ranging from 73.4% in individuals aged 41 years, who had the shortest interval from a previous smallpox vaccination, to 47.1% in individuals aged ≥100 years (Table 1 , Figure 3 ).
Table 1.
Proportion of protection against human monkeypox and smallpox in individual age groups born before 1981
| Age group | Fraction of total population | Protection against human monkeypox | Protection against smallpox |
|---|---|---|---|
| 41-50 years | 13.4% | 62.4% - 58.3% | 73.4% - 68.6% |
| 51-60 years | 13.9% | 57.8% - 54.0% | 68.1% - 63.6% |
| 61-70 years | 9.6% | 53.6% - 50.1% | 63.2% - 59.0% |
| 71-80 years | 7.4% | 49.7% - 46.5% | 58.6% - 54.7% |
| 81-90 years | 3.4% | 46.1% - 43.1% | 54.3% - 50.8% |
| 91- ≥100 years | 0.6% | 42.8% - 40.0% | 50.4% - 47.1% |
| Mean | 50.4% | 59.3% |
Figure 3.
Age-dependent protection by smallpox vaccination against human monkeypox and smallpox in the Viennese population born before 1981.
Using the estimated 85% smallpox vaccine-induced protection against MPXV (Fine et al., 1988), the residual protection against human MPX in the cohort born before 1981 was calculated lower, with an overall rate of 50.4%, ranging from 62.4% for those aged 41 years and gradually decreasing to 40.0% for those aged ≥100 years (Table 1, Figure 3). In contrast, for the population born during or later than 1981, compulsory mass vaccination was discontinued, and the vaccine has not been available since this time. Hence, a vaccination rate of zero was assumed for the younger age group, accounting for 933,783 unvaccinated individuals.
Modeling of the early exponential growth of human monkeypox infection
In the present cohort, comprising vaccinated and unvaccinated individuals, the average age distribution-dependent vaccine-induced protection against human MPX was calculated at 26.24%. This corresponded to an average age distribution-dependent susceptibility rate for MPXV of 73.76%. Based on these parameters, the growth dependencies of human MPX were determined. The basic reproduction number R0 had a strong impact on the number of resulting incidences per infection cycle. Importantly, under the assumption of a susceptibility fraction of 73.76% in the population of Vienna, the threshold for an increase in the number of infected cases was calculated at an R0 of approximately 1.4.
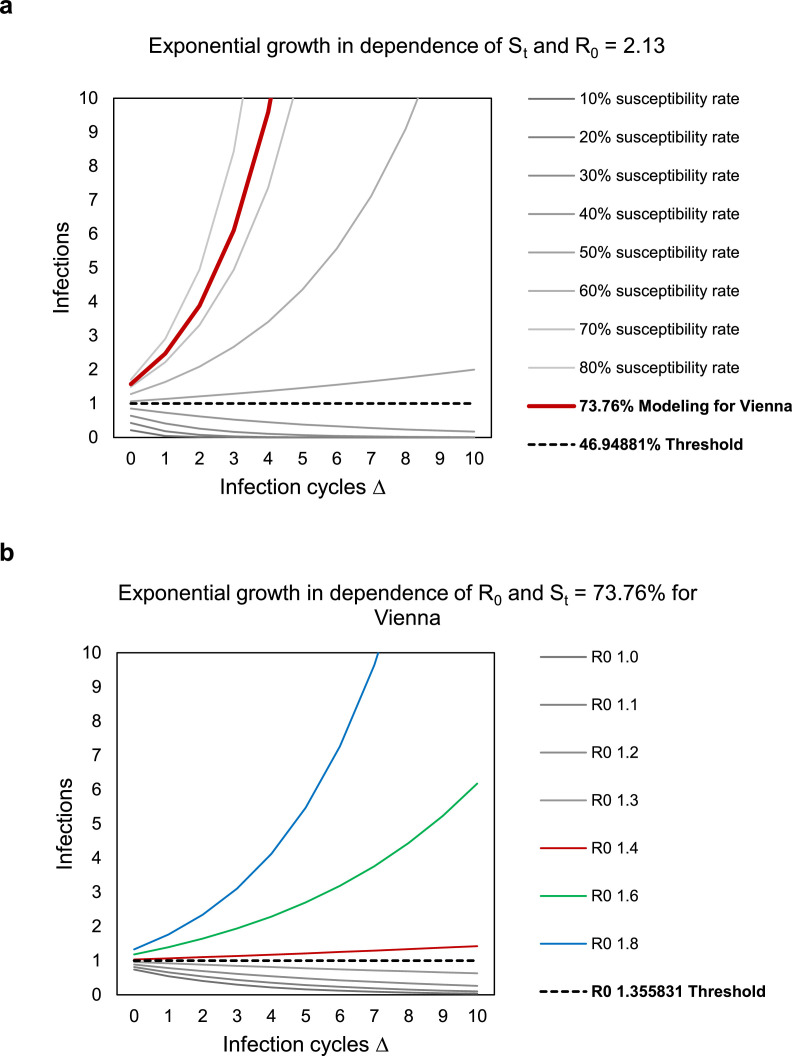
For the extrapolation, human MPX was assumed to have an overall basic reproduction rate of R0 = 2.13 in the general population, as determined previously from the outbreak in the Democratic Republic of the Congo in 1980-1984 (Grant et al., 2020). With an R0 = 2.13, an epidemic growth would occur if the susceptible population exceeds 47%. Under the assumption of R0 = 2.13 and a susceptibility fraction St of 73.76%, an exponential growth with a reproduction factor of 1.57 cases per infection cycle would be estimated (Figure 4 a).
Figure 4.
a. Modeling of the exponential growth of infection in dependence of the total proportion of susceptible individuals in Vienna, St, and a basic reproduction number R0 of 2.13. b. Modeling of the exponential growth of infection in dependence of R0, employing the calculated susceptibility rate St of 73.76% for Vienna.
Using the recently reported estimates for R0 obtained from the affected populations of different countries in Europe (Kwok et al., 2022) and the calculated susceptibility rate St of 73.76% for human MPX in Vienna, our model of the exponential growth showed that the threshold R0 of approximately 1.4 for an epidemic growth would rapidly be exceeded and an exponential increase of cases would have to be anticipated in this population (Figure 4b).
Consequently, in a worst-case scenario, that is if no additional measures are taken, our model illustrates that human MPX can have the potential capacity for a large-scale distribution in Vienna owing to the incomplete protection of the population by the previous compulsory smallpox vaccination.
Discussion
In this study, we modeled a possible scenario of introduction of human MPX into a major European capital of about 1.9 million inhabitants using population-based smallpox vaccine-induced immunity data. Smallpox vaccination has been reported to elicit long-lasting immunity against smallpox in the vaccinees (Amanna et al., 2007; Hammarlund et al., 2003; Taub et al., 2008). For human MPX, the vaccine-induced cross-protective effectiveness was inferred from previous smallpox clinical studies and MPXV challenges in nonhuman primates (Earl et al., 2004; Fine et al., 1988; Hatch et al., 2013; Rimoin et al., 2010). The model presented herein revealed that in the potential case of increasing dissemination of MPXV, the collective immunization status of the population of Vienna would not suffice to effectively contain an epidemic outbreak, at least if no additional measures to control the spread of human MPX are performed. We have not incorporated country- and climate-specific circumstances and individual drivers (e.g. lifestyle habits, sexual orientation, health conditions of persons at risk, household composition, access to health care) into the calculations but aimed for a comprehensive risk assessment based on the age-dependent immunity to be applicable to a general population. Hence, this model could easily be adapted to other populations and cohorts for specific risk assessment and might be useful for risk management initiatives in other countries where smallpox vaccination was discontinued around the same time or even earlier.
In our population, it cannot be excluded that some individuals, particularly military personnel and health care workers, who were sent to countries known to be endemic for smallpox, were immunized even after compulsory vaccination had ceased. However, these numbers are very low and were considered negligible in our calculations of the general Viennese population. Contrary, some individuals, particularly of the generations born between 1975 and 1981, may have escaped from mandatory smallpox vaccination or in some cases, the vaccine might not have been “taken” due to improper vaccine administration and resulting lack of live virus replication. The exact numbers, however, are unknown owing to the lack of a nationwide register.
In our model of the early growth period of infection, we first used the reported estimate of R0 = 2.13 for human MPX derived from the population data of the Democratic Republic of Congo (Grant et al., 2020) to be applicable to a general population. During this period, however, the smallpox vaccination coverage and the subsequent protective immunity were still high in the population, contrary to the current 2022 situation four decades later, where vaccine-induced immunity is absent in individuals below the age of 40 years. Contrary to previous data derived from the surveillance programs in endemic African countries between the periods 1980-1987 and 2005-2007, where human-to-human transmission through airborne, household and nosocomial routes, and animal-to-human transmission were the main routes for the acquisition of infection (Beer and Rao, 2019; Fine et al., 1988; Jezek et al., 1988), in the present outbreak, most human MPX cases were associated with sexual transmission of the MPXV and were identified among the social and sexual networks of GBMSM of younger ages, in whom smallpox vaccine-induced immunity is mostly lacking (Bragazzi et al., 2022; Philpott et al., 2022; Tarín-Vicente et al., 2022; Thornhill et al., 2022). A recent study modeled the spread of MPXV infection in the currently predominantly affected subpopulation of GBMSM and concluded that in the absence of effective interventions or behavioral changes, a sustained outbreak was likely for this subpopulation but not for the non-GBMSM population (Endo et al., 2022). Subsequent estimates of the transmissibility of the MPXV based on data obtained from the 2022 outbreak in the GBMSM subpopulation within different European countries have calculated a R0 rate in the range of 1.4 to 1.8 (Kwok et al., 2022). So far, the numbers of human MPX cases in the non-GBMSM population have not yet been high enough for a valid statement of R0. However, our model of the exponential growth revealed that with an R0 of approximately 1.4 or higher, as currently reported in other European countries (Kwok et al., 2022), an epidemic growth could be possible in Vienna, mainly owing to the declines in the population-level immunity. A recent elaborate study simulated an outbreak of human MPX in a fictional high-income population of 50 million by taking the population's sociocultural and movement processes into account (Bisanzio and Reithinger, 2022). In their intervention scenarios, MPXV spread was reduced by the implementation of effective preventive public health measures, contrary to the baseline scenario, in which no concomitant measures were performed. Our study complements the studies by taking the age-specific immunity induced by the previous smallpox vaccination into account and applying it to an existing population of a major European capital. Another aspect is that sexual activity gradually wanes with increasing age due to physiologic changes, declining health, and/or social factors. A limitation in our study is that the individuals belonging to the older cohort, who were born during or later than 1981 and represent the group that had received smallpox vaccination, would presumably be less sexually active than the individuals of the younger cohort, who were born after 1981 and were not vaccinated. Although this poses a potential confounder in our assessment of the smallpox vaccine's protective efficacy over different age groups, unfortunately an exact estimation of the sexual activity in our population is not available.
Although most human MPX cases in the present outbreak have been sexually transmitted, it is of utmost importance to keep in mind that the MPXV is not a disease that is inherent to GBMSM but can be transmitted to anyone after contact with an infectious case or contaminated material, regardless of sex or sexual orientation. In addition, individual superspreaders or superspreading events as well as unanticipated mutational evolution (Wang et al., 2022) may potentiate the risk of viral distribution. Furthermore, the presence of detectable viremia during symptomatic infection and sometimes lasting up to 3 weeks after resolution of infective lesions (Adler et al., 2022) has raised the concern for a potential viral transmission through blood transfusion (Jacobs et al., 2022). In this regard, lessons learned from HIV show that a disease first confined to a certain subgroup has the potential to spread to the general population (Gonsalves et al., 2022), which has already occurred in few instances with the MPXV (Bruno et al., 2022; Tutu van Furth et al., 2022).
In this study, we illustrated a possible, albeit a worst-case, scenario that could hypothetically become true if no additional adequate measures are performed. Given the steadily increasing numbers of human MPX cases in the past weeks and months, the possibility that MPXV infections might spill over to the general population exists, and it is uncertain whether the low numbers in the general population will be sustained in the nearest future. Hence, consistent and strict public health interventions, such as early identification and isolation of index cases at the community and household level, tracing of contact persons, and active surveillance of potential clusters, are needed to control the dissemination of the disease. Investigations addressing open questions, such as the determination of the transmission rates of the MPXV by different routes and the identification of the still unknown natural host, are urgently warranted to contain further spread. Furthermore, controlled clinical trials for the evaluation of the protection rates of the newer generation smallpox vaccines and antivirals against human MPX are required and the supply of sufficient vaccine doses for the recommended pre- and postexposure immunizations (Rao et al., 2022) must be secured for all countries worldwide. Ultimately, as increasing introduction of the virus from animal habitats into the human population can be assumed to be caused by the continuous reduction of natural wildlife reservoirs by deforestation, climate change, and population movement to animal habitats to escape ongoing wars and political conflicts, global efforts for the preservation of natural wildlife-reserves and restrictions on animal trades, especially of wild rodents and nonhuman primates, should be established worldwide to prevent zoonotic transmission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical approval was not required for this study because it did not include medical aspects, person-identifiable data, or sensitive or confidential data.
Author contributions
Conceptualization and study design: Tibor Spath, Florian Thalhammer, Alessandra Handisurya. Acquisition, analysis and interpretation of the data: Tibor Spath, Sophie Brunner-Ziegler, Tanja Stamm, Michael Kundi, Kim Purkhauser. Writing - original draft: Tibor Spath, Sophie Brunner-Ziegler, Tanja Stamm, Alessandra Handisurya. Writing - review and editing: Florian Thalhammer, Michael Kundi, Kim Purkhauser, Alessandra Handisurya. All authors revised the manuscript and approved the final version of the manuscript.
Data availability statement
The database used in this study is publicly available from the Federal Statistical Office of Austria (“Statistics Austria”).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.09.022.
Appendix. Supplementary materials
References
- Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisanzio D, Reithinger R. Projected burden and duration of the 2022 monkeypox outbreaks in non-endemic countries. Lancet Microbe. 2022;3:e643. doi: 10.1016/S2666-5247(22)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragazzi NL, Kong JD, Mahroum N, Tsigalou C, Khamisy-Farah R, Converti M, et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J Med Virol. 2022 doi: 10.1002/jmv.27931. [DOI] [PubMed] [Google Scholar]
- Bruno G, Fabrizio C, Rodano L, Buccoliero GB. Monkeypox in a 71-year-old woman. J Med Virol Forthcoming. 2022 doi: 10.1002/jmv.27993. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2022 Monkeypox Outbreak Global Map. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html, 2022 (accessed 5 September 2022).
- Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- Endo A, Murayama H, Abbott S, Ratnayake R, Pearson CAB, Edmunds WJ, et al. Heavy-tailed sexual contact networks and the epidemiology of monkeypox outbreak in non-endemic regions. medRxiv. 13 June 2022. https://www.medrxiv.org/content/10.1101/2022.06.13.22276353v1 (accessed 4 August 2022).
- Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- Gonsalves GS, Mayer K, Beyrer C. Déjà vu all over again? Emergent monkeypox, delayed responses, and stigmatized populations. J Urban Health. 2022;99:603–606. doi: 10.1007/s11524-022-00671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R, Nguyen LL, Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98:638–640. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Happi C, Adetifa I, Mbala P, Njouom R, Nakoune E, Happi A, et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GJ, Graham VA, Bewley KR, Tree JA, Dennis M, Taylor I, et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol. 2013;87:7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JW, Filkins L, Booth GS, Adkins BD. The potential impact of monkeypox infection and vaccination on blood donor deferrals and the blood supply. Br J Haematol Forthcoming. 2022 doi: 10.1111/bjh.18388. [DOI] [PubMed] [Google Scholar]
- Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465–470. [PMC free article] [PubMed] [Google Scholar]
- Kermack WO, McKendrick AG. A contribution to the mathematical theory of epidemics. Proc R Soc Lond A. 1927;115:700–721. [Google Scholar]
- Kwok KO, Wei WI, Tang A, Wong SYS, Tang JW. Estimation of local transmissibility in the early phase of monkeypox epidemic in 2022. Clin Microbiol Infect. 2022 doi: 10.1016/j.cmi.2022.06.025. S1198-743X(22)00340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu E, Spooner F, Dattani S, Ritchie H, Roser M. Monkeypox, 2022. https://ourworldindata.org/monkeypox, 2022 (accessed 4 August 2022).
- Metzger W, Mordmueller BG. Vaccines for preventing smallpox. Cochrane Database Syst Rev. 2007;2007 doi: 10.1002/14651858.CD004913.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen LD, Osadebe L, Katomba J, Likofata J, Mukadi D, Monroe B, et al. Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg. 2015;93:410–415. doi: 10.4269/ajtmh.15-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörz D, Pfefferle S, Brehm TT, Franke G, Grewe I, Knobling B, et al. Evidence of surface contamination in hospital rooms occupied by patients infected with monkeypox, Germany, June 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.26.2200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, Marcos MÁ, Vilella A, Navarro M, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E, Kantele A, Koopmans M, Asogun D, Yinka-Ogunleye A, Ihekweazu C, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E, Zumla A, Hui DS, Blumberg L, Valdoleiros SR, Amao L, et al. Vaccination for monkeypox prevention in persons with high-risk sexual behaviours to control on-going outbreak of monkeypox virus clade 3. Int J Infect Dis. 2022;122:569–571. doi: 10.1016/j.ijid.2022.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott D, Hughes CM, Alroy KA, Kerins JL, Pavlick J, Asbel L, et al. Epidemiologic and clinical characteristics of monkeypox cases - United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018–1022. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AK, Petersen BW, Whitehill F, Razeq JH, Isaacs SN, Merchlinsky MJ, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistik Austria. Population by age/sex, 2022. https://www.statistik.at/statistiken/bevoelkerung-und-soziales/bevoelkerung/bevoelkerungsstand/bevoelkerung-nach-alter/geschlecht, 2022 (accessed 23 May 2022).
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, Ubals M, Suñer C, Antón A, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400:661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008;121:1058–1064. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- Tutu van Furth AM, van der Kuip M, van Els AL, Fievez LC, van Rijckevorsel GG, van den Ouden A, et al. Paediatric monkeypox patient with unknown source of infection, the Netherlands, June 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.29.2200552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shang J, Weng S, Aliyari SR, Ji C, Cheng G, et al. Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022. J Med Virol Forthcoming. 2022 doi: 10.1002/jmv.28036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The database used in this study is publicly available from the Federal Statistical Office of Austria (“Statistics Austria”).