Abstract
Purpose
The aim was to verify the impact of obesity on the long-term outcome of patients with severe SARS-CoV-2 ARDS.
Materials and methods
The retrospective study included patients admitted to the high-volume ECMO centre between March 2020 and March 2022. The impact of body mass index (BMI), co-morbidities and therapeutic measures on the short and 90-day outcomes was analysed.
Results
292 patients were included, of whom 119(40.8%) were treated with veno-venous ECMO cannulated mostly (73%) in a local hospital. 58.5% were obese (64.7% on ECMO), the ECMO was most frequent in BMI > 40(49%). The ICU mortality (36.8% for obese vs 33.9% for the non-obese, p = 0.58) was related to ECMO only for the non-obese (p = 0.04). The 90-day mortalities (48.5% obese vs 45.5% non-obese, p = 0.603) of the ECMO and non-ECMO patients were not significantly influenced by BMI (p = 0.47, p = 0.771, respectively). The obesity associated risk factors for adverse outcome were age <50 (RR 2.14) and history of chronic immunosuppressive therapy (RR 2.11, p = 0.009). The higher dosage of steroids (RR 0.57, p = 0.05) associated with a better outcome.
Conclusions
The high incidence of obesity was not associated with worse short and long-term outcomes. ECMO in obese patients together with the use of steroids in the later stage of ARDS may improve survival.
Keywords: SARS-CoV-2 ARDS, Extracorporeal membrane oxygenation, Covid-19, Obesity, Morbid obesity, Corticosteroids
Abbreviations: SARS-CoV-2, pandemic coronavirus; ARDS, acute respiratory distress syndrome; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; ECLS, extracorporeal life support; ELSO, extracorporeal life support organisation; E-CPR ECMO, assisted cardiopulmonary resuscitation; LOS, length of stay; SOFA, sequential organ failure assessment; APACHE IV, acute physiology and chronic health evaluation; IPPV, intermittent positive pressure ventilation; PEEP, positive end-expiratory pressure
1. Introduction
The prevalence of obesity is rising worldwide and currently represents around 20% of the critically ill [1]. According to the British ICNARC registry [2] during the SARS-CoV-2 pandemic 72% of general ICU patients had a BMI > 25 whilst 38% had a BMI > 30, highlighting the problem of obesity in intensive care units [2,3].
Obese patients are fraught with multiple co-morbidities including diabetes, hypertension, cor pulmonale, chronic kidney disease, ischemic heart disease, obstructive sleep apnea and obesity-hypoventilation syndrome, which lead to a more severe hospital and ICU course [1,[4], [5], [6]]. A body mass index (BMI) > 30 and 35 kg/m2, respectively, doubles the odds ratio for invasive mechanical ventilation and identifies a population of patients at high risk of severe illness and death with Covid-19 [[7], [8], [9]]. Obese patients have an increased risk of ARDS owing to a baseline ventilation-perfusion mismatch, atelectrauma from alveolar collapse due to higher chest elastance, increased intrabdominal pressure with a possible impact of an inflammatory response from adipose tissue [10]. IPPV may require aggressive settings in obese patients with ARDS. Nonetheless, the open lung concept with higher PEEP tested on perioperative patients may not lead to survival with regards to the adverse impact of IPPV on lung parenchyma, cor pulmonale and pulmonary hypertension [[11], [12], [13]]. In addition, eventual muscle paralysis, steroids, together with hypoxia contribute to critical illness polyneuropathy and declining muscle strength limiting mobilisation and recovery of obese ICU patients with Covid-19. The demand for ICU care is further stretched to the limits with higher grades of obesity, also due to positioning, proning, airway management, care for vascular access and drain sutures to skin flaps [14,15].
Regardless of all adverse prognostic indicators, the paradox of obesity has been described in intensive care suggesting that mild-to-moderate obesity is protective compared to normal BMI or morbid obesity. The so called “U shape” mortality curve has been proposed to describe the relationship between clinical outcome and BMI [16,17]. Obese patients may demonstrate improved survival and shorter hospital length-of-stay (LOS), possibly explained by a larger metabolic reserve for the recovery stage of critical illness [1,18].
Extracorporeal membrane oxygenation (ECMO) is an established modality in severe respiratory failure not responding to conventional therapy. For SARS-CoV-2-ARDS, the recommended ECMO indications are similar to other ARDS etiologies and are based on EOLIA criteria [19]. The limitations of ECMO therapy are related to BMI when patients with a large circulating blood volume (e.g. in the morbidly obese with a BMI > 40) may showcase difficult oxygenation with a relatively inadequate veno-venous ECMO (VV-ECMO) blood flow and persisting hypoxia [20,21]. Moreover, an increased risk of a decompensated cor pulmonale due to aggressive IPPV in the morbidly obese or/and pulmonary embolism as part of severe SARS-CoV-2-ARDS allude to the limits of veno-venous ECMO.
Various case series available on not homogenous non-Covid-19 cohorts of patients with indications for ECMO demonstrated the tendency to contraindicate ECMO in high-grade obesity, also raising the question of an “obesity paradox” in the critically ill [[22], [23], [24], [25], [26], [27]]. The high volume ECMO centres provide care for extensive catchment areas of care and as thus centralized the most difficult cases of respiratory failure during the SARS-CoV-2 pandemic.
The primary outcome of the retrospective study was an impact of obesity on short and long term survivals of the ECMO and non-ECMO patients with severe SARS-CoV-2 ARDS admitted to the high volume ECMO centre over the two years of the pandemic. The aims of the study were to verify the assumed adverse impact of obesity and to clarify the issue of “obesity paradox” [13]. The secondary outcomes included the prevalence of various grades of obesity and the impacts of various pathologies and therapeutic interventions compared between the obese (BMI > 30) and non-obese (BMI ≤ 30) patients.
2. Methods
Patients with severe SARS-CoV-2 ARDS according to the Berlin 2011 criteria admitted to a single high-volume ECMO centre between March 2020 and March 2022 were retrospectively analysed. The overweight status was defined as a BMI between 25 and 30 kg/m2, or equal to 30 kg/m2, obesity as a BMI > 30 and the morbid obesity as a BMI > 40 kg/m2. The authors sought to determine the impact of BMI, co-morbidities, therapeutic measures on the outcome including the 90-day mortality and the distribution of BMI across the cohort of patients with severe ARDS. Selected variables were compared between obese (BMI > 30) and non-obese (BMI ≤ 30) patients.
The data was collected from the patients' medical records including demographic characteristics (BMI, comorbidities, clinical presentation at ICU admission and severity scores [APACHE IV, Sequential Organ Function (SOFA)]. Specific treatments for COVID-19 were recorded before and after admission as well as parameters including ECMO. In the cases of the ECMO retrievals the data was transferred from the referring hospitals. Length of stay (LOS), ICU, hospital and 90-day mortality as well as the dates of SARS-CoV-2 positivity, duration and type of therapy prior to admission to the ICU, dates of ECMO explant and IPPV duration were entered into the database. The patients were followed for a minimum of 90 days, and for those discharged from the ECMO centre to local hospitals the hospital outcome was defined as the discharge of the patient from the hospital. Complications of the ICU stay were recorded including the septic shock, barotrauma, pneumothorax, deep venous thrombosis, pulmonary embolism, stress cardiomyopathy, renal failure requiring replacement therapy. Severe bleeding was defined by the need of ≥2 packed red cells over a period of 24 h.
The ECMO centre at the Prague General University Hospital treats approximately 120 ECMO patients annually since 2009 with numbers increasing during the 2020–22 pandemic. ECLS patients have been entered into the ELSO database, 80% of all emergency ECMO cannulations are performed percutaneously by the intensive care or interventional cardiology teams. The centre provides an emergency service for cardiorespiratory failure for the whole country as well as an E-CPR service for the Prague metropolitan area.
The research qualified as a retrospective evaluation of cases prospectively recorded into our ECMO and ICU database. The study (No. 100/21 Grant AZV VES VFN Covid) was registered by the University Hospital Ethical Board which waived a need for an informed consent All patients with mild ARDS and/or the absence of a weighted bed for BMI measurement were excluded.
After verifying the distribution of data, the differences between the groups were tested with the Mann-Whitney U test for continuous variables and with the chi-square test, followed by the Fischer's exact test as appropriate for categorical variables. The data are expressed as median and interquartile range (IQR) or as numbers and percentages. The Kaplan-Meier curves of the outcome data were created and compared with the log-rank test between the groups. A subgroup analysis was calculated using Mantel-Haenszel analysis between a given stratification and the study group. The Cox proportional-hazards regression analysis was utilized to test the various risk factors and their relationships to the outcomes of the obese and non-obese patients.
3. Results
3.1. Characteristics of the obese patients and the impact of obesity
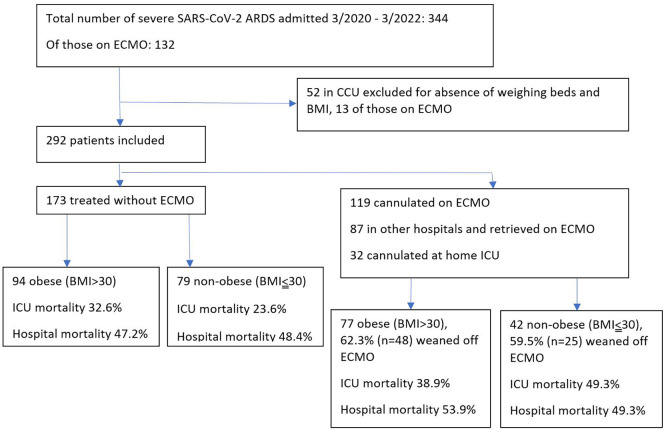
For a period of over 24 months, a total of 292 patients with median age of 57 years were included in the analysis, with men comprising a total of 194 (66.4%) (Fig. 1 ).
Fig. 1.
Study flow chart.
The characteristics of the patients including medical history and the admission parameters are summarized in Table 1 . The obese patients were younger (p = 0.01), significantly more often hypertensive (p < 0.001), with more frequent coronary artery disease (p = 0.01). Patients with a normal BMI and those overweight who developed severe SARS-CoV-2 ARDS were more often with history of chronic immunosuppressive therapy (corticosteroids or combined, p = 0.005). The intervals between a positive SARS-CoV-2 test and the ICU admission in the obese and the non-obese patients were not different. With the same degree of severity of the illness and hypoxia the admission ventilation settings required a higher PEEP (p = 0.048), a higher driving pressure (p < 0.001) and a higher plateau pressure (p = 0.002) in the obese patients.
Table 1.
Characteristics of the patients on admission to the ICU.
| BMI > 30 (n = 171) | BMI ≤ 30 (n = 121) | P-value | |
|---|---|---|---|
| Age (years) | 56 (48–65) | 61 (51–68) | 0.013 |
| Weight (kg) | 110 (100−120) | 82 (75–90) | <0.001 |
| Height (m) | 1.74 (1.68–1.8) | 1.77 (1.7–1.8) | 0.024 |
| BMI | 35.1 (32.1–40.1) | 26.3 (24.8–27.8) | <0.001 |
| Gender (males) | 63.7% (109) | 70.2% (85) | 0.246 |
| APACHE IV | 87 (77–100) | 90 (77–100) | 0.371 |
| SOFA | 10 (8–12) | 11 (8–12) | 0.480 |
| Hypertension | 59.1% (101) | 38.8% (47) | <0.001 |
| Diabetes mellitus | 26.3% (45) | 17.4% (21) | 0.071 |
| CAD | 9.4% (16) | 19.8% (24) | 0.01 |
| COPD | 7,6% (13) | 3.3% (4) | 0.122 |
| Smoking | 18.1% (31) | 22.3% (27) | 0.404 |
| Immunosuppression | 5.3% (9) | 14.9% (18) | 0.005 |
| Cancer | 5.3% (9) | 7.4% (9) | 0.436 |
| Time from SARS-CoV-2 PCR positivity to admission (days) | 9 (3–13) | 8 (4–14.5) | 0.544 |
| PaO2/FiO2 (at admission) | 75 (62–101) | 75.5 (60–105) | 0.883 |
| Orotracheal intubation on admission | 95.9% (164) | 94.2% (114) | 0.505 |
| MV parameters (at admission) | |||
| - PEEP | 12 (10–14) | 11 (8–14) | 0.048 |
| - driving pressure | 18 (15–20) | 16 (12–18) | <0.001 |
| - plateau pressure | 30 (26–34) | 28 (24–30) | 0.002 |
APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease, HFNO, high flow nasal oxygen; NIV, non-invasive ventilation; MV, mechanical ventilation; PEEP, positive end-expiratory pressure.
Table 1: Comparison of the obese versus non-obese patients on admission to the ICU (numbers and percentages or medians with interquartile ranges, compared with Mann-Whitney U or the Fischer's exact tests).
Regarding patient treatment (Table 2 ) the differences between obese and non-obese groups were found for a low dosage of steroids, which were more frequent in the non-obese group (81.8% vs 71.3%, p = 0.04). On the contrary, the higher dosage of steroids (>6 mg of dexamethasone or 40 mg methylprednisolone) was more often administered in the obese group (p < 0.001). Differences were also found for the treatments with remdesivir and monoclonal antibodies against SARS-CoV-2, often started pre-admission to the ICU and both more frequent in the non-obese group (p < 0.001 and p = 0.018, respectively). With similar intervals between intubation and tracheostomy times (i.e. 7–8 days), the obese patients reached spontaneous pressure support ventilation after intubation slightly later (5.5 days vs 5 days, p = 0.02) than in the non-obese patients.
Table 2.
Patient's treatment.
| BMI > 30 (n = 171) | BMI ≤ 30 (n = 121) | P-value | |
|---|---|---|---|
| Treatment of COVID-19 | |||
| - Low dose corticosteroids (up to 8 mg dexamethasone/day) | 71.3% (122) | 81.8% (99) | 0.04 |
| - High dose corticosteroids (>8 mg dexamethasone or >40 mg methylprednisolone/day) | 28.1% (48) | 6.6% (8) | <0.001 |
| - Isoprinosine | 70.8% (121) | 67.8% (82) | 0.584 |
| - Remdesivir | 41.5% (71) | 61.2% (74) | <0.001 |
| - Monoclonal antibodies⁎ | 9.4% (16) | 19% (23) | 0.018 |
| - Baricitinib | 2.9% (5) | 5% (6) | 0.368 |
| - Tocilizumab | 1.2% (2) | 1.7% (2) | 0.726 |
| NIV/HFNO (days) | 2 (1–4) | 2 (0–5) | 0.702 |
| Tracheostomy | 75.4% (129) | 69.4% (84) | 0.254 |
| Intubation to tracheostomy interval (days) | 8 (5–9) | 7 (6–9) | 0.552 |
| Initiation of PSV post intubation (days) | 5.5 (3–9) | 5 (2–7) | 0.023 |
| Prone position | 62% (106) | 59.5% (72) | 0.623 |
| VV ECMO | 45% (77) | 34.7% (42) | 0.087 |
|
29 (25–29) | 27 (25–29) | 0.503 |
|
23 (21−23) | 23 (21–23) | 0.726 |
|
4.7 (4.2–5.2) | 4.6 (4.2–5) | 0.631 |
|
3 (2.5–4) | 3 (2.5–3.5) | 0.459 |
| ECMO retrieval | 34.5% (59) | 23.1% (28) | 0.85 |
|
5 (2–9) | 7 (4–10) | 0.021 |
| ECMO duration (days) | 14 (8–24) | 14 (9–23) | 0.775 |
|
62.3% (48) | 59.5% (25) | 0.845 |
| circuit thrombosis | 3.5% (6) | 0 | 0.519 |
| number of circuits used | 2 (1–3) | 2 (1–2) | 0.570 |
| duration of MV or NIV prior to ECMO initiation (days) | 3 (1–5) | 2 (1–4) | 0.201 |
| prone on ECMO | 30.7% (23) | 28.6% (12) | 0.837 |
|
6 (5–13) | 7 (3−10) | 0.447 |
Table 2: Comparison of therapy in the obese versus non-obese groups (numbers and percentages or medians with interquartile ranges, compared with Mann-Whitney U or the Fischer's exact tests).
Monoclonal antibodies available since 2021; NIV, noninvasive ventilation; HFNO, high flow nasal oxygen; PSV, pressure support ventilation; MV, mechanical ventilation; circuit thrombosis = pump head thrombosis requiring urgent resetting. Interval from ICU admission to ECMO starts at the referring hospital ICU admission. Initial fraction of oxygen on the ECMO blender (FsO2) was 100% in all patients.
ICU related factors (Table 3 ) did not demonstrate significant differences between the obese and non-obese groups except for the need for a renal replacement therapy, which was more frequent in the obese group (p = 0.046). In addition, the time interval to a negative SARS-CoV-2 test was longer by one day (14 days) in the obese than in the non-obese patients (13 days, p = 0.049).
Table 3.
Clinical outcomes.
| BMI > 30 (n = 171) | BMI ≤ 30 (n = 121) | P-value | |
|---|---|---|---|
| Pneumothorax | 18.1% (31) | 19.8% (24) | 0.749 |
| Pneumothorax in ECMO patients | 26% (20) | 33.3% (14) | 0.404 |
| Pneumomediastinum | 8.8% (15) | 9.9% (12) | 0.763 |
| Pneumomediastinum in ECMO patients | 18.2% (14) | 19% (8) | 1.00 |
| Ventilator free days | 11 (6–19) | 14 (4–22) | 0.77 |
| Septic shock | 61.4% (105) | 61.2% (74) | 0.866 |
| CRRT | 31.6% (54) | 21.5% (26) | 0.046 |
| Major bleeding | 17.5% (30) | 19% (23) | 0.777 |
| DVT (pre- or non-ECMO) | 5.8% (10) | 11.6% (14) | 0.090 |
| DVT (post ECMO) | 21.6% (37) | 11.6% (14) | 0.290 |
| Pulmonary embolism | 7.6% (13) | 9.1% (11) | 0.645 |
| Septic cardiomyopathy | 5.8% (10) | 4.1% (5) | 0.513 |
| Interval from SARS-CoV-2 PCR positivity to negative test (days) | 14 (10–19) | 13 (7–18) | 0.049 |
| ICU LOS (days) | 13 (7–22) | 13 (6–20) | 0.432 |
| Hospital LOS (days) | 27 (14–51) | 27 (13–66) | 0.639 |
| ICU mortality | 36.8% (63) | 33.9% (41) | 0.578 |
| Hospital mortality | 49.7% (85) | 48.8% (59) | 0.873 |
| 90-day mortality | 48.5% (83) | 45.5% (55) | 0.603 |
CRRT, continuous renal replacement therapy; DVT, deep venous thrombosis; LOS, length of stay; ICU, intensive care unit.
Table 3: Comparison of complications, length-of-stay and the ICU, hospital and 90-day outcomes between the obese and non-obese patients (numbers and percentages or medians with interquartile ranges, compared with Mann-Whitney U or the Fischer's exact tests).
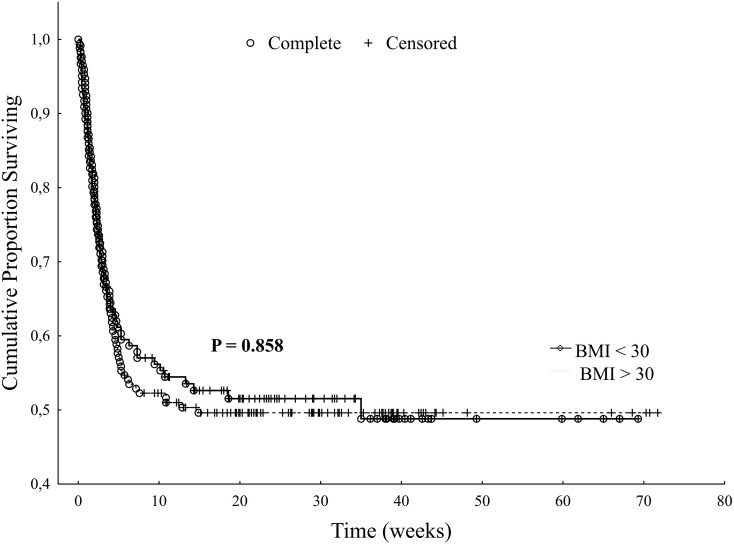
The ICU and hospital outcomes were not different between the obese and non-obese groups (ICU mortality was 36.8% for the obese group vs 33.9% for the non-obese group, p = 0.58, and hospital mortality 49.7% vs 48.8%, respectively, p = 0.87, Table 3). The long-term survival of the obese and non-obese groups is shown in Fig. 2 with no significant differences (p = 858).
Fig. 2.
Comparison of the long-term survival of obese patients vs non-obese patients (Kaplan-Meier curves with log rank test, p = 0.858).
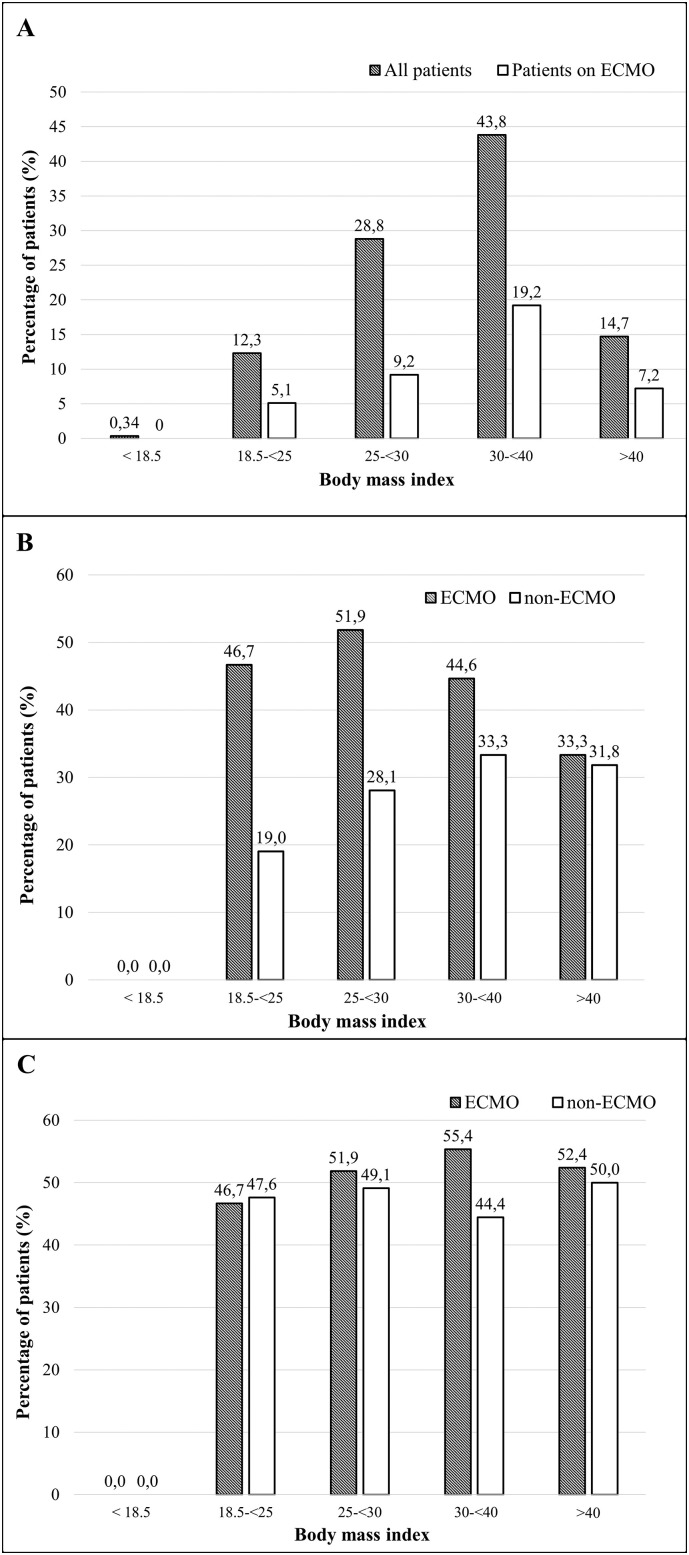
The distribution of BMI among patients with severe SARS-CoV-2 related ARDS showed that 58.5% of the patients were obese and 14.7% were morbidly obese which is higher than in the British ICNARC registry (48.3% and 11.7%, respectively) (Fig. 3A) [2]. The difference is more evident for the group of patients with severe SARS-CoV-2-ARDS on ECMO (64.7% and 17.6%, respectively) especially when compared to the distribution of BMI across the average European population (31.7% with a BMI > 30, and 3% with a BMI > 40) [2,16].
Fig. 3.
Distribution of the body mass indexes (3A) among the 292 severe SARS-CoV-2 ARDS patients and their relationships to application of ECMO and the ICU mortality (3B) and the hospital mortality (3C).
3.2. Impact of the ECMO therapy
From the 292 patients, 173 were treated conservatively and 119 (40.8%) were treated with ECMO (Fig. 1). 87 of 119 patients (73%) on ECMO were rescued and cannulated in 46 other hospitals within a radius of 250 km from the centre, whilst 32 patients were cannulated in the department (27%). The 119 ECMO patients were primarily treated with the veno-venous modality (VV-ECMO, n = 110), the veno-arterial and hybrid modalities (VA-VAV-VVA ECMO, n = 9), and with the veno-arterial and Impella (VA + Impella, n = 1) modality. The VA and Impella modalities were indicated for stress cardiomyopathy (n = 7) or pulmonary embolism (n = 2) complicating severe ARDS, all of those patients were later weaned through a hybrid modality to the VV-ECMO due to their pulmonary disease. Five patients had to be re-cannulated, four of those for bacterial superinfection and one peri-procedurally for bronchial stenting. The awake ECMO was cannulated in 9 patients while on HFNO or NIV, all of them had to be intubated for at least 24 h either during the ECMO run or after the ECMO explant. The time from positive Covid-19 test to ECMO cannulation was two days shorter in the obese ECMO group (5 days) compared to the non-obese ECMO patients (7 days, p = 0.02). The initial ECMO settings, rates of proning and the successful ECMO weanings are shown in Table 2. With comparable rates of ECMO retrievals in both groups the median duration of the typical VV-ECMO support was 14 days requiring two ECMO circuits.
After the commencement of ECMO the standard IPPV setting was always pulmo-protective, e.g. PEEP 3–10 cmH2O, Pplat up to 24–26 cmH2O with a spontaneous modality (PSV, BIPAP) whenever possible. Barotrauma represented by a pneumothorax was significantly more frequent in the obese and non-obese ECMO patients (26% and 33%, respectively) than for the obese (11.7%, p = 0.018) and the non-obese patients treated without ECMO (12.7%, p = 0.009). The pneumomediastinum was more frequent among the obese and non-obese ECMO patients (18.2% and 19%, respectively) than for the non-ECMO obese patients (1.1%, p < 0.001) and the non-ECMO and not obese controls (5.1%, p = 0.023). Nonetheless, the rates of barotrauma were similar comparing the obese and non-obese patients (Table 3).
The patients (n = 87) cannulated in local hospitals during the ECMO retrievals neither differed in age (53 (47–60) from the in-house cannulations (54 (48–59) years, p = 0.885), nor in the ICU mortality (46% vs 40.6%, p = 0.602) and the hospital mortality (51.7% vs 53.1, p = 0.892).
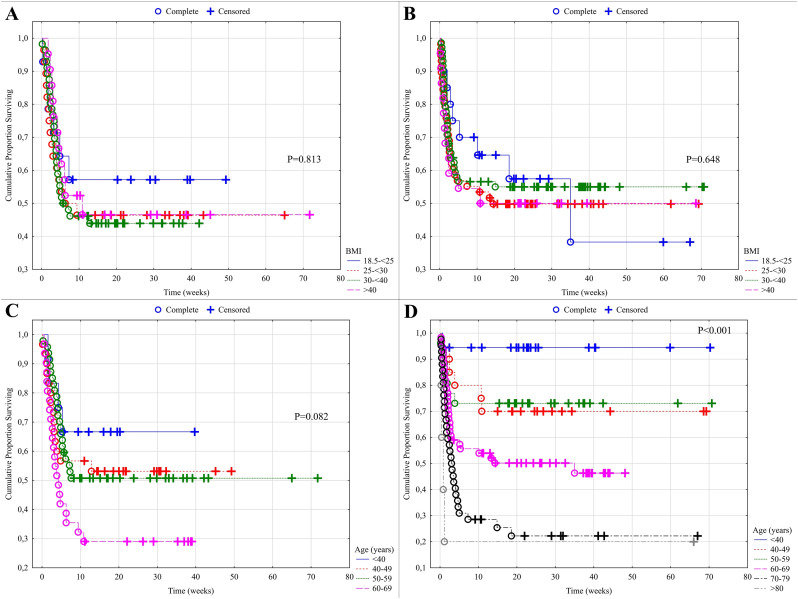
The proportion of ECMO therapy was the highest among the morbidly obese with BMI > 40 (49%), as compared to patients with a lower BMI (43.8% in BMI 30–40, 31.9% in BMI 25–30, Fig. 3A). ICU mortality for patients on ECMO (44.5%, n = 53) was significantly higher than that of patients treated without ECMO (30.1%, n = 52, p = 0.01), yet the hospital mortality was not significantly different (ECMO 52%, n = 62) compared to the group not on ECMO (46.8%, n = 81, p = 0.37)(Fig. 3B,C). ICU mortality of patients on ECMO was highest among the patients with a normal BMI and those who were overweight (BMI 25–30) in comparison to the patients not on ECMO (Fig. 3B, p = 0.013). The differences were not significant with a BMI above 30 (p = 0.268, Fig. 3B). This is confirmed by the following observations. The mortality beyond the 90-days for the ECMO patients (p = 0.813, Fig. 4A, ESM) was not significantly influenced by BMI and the same applied to the patients not on ECMO (p = 0.648, Fig. 4B ESM). 90-day mortality for the non-ECMO patients was significantly influenced by age (p < 0.001, Fig. 4D ESM), while the differences were not significant for the ECMO patients (p = 0.082, Fig. 4C ESM).
3.3. Linear regression analysis
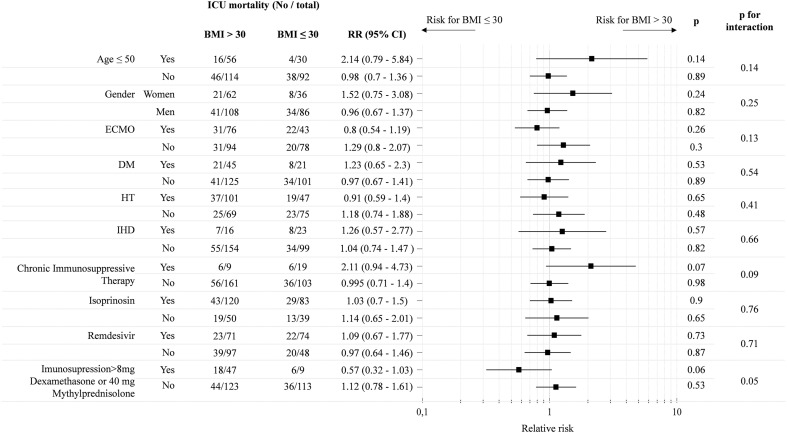
The linear regression (Cox) analysis (Table 4 ) evaluated risk factors for the obese and non-obese subgroups. The favourable impact of age <50 for a severe SARS-CoV-2 ARDS was confirmed, but it was four times less significant for the obese than for the non-obese patients (p = 0.02). Non-obese patients on ECMO had two times worse ICU prognosis than patients who did not require ECMO therapy (p = 0.04) however, the odds ratio was approaching one for the obese, suggesting a potential benefit of ECMO with increasing BMI in the most severe forms of ARDS. The risk of an adverse outcome was more than three times higher in the obese patients with chronic immunosuppression (p = 0.009), and not significantly reduced in patients given remdesivir and isoprinosin. Similarly, as for ECMO therapy, the newly administered higher dose of steroids doubled the risk for an adverse outcome for non-obese patients which did not apply to patients with obesity. Moreover, the potential positive impact of higher dose steroids was further supported by the Mantel-Haenszel estimate of relative risk (RR) of adverse outcome (ICU mortality) comparing obese and non-obese patients (Fig. 5 ). A higher dose of steroids in the later stage of therapy showed reduced RR with a borderline statistical significance (0.57, p = 0.05). The most important risk factors associated with a BMI > 30 in a high volume ECMO centre were age <50, female gender and a history of chronic immunosuppressive therapy.
Table 4.
Cox proportional-hazards regressions for the obese and non-obese patients.
| BMI > 30 (n = 170) |
BMI ≤ 30 (n = 122) |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| age ≤ 50 | 0.59 | 0.31 to 1.12 | 0.11 | 0.28 | 0.09 to 0.81 | 0.02 |
| gender female | 0.96 | 0.56 to 1.66 | 0.89 | 0.6 | 0.26 to 1.39 | 0.24 |
| ECMO | 1.226 | 0.71 to 2.09 | 0.48 | 1.97 | 1.03 to 3.78 | 0.04 |
| DM | 1.63 | 0.92 to 2.9 | 0.09 | 1.01 | 0.43 to 2.38 | 0.98 |
| HT | 0.7 | 0.39 to 1.27 | 0.24 | 1.24 | 0.63 to 2.41 | 0.53 |
| IHD | 1.38 | 0.57 to 3.32 | 0.48 | 0.86 | 0.37 to 2.02 | 0.73 |
| chronic immunosuppressive therapy | 3.3 | 1.35 to 8.11 | 0.009 | 0.79 | 0.3 to 2.09 | 0.63 |
| isoprinosin | 0.88 | 0.51 to 1.54 | 0.67 | 1.26 | 0.64 to 2.5 | 0.5 |
| remdesivir | 0.81 | 0.48 to 1.39 | 0.45 | 0.85 | 0.441to 1.65 | 0.64 |
| imunosupression>8 mg dexamethasone or 40 mg methylprednisolone | 1.03 | 0.59 to 1.8 | 0.92 | 2.05 | 0.78 to 5.38 | 0.14 |
DM diabetes mellitus, HT hypertension, IHD ischemic heart disease.
Table 4: Results of the multivariable Cox analysis separated for BMI > 30 and BMI ≤ 30.
Fig. 5.
The Mantel–Haenszel estimate of relative risk (RR) for adverse outcome (ICU mortality) compared between obese and non-obese patients.
4. Discussion
The research shows that obesity and morbid obesity do not associate with worse short and long-term outcomes of the severe SARS-CoV-2 ARDS when admitted to a high volume ECMO centre. The use of ECMO in the obese patients was not associated with worse outcomes – in contrast to the non-obese patients. Moreover, for the morbidly obese patients with BMI > 40, the study has not confirmed the relative contraindication to ECMO. Their outcome was better than for the overweight patients, likely linked to the highest proportion of patients on ECMO in higher grades of obesity. The study shows that a high-volume ECMO centre admitted an extraordinarily high number of obese and morbidly obese patients during the pandemic.
The ECMO indicators in our study group were in accordance with the established ELSO criteria [28]. Except for the shortened time from PCR positivity to ECMO cannulation of the obese patients, there were no other differences in the timing of the ECLS intervention between the groups. ECMO in the obese patients was not indicated earlier in relation to IPPV or oxygen therapy prior to cannulation, and the proportion of the ECMO retrievals was not different between the obese and non-obese patients. Mechanical ventilation parameters were different with higher PEEP, Paw and Pplat suggesting higher thoracic elastance in the obese group. We cannot confirm better lung compliance and PEEP benefits in obese patients as compared to the non-obese [27]. In obese patients on ECMO the relative benefit could be the consequence of earlier respiratory failure due to altered respiratory mechanics leading to fewer lung parenchymal lesions at the time of ECMO initiation and later to a faster recovery compared to the conservatively treated patients without ECMO [29]. The barotrauma was frequent among the ECMO patients and represented one of the indications for the extracorporeal support, yet we did not register higher rates among the obese patients compared to the non-obese. Considering the limits of IPPV, the rates of an awake ECMO were very low (9 out of 119, 7.6%), which is explained by a specific case mix of prevailing (73%) ECMO retrievals of patients failing therapy of severe ARDS in local hospitals. An established system is based on a 24/7 presence of qualified intensive care team, which receives requests for consultations including internet transmissions of patient's imaging and laboratory results. Many referrals are improved by remote consultations and guidance however, approximately one third of annual referrals to ECMO require an urgent travel to a local hospital and cannulation on site. Centralisation of care to high-volume ECMO centres meets the requirements for a minimum annual number of ECMO cases per centre [30] and is supported by the payment-per-diagnosis from the insurance system. The outcome data for the retrievals do not significantly differ from the in-house cannulations. A comparison to the systems with a proportional reimbursement for care not regulated by medical societies and medical insurance [31] has been demonstrated particularly during the pandemic of SARS-CoV-2 [32,33].
Most of the up-to-date published articles on the outcome of severe SARS-CoV-2-ARDS ECMO and non-ECMO patients have found similar mortality rates between 31% and 51% [21,34,35], however, the novelty of our paper is in assessing the outcome beyond 90 days and tracking of the patients until discharged home from the local hospitals, which was carried out for the two years of the pandemic. In the established ECMO centres, the survival rates of patients with severe ARDS related to Covid-19 are close to those reported in the EOLIA trial [36] and similar to those of patients with severe ARDS supported by ECMO for influenza [37]. The authors also found different outcomes for a time period until June 2021 and after, until March 2022, with better outcomes for the later stage (ICU mortality 29.6%, hospital mortality 42.3%). This was likely due to the presence of multidrug resistant bacteria, fungi and viral superinfections (pan-drug resistant acinetobacter baumannii n = 29; pan-drug resistant klebsiella pneumoniae n = 9, resistant pseudomonas aeruginosa n = 6, vancomycin resistant enterococcus n = 25, aspergillus species n = 27, MRSA n = 3, herpes simplex n = 27, cytomegalovirus n = 4) complicating severe SARS-CoV-2 ARDS and the incidence of septic shock [38] in the obese and non-obese groups shown in Table 3. (overall 61.3%, 65% until June 2021 and 46.5% for the later stage). The monoclonal antibodies were administered since the second half of 2021 to the seronegative patients and might have also improved the outcomes [39].
In addition to ECMO, another factor that could have improved the outcome was the newly administered higher dose of steroids. The meta-analysis on steroid administration demonstrated its benefit in a severe Covid-19 [40], yet their timing and exact dosage are matter of debate [41,42]. In our setting, a higher dose of steroids, e.g. 4–5 mg/kg of methylprednisolone was applied and de-escalated to 1 mg/kg.day for up to one week in the late phase of ARDS and, after excluding superinfection. The authors tend to personalize the therapy with corticosteroids avoiding an early administration of the low-dosage (e.g. 6 mg dexamethasone/day) on everyone [43]. In contrast to the newly administered steroids the study confirms an adverse effect of previous chronic immunosuppressive therapy including steroid administration. Regarding other drugs, we did not see a significant impact on outcome of a virostatic remdesivir or a lymphocyte stimulation with isoprinosine. The rates of anti-inflammatory therapy with tocilizumab and baricitinib were low largely due to absence of the so called “cytokine storm” which fits into the published data [[44], [45], [46]]. Another reason for a restrained policy with anti-interleukin therapies was the high incidence of bacterial, fungal, and viral superinfections of ARDS patients mainly imported from various ICUs across the country.
The presented cohort of severe ARDS confirms the prognostic importance of age across the BMI groups however, with a growing BMI, the favourable impact of young age is surpassed by obesity. The result confirms the output of large databases showing a risk of death related to obesity particularly for patients under the age of 40–50 years [9,47].
Two thirds of the patients with severe ARDS were males, nonetheless, the results point towards a non-negligible risk of adverse outcome for obese women. High BMI requires high blood flow in severe oxygenation failure and the small size vessels especially in obese women may limit the cannulation strategy, which is predominantly double site wide bore cannulas rather than a single site access allowing only for lower blood flows [21,28].
Regarding already reported risk factors associated with obesity, we may confirm the increased rates of renal replacement therapy among the obese patients [15,47]. Ventilator induced acute on chronic cor pulmonale in obese patients with severe ARDS may lead to hemodynamic instability, cardiorenal syndrome and a need for renal replacement therapy.
In contrast to the published data [15,47], our rates of deep venous thrombosis and pulmonary embolism were not different between the obese and non-obese patients which may be related to moderate therapeutic anticoagulation already administered to all mechanically ventilated patients from the beginning of the pandemic.
This study has several limitations. First, it is a monocentric retrospective study, making it difficult for our results to be extrapolated to other ICUs with less ECLS experience. Eventual power analysis should stem from the existing dataset and may serve as a guidance for a prospective research verifying author's conclusions. Second, our cohort has a relatively low number of patients, which leads to difficulties in the interpretation of univariate and multivariate analyses, especially with an input of multiple therapeutic interventions. Small samples can lead to lack of power and some risk factors could have been missed or under-estimated. Regarding an impact of age, the authors indicated ECMO in a limited number of patients older than 60 years. This numerical disproportion might have influenced the statistical significance (Fig. 4C ESM). Third, our study included a vast majority of white Caucasian patients (288 out of all 292 patients) who may show different outcomes when compared, for example, to obese black Americans [48]. Fourth, unless a deep analysis within the morbidly obese patients group (BMI > 40) is performed, we cannot confirm the “J” shape of the obesity paradox that is demonstrated in our study, compared to the proposed “U” shape with worse outcomes in the morbidly obese cohort [16,17].
5. Conclusions
In this monocentric cohort of patients with severe COVID-19 ARDS, the very high incidence of obesity in a high-volume ECMO centre was not associated with worse short and long-term outcomes as compared to non-obese patients. ECMO in obese patients together with the use of steroids in higher doses at later stage of ARDS may be associated with better survival than expected.
Author statement
Balik M: design, conceptualization, methodology, data collection, formal analysis, writing and editing, supervision, funding acquisition; Svobodova E: design, conceptualization, methodology, data curation, writing; Porizka M: design, methodology, formal analysis, data curation, writing; Maly M: data collection; Brestovansky P: data collection; Volny L: data collection; Brozek T: data collection; Bartosova T: data collection; Jurisinova I: data collection, methodology; Mevaldova Z: data collection; Misovic O: data collection; Novotny A: data collection; Horejsek J: data collection; Otahal M: data collection; Flaksa M: data collection; Stach Z: data collection; Rulisek J: data collection; Trachta P: data collection; Kolman J: data collection; Sachl R: data collection; Kunstyr J: data collection; Kopecky P: data collection; Romaniv S: data collection; Huptych M: methodology, data curation, validation, formal analysis; Svarc M: data collection; Hodkova G: data collection; Fichtl J: data collection; Mlejnsky F: data collection; Grus T: data collection; Belohlavek J: data collection, methodology, validation; Lips M: data collection; Blaha J: methodology, supervision.
Financial disclosure statement
The research was supported in part by the grant from the Czech Ministry of Health NU-22-B-147.
The following is the supplementary data related to this article.
Supplementary Fig. 4.
(ESM): Impact of body mass index on the long-term outcome among ECMO patients (4A, p = 0.813) and the non-ECMO patients (4B, p = 0.648). Impact of age on the long-term outcome in the ECMO patients is shown in 4C (p = 0.082) and among the non-ECMO patients in 4D (p < 0.001), all Kaplan-Meier curves with log rank test.
Conflict of interest
None.
References
- 1.Schetz M., De Jong A., Deane A.M., Druml W., Hemelaar P., Pelosi P., et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 2.Centre ICNAR ICNARC registry. 2021. https://wwwicnarcorg/Our-Audit/Audits/Cmp/Reports
- 3.Chiumello D., Pozzi T., Storti E., Caccioppola A., Pontiroli A.E., Coppola S. Body mass index and acute respiratory distress severity in patients with and without SARS-CoV-2 infection. Br J Anaesth. 2020;125(4) doi: 10.1016/j.bja.2020.07.006. e376-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legrand M. Complications in patients with COVID-19. JAMA Cardiol. 2021;6(3):359. doi: 10.1001/jamacardio.2020.5788. [DOI] [PubMed] [Google Scholar]
- 5.Madjid M., Solomon S.D., Vardeny O. Complications in patients with COVID-19-reply. JAMA Cardiol. 2021;6(3):360. doi: 10.1001/jamacardio.2020.5794. [DOI] [PubMed] [Google Scholar]
- 6.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 7.Rottoli M., Bernante P., Belvedere A., Balsamo F., Garelli S., Giannella M., et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian Centre. Eur J Endocrinol. 2020;183(4):389–397. doi: 10.1530/EJE-20-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim T.S., Roslin M., Wang J.J., Kane J., Hirsch J.S., Kim E.J. BMI as a risk factor for clinical outcomes in patients hospitalized with COVID-19 in New York. Obesity (Silver Spring, Md) 2021;29(2):279–284. doi: 10.1002/oby.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao M., Piernas C., Astbury N.M., Hippisley-Cox J., O’Rahilly S., Aveyard P., et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes & Endocrinol. 2021;9(6):350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson M.R., Shashaty M.G.S. Impact of obesity in critical illness. Chest. 2021;160(6):2135–2145. doi: 10.1016/j.chest.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Jong A., Wrigge H., Hedenstierna G., Gattinoni L., Chiumello D., Frat J.P., et al. How to ventilate obese patients in the ICU. Intensive Care Med. 2020;46(12):2423–2435. doi: 10.1007/s00134-020-06286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh R., Garcia M.A., Rajendran I., Johnson S., Mesfin N., Weinberg J., et al. ICU outcomes in Covid-19 patients with obesity. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620971146. 1753466620971146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jose R.J., Manuel A. Does coronavirus disease 2019 disprove the obesity paradox in acute respiratory distress syndrome? Obesity (Silver Spring, Md) 2020;28(6):1007. doi: 10.1002/oby.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buetti N., Souweine B., Mermel L., Mimoz O., Ruckly S., Loiodice A., et al. Obesity and risk of catheter-related infections in the ICU. A post hoc analysis of four large randomized controlled trials. Intensive Care Med. 2021;47(4):435–443. doi: 10.1007/s00134-020-06336-4. [DOI] [PubMed] [Google Scholar]
- 15.Lemyze M., Courageux N., Maladobry T., Arumadura C., Pauquet P., Orfi A., et al. Implications of obesity for the management of severe coronavirus disease 2019 pneumonia. Crit Care Med. 2020;48(9) doi: 10.1097/CCM.0000000000004455. e761-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dana R., Bannay A., Bourst P., Ziegler C., Losser M.R., Gibot S., et al. Obesity and mortality in critically ill COVID-19 patients with respiratory failure. Int J Obes (Lond) 2005;45(9):2028–2037. doi: 10.1038/s41366-021-00872-9. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutagalung R., Marques J., Kobylka K., Zeidan M., Kabisch B., Brunkhorst F., et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011;37(11):1793–1799. doi: 10.1007/s00134-011-2321-2. [DOI] [PubMed] [Google Scholar]
- 19.Badulak J., Antonini M.V., Stead C.M., Shekerdemian L., Raman L., Paden M.L., et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 2021;67(5):485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Organisation ELS ELSO guidelines. 2020. https://wwwelsoorg/ecmo-resources/elso-ecmo-guidelinesaspx
- 21.Lebreton G., Schmidt M., Ponnaiah M., Folliguet T., Para M., Guihaire J., et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in greater Paris, France: a multicentre cohort study. The lancet. Respir Med. 2021;9(8):851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merritt-Genore H., Lyden E., Ryan T., Kwapnoski Z. The effect of patient obesity on extracorporeal membrane oxygenator outcomes and ventilator dependency. J Card Surg. 2020;35(6):1283–1286. doi: 10.1111/jocs.14579. [DOI] [PubMed] [Google Scholar]
- 23.Galvagno S.M., Jr., Pelekhaty S., Cornachione C.R., Deatrick K.B., Mazzeffi M.A., Scalea T.M., et al. Does weight matter? Outcomes in adult patients on venovenous extracorporeal membrane oxygenation when stratified by obesity class. Anesth Analg. 2020;131(3):754–761. doi: 10.1213/ANE.0000000000004454. [DOI] [PubMed] [Google Scholar]
- 24.Kon Z.N., Dahi S., Evans C.F., Byrnes K.A., Bittle G.J., Wehman B., et al. Class III obesity is not a contraindication to venovenous extracorporeal membrane oxygenation support. Ann Thorac Surg. 2015;100(5):1855–1860. doi: 10.1016/j.athoracsur.2015.05.072. [DOI] [PubMed] [Google Scholar]
- 25.Zaidi S.A.A., Saleem K. Obesity as a risk factor for failure to wean from ECMO: a systematic review and meta-analysis. Can Respir J. 2021;2021:9967357. doi: 10.1155/2021/9967357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho W.H., Oh J.Y., Yeo H.J., Han J., Kim J., Hong S.B., et al. Obesity survival paradox in pneumonia supported with extracorporeal membrane oxygenation: analysis of the national registry. J Crit Care. 2018;48:453–457. doi: 10.1016/j.jcrc.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Daviet F., Guilloux P., Hraiech S., Tonon D., Velly L., Bourenne J., et al. Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort. Ann Intensive Care. 2021;11(1):157. doi: 10.1186/s13613-021-00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonna J.E., Abrams D., Brodie D., Greenwood J.C., Rubio Mateo-Sidron J.A., Usman A., et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the extracorporeal life support organization (ELSO) ASAIO J. 2021;67(6):601–610. doi: 10.1097/MAT.0000000000001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vera M., Kattan E., Born P., Rivas E., Amthauer M., Nesvadba A., et al. Intubation timing as determinant of outcome in patients with acute respiratory distress syndrome by SARS-CoV-2 infection. J Crit Care. 2021;65:164–169. doi: 10.1016/j.jcrc.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combes A., Brodie D., Bartlett R., Brochard L., Brower R., Conrad S., et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 31.Bercker S., Petroff D., Polze N., Karagianidis C., Bein T., Laudi S., et al. ECMO use in Germany: an analysis of 29,929 ECMO runs. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0260324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karagiannidis C., Slutsky A.S., Bein T., Windisch W., Weber-Carstens S., Brodie D. Complete countrywide mortality in COVID patients receiving ECMO in Germany throughout the first three waves of the pandemic. Crit Care. 2021;25(1):413. doi: 10.1186/s13054-021-03831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann J., Lotz C., Karagiannidis C., Weber-Carstens S., Kluge S., Putensen C., et al. Key characteristics impacting survival of COVID-19 extracorporeal membrane oxygenation. Crit Care. 2022;26(1):190. doi: 10.1186/s13054-022-04053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt M., Hajage D., Lebreton G., Monsel A., Voiriot G., Levy D., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbaro R.P., MacLaren G., Boonstra P.S., Combes A., Agerstrand C., Annich G., et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international extracorporeal life support organization registry. Lancet (London, England) 2021;398(10307):1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Combes A., Hajage D., Capellier G., Demoule A., Lavoué S., Guervilly C., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 37.Zangrillo A., Biondi-Zoccai G., Landoni G., Frati G., Patroniti N., Pesenti A., et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17(1):R30. doi: 10.1186/cc12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet (London, England) 2022;399(10325):665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhuri D., Sasaki K., Karkar A., Sharif S., Lewis K., Mammen M.J., et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47(5):521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munch M.W., Myatra S.N., Vijayaraghavan B.K.T., Saseedharan S., Benfield T., Wahlin R.R., et al. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. Jama. 2021;326(18):1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maláska J., Stašek J., Duška F., Balík M., Máca J., Hruda J., et al. Effect of dexamethasone in patients with ARDS and COVID-19 - prospective, multi-centre, open-label, parallel-group, randomised controlled trial (REMED trial): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22(1):172. doi: 10.1186/s13063-021-05116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres A., Motos A., Cillóniz C., Ceccato A., Fernández-Barat L., Gabarrús A., et al. Major candidate variables to guide personalised treatment with steroids in critically ill patients with COVID-19: CIBERESUCICOVID study. Intensive Care Med. 2022;48(7):850–864. doi: 10.1007/s00134-022-06726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 45.Kox M., Waalders N.J.B., Kooistra E.J., Gerretsen J., Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. Jama. 2020;324(15):1565–1567. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hendren N.S., de Lemos J.A., Ayers C., Das S.R., Rao A., Carter S., et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 cardiovascular disease registry. Circulation. 2021;143(2):135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 48.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]