Abstract
Idiopathic multicentric Castleman disease (iMCD) is a rare immunologic disorder characterized by systemic inflammation, multicentric lymphadenopathy, and organ dysfunction. Enlarged lymph nodes demonstrate a spectrum of characteristic but variable histopathologic features historically categorized into hyaline vascular (HV) (or hypervascular (HyperV) more recently), plasmacytic, or “mixed.” Though the etiology is unknown, a pro-inflammatory cytokine storm, often involving interleukin-6 (IL-6), contributes to pathogenesis. Anti-IL-6 therapy with siltuximab is the only FDA- or EMA-approved treatment based on efficacy and safety in multiple studies. Importantly, no patients considered to have HV histopathology achieved the primary endpoint in the Phase II study. NCCN currently recommends siltuximab first-line for iMCD except for patients considered to have HV histopathology. We investigated whether histopathologic subtype should guide siltuximab treatment decisions. Secondary analyses of clinical trial and real-world data revealed similar clinical benefit across histopathologic subtypes. Notably, only 18/79 patients in the Phase II study were consistently classified into histopathologic subtype by three independent review panels, demonstrating limited reliability to guide treatment decisions. Real-world data further demonstrate siltuximab’s effectiveness in patients considered to have HV (or HyperV). Though histopathology is a critical component for diagnosis, there is insufficient evidence to guide treatment based solely on lymph node histopathologic subtype.
Keywords: Regular Article, Idiopathic Multicentric Castleman Disease, Histopathology, Anti-IL6 Therapy
Introduction
Multicentric Castleman disease (MCD) is a multisystem immunologic disorder characterized by systemic inflammation, cytopenias, multicentric lymphadenopathy, and organ dysfunction. MCD can be caused by uncontrolled human herpesvirus-8 (HHV-8) infection, often in immunocompromised individuals. This variant of MCD is commonly referred to as HHV-8-associated MCD. MCD can also occur in the setting of POEMS syndrome, which is known as POEMS-associated MCD. Approximately 50% of MCD cases occur without HHV-8 infection or POEMS syndrome, and the etiology is unknown.1 In these idiopathic MCD (iMCD) cases, the specific etiology is the subject of active research and a recent study failed to identify a causative viral etiology.2
Enlarged lymph nodes in iMCD demonstrate a spectrum of characteristic but variable histopathologic features, including atrophic germinal centers, expanded mantle zones, hypervascularization, and interfollicular plasmacytosis.1 Patients demonstrating a continuous spectrum of these features without clear divisions have been historically categorized into hyaline vascular (HV) on one end and plasmacytic (PC) on the other with a “mixed” histopathologic subgroup in between. In 2017, we established international, consensus diagnostic criteria for iMCD that introduced a few key changes.3 First, since HV histopathology is more frequently reported to occur in unicentric Castleman disease and the overlapping histopathology observed in iMCD has even more pronounced vascularization4, we recommended using hypervascular (HyperV) instead of HV when referring to these features in the setting of iMCD. Second, due to the sometimes-subjective nature of the histologic features and the varying degrees of tissue involvement, it is currently challenging to reproducibly classify these subtypes resulting in discrepancies even among expert pathologists, and the clinical implications of this classification are unclear. Therefore, we recommended using histopathologic features for diagnosing iMCD, but we de-emphasized the importance of determining where on the spectrum cases may lie from HyperV to PC. Third, we recommended transitioning from categorizing patients by histopathologic subgroups towards subclassifying iMCD into two clinicopathologic subgroups3: iMCD-TAFRO (defined by thrombocytopenia [T], anasarca [A], fever [F], reticulin fibrosis [R], and organomegaly [O]) and iMCD-NOS (not otherwise specified, typically have thrombocytosis and hypergammaglobulinemia).5,6 iMCD-TAFRO cases are more acutely ill, often demonstrate HyperV or mixed histopathology, and have an inferior 2-year overall survival.7,8 In our experience, iMCD patients classified as having HyperV or HV histopathology typically present as either the most acutely ill iMCD-TAFRO cases or with a milder iMCD-NOS clinical phenotype.
Though the etiology of iMCD is currently unknown, a pro-inflammatory cytokine storm, most frequently including interleukin-6 (IL-6), is recognized as an important contributor to disease pathogenesis.1 The anti-IL-6 monoclonal antibody, siltuximab, became the first FDA- and EMA-approved treatment for iMCD in 2014 based on a durable radiologic and symptomatic response in 18/53 (34%) siltuximab-treated patients compared to 0/26 placebo-treated controls in a Phase II randomized controlled trial.9 Based on efficacy and safety data from the only randomized controlled trial performed in iMCD as well as a Phase I open-label study10, the Castleman Disease Collaborative Network (CDCN) published guidelines in 2018 recommending siltuximab as first-line therapy for iMCD.11 Tocilizumab, which neutralizes the IL-6 receptor, was approved for treating iMCD in Japan based upon an open-label study.12 It is also used off-label around the world. The anti-IL-6 targeted therapies siltuximab and tocilizumab remain the only approved therapies worldwide for iMCD. However, not all iMCD patients respond to anti-IL-6 therapy with siltuximab or tocilizumab and attempts to further define optimal target populations are important priorities.
The National Comprehensive Cancer Network (NCCN) recently issued guidance recommending siltuximab first-line for iMCD except for patients with HV lymph node histopathology.13 Recognizing that the uniform diagnostic criteria terminology from 2017 have not been uniformly adopted by guiding bodies and clinicians, we expect this to have implications for patients characterized as having HV (under the old schema) or HyperV (under the new schema) histopathological subtype. This decision was based on data from the pivotal siltuximab Phase II randomized controlled trial, in which none of the patients who achieved the primary endpoint of a durable tumor and symptomatic response to siltuximab were classified as having HV histopathology by central review; instead, all responders had PC or mixed histopathology.9 As both published and unpublished data from other primary and secondary endpoints in the Phase I and II studies, long-term extension studies, expert experience, and real-world data conflicted with this particular result, the CDCN consensus treatment guidelines did not recommend the use of histopathologic subtyping to guide therapeutic decisions. Patients considered to have HV histopathology were deliberately included as part of the recommendation for siltuximab first-line for all iMCD patients. These guidelines were developed by a working group comprising 42 experts from 10 countries based on data from 344 cases.11 Further, none of the existing approvals for siltuximab or tocilizumab has been limited to certain histopathologic or clinical subtypes of iMCD. Herein, we asked whether iMCD patients with each histopathologic subtype benefit from siltuximab and whether histopathology alone should be used to guide treatment decisions.
Methods
Clinical Trial Data
Secondary analyses of data from the Phase I, Phase II, and long-term safety study of siltuximab are presented in this manuscript.9,10,14 Specifically, we calculated the number of patients considered to have HV histopathology in the Phase I and II studies that went on to the long-term safety study, proportion of patients who achieved clinical benefit response (CBR) at their last assessment, overall response at last evaluation, median time on study drug, and number of administrations from the study data files. See Supplementary Table 1 for response criteria from Phase I and II studies.
Among siltuximab-treated patients in the Phase II study, we also calculated median C-reactive protein (CRP) levels, a biomarker of disease activity, number of iMCD clinical and laboratory diagnostic criteria met, and general MCD-related signs and symptoms score between patients assigned HV, PC, and mixed histopathology. Mann-Whitney U test was used to compare baseline CRP and diagnostic criteria between HV and non-HV. A negative binomial model was used to compare MCD-related signs-and-symptoms score between HV and non-HV. Alpha=0.05. Data from the supplementary materials from the Phase II study were included in this manuscript.9 The histopathologic subtype according to local site, central pathology, and CDCN expert panel review were compared across all 79 patients in the Phase II study from study data files and Fajgenbaum et al, 2017.
Histopathologic Subtype Assignment
All 79 cases in the Phase II siltuximab clinical trial were previously reviewed by the local site pathologist (local site) and central pathology (central review) for the Phase II siltuximab trial,9 as well as by a CDCN expert panel (CDCN panel). Review by the local site pathologist had been initially performed on H&E stained lymph node tissue to determine if the case appeared to be consistent with Castleman disease. Central pathology review had been performed by a group of academic, tertiary care, board-certified hematopathologists (including author, DW) whose practice is limited to hematopathology to determine if patients met inclusion criteria for the study and to assign histopathologic subtype. Agreement between at least two of three reviewers was required for trial enrolment.9 Finally, a CDCN expert panel of four academic, tertiary care, board-certified hematopathologists (including authors, ML, AB) re-reviewed every case as part of an effort to establish the histopathologic features to be included in the diagnostic criteria for iMCD.3 An expanded panel assembled by the CDCN of academic, tertiary care, board-certified hematopathologists reviewed cases for which there was disagreement among the four-member panel. The comparison of the histopathologic subtype assignments of each individual case by local site, central review, and CDCN expert panel is new and reported for the first time in this study.
Real-World Data
A search of PubMed was performed for “TAFRO AND siltuximab,” “TAFRO AND tocilizumab,” and “TAFRO” (March 2, 2020) to identify potential iMCD-TAFRO cases treated with anti-IL-6 therapy. Each case was reviewed to identify cases reported to have HyperV or HV histopathologic subtype. Data were abstracted on treatments, author-assessed clinical response, and if relapse occurred before publication.
All cases of iMCD treated at the University of Arkansas for Medical Sciences (UAMS) were evaluated to identify iMCD patients classified as having HyperV or HV histopathology who were also treated with siltuximab or tocilizumab. Data were abstracted on the treatments used, the clinical response, and if the patient relapsed while on treatment by the time of publication. All these patients met the diagnostic criteria for iMCD as stipulated by the CDCN and their pathology slides were reviewed by a member of the expert panel.
Results
iMCD patients considered to have HV histopathology benefitted from siltuximab in the Phase I, Phase II, and long-term extension trials
The Phase I trial included 34 iMCD patients; 16 were classified as having HV histopathologic subtype.10 31% (5/16) of iMCD patients considered to have HV histopathology met radiologic response criteria and 88% (14/16) achieved a CBR, a score summarizing symptomatic and biochemical response criteria (Table 1). These response rates were similar to those seen for iMCD patients classified as having PC histopathology where 35% (6/17) of patients met radiologic response criteria and 88% (15/17) achieved a CBR. 10/16 (63%) patients considered to have HV histopathology from the Phase I study transitioned to the long-term safety study,15 and 90% (9/10) of these patients maintained CBR (8/10, 80% complete responses) at their last assessment. 9/17 (53%) patients in the Phase I study considered to have PC histopathology went on to the long-term safety study, and 6/9 (67%) of these patients maintained CBR (6/9, 67% complete responses). In total, patients considered to have HV histopathology that went on the long-term study received a median of 121.5 administrations for a median duration of 8.3 years (Table 2).
Table 1.
Comparison of response to siltuximab by patient histopathologic subtype in Phase I trial
| Hyaline vascular (N=16) |
Plasmacytic (N=17) |
Mixed (N=2) |
|
|---|---|---|---|
| Best overall radiologic response (N, %)* | |||
| Not Evaluable | 0 | 1 (6%) | 0 |
| Progressive Disease (PD) | 0 | 1 (6%) | 0 |
| Stable Disease (SD) | 10 (63%) | 7 (41%) | 1 (50%) |
| Unconfirmed Partial Response (uPR) | 1 (7%) | 2 (12%) | 0 |
| Partial Response (PR) | 5 (31%) | 5 (29%) | 1 (50%) |
| Complete Response (CR) | 0 | 1 (6%) | 0 |
| Clinical benefit response (CBR) (N, %) † | |||
| No improvement or worsening of any components | 2 (13%) | 2 (12%) | 0 |
| Improvement in ≥1 component | 14 (88%) | 15 (88%) | 2 (100%) |
| Improvement in ≥2 components | 13 (81%) | 13 (76%) | 2 (100%) |
| Improvement in ≥3 components | 11 (69%) | 9 (53%) | 1 (50%) |
| Overall response at last evaluation (N, %) †† | |||
| Not Evaluable | 1 (6%) | 1 (6%) | 0 |
| Progression | 0 | 3 (18%) | 1 (50%) |
| Stable | 4 (25%) | 0 | 0 |
| Responder | 11 (69%) | 13 (76%) | 1 (50%) |
Best overall radiologic response was evaluated using Cheson criteria (Cheson et al, 1999), modified to include the assessment of measurable cutaneous lesions.
Clinical benefit response (CBR) is defined as improvement in any number of the following components: hemoglobin, fatigue, anorexia, fever, weight and size of largest lymph node (CT or physical examination) and/or cutaneous disease, with no worsening of other components.
Overall response at last evaluation: responder was defined as patient who demonstrated an improvement, compared to baseline, in at least one of anorexia, fatigue, hemoglobin, fever, weight, and size of largest lymph node at the time of final study evaluation. Not Evaluable was chosen if any one of the 6 criteria were not available at the time of final study evaluation. Progression was chosen if any one of the 6 criteria has worsened at the time of final study evaluation compared to baseline. Stable was chosen if there was no worsening or improvement in any of the 6 criteria at the time of final study evaluation compared to baseline. Data for this table were obtained from study files.
Table 2:
Follow up in the long-term safety study of siltuximab-treated iMCD patients from the Phase I and Phase II studies of siltuximab
| Hyaline vascular (HV) | Plasmacytic (PC) |
Mixed | |
|---|---|---|---|
| Patients in Phase I study that went on to the long-term safety study | 10/16 (63%) | 9/17 (53%) | 0/2 (0%) |
| Patients in Phase I study maintaining CBR at the last assessment of the long-term safety study | 8/10 (80%) | 6/9 (67%) | N/A |
| Median (range) administrations of siltuximab from beginning of Phase I study through long-term safety study | 121.5 (85–184) | 120 (87–197) | N/A |
| Median (range) duration of siltuximab therapy among Phase I patients on the long-term safety study, years | 8.3 (5.1–10.6) | 8.5 (5.1–10.8) | N/A |
| Siltuximab-treated patients in Phase II study that went on to the long-term safety study* | 6/18 (33%) | 19/22 (86%) | 13/13 (100%) |
| Patients in Phase II study maintaining durable stable response at the last assessment of the long-term safety study** | 5/5 (100%) | 20/21 (95%) | 14/15 (93%) |
| Median (range) administrations of siltuximab from beginning of Phase II study through long-term safety study | 58 (50–108) | 64 (12–119) | 74 (34–110) |
| Median (range) duration of siltuximab therapy among Phase II patients on the long-term safety study, years | 4.8 (2.8–6.1) | 4.9 (0.8–6.7) | 4.9 (1.9–6.2) |
HV, hyaline vascular; PC, plasmacytic; CBR, clinical benefit response
The values in the denominators of this row add up to 38 rather than 42 (total number in the long-term safety study), because 4 patients from the placebo arm of the Phase II study went on to the long-term safety study.
The values in the denominators of this row do not match the values in the numerators of the row above, because 1 patient considered to have HV histopathology in the Phase I study that went on to the long-term safety study failed screening, 2 patients in the placebo arm of the Phase II study considered to have PC histopathology went on to the long-term safety study, and 2 patients in the placebo arm of the Phase II study considered to have mixed histopathology went on to the long-term safety study.
The Phase II randomized, placebo-controlled trial of siltuximab included 79 patients, 26 of which were classified as having HV histopathology as assessed by central review.9 None of the patients considered to have HV histopathology met the criteria for durable combined radiologic (by modified Cheson criteria according to central independent review) and symptomatic (by investigator-assessed disease symptoms) response to siltuximab, as defined by the study. However, further review of the data in the supplement to van Rhee et al 2014 suggest siltuximab activity in a relevant number of these patients. In fact, 6/18 (33%) of patients considered to have HV histopathology treated with siltuximab achieved a durable symptomatic response (3/18, 17% complete durable symptomatic response) compared to 1/8 (13%) of placebo-treated patients considered to have HV histopathology achieving a durable symptomatic response (0/8, 0% complete durable symptomatic response). Furthermore, 3/18 (17%) patients considered to have HV histopathology met criteria for durable combined radiologic (modified Cheson criteria, according to investigator-assessment) and symptomatic response by investigator-assessment (versus 0/8 placebo-treated patients). 4/18 (22%) patients considered to have HV histopathology achieved a radiologic response by investigator-assessment (versus 0/8 placebo-treated patients). Median time to treatment failure for patients considered to have HV histopathology was nearly three-times longer for siltuximab-treated patients (206 days) than placebo (70 days).9 6/18 (33%) siltuximab-treated individuals considered to have HV histopathology from the Phase II trial continued into the long-term safety study;14 one failed screening, but the remaining five showed durable stable disease control at their last on-study assessment (median number of siltuximab administrations from the start of Phase II study: 58; median duration of treatment: 4.8 years) (Table 2). Remaining on study drug without initiating another treatment for this extended duration suggests that these patients experienced a clinical and quality of life benefit, which may have also led to broader benefits such as decreased healthcare utilization.
Differences between iMCD patients considered to have HV, PC, and mixed histopathology
The Phase II study’s inclusion and exclusion criteria may have contributed to differences between the phenotypes of patients with various histopathology patterns. Only patients with Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, platelet count >75,000/μL, and laboratory values not observed in critically ill patients were eligible for the study. Thus, all iMCD patients with the more aggressive iMCD-TAFRO clinico-pathologic subtype, who often have HyperV (or HV) histopathology, would have been excluded. In fact, iMCD patients considered to have HV histopathology in the Phase II study trended towards having lower median number of abnormal iMCD clinical and laboratory diagnostic criteria and significantly lower MCD-related signs-and-symptoms scores at baseline compared with non-HV cases (Supplementary Table 2). We have previously shown that patients with greater clinical disease burden and more abnormal laboratory tests tend to have an increased likelihood of response to siltuximab.3,16 Therefore, a subgroup with lower disease activity would be expected to have a lower response rate.
Histopathologic subtypes inconsistently selected between hematopathologists
Based on anecdotal reports of inconsistency between hematopathologists in determining histopathologic subtypes, we investigated subgroup assignment in the Phase II study. Hematopathologists at the local study sites for the Phase II trial classified patients into PC, HV, or mixed histopathologic subtypes based on review of lymph node tissue. All 79 cases included in the Phase II trial were also independently reviewed and assigned a histopathologic subtype by central review by at least three board-certified hematopathologists at the University of Washington, Seattle.9 In 2015–2016, an expert panel assembled by the CDCN, including four hematopathologists that reviewed every case and additional panelists that reviewed cases for which there were disagreements, re-reviewed all 79 cases as part of the development of the consensus diagnostic guidelines (Supplementary Figure 1, previously published in Blood, is provided for reference).3 Thus all 79 cases were assessed at three different levels. Of note, the local site was unable to determine a histopathologic subtype for 7/79 (9%) cases, and the CDCN panel felt that 14/79 (18%) cases were not consistent with the newly-developed iMCD diagnostic criteria, so no subtype was assigned for those cases. Only 18/79 (23%) patients had the same iMCD histopathologic subtype selected by all three groups of evaluators (Figure 1). This result underscores the challenge faced by pathologists in placing a patient into a histopathologic subgroup when there are overlapping features that can be seen across the entire spectrum and the difficulty of using a single reviewer’s classification to guide treatment decisions.
Figure 1: Histopathologic subtype selected by local site pathologist (local site), central pathology (central review), and CDCN expert panel (CDCN panel).
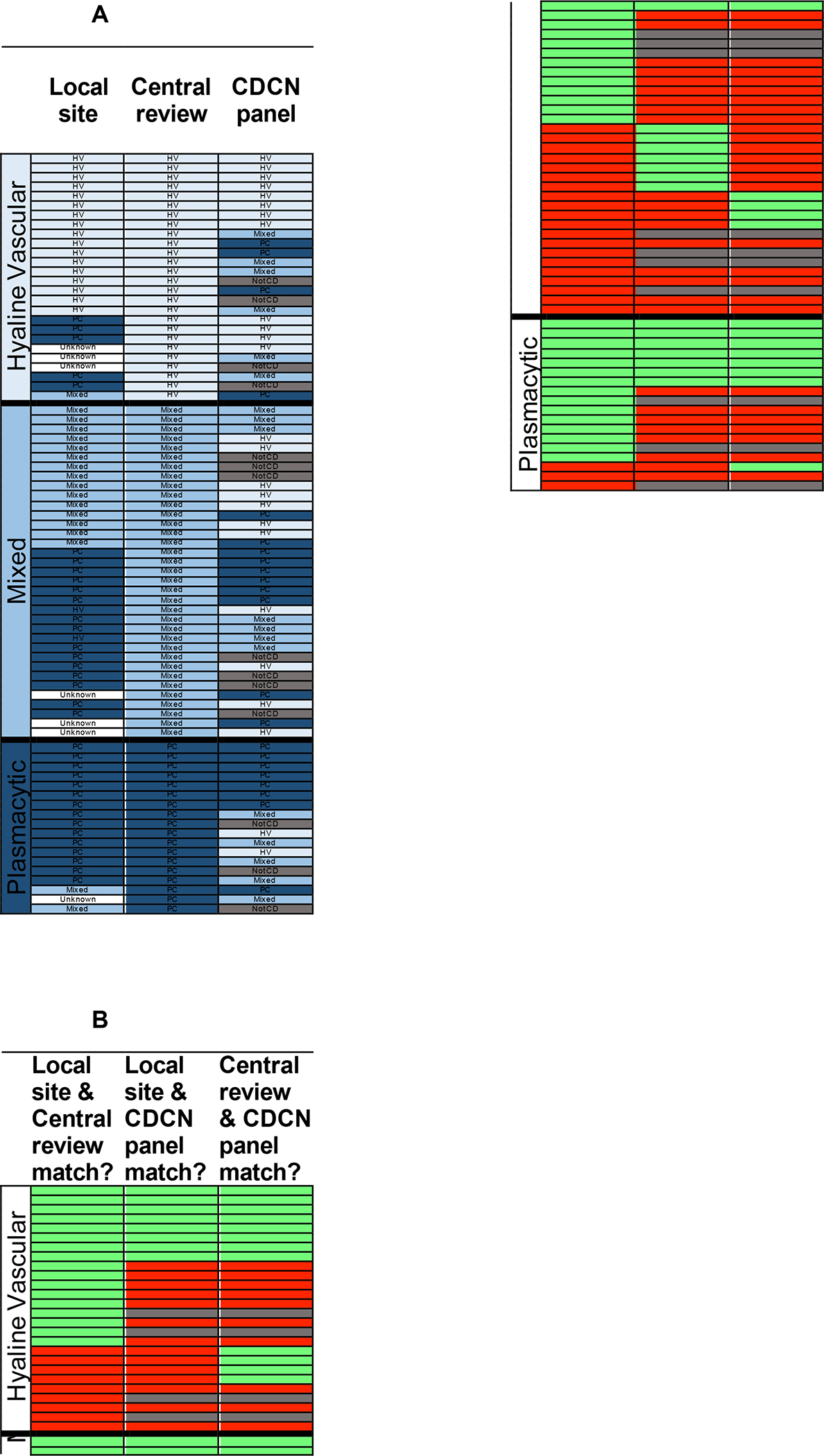
All 79 cases in the Phase II siltuximab clinical trial that were determined to meet all inclusion criteria, including histopathology review, are sorted based on the histopathologic subtype assigned by central pathology. A. The histopathologic subtype assigned by the three groups (columns) are provided for each case (row). Patients are sorted within histopathologic subgroups by descending number of matches between different assessors. Cells are colored according to the following scheme: white (unknown), grey (not CD, Castleman disease), light blue (HV, hyaline vascular), blue (Mixed), and dark blue (PC, plasmacytic). B. Rows in 1A correspond with rows in 1B. Cells are colored according to the following scheme: red (no match), green (match between the two groups in the selection of histopathologic subtype), and grey (not CD). Of note, 14 cases were assessed as ‘not CD’ by the CDCN panel and these cases were considered to not represent a match when compared with either local site or central review.
Real-world data reveal severe patients considered to have HV histopathology responding to anti-IL-6 therapy
Recognizing the limitation of interpreting histopathologic subtype, per above, we reviewed the published literature for iMCD-TAFRO cases with HyperV or HV histopathology that received siltuximab or tocilizumab and identified 18 documented cases. Notably, 14/18 (78%) iMCD-TAFRO patients responded to a combination including anti-IL-6 therapy (siltuximab or tocilizumab), and 9/13 (69%) responded to anti-IL-6 therapy±corticosteroids, according to the case report authors’ assessments; three patients that responded to anti-IL-6±corticosteroids relapsed before the time of publication (Table 3).17–29 We also assessed all of the iMCD-TAFRO and iMCD-NOS cases with HyperV or HV histopathology at UAMS that were treated with anti-IL-6 therapy±corticosteroids. Here, 4/4 iMCD-TAFRO and 7/7 iMCD-NOS patients responded, according to the investigator’s assessment of clinical and laboratory abnormalities. With possible differences in response criteria and clinical management between real-world settings and the published Phase II trial, these data do support a clinically significant response to anti-IL-6 therapy in iMCD patients with HyperV or HV histopathology. The real-world data may even suggest higher response rates in iMCD-TAFRO with HyperV histopathology than in the clinically less aggressive cases considered to have HV histopathology in the Phase II trial.
Table 3:
iMCD patients considered to have HV histopathology treated with anti-IL-6 therapy in the real world
| Published literature* | Clinical subtype | Responses | Total cases | Responders that relapsed on therapy prior to publication |
|---|---|---|---|---|
| Anti-IL-6 ± CS | iMCD-TAFRO | 9 | 13 | 3 |
| Anti-IL-6 + rituximab or cyclosporine ± CS | iMCD-TAFRO | 5 | 5 | 3 |
| UAMS experience | ||||
| Anti-IL-6 ± CS | iMCD-TAFRO | 4 | 4 | 0 |
| Anti-IL-6 ± CS | iMCD-NOS | 7 | 7 | 0 |
CS, corticosteroids; anti-IL-6, anti-interleukin-6 therapy including siltuximab or tocilizumab
Citations: Sakai et al, 2018; Fujiwara et al, 2016; Fajgenbaum et al, 2019; Pai et al, 2020; Smith et al, 2014; Mizuno et al, 2018; Sakashita et al, 2016; Angenendt et al, 2015; Iwaki et al, 2013; José et al, 2017; Behnia et al, 2017; Takayama et al, 2018; Miatech et al, 2019.
Discussion
Overall, there was clinically relevant efficacy of siltuximab among individuals considered to have HV histopathology in the Phase I, Phase II, and long-term extension studies. Activity of anti-IL-6 targeted therapies was further demonstrated in iMCD patients with HyperV or HV histopathology in real-world data. Potential explanations for the lower-than-expected response rate observed in the Phase II study of siltuximab among patients reported to have HV histopathology include recruitment of patients with less aggressive disease, differences in prior therapies, which can be more pronounced in studies with small sample sizes, and the inconsistency with which histopathologic subtype is determined.
It remains unknown whether there is a true biologic difference between patients who demonstrate more HyperV or HV (many atrophic germinal centers, highly increased vascularity) versus more PC (some atrophic and hyperplastic germinal centers, some increased vascularity, many interfollicular plasma cells) versus mixed (combinations of the two) histopathologic features. Our experience with the 79 patients in the phase II trial shows that it is difficult to consistently and reliably separate patients based on these histopathologic features. It also evident that the histopathology of iMCD has a spectrum of features, which can render subtyping challenging even in the hands of skilled hematopathologists. It is possible that certain as-yet-identified histopathological features are strongly associated with differences in biology. Recent research suggests that iMCD can be further segmented into biologically relevant subgroups based on serum proteomics, but further research is needed.30 Therefore, histopathologic subtype alone is a suboptimal criterion from which to make siltuximab treatment decisions. Though not approved for the treatment of iMCD, other drugs used off-label in iMCD appear to demonstrate benefit across histopathologic subtypes as well, further suggesting that histopathology should not be used to guide iMCD treatment.31,32 Though the histopathologic subtype should not guide treatment, histopathology is nevertheless critical to establishing an iMCD diagnosis. These data emphasize the need for histopathologic review by multiple, experienced pathologists to establish an iMCD diagnosis.
There are several limitations to this study. First, these studies include relatively small sample sizes. Though further randomized controlled trials are needed, it is unlikely that they will be performed in this rare disorder. Thus, real-world data from published cases and the ACCELERATE natural history registry provide us with most effective tools for continuing to investigate the effectiveness of anti-IL-6 therapies in iMCD. Second, no consensus existed on diagnostic criteria or histopathologic subtyping before 2017. Therefore, older studies likely used different definitions than more recent studies. However, even following the establishment of consensus guidelines, clinicians and hematopathologists continue to use differing disease definitions and sub classification schemes. This further supports the importance of not relying on a single reviewer’s classification to guide treatment decisions. Lastly, there are no alternative tools or validated biomarkers available to guide treatment decisions. Further research is underway to identify validate biomarkers with strong sensitivity and specificity.
Taken together, a predominance of evidence suggests that anti-IL-6 therapies should be considered as an important treatment option across all cases of iMCD, including iMCD-TAFRO patients, even when histopathology suggests HyperV or HV morphology. The data summarized here conflict with the current NCCN guidelines to use histopathologic subtypes as the primary driver of treatment decisions and to avoid anti-IL-6 therapy in patients considered to have HV histopathology. Failure to apply effective anti-IL-6 therapy in a timely manner may present a serious risk to the sickest iMCD-TAFRO patients who often demonstrate HyperV or HV histopathology and are most in need of urgent effective therapy. Furthermore, there are no therapies other than anti-IL-6 therapies currently licensed for iMCD anywhere in the world. At this time, we consider that siltuximab (or tocilizumab, if siltuximab is not available) remains the preferred therapy for both non-severe and severe iMCD, regardless of histopathology, as per consensus guidelines11 and consistent with the FDA-approval of siltuximab for all iMCD patients. Patients with severe iMCD should be monitored closely for progressive organ dysfunction and immediately started on chemotherapy.11 Though iMCD histopathology is not supported as a reliable predictor of response to siltuximab at this time, further research is needed to elucidate effective predictive biomarkers.
Supplementary Material
Acknowledgements:
We wish to thank Sheila Pierson for her contributions to statistical analysis and interpretation of the data. We wish to thank Amy Greenway for her assistance with clinical data extraction. We wish to thank EUSA Pharma for providing access to data and study files for the siltuximab Phase I, Phase I, and long-term extension studies.
Footnotes
Relevant conflicts of interest:
DCF receives research support from EUSA Pharma for the ACCELERATE study (formerly sponsored by Janssen Pharmaceuticals) and study drug from Pfizer for NCT03933904 without corresponding financial support. DCF has a provisional patent Methods Of Treating Idiopathic Multicentric Castleman Disease with JAK1/2 inhibition (62/989,437) pending. FvR receives research support from Janssen Pharmaceuticals and consultant fees from EUSA Pharma. AG receives consultant fees from EUSA Pharma. CH receives research support from Janssen Pharmaceuticals and consultant fees from EUSA Pharma. SF receives consulting fees, speaker honoraria, and serves an advisory board member for Janssen, receives speaker honoraria and is an advisory board member for EUSA Pharma, receives research funding from Gilead, serves on an advisory board for Sandoz, and receives speaker honoraria from Servier. RW has received research funding from Janssen Pharmaceuticals and served on advisory boards for Janssen Pharmaceuticals. PLZ has served on advisory boards and speaker’s bureaus for Verastem, Celltrion, Gilead, Janssen, BMS, Servier, Immune Design, Celgene, Portola, Roche, Kyowa Kirin, as well as consulting fee, speaker’s bureaus, and advisory board membership for Veratem, MSD, and EUSA Pharma, advisory board membership with Sandoz and consulting fees with Sanofi. NP receives consulting fees/honorarium from Celgene, Stemline, Incyte, Novartis, MustangBio, Roche Diagnostics, LFB, Pacylex, and research funding/clinical trials support from Stemline, Novartis, Abbvie, Samus, Cellectis, Plexxikon, Daiichi-Sankyo, Affymetrix, and SagerStrong Foundation. EO receives consulting fees and serves on an advisory board for EUSA Pharma. AD receives research support from Celgene, Takeda, Alnylam, and Pfizer and serves in an advisory capacity for Janssen, Intellia, and Akcea. SM serves on Advisory Boards for BMS (formerly Celgene)/Acceleron and Novartis, receives honoraria from the Aplastic Anemia and MDS International Foundation, Bristol Myers Squib, McGraw Hill, Partnership for Health Analytic Research, and LLC (PHAR, LLC), receives consulting fees from BioPharm, BMS (formerly Celgene), Novartis, and research funding from BMS (formerly Celgene) and Novartis. DW, on behalf of the University of Washington, received research support from Janssen Pharmaceuticals for role in Central Pathology review for the 2014 van Rhee Lancet Oncology work. The remaining authors declares no relevant conflicts of interest.
References
- 1.Fajgenbaum DC. Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease. Blood. 2018;132(22):2323–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nabel CS, Sameroff S, Shilling D, et al. Virome capture sequencing does not identify active viral infection in unicentric and idiopathic multicentric Castleman disease. PLoS One. 2019;14(6):e0218660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129(12):1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HW, Pittaluga S, Jaffe ES. Multicentric Castleman disease: Where are we now? Semin Diagn Pathol. 2016;33(5):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igawa T, Sato Y. TAFRO Syndrome. Hematol Oncol Clin North Am. 2018;32(1):107–118. [DOI] [PubMed] [Google Scholar]
- 6.Iwaki N, Fajgenbaum DC, Nabel CS, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91(2):220–226. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto S, Sakai T, Kawabata H, et al. Is TAFRO syndrome a subtype of idiopathic multicentric Castleman disease? Am J Hematol. 2019;94(9):975–983. [DOI] [PubMed] [Google Scholar]
- 8.Liu AY, Nabel CS, Finkelman BS, et al. Idiopathic multicentric Castleman’s disease: a systematic literature review. The Lancet Haematology. 2016;3(4):e163–175. [DOI] [PubMed] [Google Scholar]
- 9.van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15(9):966–974. [DOI] [PubMed] [Google Scholar]
- 10.Kurzrock R, Voorhees PM, Casper C, et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(13):3659–3670. [DOI] [PubMed] [Google Scholar]
- 11.van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018;132(20):2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627–2632. [DOI] [PubMed] [Google Scholar]
- 13.Abramson JS. Diagnosis and Management of Castleman Disease. Journal of the National Comprehensive Cancer Network : JNCCN. 2019;17(11.5):1417–1419. [DOI] [PubMed] [Google Scholar]
- 14.van Rhee F, Casper C, Voorhees PM, et al. Long-term safety of siltuximab in patients with idiopathic multicentric Castleman disease: a prespecified, open-label, extension analysis of two trials. The Lancet Haematology. 2020;7(3):e209–e217. [DOI] [PubMed] [Google Scholar]
- 15.van Rhee F, Casper C, Voorhees PM, et al. A phase 2, open-label, multicenter study of the long-term safety of siltuximab (an anti-interleukin-6 monoclonal antibody) in patients with multicentric Castleman disease. Oncotarget. 2015;6(30):30408–30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morra DE, Pierson SK, Shilling D, et al. Predictors of response to anti-IL6 monoclonal antibody therapy (siltuximab) in idiopathic multicentric Castleman disease: secondary analyses of phase II clinical trial data. Br J Haematol. 2019;184(2):232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai K, Maeda T, Kuriyama A, Shimada N, Notohara K, Ueda Y. TAFRO syndrome successfully treated with tocilizumab: A case report and systematic review. Mod Rheumatol. 2016:1–6. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara S, Mochinaga H, Nakata H, et al. Successful treatment of TAFRO syndrome, a variant type of multicentric Castleman disease with thrombotic microangiopathy, with anti-IL-6 receptor antibody and steroids. Int J Hematol. 2016;103(6):718–723. [DOI] [PubMed] [Google Scholar]
- 19.Fajgenbaum DC, Langan RA, Japp AS, et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6-blockade-refractory idiopathic multicentric Castleman disease. The Journal of clinical investigation. 2019;129(10):4451–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith C, Lee-Miller C, Dishop MK, Cost C, Wang M, Asturias EJ. Multicentric Castleman disease presenting with fever. The Journal of pediatrics. 2014;165(6):1261–1265. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno H, Sekine A, Oguro M, et al. Renal histology in a patient with TAFRO syndrome: a case report. Hum Pathol. 2018;82:258–263. [DOI] [PubMed] [Google Scholar]
- 22.Sakashita K, Murata K, Inagaki Y, Oota S, Takamori M. An anterior mediastinal lesion in TAFRO syndrome showing complete remission after glucocorticoid and tocilizumab therapy. Respirol Case Rep. 2016;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angenendt L, Kerkhoff A, Wiebe S, et al. Remissions of different quality following rituximab, tocilizumab and rituximab, and allogeneic stem cell transplantation in a patient with severe idiopathic multicentric Castleman’s disease. Ann Hematol. 2015;94(7):1241–1243. [DOI] [PubMed] [Google Scholar]
- 24.Iwaki N, Sato Y, Takata K, et al. Atypical hyaline vascular-type castleman’s disease with thrombocytopenia, anasarca, fever, and systemic lymphadenopathy. Journal of clinical and experimental hematopathology : JCEH. 2013;53(1):87–93. [DOI] [PubMed] [Google Scholar]
- 25.Jose FF, Kerbauy LN, Perini GF, et al. A life-threatening case of TAFRO syndrome with dramatic response to tocilizumab, rituximab, and pulse steroids: The first case report in Latin America. Medicine. 2017;96(13):e6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behnia F, Elojeimy S, Matesan M, Fajgenbaum DC. Potential value of FDG PET-CT in diagnosis and follow-up of TAFRO syndrome. Ann Hematol. 2017;96(3):497–500. [DOI] [PubMed] [Google Scholar]
- 27.Takayama Y, Kubota T, Ogino Y, Ohnishi H, Togitani K, Yokoyama A. TAFRO Syndrome with Disseminated Intravascular Coagulation Successfully Treated with Tocilizumab and Recombinant Thrombomodulin. Internal medicine (Tokyo, Japan). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miatech JL, Patel NR, Latuso NQ, Ellipeddi PK. TAFRO Syndrome: A Case of Significant Endocrinopathy in a Caucasian Patient. Cureus. 2019;11(6):e4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pai RL, Japp AS, Gonzalez M, et al. Type I IFN response associated with mTOR activation in the TAFRO subtype of idiopathic multicentric Castleman disease. JCI Insight. 2020;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierson SK, Stonestrom AJ, Shilling D, et al. Plasma proteomics identifies a ‘chemokine storm’ in idiopathic multicentric Castleman disease. Am J Hematol. 2018;93(7):902–912. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y, Zhang L, Nong L, et al. Effectiveness of rituximab-containing treatment regimens in idiopathic multicentric Castleman disease. Ann Hematol. 2018;97(9):1641–1647. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Zhao AL, Duan MH, et al. Phase 2 study using oral thalidomide-cyclophosphamide-prednisone for idiopathic Multicentric Castleman disease. Blood. 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


