Abstract
Antimicrobial treatments for extensively drug-resistant Acinetobacter baumannii (XDR-AB) infections have proven lackluster, while dosing challenges in patients receiving continuous renal replacement therapy continue. We describe a patient receiving cefiderocol, ampicillin/sulbactam, and tigecycline for XDR-AB while undergoing continuous venovenous hemodiafiltration. The clinical course, cefiderocol and sulbactam pharmacokinetics, and synergy assessments are described.
Keywords: cefiderocol, Acinetobacter baumannii, pharmacokinetics, synergy
Carbapenem-resistant Acinetobacter baumannii (CRAB) infections are difficult to manage given a myriad of resistance mechanisms that give rise to extensive drug resistance (XDR; ie, nonsusceptible to ≥1 agent in all but ≤2 antibiotic classes) or pan drug resistance (PDR; ie, nonsusceptible to all classes), which leads to treatment failure and mortality [1–4]. Recent guidance from the Infectious Diseases Society of America (IDSA) on treatment of CRAB infections states that “there is no standard of care” antibiotic regimen. Unfortunately, definitive data assessing the use of 1 agent vs a combination of agents are lacking; the use of 2 or more agents is currently suggested for severe CRAB infections [5, 6].
Cefiderocol is a novel siderophore cephalosporin that has in vitro activity against many gram-negative bacteria including A. baumannii and has received approval for treatment of such infections [7]. However, during the CREDIBLE-CR trial, patients with CRAB demonstrated higher microbiologic failure and mortality when treated with cefiderocol monotherapy vs best available therapy [8]. Additionally, a case series where cefiderocol was predominately used as monotherapy for XDR-AB reported that 54% of critically ill patients with ventilator-associated pneumonia (VAP) experienced microbiologic failure [4]. While the use of cefiderocol as part of a combination regimen appears advisable in the setting of critical illness and severe infection with XDR-AB, limited in vitro data are available to support the choice of an agent(s) [9].
Herein, we report the use of a combination regimen including cefiderocol, ampicillin-sulbactam, and tigecycline in a critically ill patient with XDR-AB VAP on continuous venovenous hemodiafiltration (CVVHDF). Given previous reports of higher risk of clinical failure with novel beta-lactams in critically ill patients receiving continuous renal replacement therapy (CRRT) and the dearth of data on combination therapy, we characterized the cefiderocol and sulbactam plasma profiles and pharmacokinetic/pharmacodynamic (PK/PD) attainment and assessed synergy [10, 11].
CASE
A 65-year-old Caucasian male with a medical history of hypertension and mild chronic obstructive pulmonary disease (COPD) was admitted for a planned operative repair of a thoracoabdominal aortic aneurysm (TAAA) in December 2021. Postoperatively, he was found to have absent pulses on his left lower extremity (LLE) with mottling and had to have bilateral femoral cut-downs and LLE thrombectomy with stent placement in the left external iliac. He was transferred to the intensive care unit, intubated, and in hemorrhagic shock due to significant operative blood loss that was further complicated by acute renal failure and anuria leading to initiation of CVVHDF. Further evaluation also revealed that the patient had bilateral (b/l) renal artery partial occlusion, for which he had b/l renal artery stenting and a right renal artery nonocclusive thrombus that was suctioned. On post-op day 3, there were increased lower lobe infiltrates, and bronchoalveolar lavage (BAL) culture revealed only normal flora. On post-op day 7, the patient developed Escherichia coli pneumonia that was pan-susceptible, and he was treated with cefazolin. He remained hypoxemic with high ventilator requirements. On day 12, BAL grew XDR-AB, and he was empirically given cefiderocol 2 g every 12 hours (3-hour infusion) and minocycline 200 mg every 12 hours (1-hour infusion). Cefiderocol dosing started as 2 g every 12 hours (3-hour infusion); however, on day 3 of therapy, minocycline susceptibility results were resistant per Clinical and Laboratory Standards Institute (CLSI) guidelines, with a minimum inhibitory concentration (MIC) >16 mg/L (Table 1), while tigecycline susceptibility results were uninterpretable, with an MIC of 2 mg/L as the CLSI does not provide breakpoints for A. baumannii. With the patient still critically ill on pressors and cefiderocol susceptibilities pending due to a 7–10-day turnaround time for send-outs, therapy modifications were made by increasing cefiderocol to 2 g every 8 hours (3-hour infusion) while an effluent rate of 3.5 L/h continued. Further modifications were made, switching minocycline to tigecycline and adding ampicillin-sulbactam. Despite a resistant ampicillin-sulbactam (MIC > 16 mg/L), a high-dose regimen of 9 g every 8 hours over 4 hours was selected per the recent IDSA's guidance on the treatment of severe CRAB even if the isolate is not susceptible [5]. The patient improved, completing an 8-day course of cefiderocol combination therapy for VAP, and appeared to achieve microbiologic cure, with a repeat BAL finalizing as oropharyngeal flora on day 27 of admission.
Table 1.
Phenotypic Profile of A. baumannii Isolates From the Case Patient
| Day of Hospitalization | Source | Isolate | Drug MIC and CLSI Interpretations | ||||
|---|---|---|---|---|---|---|---|
| Cefiderocol | A/Sa | IPM | TIG | MIN | |||
| 12 | BAL | A. B # 1 | 1 (S) | 64 (R) | >8 (R) | 2 | 32 (R) |
| 34 | BAL | A. B # 2 | 0.25 (S) | 64 (R) | >8 (R) | 2 | ND |
| 44 | Sputum/endotracheal aspirate | A. B # 3 | ND | 64 (R) | >8 (R) | ND | ND |
| 45 | Peritoneal fluid | A. B #4 | ND | ND | >8 (R) | ND | ND |
Abbreviations: A/S, ampicillin/sulbactam; CLSI, Clinical and Laboratory Standards Institute; IPM, imipenem; MIC, minimum inhibitory concentration; MIN, minocycline; ND, not determined; (R), resistant; (S), susceptible; TIG, tigecycline.
MICs are representative of sulbactam concentrations.
Unfortunately, the patient's clinical course was further complicated when Candida albicans grew from the blood, prompting concern that the aortic graft had become infected. The patient remained ventilated throughout the course of his hospital stay, and on day 34, gram-negative rods were identified on BAL, prompting re-initiation of cefiderocol, tigecycline, and ampicillin-sulbactam. Escherichia coli and XDR-AB were identified from the BAL culture. A few days later, a Helicobacter pylori antigen test was positive, and on day 45 the patient was taken to the operating room, where a gastric perforation was found with frank purulent fluid. Cultures from a peritoneal fluid sample speciated Candida parapsilosis & XDR-AB. The patient's family requested that he be transitioned to comfort care. No adverse events were attributed to the administration of cefiderocol, tigecycline, or the ampicillin–sulbactam combination.
PHARMACOKINETIC ANALYSIS
Plasma samples were collected while the patient was undergoing CVVHDF following the 15th and 8th doses, respectively, of cefiderocol and ampicillin/sulbactam. Samples were drawn at 0, 1, 2, 3, 5, 6, and 8 hours. Additionally, 2 post-hemodialysis filter plasma samples were collected at 4 and 8 hours concurrently with prefilter plasma samples to assess CVVHDF clearance (CLCVVHDF). Plasma samples were stored at −80°C and shipped on dry ice to Keystone Bioanalytical, Inc. (North Wales, PA, USA), for cefiderocol concentrations using a validated LC/MS/MS analysis, while sulbactam concentrations were assayed at the Center for Anti-Infective Research and Development (Hartford, CT, USA) using a validated method. Tigecycline PK analysis was not undertaken as previous reports have described its PK in patients undergoing CRRT [12, 13].
Plasma concentrations of cefiderocol and sulbactam were modeled using Phoenix WinNonLin (version 8.3; CERTARA, Princeton, NJ, USA). Free plasma concentrations were corrected using the reported percent protein binding in each drug's package insert (cefiderocol: 50% [7]; and sulbactam: 38% [14]). The CLCVVHDF was calculated using as previously described, where Cpre is the prefilter concentration, Cpost is the postfilter concentration, Qplasma is the blood flow corrected for hematocrit, and QUF is the ultrafiltration flow rate [15]. CVVHDF was conducted using the Prismaflex filter AN69 high flux, M100 membrane set (Gambro, Meyzieu Cedex, France).
The derived PK parameters were then used to simulate the plasma time profile. The pharmacodynamic assessment of the cefiderocol pharmacokinetic simulation was evaluated using a pharmacodynamics target of 88.1% fT > MIC. This target was derived from an A. baumannii in vivo pneumonia murine infection model [16]. Against A. baumannii, sulbactam is the active agent, with no synergy upon ampicillin addition; thus only this compound was modeled for efficacy [17].
The patient's CVVHDF settings were as follows: Blood flow, dialysate, replacement fluid (continuous pre- and postfilter replacement), prepump blood flow, and ultrafiltrate flow rates were 200 mL/min, 2.5 L/h, 0.5 L/h, 0.5 L/h, and 1 L/h, respectively. The patient had no urine output during the dosing and sampling time periods, indicating that no residual renal function was present.
Cefiderocol PK was best described by a 1-compartment model. Half-life, clearance, and volume of distribution (Vd) were 6.8 hours, 3.4 L/h, and 33.8 L, respectively. Cefiderocol maximum concentration (Cmax) and free area under the concentration time curve (fAUC0–24) were 50.1 mg/L and 847.82 mg h/L, respectively. Cefiderocol CVVHDF clearance was 3.53 L/h. Sulbactam PK was similarly described by a 1-compartment model, with the following parameters displayed in Table 2.
Table 2.
Pharmacokinetic Parameters and CVVHDF CL of Cefiderocol and Sulbactam
| Parameter | Cefiderocol 2g q8 (3-h Infusion) | Sulbactam 3g q8a (4-h Infusion) |
|---|---|---|
| Vd, L | 33.80 | 33.36 |
| K10, h−1 | 0.10 | 0.16 |
| Half-life, h | 6.80 | 4.27 |
| Cmax, mg/L | 50.10 | 66.15 |
| CL, L/h | 3.40 | 5.41 |
| CLCVVHDF, L/h | 3.53 | 3.72 |
Abbreviations: CL, clearance; CVVHDF, continuous venovenous hemodiafiltration.
Administered as ampicillin 6 g/sulbactam 3 g given every 8 h via a 4-h infusion.
The simulated plasma time profile of cefiderocol 2 g q8h (3-hour infusion) provided target attainment for isolates with cefiderocol MICs up to 16 mg/L (Table 3). Our analysis accounted for 50% cefiderocol protein binding, which is the midpoint of 40%–60% protein binding included in the package insert. A follow-up sensitivity analysis accounting for 60% protein binding of cefiderocol provided similar results, with 100% fT > MIC 16 mg/L and 24% fT > MIC 32 mg/L. Sulbactam % fT > MIC remained 100% for isolates with MICs up to 16 mg/L. Additionally, sulbactam % fT > MIC was 90% for isolates with an MIC of 32 mg/mL, while it was 0% for isolates with an MIC of ≥64 mg/mL.
Table 3.
Plasma Pharmacodynamic Profile of Cefiderocol and Sulbactam in the Case Patient
| % fT > MIC, mg/L | fAUC0–24, mg h/L | Total AUC0–24, mg h/L | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Regimen | 1 | 2 | 4 | 8 | 16 | 32 | 64 | ||
| Cefiderocol | 2 g q8 (3-h inf) | 100 | 100 | 100 | 100 | 100 | 67 | 0 | 847.82 | 1695.64 |
| Sulbactam | 3 g q8 (4-h inf) | 100 | 100 | 100 | 100 | 100 | 90 | 0 | 1028.72 | 1659.23 |
Abbreviations: AUC, area under the curve; MIC, minimum inhibitory concentration.
TIME-KILL ASSAYS
The susceptibility of the A. baumannii clinical isolates was determined by the broth microdilution method according to CLSI recommendations (Table 1). Additionally, cefiderocol susceptibility was confirmed by disc diffusion (disc zone 22 mm).
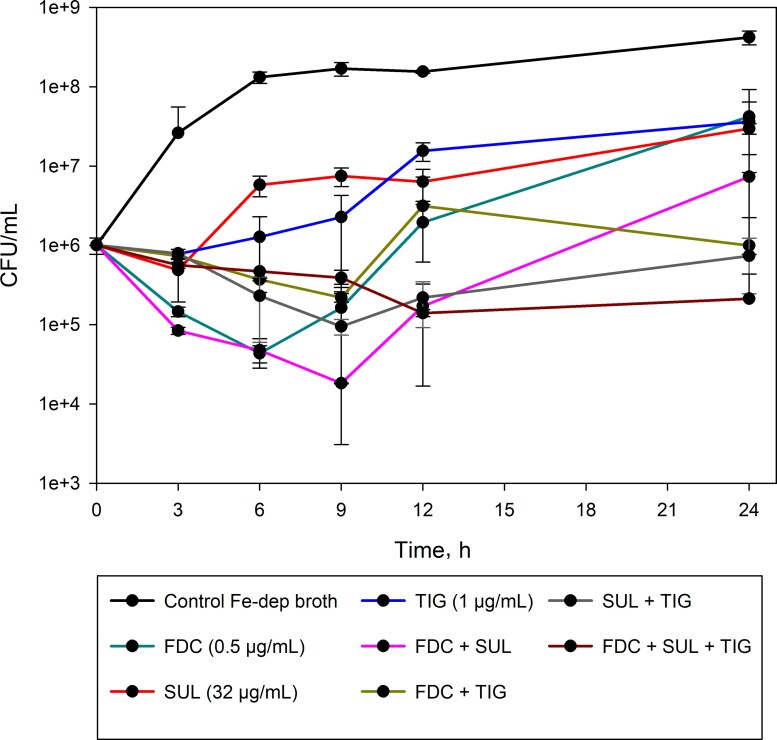
Synergy among the components of the clinical regimen was assessed using time-kill methodology in iron-depleted broth, as recommended for cefiderocol susceptibility testing. Preliminary experiments confirmed that iron-depleted broth did not adversely affect the activity of tigecycline or sulbactam. To determine synergy, test drugs were added to a bacterial inoculum of ∼107 CFU/mL at concentrations equivalent to half of their corresponding MIC. These concentrations were chosen because subinhibitory concentrations would allow for a better demonstration of synergy. Additionally, selected sulbactam and tigecycline concentrations emulate in vivo steady-state concentrations (Css). PK analysis of sulbactam in this patient revealed a Css of 43 µg/mL, while tigecycline Css was estimated to be ∼0.6 µg/mL as previously reported [18]. Therefore, 32 mg/L (half-MIC of 64 mg/L) of sulbactam and 1 mg/L of tigecycline (half-MIC of 2 mg/L) were selected. At 0, 3, 6, 9, 12, and 24 hours after incubation, an aliquot of 100 µL was obtained, and colony-forming units/mL (CFU/mL) was determined. The combination was considered synergistic if the CFU/mL reduction was >2-log at 24 hours relative to the most active single agent [19]. CFU/mL for cefiderocol, sulbactam, and tigecycline alone was ∼7 logs (Figure 1). The combination of any 2 drugs did not result in synergy as the log CFU reduction varied from 0.61 to 1.6. On the other hand, when cefiderocol, tigecycline, and sulbactam were studied together, a synergistic 2.15-CFU/mL log reduction was observed (Figure 1).
Figure 1.
Time-kill assay to assess the synergy between cefiderocol (FDC), sulbactam (SUL), and tigecycline (TIG) against A. baumannii AB#1.
DISCUSSION
XDR A. baumannii are challenging infections to treat. To date, the preferred regimen for XDR A. baumannii has not yet been defined; however, combination therapy is highly recommended [20]. Adding to the challenge, obtaining optimal antibiotic concentrations in critically ill patients undergoing CRRT is crucial, as insufficient dosing can lead to therapeutic failure [21]. Therefore, understanding drug pharmacokinetics and evaluating drug synergy could guide future clinical choices.
Cefiderocol, a siderophore cephalosporin with activity against drug-resistant gram-negative pathogens, has a well-described PK in healthy subjects and in patients with impaired renal function [22–24]. Although detailed dosing recommendations in patients with CRRT are enclosed in its package insert, very few real-world published data exist. We previously described cefiderocol PK in a patient with Pseudomonas aeruginosa pneumonia and bacteremia undergoing CVVHDF [25]. Another report by Fratoni and colleagues described cefiderocol PK in a patient with Stenotrophomonas maltophilia undergoing CVVHDF [26]. To our knowledge, this is the first report to evaluate cefiderocol and sulbactam PK in a patient with XDR-AB receiving CVVHDF.
PK analysis of cefiderocol revealed that its exposure exceeded the 88% fT > MIC necessary for 1-log kill for isolates with MICs up to 16 mg/L (100% fT > MIC). In a sensitivity analysis accounting for highest protein binding reported (60%), cefiderocol exposure remained above the 88% fT > MIC of 16 mg/L. Given that the patient's XDR-AB had a cefiderocol MIC of 1 mg/L, cefiderocol exposure was sufficiently high. Indeed, microbiologic eradication was seen with initial treatment of XDR-AB VAP, with a repeat BAL that finalized with oropharyngeal flora on day 8 of combination therapy; however, the patient remained on the ventilator during their entire hospital stay, and in the presence of uncontrolled sources, infection recurred.
Sulbactam exposure at the recommended IDSA dosing of a 3 g q8h 4-hour infusion was not sufficient to achieve target attainment in this patient given that the isolate is highly resistant (sulbactam MIC of 64 mg/L). The current regimen provided sufficient exposure for target attainment in isolates with MICs ≤16 mg/L. At an MIC of 32 mg/L, the sulbactam % fT > MIC was 90%; although the exact % fT > MIC of sulbactam necessary for optimal kill is not yet defined, this exposure would be considered sufficiently high when considering the efficacy of other related compounds.
As a result of the 4-drug clinical regimen, we evaluated the synergy between cefiderocol, sulbactam, and tigecycline. Given that this isolate was susceptible to cefiderocol, it would be challenging to demonstrate an additional benefit of combination therapy using the steady-state plasma profile observed in our patient (ie, 35 mg/L); therefore, we chose subinhibitory concentrations, 0.5× MIC, of cefiderocol to characterize synergy. As a result of the high MICs of the isolate to both sulbactam and tigecycline, the steady-state concentration of sulbactam in our patient (ie, 43 mg/L) and that typically observed in VAP patients receiving tigecycline (ie, 0.6 mg/L) improved the sensitivity of the synergy assessment as subinhibitory concentrations of all agents were utilized [18]. Using this highly cefiderocol-susceptible isolate, only the combination of cefiderocol, tigecycline, and sulbactam demonstrated synergy.
This report is not without limitations. While the study included only a single patient, given the sparse real-life evidence of cefiderocol and sulbactam in A. baumannii infections, these data broaden current knowledge. Second, although the patient received tigecycline, exposure in this patient was not quantified as other data are available in patients undergoing CRRT [12, 13]. Third, given that the isolate was susceptible to cefiderocol, we tested a very low concentration of cefiderocol in time-kill assays relative to the human exposure profile with the indicated dosing regimen; thus additional studies should be undertaken with cefiderocol-nonsusceptible isolates and conventional plasma concentrations to fully assess the synergistic potential of combination therapy against XDR-AB.
In conclusion, in absence of a standard-of-care treatment for CRAB, the use of multiple agents appears prudent. This study described cefiderocol PK as a part of combination therapy with tigecycline and sulbactam in a patient undergoing CVVHDF to achieve microbiological cure. As would be expected due to the susceptibility of the pathogen and the dosing regimen of cefiderocol, exposures were high and achieved robust target attainment in this patient. Cefiderocol, sulbactam, and tigecycline administered together may have an additional benefit when combined even in highly resistant isolates. Further evaluations of drug synergy are warranted with cefiderocol in XDR A. baumannii.
Acknowledgments
We would like to thank Margaret Bertrandt for their expertise in the conduct of patient care for this patient.
Patient consent. Written informed consent was obtained from the patient's legally authorized representative and Banner Health's nonresearch data use committee (NRDUC).
Contributor Information
Emir Kobic, Department of Pharmacy, Banner University Medical Center, Phoenix, Arizona, USA.
Yasmeen Abouelhassan, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, Connecticut, USA.
Kumara Singaravelu, Division of Infectious Diseases, Banner University Medical Center, Phoenix, Arizona, USA.
David P Nicolau, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, Connecticut, USA; Division of Infectious Diseases, Hartford Hospital, Hartford, Connecticut, USA.
References
- 1. Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017; 30:409–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magiorakos AP, Srinivasan A, Carey RB, et al. . Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 3. Shlaes DM, Sahm D, Opiela C, Spellberg B. The FDA reboot of antibiotic development. Antimicrob Agents Chemother 2013; 57:4605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gatti M, Bartoletti M, Cojutti PG, et al. . A descriptive case series of pharmacokinetic/pharmacodynamic target attainment and microbiological outcome in critically ill patients with documented severe extensively drug-resistant Acinetobacter baumannii bloodstream infection and/or ventilator-associated pneumonia treated with cefiderocol. J Glob Antimicrob Resist 2021; 27:294–8. [DOI] [PubMed] [Google Scholar]
- 5. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 2022; 74:2089–114. [DOI] [PubMed] [Google Scholar]
- 6. Bavaro DF, Belati A, Diella L, et al. . Cefiderocol-based combination therapy for “difficult-to-treat” gram-negative severe infections: real-life case series and future perspectives. Antibiotics (Basel) 2021; 10:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shionogi & Co. Cefiderocol: product information. Available at: www.fetroja.com. Accessed May 5, 2022.
- 8. Bassetti M, Echols R, Matsunaga Y, et al. . Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21:226–40. [DOI] [PubMed] [Google Scholar]
- 9. Abdul-Mutakabbir JC, Nguyen L, Maassen PT, et al. . In vitro antibacterial activity of cefiderocol against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2021; 65:e0264620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shields RK, Potoski BA, Haidar G, et al. . Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bassetti M, Castaldo N, Cattelan A, et al. . Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: a multicentre nationwide clinical experience. Int J Antimicrob Agents 2019; 53:408–15. [DOI] [PubMed] [Google Scholar]
- 12. Broeker A, Wicha SG, Dorn C, et al. . Tigecycline in critically ill patients on continuous renal replacement therapy: a population pharmacokinetic study. Crit Care 2018; 22:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao HH, Tang WJ, Yang YX, Cen ZR, Wang LQ. PK/PD study of tigecycline in severely infected patients with continuous renal replacement therapy. Int J Clin Pharmacol Ther 2020; 58:531–8. [DOI] [PubMed] [Google Scholar]
- 14. Pfizer . Unasyn medical information. Available at: www.pfizermedicalinformation.com/en-us/unasyn/other. Accessed May 5, 2022.
- 15. Bremmer DN, Nicolau DP, Burcham P, Chunduri A, Shidham G, Bauer KA. Ceftolozane/tazobactam pharmacokinetics in a critically ill adult receiving continuous renal replacement therapy. Pharmacotherapy 2016; 36:e30–3. [DOI] [PubMed] [Google Scholar]
- 16. Nakamura R, Ito-Horiyama T, Takemura M, et al. . In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother 2019; 63:e02031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corbella X, Ariza J, Ardanuy C, et al. . Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J Antimicrob Chemother 1998; 42:793–802. [DOI] [PubMed] [Google Scholar]
- 18. Dimopoulos G, Almyroudi MP, Kapralos I, et al. . Intrapulmonary pharmacokinetics of high doses of tigecycline in patients with ventilator-associated pneumonia. Int J Antimicrob Agents 2022; 59:106487. [DOI] [PubMed] [Google Scholar]
- 19. White RL, Burgess DS, Manduru M, Bosso JA. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother 1996; 40:1914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdul-Mutakabbir JC, Griffith NC, Shields RK, Tverdek FP, Escobar ZK. Contemporary perspective on the treatment of Acinetobacter baumannii infections: insights from the Society of Infectious Diseases Pharmacists. Infect Dis Ther 2021; 10:2177–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Honore PM, Jacobs R, De Waele E, Spapen HD. Applying pharmacokinetic/pharmacodynamic principles for optimizing antimicrobial therapy during continuous renal replacement therapy. Anaesthesiol Intensive Ther 2017; 49:412–8. [DOI] [PubMed] [Google Scholar]
- 22. Ito A, Kohira N, Bouchillon SK, et al. . In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting gram-negative bacteria. J Antimicrob Chemother 2016; 71:670–7. [DOI] [PubMed] [Google Scholar]
- 23. Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C. Cefiderocol, a siderophore cephalosporin for gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol 2017; 57:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother 2018; 62:e02163-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobic E, Gill CM, Mochon AB, Nicolasora NP, Nicolau DP. Cefiderocol pharmacokinetics in a patient receiving continuous venovenous hemodiafiltration. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fratoni AJ, Kuti JL, Nicolau DP. Optimised cefiderocol exposures in a successfully treated critically ill patient with polymicrobial Stenotrophomonas maltophilia bacteraemia and pneumonia receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 2021; 58:106395. [DOI] [PubMed] [Google Scholar]