Abstract
Background
Acute myeloid leukemia (AML) is associated with poor prognosis, particularly in elderly patients with comorbidities. Combining azacitidine (AZA) with BCL-2 inhibitor venetoclax (VEN) demonstrated significant improvement in outcomes for newly-diagnosed AML patients compared to AZA alone. However, this regimen is myelosuppressive, and the incidence of invasive fungal infections (IFIs) and impact of antifungal prophylaxis are not well defined.
Methods
This retrospective cohort study evaluated newly-diagnosed AML patients treated with VEN/AZA at the University of Colorado Hospital from January 2014 to August 2020. Patients with history of prior IFI were excluded. Primary outcome was IFI incidence during VEN/AZA therapy. χ2 and Fisher exact tests assessed the impact of patient demographics, AML-specific risk factors, and receipt of antifungal prophylaxis on IFI incidence.
Results
144 VEN/AZA-treated AML patients were included in the study. 25 (17%) patients developed IFI: 8% (n = 2) “proven,” 24% (n = 6) “probable,” and 68% (n = 17) “possible” per European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium criteria. There was no statistically significant association between IFI incidence with age, sex, or European LeukemiaNet classification. 10 patients received antifungal prophylaxis; none developed IFI. IFI incidence rate per 1000 patient-days was greatest 0–9 days after starting VEN/AZA, at 8.39.
Conclusions
Incidence of “proven” and “probable” IFI in our VEN/AZA-treated AML cohort was 5.6%, in-line with incidence rates reported by recent similar studies. Furthermore, IFI incidence decreased as days from starting VEN/AZA therapy increased.
Keywords: acute myeloid leukemia, azacitidine, invasive fungal infection, prophylaxis, venetoclax
Acute myeloid leukemia (AML) is the most common acute leukemia in adults, with a median age at diagnosis of 68 years [1]. For patients who can tolerate it, the standard of care is to treat newly diagnosed patients with intensive chemotherapy containing an anthracycline and cytarabine [2]. However, many AML patients are not candidates for this therapy due to an increased likelihood of having medical comorbidities, increased potency of cytotoxic effects, and increased probability of genetic abnormalities, all of which are associated with older age [3]. In these cases, the standard of care is to administer the hypomethylating agent azacitidine (AZA) with the BCL-2 inhibitor venetoclax (VEN).
The myelosuppressive properties of this regimen are well known. When comparing VEN/AZA treatment with AZA and a placebo, the incidence of anemia (26% vs 20%), thrombocytopenia (45% vs 38%), and neutropenia (42% vs 29%) were all greater in patients who received VEN/AZA treatment [4]. The median duration of neutropenia in the first cycle of treatment is 25 days; during subsequent cycles, this decreases to 14.5, 9.5, 12.5, and 7 days for cycles 2, 3, 4, and 5, respectively [5]. Use of a prophylactic antifungal agent for patients receiving AZA alone is not recommended, as it has been shown that the number of AML or myelodysplastic patients needed to treat to prevent 1 case of invasive fungal infection (IFI) in patients with severe neutropenia was 24 patients [6]. Given the increased myelosuppressive properties of this combination, the value of routine use of a prophylactic antifungal agent is not clear.
While the use of antifungal prophylaxis is effective at reducing the incidence of IFI in immunocompromised patients, prophylactic azoles inhibit CYP3A4, which requires significant dose reductions of VEN [7]. Previous studies demonstrated that 84% of patients receiving VEN/AZA subsequently developed infections, compared to only 67% of patients in the control group [8]. In contrast, multiple studies have since shown that not only does use of VEN with a hypomethylating agent demonstrate incidence rates as low as 5.1% in proven and probable IFI for AML patients, but also that incidence rate does not significantly change with and without the use of antifungal prophylaxis [9, 10]. This study evaluated the incidence of IFIs in AML patients treated with VEN/AZA to determine if antifungal prophylaxis is beneficial for this patient population.
METHODS
This was a retrospective cohort study conducted at the University of Colorado Hospital (UCH). Patients with newly diagnosed AML treated at UCH between January 2014 and August 2020 with VEN/AZA were included. This study was approved by the Colorado Multiple Institutional Review Board prior to study initiation. Data collected included demographic and clinical characteristics such as age, sex, death, AML classification, and European LeukemiaNet (ELN) classification. Risk factors for IFI evaluated were age ≥65 years, sex, unfavorable ELN classification, and receipt of antifungal prophylaxis.
Duration of VEN/AZA therapy was defined as the time from initiation of induction therapy through 1 of the 4 endpoints: (1) patient discontinuation, (2) progression of disease, (3) bone marrow transplantation, or (4) death. If a patient were to reach any of the 4 endpoints during VEN/AZA therapy, the day the endpoint was reached was used as the end date. Antifungal prophylaxis was defined as receipt of ≥1 day of systemic antifungal for preventive purposes while receiving therapy with VEN/AZA.
The primary outcome was incidence of IFI as defined by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) [11]. IFIs were further classified as “proven,” “probable,” or “possible” based on defined clinical, radiologic, and laboratory criteria. Subgroup analysis included incidence of IFI based on age, sex, ELN classification, and receipt of antifungal prophylaxis.
Patient baseline characteristics were evaluated using descriptive statistics. A χ2 test was used to calculate P values for categorical outcomes, with a significance threshold of .05. In the event of small cell counts, Fisher exact test was used to determine P values. To account for possible confounding by death, Cochran-Mantel-Haenszel adjustment was carried out for categorical variables of interest, and a death-adjusted P value and odds ratio were reported.
RESULTS
In total, 144 patients who received VEN/AZA therapy were evaluated. Patients with history of prior IFI were excluded from the total patient count (n = 5), as they are at a higher likelihood of developing subsequent IFIs compared to patients without history of prior IFI. Median age was 72 (interquartile range [IQR], 66–76) years and 49% of patients were female (Table 1). The median duration of VEN/AZA therapy was 137 (IQR, 56–268) days. Ten (6.9%) patients received antifungal prophylaxis during therapy. Eighty-one (56%) patients developed neutropenia, with a median duration of 35 (IQR, 19–58) days.
Table 1.
Demographics and Classification of Patients With Acute Myeloid Leukemia
| Characteristic | All Patients (N = 144) |
IFI (n = 25) |
No IFI (n = 119) |
|---|---|---|---|
| Age, y, median (IQR) | 72 (66–76) | 73 (65–78) | 72 (66–76) |
| Sex, female | 71 (49) | 10 (40) | 61 (51) |
| Death | 38 (26) | 10 (40.0) | 28 (23.5) |
| AML classification | |||
| Secondary AML | 59 (41) | 13 (52) | 46 (39) |
| De novo AML | 35 (24) | 7 (28) | 28 (24) |
| ELN classification | |||
| Unknown/unable to assess | 5 (3.5) | 1 (4) | 4 (3.4) |
| Unfavorable | 92 (64) | 18 (72) | 74 (62) |
| Intermediate | 23 (16) | 2 (8) | 21 (18) |
| Favorable | 24 (17) | 4 (16) | 20 (17) |
| VEN/AZA duration, d, median (IQR) | 137 (56–268) | 206 (62–292) | 133 (55–256) |
| Duration of neutropenia, d, median (IQR) | 35 (19–58) | 55 (38–68) | 32 (14–55) |
| Antifungal prophylaxisa | 10 (6.9) | 0 (0) | 10 (8.4) |
| Anidulafungin | 6 (60) | 0 (0) | 6 (60) |
| Azole | 5 (50) | 0 (0) | 5 (50) |
| Fluconazole | 4 (80) | 0 (0) | 4 (80) |
| Isavuconazole | 1 (20) | 0 (0) | 1 (20) |
| Antifungal prophylaxis duration, d, median (IQR) | 31 (9–63) | NA | 31 (9–63) |
Data are presented as No. (%) of patients unless otherwise indicated.
Abbreviations: AML, acute myeloid leukemia; ELN, European LeukemiaNet; IFI, invasive fungal infection; IQR, interquartile range; NA, not assessed; VEN-AZA, venetoclax/azacitidine.
May have received >1 antifungal agent.
Of the 144 VEN/AZA-treated AML patients, 25 cases (17%) of IFI were identified (Table 2). Of these, only 8 (5.6%) cases were classified as “proven” or “probable” according to the EORTC/MSGERC definition. Among patients with proven or probable IFI (n = 8), all cases were most consistent with invasive pulmonary aspergillosis (IPA). One patient with proven IPA ultimately died, with evidence of Aspergillus seen on autopsy.
Table 2.
Patients Categorized Based on Neutropenia, Age, Sex, European LeukemiaNet Classification, and Antifungal Prophylaxis Versus Incidence or No Incidence of Invasive Fungal Infection
| Variable | IFI (n = 25) |
No IFI (n = 119) |
Odds Ratio (95% CI) |
P Value | Death-Adjusted OR (95% CI) | Death-Adjusted P Value |
|---|---|---|---|---|---|---|
| Age ≥65 y | 18 (72) | 92 (77) | 0.75 (.29–2.00) | .57 | 0.68 (.25–1.82) | .438 |
| Sex, female | 10 (40) | 61 (51) | 1.58 (.66–3.79) | .30 | 1.69 (.69–4.15) | .250 |
| Unfavorable ELN classification | 13 (52) | 55 (46) | 1.26 (.53–2.99) | .74a | 1.41 (.58–3.41) | .454 |
| Antifungal prophylaxis | 0 (0) | 10 (8.4) | Cannot be computed | .21a | 0.36 (.04–2.92) | .126 |
| Proven/Probable IFI (n = 8) | No Proven/Probable IFI (n = 136) | Odds Ratio (95% CI) |
P Value | Death-Adjusted OR (95% CI) | Death-Adjusted P Value | |
| Age ≥65 y | 7 (87.5) | 103 (75.7) | 2.24 (.27–18.90) | .68a | 1.97 (.23–17.18) | .537 |
| Sex, female | 4 (50.0) | 67 (49.3) | 0.97 (.23–4.04) | 1.00a | 1.06 (.25–4.53) | .943 |
| Unfavorable ELN classification | 3 (37.5) | 65 (47.8) | 0.66 (.15–2.85) | .63a | 0.75 (.17–3.35) | .705 |
| Antifungal prophylaxis | 0 (0) | 10 (7.4) | Cannot be computed | 1.00a | 1.20 (.14–10.47) | .415 |
χ2 test was performed unless otherwise indicated.
Abbreviations: CI, confidence interval; ELN, European LeukemiaNet; IFI, invasive fungal infection; OR, odds ratio.
Fisher exact test.
For each secondary outcome of interest (Table 2), patients were categorized by the presence or absence of IFI. After calculating both a standard and death-adjusted odds ratio and P value for each outcome of interest, age, sex, ELN classification, and receipt of antifungal prophylaxis were all not significantly associated with incidence of IFI or incidence of proven/probable IFI.
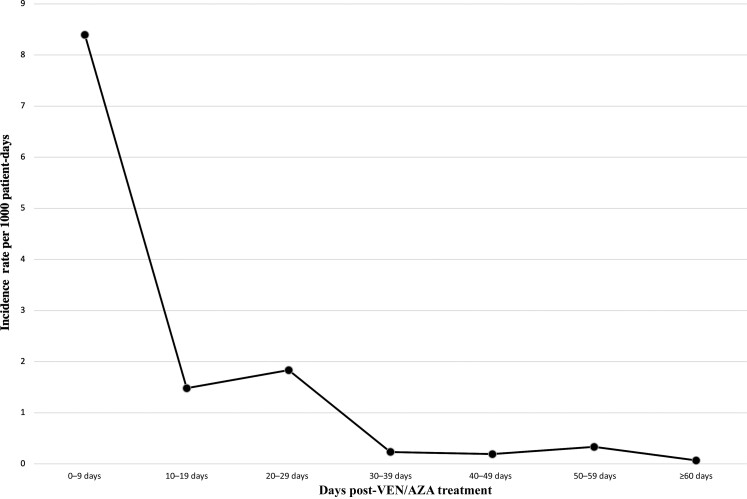
For patients with proven, probable, and possible IFI, the incidence rate per 1000 patient-days was plotted versus the number of days from starting VEN/AZA treatment (Figure 1). The incidence rates per 1000 patient-days for each categorization of days post–VEN/AZA treatment were 8.39 (0–9 days), 1.48 (10–19 days), 1.83 (20–29 days), 0.23 (30–39 days), 0.19 (40–49 days), 0.33 (50–59 days), and 0.07 (≥60 days).
Figure 1.
Incidence rate of invasive fungal infections (IFIs) after starting venetoclax/azacitidine (VEN/AZA) treatment. Patients diagnosed with proven, probable, and possible IFI were categorized by the number of days between starting VEN/AZA treatment and starting antifungal treatment.
DISCUSSION
Of the 144 AML patients treated with VEN/AZA included in this study, 25 (17%) were diagnosed with either a proven, probable, or possible IFI. The rate of proven and probable IFI in our cohort was 5.6%. Our incidence rate was very similar to the 5.1% incidence rate of probable or confirmed IFI from a recent study that also looked at the use of VEN with either AZA or decitabine in patients with AML [9]. These consistent rates suggest a lower likelihood of IFI incidence than previously reported rates for newly diagnosed AML cohorts that were similarly treated, where the incidence of proven and possible IFIs was 16% for patients treated with hypomethylating agent–based induction therapy [12]. In other studies, cohorts that received induction, reinduction, or consolidation chemotherapy plus antifungal prophylaxis reported incidences of proven and probable IFI of 5.9% with antimold prophylaxis and 8.7% without antimold prophylaxis [13].
In our cohort, the incidence rate of IFI per 1000 patient-days was greater when the timeframe between start of VEN/AZA therapy and diagnosis of IFI was shorter. Our results suggest that the risk of developing an IFI during VEN/AZA therapy is not associated with increased duration of therapy. Therefore, although VEN/AZA therapy does have significant myelosuppressive properties, the greatest incidence of IFI being within 10 days of starting VEN/AZA therapy suggests that prolonged neutropenia as a result of VEN/AZA therapy is not a significant risk factor of developing an IFI in our patient cohort [4]. However, existing literature has shown that prolonged neutropenia for >10 days is associated with invasive aspergillosis [14]. A possible explanation for our patient cohort developing IFIs early on during VEN/AZA therapy is the existence of neutropenia before VEN/AZA therapy was started. The median duration of neutropenia in patients diagnosed with IFI from the date of their AML diagnosis to their respective endpoint date was 55 days, compared to 32 days for patients who were not diagnosed with IFI. Given that neutropenia is a known clinical marker of underlying hematological diseases and is associated with AML, prolonged neutropenia before VEN/AZA therapy could have contributed to our cohort patients who developed an IFI [15, 16]. Thus, VEN/AZA therapy potentially contributes to the severity of neutropenia and ultimately the acute development of IFI in these patients. Establishing benchmarks for neutrophil counts or duration of neutropenia that identify AML patients at risk for developing an IFI acutely after starting VEN/AZA therapy warrants future research and discussion.
Current Infectious Diseases Society of America guidelines recommend antifungal prophylaxis for patients likely to have prolonged neutropenia [17]. Most recommendations have been developed with standard intensive chemotherapy regimens as the treatment modality; indeed, previous studies using conventional AML therapies have shown decreased incidence of IFI when antimold prophylaxis is utilized, supporting these recommendations [13]. However, in the AML field, VEN-based therapies, with a distinct mechanism of action, are becoming more prevalent. Whether different antifungal recommendations should be made in the context of this novel therapy is unclear. The severity and prevalence of IFIs in this patient population is evident, as they have been shown to have the highest incidence of occurring in AML patients, with mortality rates of up to 40%, making IFI treatment especially important [18]. Given in the literature that neutropenia is a significant risk factor for IFI, judicious use of granulocyte colony-stimulating factor for patients who achieve a morphologic remission should be considered [19]. However, antifungal prophylaxis is not necessarily the right fit for all patients, such as the vast majority of those planning to start VEN/AZA therapy. Preemptive antifungal therapy results in similar mortality rates as prophylaxis and decreases the incidence of treatment toxicity by significantly decreasing the use of antifungal agents; whereas incidence of IFI was significantly higher with preemptive antifungal therapy, there was no significant difference in rates of survival between the 2 therapies [20]. Additionally, the same study found that patients treated with preemptive therapy for IFI had a significant decrease in antifungal drug costs, including some therapies that would have decreased costs by 40% if used by all patients [20].
Local rates of IFI should influence the decision to utilize a universal antifungal prophylaxis protocol versus a preemptive monitoring protocol; therefore, multiple factors outside of VEN/AZA therapy potentially contribute to our low incidence of IFI, one being geographical location. In the United States, predictive models of coccidioidomycosis and climate change warn that warmer temperatures may increase the incidence and area of at-risk regions of Valley fever by up to 50%, which is typically only found in the southwestern United States [21]. Other mycoses such as histoplasmosis are known to be found in regions of 35–50 inches of rainfall along with temperatures around 22°C–29°C [22]. Given Colorado's typically dryer weather along with cold winter months, climate potentially decreases the incidence of IFI. Further investigation on specific geographical and climate features of Colorado should be considered in the future to better understand what specific factors about Colorado's location possibly contribute to the lower incidence of IFI. Another factor to consider is that variation in the backbone therapy with VEN may impact incidence of IFI. The use of AZA and a similar hypomethylating agent, decitabine, in combination with VEN results in similar overall survival (16.4 months and 16.2 months, respectively), yet the incidence of febrile neutropenia was 39% with AZA compared to 65% for decitabine [23]. When comparing AZA with decitabine, decitabine is known to cause significantly higher risks of anemia, febrile neutropenia, and leukemia in AML patients; thus, it is thought to have a higher level of toxicity that is considered when choosing a hypomethylating agent [24]. Other studies that used AZA for patients as maintenance therapy after remission of AML have similarly found neutropenia as common severe adverse events, with incidence rate of 41% compared to 24% in a placebo group [25]. Given the variability in IFI incidence based on geographical location and combination regimen with VEN, both of which likely play a role in antifungal prophylaxis protocol at different healthcare institutions, a blanket recommendation to use or not use antifungal prophylaxis is not suitable for every medical practice. Thus, there should be unique discussions on the benefits, risks, and alternatives of prophylaxis regimens at each institution.
There were several limitations to our retrospective cohort study. In this study, 10 (6.9%) patients received antifungal prophylaxis, and none developed an IFI; we determine that the number needed to treat with antifungal prophylaxis to avoid IFI is 12 patients. These 10 patients were included so that our retrospective cohort study could be comprehensive and include all AML patients treated with VEN/AZA in our patient population, whether they were on antifungal prophylaxis or not. Inclusion of these patients allowed us insight into whether there was benefit in using antifungal prophylaxis in preventing IFIs. However, given our small sample size, our study was not powered to determine if antifungal prophylaxis impacted incidence of proven and probable IFI in AML patients compared to no antifungal prophylaxis. A prospective controlled study would be the optimal way to answer the question of whether antifungal prophylaxis prevents IFI in this population. Our AML patient population treated with VEN/AZA was also limited to the University of Colorado, and thus our conclusions may not apply to a more widespread patient population nationally and globally, given the potential impact of climate.
IFIs can cause serious morbidity and mortality for AML patients; thus, universal antifungal prophylaxis is often considered a standard alongside many AML-directed therapies. However, drug–drug interactions and adverse side effects may negatively impact patient well-being and treatment efficacy. Newer therapies, such as VEN/AZA, show promising results of lower incidences of proven and probable IFI. Further understanding of patient-specific circumstances could lead treatment centers to internally evaluate their specific rates of IFI to determine whether universal prophylaxis or preemptive monitoring would be more appropriate in caring for VEN/AZA-treated AML patients.
Contributor Information
Alexander Zhang, School of Medicine, University of Colorado, Anschutz Medical Campus, Aurora, Colorado, USA.
Tanner Johnson, Department of Pharmacy, University of Colorado, Anschutz Medical Campus, Aurora, Colorado, USA.
Diana Abbott, Department of Biostatistics and Informatics, Center for Innovative Design and Analysis, Colorado School of Public Health, Aurora, Colorado, USA.
Tanit Phupitakphol, Division of Infectious Diseases, University of Colorado, Anschutz Medical Campus, Aurora, Colorado, USA.
Jonathan A Gutman, Division of Hematology, University of Colorado, Anschutz Medical Campus, Aurora, Colorado, USA.
Daniel A Pollyea, Division of Hematology, University of Colorado, Anschutz Medical Campus, Aurora, Colorado, USA.
Yiannis Koullias, Division of Infectious Diseases, University of Colorado, Anschutz Medical Campus, Aurora, Colorado, USA.
Notes
Patient consent. This retrospective analysis was approved by the University of Colorado Anschutz Medical Campus institutional review board; this study was not a clinical trial.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. American Cancer Society (ACS) . Cancer facts and figures 2022. Atlanta, GA: ACS, 2022. [Google Scholar]
- 2. Murphy T, Yee KWL. Cytarabine and daunorubicin for the treatment of acute myeloid leukemia. Expert Opin Pharmacother 2017; 18:1765–80. [DOI] [PubMed] [Google Scholar]
- 3. Krug U, Buchner T, Berdel WE, Muller-Tidow C. The treatment of elderly patients with acute myeloid leukemia. Dtsch Arztebl Int 2011; 108:863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DiNardo CD, Jonas BA, Pullarkat V, et al. AML-063: a randomized, double-blind, placebo-controlled study of venetoclax with azacitidine versus azacitidine in treatment-naive patients with acute myeloid leukemia ineligible for intensive therapy—VIALE-A. Clin Lymphoma Myeloma Leuk 2020; 20:S179. [Google Scholar]
- 5. Pratz KW, Wei AH, Pollyea DA, et al. Management of neutropenia during venetoclax-based combination treatment in patients with newly diagnosed acute myeloid leukemia. Blood 2019; 134:3897. [Google Scholar]
- 6. Pomares H, Arnan M, Sanchez-Ortega I, Sureda A, Duarte RF. Invasive fungal infections in AML/MDS patients treated with azacitidine: a risk worth considering antifungal prophylaxis? Mycoses 2016; 59:516–9. [DOI] [PubMed] [Google Scholar]
- 7. Dvorak Z. Drug–drug interactions by azole antifungals: beyond a dogma of CYP3A4 enzyme activity inhibition. Toxicol Lett 2011; 202:129–32. [DOI] [PubMed] [Google Scholar]
- 8. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 2020; 383:617–29. [DOI] [PubMed] [Google Scholar]
- 9. On S, Rath CG, Lan M, et al. Characterisation of infections in patients with acute myeloid leukaemia receiving venetoclax and a hypomethylating agent. Br J Haematol 2022; 197:63–70. [DOI] [PubMed] [Google Scholar]
- 10. Chen EC, Liu Y, Harris CE, et al. Outcomes of antifungal prophylaxis for newly diagnosed AML patients treated with a hypomethylating agent and venetoclax. Leuk Lymphoma 2022; 63:1934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ozga M, Huang Y, Blachly JS, Grieselhuber NR, Wall S. The incidence of invasive fungal infections in patients with AML treated with a hypomethylating agent. Clin Lymphoma Myeloma Leuk 2021; 21:e76–83. [DOI] [PubMed] [Google Scholar]
- 13. Oh SM, Byun JM, Chang E, Kang CK, Shin DY. Incidence of invasive fungal infection in acute lymphoblastic and acute myelogenous leukemia in the era of antimold prophylaxis. Sci Rep 2021; 11:22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pagano L, Caira M, Candoni A, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica 2010; 95:644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen BA, Wendelbo O, Bruserud O, Hemsing AL, Mosevoll KA, Reikvam H. Febrile neutropenia in acute leukemia. Epidemiology, etiology, pathophysiology and treatment. Mediterr J Hematol Infect Dis 2020; 12:e2020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmblad J, Siersma V, Lind B, Bjerrum OW, Hasselbalch H, Andersen CL. Age-related prevalence and clinical significance of neutropenia—isolated or combined with other cytopenias: real world data from 373 820 primary care individuals. Am J Hematol 2020; 95:521–8. [DOI] [PubMed] [Google Scholar]
- 17. Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol 2018; 36:3043–54. [DOI] [PubMed] [Google Scholar]
- 18. Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006; 91:1068–75. [PubMed] [Google Scholar]
- 19. Jonas BA, Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia 2019; 33:2795–804. [DOI] [PubMed] [Google Scholar]
- 20. Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis 2009; 48:1042–51. [DOI] [PubMed] [Google Scholar]
- 21. Gorris ME, Treseder KK, Zender CS, Randerson JT. Expansion of coccidioidomycosis endemic regions in the United States in response to climate change. Geohealth 2019; 3:308–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maiga AW, Deppen S, Scaffidi BK, Baddley J, Aldrich MC. Mapping histoplasma capsulatum exposure, United States. Emerg Infect Dis 2018; 24:1835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pollyea DA, Pratz K, Letai A, Jonas BA, Wei AH. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol 2021; 96:208–17. [DOI] [PubMed] [Google Scholar]
- 24. Ma J, Ge Z. Comparison between decitabine and azacitidine for patients with acute myeloid leukemia and higher-risk myelodysplastic syndrome: a systematic review and network meta-analysis. Front Pharmacol 2021; 12:701690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei AH, Dohner H, Pocock C, Montesinos P, Afanasyev B. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med 2020; 383:2526–37. [DOI] [PubMed] [Google Scholar]