Abstract
Background:
Appendiceal cancer with peritoneal metastases (ACPM) is a complex disease requiring multidisciplinary care. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion (CRS HIPEC) can significantly improve survival but requires evaluation by a surgical oncologist and significant treatment endurance. The impacts of socioeconomic status (SES) and other social determinants of health on rates of surgical evaluation and treatment have not been examined.
Methods:
We conducted a retrospective cohort study examining all patients with ACPM from 2010 to 2018 in a regional healthcare system. Patient characteristics, oncologic details, treatment strategies, and survival were examined. The primary outcomes of interest were referral to Surgical Oncology, receipt of CRS HIPEC, and survival.
Results:
Of 194 patients identified, 94% had synchronous ACPM. The majority of patients (95%) were referred to surgical oncology. Advanced age was the only predictor of nonreferral (p <0.001). A total of 147 patients (76%) ultimately underwent CRS HIPEC. After adjusting for medical and tumor characteristics, CRS HIPEC was less likely for patients who were unmarried [odds ratio (OR) 0.253, p = 0.004] or of low SES (OR 0.372, p = 0.03). On subanalysis of patients undergoing CRS HIPEC, median overall survival was worse for patients of low SES [51 months versus not reached (NR), p = 0.05], and this disparity persisted on multivariate analysis [hazard ratio (HR) = 2.278, p = 0.001].
Conclusions:
This analysis is the first to evaluate barriers to CRS HIPEC for ACPM. While most patients were evaluated by a multidisciplinary team, nonmedical factors may play a role in the treatment received and ultimate outcomes. Addressing these disparities is crucial for ensuring equitable outcomes and improving patient care.
Introduction
Disparities in treatment and survival for complex gastrointestinal cancers exist owing to nonmedical patient factors such as race, rural location, and Medicaid or lack of health insurance.1-4 Socioeconomic status (SES), most often approximated using location-based US census data, underlies many of these characteristics and has also been linked to inferior treatment and survival.5-7
Appendiceal cancer with peritoneal metastases (ACPM)is among the most complex gastrointestinal malignancies, requiring multidisciplinary treatments and care planning. The surgical treatment, cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion (CRS HIPEC), is complicated and high risk owing to the physiologic stress on patients. We recently studied our large institutional CRS HIPEC database and found patients of low SES had more preoperative comorbidities and postoperative complications than patients of high SES.8 Despite similar tumor characteristics and recurrence patterns, patients of low SES were less likely to receive adjuvant chemotherapy or repeat CRS HIPEC and had worse long-term survival. This study was based on a surgical dataset, and we were unable to analyze disparities in CRS HIPEC referral and treatment owing to SES and other patient factors.9
The aim of this study was to identify barriers to surgical evaluation and treatment for patients with ACPM who were diagnosed across a large regional hospital system. We used our institutional cancer registry that draws from multiple hospitals within a single network to identify patients and chart reviews to understand their evaluation and management. We hypothesized that low-SES patients would less frequently be referred to surgical oncology and less frequently undergo CRS HIPEC after controlling for medical and oncologic factors.
Methods
Study Design and Population
We conducted a retrospective cohort study for all patients diagnosed with ACPM from 2010–2018 in a hospital network that includes a high-volume CRS HIPEC center and 15 affiliated hospitals. Our institutional cancer registry captures all patients diagnosed at participating network hospitals. The hospital system employs a hub-andspoke system for advanced cancer care, whereby rare cancers diagnosed at outlying facilities are referred to the central cancer center for evaluation and surgical care. This study was approved by our institutional review board (IRB20100475).
The following demographics were collected: age, sex, race (white, Black, or Asian), marital status (married, single, separated/divorced, or widowed), primary insurance status (private, Medicare, Medicaid, or none), employment status, age adjusted Charlson–Deyo comorbidity index score (AA-CCI),10 and body mass index (BMI). Driving time (hours) and distance (miles) from CRS HIPEC facility and nearest hospital network facility were calculated using patient addresses in ArcGis.
The following oncologic variables were examined: presence of peritoneal metastases at diagnosis of primary tumor or in subsequent follow-up (synchronous or metachronous), tumor grade (G1–G3), and the presence of signet ring cells.
The following treatment details were collected: setting of diagnosis [outpatient, emergency department (ED), or inpatient], physician specialty making the diagnosis, initial treatment plan, evaluation by surgical oncology, time to surgical oncology evaluation, and treatment received (chemotherapy, CRS HIPEC).
For patients who underwent CRS HIPEC we collected: peritoneal cancer index (PCI), operative duration (hours), completeness of cytoreduction score (CC score 0,1,2+),11 number of visceral resections, ostomy creation, length of stay (days) in hospital, comprehensive complication index score (CCI),12 major complication rate (Clavien–Dindo grade III or higher), readmission within 90 days of discharge to index or other hospital, and death within 90 days of CRS HIPEC.
Exposure
Patient SES was the primary exposure of interest. SES data for patients were assessed via the national Area Deprivation Index (ADI) percentile at the census block neighborhood level obtained by patient nine-digit zip code. ADI is a composite measure of income, education, employment, and housing quality.13 Scores range from 1–100 with higher scores indicating more disadvantage. The median ADI of the study population was used to group patients into cohorts for high SES and low SES.14,15
Outcomes
The primary outcomes of interest for this analysis were referral to surgical oncology and receipt of CRS HIPEC. The secondary outcome of interest was overall survival (OS) from time of diagnosis of ACPM
Statistical Methods
Descriptive statistics were used to examine the whole cohort and compare characteristics between SES cohorts. Continuous data were reported as median with interquartile range (IQR) and compared using Wilcoxon rank sum test. Categorical data were reported as frequencies and percentages and compared using chi-squared test or Fisher’s exact test.
Predictors of referral to surgical oncology were assessed by logistic regression within the whole cohort. Predictors of CRS HIPEC receipt were then examined within the cohort of patients referred to surgical oncology. All patient and tumor characteristics were assessed by univariate logistic regression. Variables with a p-value < 0.30 on univariate analysis were included in an initial backwards stepwise elimination multivariable logistic regression model. Using backwards elimination, variables were sequentially removed from the model until all remaining predictors had p< 0.05.
Kaplan–Meier analysis was used to examine survival by SES. Significance was determined by log rank test. To assess the impact of SES on OS, hazard ratio (HR) for low SES was examined using Cox proportional hazard models. Variables with a statistical significance of p <0.30 on univariate analysis were evaluated in an initial multivariable regression analysis. Variables were sequentially removed via backwards elimination with a prespecified pvalue cut-off of 0.05 a priori adjusting for age, treatment (chemotherapy and CRS HIPEC), and comorbidities. Impact of SES on survival was then specifically examined within the cohort of patients receiving CRS HIPEC by univariate and multivariate Cox analysis.
Subanalysis: Two Hour Drive Cohort
Given the possible confounding between increasing distance travelled and patient resources, we conducted a subanalysis of patients within 2 h of driving time to index CRS HIPEC facility. Drive times areas were generated using ArcGis. Two hours was chosen to recreate the actual catchment area of the hospital network and exclude out of network referrals. We then examined predictors of CRS HIPEC receipt and survival within this drive time cohort. Models were fit as described above.
Missingness of data was minimal (<1%). An alpha cutoff of 0.05 was used for all significance tests. The data were analyzed using STATA 16 (StataCorp LP, College Station, TX).
Results
Patient Demographics, Oncologic Features, and Treatment History
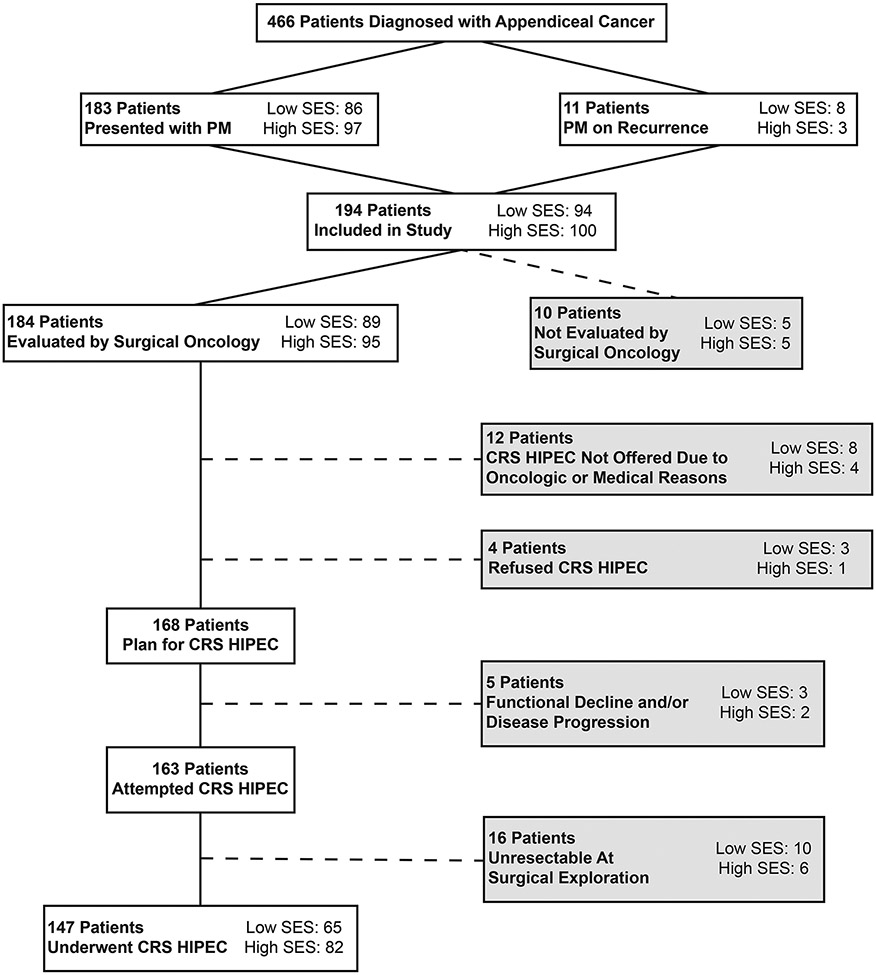
Overall, 194 patients were identified with ACPM during the study period (Fig. 1). From this cohort, 183 patients (94.3%) presented with synchronous peritoneal metastases on diagnosis while the remaining patients were found to have peritoneal recurrence during the study period. Median ADI of the study cohort was 47 (IQR 22–68) with 100 patients (51.6%) identified as high SES versus 94 (48.4%) low SES. Median follow-up time was 60.5 months (95% CI 55.0–63.5 months) for the whole cohort, with a median follow-up of 59.3 months in the cohort of high SES and 66.0 months in the cohort of low SES (p = 0.1).
Figure 1:
Study flow diagram. SES: Socioeconomic Status; CRS HIPEC: Cytoreductive Surgery Hyperthermic Intraperitoneal Chemoperfusion; PM: Peritoneal Metastases.
Patient demographics, oncologic details, and treatment summaries are presented in Table 1. There were no differences in terms of age, gender, or insurance status between groups. Patients of low SES were less often white (85.1% versus 97.0%, p = 0.001) or employed (47.9% versus 69.0%, p = 0.002), and had higher burden of comorbidities as assessed by the age adjusted CCI (8 versus 7, p = 0.02). Patients of high SES traveled significantly further for care (median 4.5 versus 1.5 h, p = 0.001).
Table 1:
Patient Characteristics
| Whole Cohort | High SES | Low SES | P Value | |
|---|---|---|---|---|
| Variable | n=194 | n=100 | n=94 | |
| Age | 58 (49-66) | 57 (49-64) | 60 (51-68) | 0.21 |
| Male | 96 (49.5%) | 53 (53.0%) | 43 (45.7%) | 0.28 |
| Race | 0.001 | |||
| White | 177 (91.2%) | 97 (97.0%) | 80 (85.1%) | |
| Black | 14 (7.2%) | 1 (1.0%) | 13 (14.1%) | |
| Asian | 3 (1.6%) | 2 (2.0%) | 1 (1.1%) | |
| Marital Status | 0.19 | |||
| Married | 146 (77.1%) | 79 (84.1%) | 67 (72.0%) | |
| Single | 18 (9.6%) | 8 (9.5%) | 10 (10.8%) | |
| Separated/Divorced | 17 (9.1%) | 5 (5.3%) | 12 (12.9%) | |
| Widowed | 6 (3.2%) | 2 (2.1%) | 4 (4.3%) | |
| Insurance Status | 0.14 | |||
| Private | 121 (65.0%) | 68 (71.6%) | 53 (58.2%) | |
| Medicare | 50 (26.9%) | 21 (22.1%) | 29 (31.9%) | |
| Medicaid | 10 (5.4%) | 3 (3.2%) | 7 (7.7%) | |
| Uninsured | 2 (1.1%) | 2 (2.1%) | 2 (2.2%) | |
| Other | 3 (1.6%) | 1 (1.0%) | ||
| Employed | 114 (58.8%) | 69 (69.0%) | 45 (47.9%) | 0.002 |
| ADI | 47 (22-68) | 23 (10-37) | 69 (57-78) | |
| Distance from Index Facility, miles | 102.6 (23.1-322.1) | 286.7 (23.4-388.1) | 64.7 (20.7-193.3) | 0.0008 |
| Drive Time from Index Facility, hours | 2.1 (0.8-5.3) | 4.5 (0.8-7.0) | 1.5 (0.8-3.6) | 0.001 |
| Drive Time from Closest Facility, hours | 1.0 (0.4-4.0) | 2.7 (0.5-4.8) | 0.7 (0.3-1.7) | 0.001 |
| CCI | 6 (6-6) | 6 (6-6) | 6 (6-7) | 0.01 |
| AA-CCI | 8 (7-9) | 7 (7-8) | 8 (7-9) | 0.02 |
| BMI | 26.2 (22.8-30.3) | 26.2 (22.3-30.2) | 26.2 (22.9-30.3) | 0.56 |
| Oncologic Variables | ||||
| Grade | 0.53 | |||
| G1 | 45 (23.2%) | 20 (20.0%) | 25 (26.6%) | |
| G2 | 55 (28.3%) | 30 (30.0%) | 25 (26.6%) | |
| G3 | 94 (48.5%) | 50 (50.0%) | 44 (46.8%) | |
| Signet Cells | 94 (48.5%) | 50 (50.0%) | 44 (46.8%) | 0.58 |
| Presentation | 0.09 | |||
| Peritoneal Metastases on Diagnosis | 183 (94.3%) | 97 (97.0%) | 86 (91.5%) | |
| Recurrence with PM | 11 (5.7%) | 3 (3.0%) | 8 (8.5%) | |
| Circumstances of Diagnosis | ||||
| Setting | 0.17 | |||
| Outpatient | 80 (41.2%) | 47 (47.0%) | 33 (35.1%) | |
| Inpatient | 111 (57.2%) | 52 (52.0%) | 59 (62.8%) | |
| Emergency | 3 (1.6%) | 1 (1.0%) | 2 (2.1%) | |
| Physician Specialty of Diagnosis | 0.07 | |||
| Emergency | 3 (1.6%) | 1 (1.0%) | 2 (2.1%) | |
| Family Medicine | 55 (28.6%) | 35 (35.7%) | 20 (21.3%) | |
| Internal Medicine | 27 (14.1%) | 16 (16.4%) | 11 (11.7%) | |
| Gynecology | 24 (12.5%) | 10 (10.2%) | 14 (14.9%) | |
| Surgery | 80 (41.7%) | 36 (36.7%) | 44 (46.8% | |
| Surgical Oncology | 3 (1.5%) | 0 (0.0%) | 3 (3.2%) | |
| Initial Treatment Plan | 0.21 | |||
| Refer to Medical Oncology | 90 (46.4%) | 48 (48.0%) | 42 (44.7%) | |
| Refer to Gynecological Oncology | 21 (10.8%) | 8 (8.0%) | 13 (13.8%) | |
| Refer to General Surgery | 19 (9.8%) | 13 (13.0%) | 6 (6.4%) | |
| Refer to Surgical Oncology | 64 (33.0%) | 31 (31.0%) | 33 (35.1%) | |
| Evaluation by Surgical Oncologist | 184 (94.9%) | 95 (95.0%) | 89 (94.7%) | 0.54 |
| Time to Surgical Oncology Evaluation, days | 37 (20-106) | 34 (17-89) | 40 (21-129) | 0.29 |
| Ultimate Treatment | ||||
| Chemotherapy | 150 (77.3%) | 79 (79.0%) | 71 (75.5%) | 0.54 |
| CRS HIPEC | 147 (75.8%) | 82 (82.0%) | 65 (69.2%) | 0.03 |
Abbreviations: SES, Socioeconomic Status; ADI, Area Deprivation Index; CCI, Charlson Comorbidity Index; AA-CCI, Age Adjusted Charlson Comorbidity Index; BMI, Body Mass Index; PM, Peritoneal Metastases; CRS HIPEC, Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy
There were no differences in histologic features of ACPM. Most patients presented with synchronous peritoneal metastases. There were no differences in setting of diagnosis or physician specialty making the diagnosis and initial treatment plan. A plurality of patients (high SES 48.0% vs low SES 44.7%, p=0.21) were initially referred to Medical Oncology.
The majority of patients (94.9%) were evaluated by surgical oncology with a median time to evaluation of 37 days (IQR 19-106), with similar timing between SES cohorts. When patient and tumor characteristics were assessed as predictors of referral to surgical oncology by logistic regression (Supplementary Table S1), only advanced age remained a significant predictor (OR 0.192 per decade, 95% CI 0.08-0.44, p<0.001).
Predictors of CRS HIPEC
Chart reviews were performed to understand the treatment course of all patients not reaching CRS HIPEC (Figure 1). Of the 184 patients evaluated by surgical oncology (95 high-SES, 89 low-SES), 12 patients were not offered CRS HIPEC (4 high SES, 8 low SES), and 4 refused surgery (1 high SES, 3 low SES). As a result, 168 patients (91.3% of those evaluated) had an initial plan for CRS HIPEC (90 high SES, 78 low SES). Documented reasons for not offering surgery included unfavorable oncologic factors (disease burden, chemotherapeutic response) or medical limitations (inability to tolerate surgery owing to comorbidity or frailty burden). Of the patients of low SES not offered surgery, two were not offered owing to intellectual disability while another was recently incarcerated and suffered from ongoing psychiatric issues. An additional four patients (2.2%) refused CRS HIPEC: one patient of high SES was elderly and elected to focus on quality of life, while the three patients of low SES did not report a specific reason.
Among the 168 patients with an initial plan for CRS HIPEC, 5 patients (3.0%) experienced preoperative functional decline or tumor progression precluding surgery. Surgical exploration revealed unresectable tumor burden in 16 patients (9.5%) for whom CRS HIPEC was aborted: ten patients of low SES and six patients of high SES. Ultimately, 147 patients (87.5%) underwent CRS HIPEC.
Rates of CRS HIPEC were lower among patients of low SES (69.2% versus 82.0%, p = 0.03). Predictors of CRS HIPEC receipt are presented in Table 2. Univariate analysis suggested patient factors (age, comorbidities), social determinants of health (race, marital status, insurance status, low SES, distance from index and nearest facility), and oncologic features (synchronous peritoneal metastases, tumor grade) were predictive of CRS HIPEC receipt. On multivariate logistic regression, low SES (OR 0.372, 95% CI 0.15–0.93), nonmarried status (OR 0.253, 95% CI 0.11–0.65), CCI score (OR 0.336 per point, 95% CI 0.18–0.62), and tumor grade (G1, reference; G2: OR 0.200, 95% CI 0.05–0.88; G3: OR 0.118, 95% CI 0.03–0.48) were all independently associated with CRS HIPEC receipt (all p <0.05).
Table 2:
Analysis of Predictors of CRS HIPEC
| Whole Cohort Analysis | 2 Hour Drive Cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| OR | 95% CI | P Value |
OR | 95% CI | P Value |
OR | 95% CI | P Value |
OR | 95% CI | P Value |
|
| Variable | ||||||||||||
| Age (per decade) | 0.767 | 0.56-1.06 | 0.10 | 0.897 | 0.61-1.32 | 0.58 | ||||||
| Male | 1.041 | 0.51-2.14 | 0.91 | 0.940 | 0.39-2.28 | 0.89 | ||||||
| Black Race | 0.110 | 0.03-0.35 | <0.001 | 0.063 | 0.01-0.54 | 0.012 | ||||||
| Non-Married | 0.089 | 0.03-0.26 | <0.001 | 0.253 | 0.11-0.65 | 0.004 | 0.304 | 0.12-0.80 | 0.02 | 0.197 | 0.06-0.66 | 0.009 |
| Insurance Status (ref=private) | 0.07 | 0.42 | ||||||||||
| Medicare | 0.429 | 0.19-0.96 | 0.526 | 0.20-1.38 | ||||||||
| Medicaid or Uninsured | 0.364 | 0.10-1.34 | 0.722 | 0.15-3.48 | ||||||||
| Employed | 1.585 | 0.77-3.28 | 0.214 | 1.171 | 0.48-2.83 | 0.73 | ||||||
| Low SES | 0.335 | 0.15-0.76 | 0.009 | 0.372 | 0.15-0.93 | 0.03 | 0.430 | 0.14-1.31 | 0.14 | 0.246 | 0.06-0.93 | 0.04 |
| Travel Time from Index Facility, per hour | 1.342 | 1.13-1.60 | 0.001 | 1.848 | 0.76-4.78 | 0.174 | ||||||
| Travel Time Nearest Facility, per hour | 1.341 | 1.09-1.65 | 0.005 | 3.429 | 0.82-14.29 | 0.09 | ||||||
| Synchronous Presentation | 3.442 | 0.88-13.52 | 0.77 | 2.568 | 0.54-12.31 | 0.24 | ||||||
| BMI | 0.928 | 0.87-0.99 | 0.02 | 0.920 | 0.85-0.99 | 0.04 | ||||||
| CCI, per point | 0.376 | 0.23-0.62 | <0.001 | 0.336 | 0.18-0.62 | <0.001 | 0.410 | 0.22-0.77 | 0.01 | 0.341 | 0.16-0.75 | 0.007 |
| Grade (ref=G1) | 0.04 | 0.01 | 0.04 | 0.004 | ||||||||
| G2 | 0.273 | 0.07-1.05 | 0.200 | 0.05-0.88 | 0.086 | 0.01-0.77 | 0.041 | 0.01-0.52 | ||||
| G3 | 0.196 | 0.06-0.69 | 0.118 | 0.03-0.48 | 0.104 | 0.01-0.86 | 0.046 | 0.01-0.55 | ||||
| Signet Cells | 0.603 | 0.29-1.23 | 0.171 | 0.988 | 0.41-2.39 | 0.98 | ||||||
Abbreviations: SES, Socioeconomic Status; BMI, Body Mass Index; CCI, Charlson Comorbidity Index; CRS HIPEC, Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy
Sensitivity Analysis: 2 Hour Driving Distance Cohort
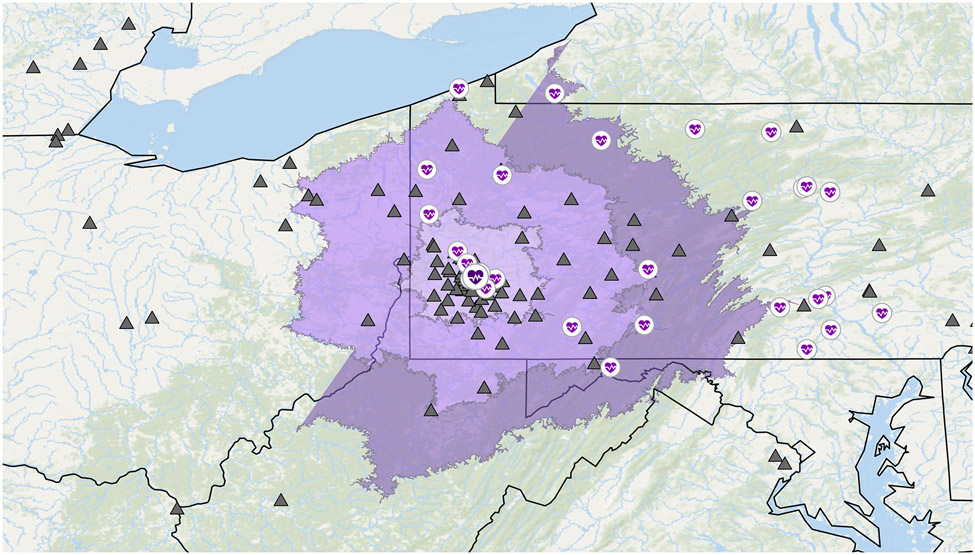
Owing to the correlation between distance travelled and patient ADI, and concern for selection bias among the long distance cohort, we conducted a subanalysis of patients within 2 h of driving time to CRS HIPEC center to recreate the actual geographic catchment area of the facility (Fig. 2). This excluded 98 patients, resulting in 96 patients within the 2-hour drive area (low SES: n = 60, 62.5%; high SES: n = 36, 37.5%). We then examined predictors of CRS HIPEC receipt in this cohort (Table 2). After adjusting for marital status, comorbidities, and tumor grade, low SES was associated with decreased odds of CRS HIPEC (OR 0.246, 95% CI 0.06–0.93, p = 0.04)
Figure 2: 2 Hour Drive Time Analysis Cohort:
Map of facilities and patients. CRS HIPEC facility (dark purple heart) and other facilities (purple heart) are shown in relation to patient zip code (black diamond). 1-, 2-, and 3-hour driving distance from CRS HIPEC facility depicted by areas shown in purple.
Survival Analysis
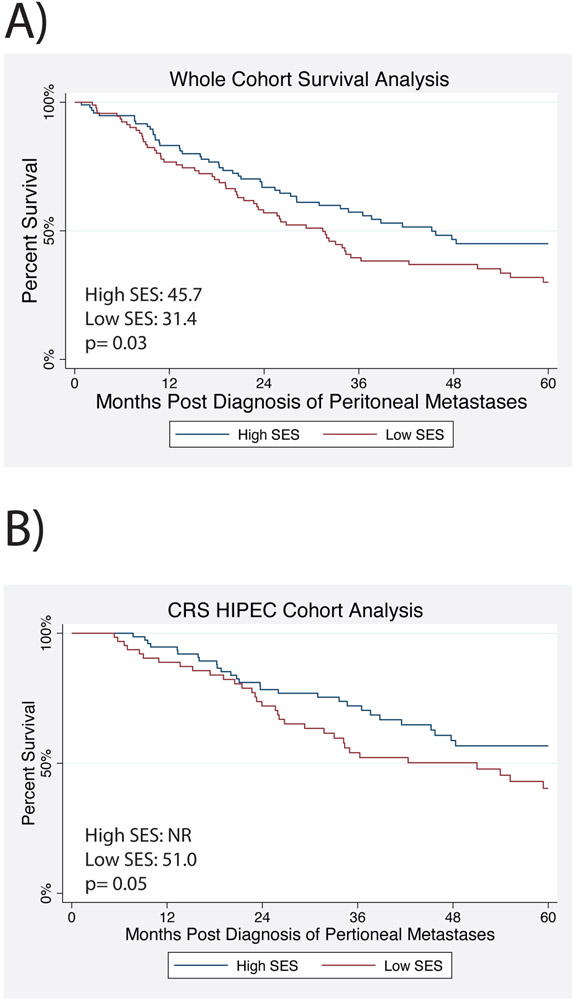
Median OS for the whole cohort was 34.7 months (95% CI 28.1–47.8), and significantly worse for patients of low SES (31.4 months, 95% CI 22.7–35.0) than patients of high SES (45.7 months, 95% CI 31.0–NR, p = 0.03; Fig. 3A). Univariate Cox analysis suggested that low SES was associated with worse survival (HR 1.536, 95% CI 1.05–2.25, p = 0.03). Additionally, age, race, marital status, comorbidities, tumor grade, signet cells, synchronous metastases, receipt of chemotherapy, and CRS HIPEC were all associated with survival (Supplementary Table S2). On multivariate Cox analysis, adjusting for age and comorbidities, tumor grade (G1, reference; G2 HR 9.632, 95% CI 3.57–25.97; G3: HR 15.682, 95% CI 5.76–42.70; p <0.001) was associated with worse overall survival, while treatment with chemotherapy (HR 0.391, 95% CI 0.19–0.80, p = 0.01) and CRS HIPEC (HR 0.266, 95% CI 0.17–0.43, p < 0.001) were associated with improved survival. Low SES trended toward predicting worse overall survival (HR 1.493, 95% CI 0.99–2.35, p = 0.05), and marital status was not significant.
Figure 3:
Survival Analysis by Socioeconomic Status (SES). A) Overall survival for the whole cohort. B) Overall survival for patients who underwent CRS HIPEC.
Perioperative Characteristics and Outcomes Following CRS HIPEC
Cohort characteristics and perioperative outcomes for patients who underwent CRS HIPEC are presented in Supplementary Table S3. Patients of low SES were less often white (90.8% versus 97.6%, p = 0.02) and trended towards higher comorbidity burden (median AA-CCI 8 versus 7, p = 0.09). There was a higher travel time among patients with high SES (4.9 versus 2.1 h, p = 0.006). There were no differences in oncologic features, presentation, or preoperative treatment between cohorts. Notably, disease burden, as reflected by PCI, was similar between cohorts (17 versus 17, p = 0.48).
Examining perioperative outcomes, there were no differences in operative duration, EBL, number or resections, or stoma creation between cohorts. However, there were higher rates of CC-0 resection in the cohort of low SES (80% versus 64.2%, p = 0.04). Postoperatively, patients had similar length of stay (high SES 14 versus low SES 12, p = 0.15), overall complication burden (high SES 24.2% versus low SES 26.2%, p = 0.96), 90-day readmissions (high SES 38.3% versus low SES 35.4%, p = 0.43) and 90-day mortality (high SES 1.2% versus low SES 0%, p = 0.56).
Given different rates of CRS HIPEC receipt between high and low SES cohort and its significant association with survival, we separately analyzed survival among patients who underwent CRS HIPEC. Median OS for patients who underwent CRS HIPEC was 59.4 months (95% CI 41.5 months to NR). Survival was worse for patients of low SES (51.02 months, 95% CI 29.3–67.3 months) than patients of high SES (median survival NR, 95% CI 45.2 months to NR, p = 0.05; Fig. 3B). On multivariate analysis adjusting for age, tumor grade, PCI, and chemotherapy receipt, low SES was associated with worse overall survival (HR 2.278, 95% CI 1.38–3.75, p = 0.001; Supplementary Table S4).
Discussion
In this analysis of a large, regional hospital network with a specialized peritoneal disease center, patients of low SES or who were unmarried were less likely to undergo CRS HIPEC. These differences in treatment contributed to disparities in overall survival observed in a population of patients with a complex malignancy. To our knowledge, this is the first study to investigate socioeconomic barriers to CRS HIPEC.
Our prior study found that patients of low SES had worse postoperative outcomes following CRS HIPEC for metastatic colorectal cancer.8 The current study analyzed a smaller cohort of only patients with metastatic appendiceal cancer and found no difference in postoperative outcomes. In both studies, low SES predicted inferior overall survival. This could be owing to higher comorbidity scores in both studies or unmeasured differences related to social determinants of health that we cannot assess in using these datasets. Indeed, low SES did not predict differences in disease recurrence, but did predict less repeat HIPEC and worse post-progression survival.8 While the current study focused on preoperative evaluation and decision-making, future qualitative studies will better highlight patient factors that affect long-term treatments and outcomes.
This analysis is unique in part in terms of the population and environment studied. Our hospital system employs a regional hub-and-spoke model with a strong referral network to a high-volume, multidisciplinary CRS HIPEC center, where physicians treat patients from all local hospital systems along with many out-of-state referrals. Using our network cancer registry, which captures all cancer diagnoses at participating hospitals within the system, provided a large number of ACPM diagnoses within our region and minimized any referral or selection bias that may have impacted our results.
Our primary finding was that patients of low SES and unmarried patients had lower rates of CRS HIPEC despite adjusting for comorbidities and tumor characteristics. Since patients of high SES traveling long distances for specialists or second opinions may have skewed these results, we performed a subanalysis of patients within a 2-hour drive of our CRS HIPEC center. Among these patients, SES, marital status, comorbidities, and tumor grade were significant predictors of undergoing CRS HIPEC. Predictors of survival included age, tumor grade, and receipt of CRS HIPEC. These findings suggest that SES and marital status influence survival for ACPM patients mostly through receipt of surgery. As such, improving access to surgery may help overcome survival disparities for patients with complex gastrointestinal cancers.16
Another strength of this analysis was the ability to perform chart reviews searching for an explanation of treatment disparities. Patient SES did not predict referral to surgical oncology or differences in tumor characteristics. Nonetheless, after surgical evaluation, patients of low SES were more likely to not be offered CRS HIPEC for psychiatric/disability reasons and more likely to refuse surgery. Unmarried patients were also less likely to undergo CRS HIPEC, perhaps owing to a lack of structural support mechanisms to advocate for their care. Refusal of surgery, which is more common among poor and minority patients, can be targeted by improved communication and education initiatives.17,18 Conversely, medically and socially vulnerable patients may not always be suitable for high risk and potentially morbid surgery such as CRS HIPEC. These findings highlight some of the modifiable and nonmodifiable barriers that patients face along the cancer care continuum.
The evaluation of patients for CRS HIPEC can be subjective, requires multidisciplinary experience with peritoneal disease, and is at times controversial. A criterion strongly associated with survival and commonly used in patient selection is the ability to achieve complete cytoreduction.19,20 While CC-0 (removal of all visible tumor) and CC-1 (residual tumor nodules B 2.5 mm) are often considered “complete cytoreduction,” CC-0 resection been shown to improve overall survival.21 In this study, low SES correlated with higher rates of CC-0 resection (80% versus 64%), which could suggest more stringent selection of these patients, as there were no differences in PCI or preoperative chemotherapy on the basis of SES. It is unclear whether a provider bias existed, either against surgery for certain patients of low SES or favoring attempted CRS HIPEC for patients of high SES who may have traveled long distances. These treatment decisions are undeniably complicated, and providers consider many medical and nonmedical factors, consciously or unconsciously, when assessing patients for high-risk surgery.
While our retrospective study cannot speak to the surgical decision to proceed with or abort borderline resectable cases, it is possible that surgeons may have been more likely to push the limits with younger, more robust, patients of high SES traveling long distances to our institution specifically for CRS HIPEC. In that sense, there is not a disparity against patients of low SES, but rather, favoring patients of high SES with borderline resectable disease.
This study is not without limitations. As a retrospective cohort analysis of patients with an uncommon disease, some of our findings may be limited in their interpretation. We were limited by the data available in the electronic records and unable to truly understand all factors that contributed to surgical evaluation for these patients. While this study was of a specialized and high-volume center, it was limited to providers at one institution and does not represent a national analysis. Finally, while our study was designed to analyze all patients with a diagnosis of ACPM in our region, we observed a rise in SES scores with increasing distance from our CRS HIPEC center, which suggests we may not have captured some patients in more remote locations. Additionally, this analysis only considers initial CRS HIPEC. Patients facing repeat CRS HIPEC may encounter additional barriers to treatment.
Conclusion
Socioeconomic factors affect surgical patient selection independent of tumor characteristics and proximity to a specialized cancer center. This disparity in receipt of surgery is an important cause of the inferior survival experienced by patients of low SES. Peritoneal carcinomatosis requires months and years of multidisciplinary management and considerable treatment endurance. As such, our findings can be extrapolated to help explain socioeconomic disparities that are increasingly recognized for many complex malignancies.
Supplementary Material
Acknowledgement:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA113263. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: There are no conflicts of interest for the authors of this manuscript.
Ethical Approval Statement: This study was approved by our institutional review board (IRB20100475).
Data Sharing and Data Accessibility:
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Kirkegård J, Ladekarl M, Fristrup CW, Hansen CP, Sall M, Mortensen FV. Urban versus rural residency and pancreatic cancer survival: A Danish nationwide population-based cohort study. PLoS One. 2018;13(8):e0202486. doi: 10.1371/journal.pone.0202486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee RM, Liu Y, Gamboa AC, et al. Race, ethnicity, and socioeconomic factors in cholangiocarcinoma: What is driving disparities in receipt of treatment? J Surg Oncol. Sep 2019;120(4):611–623. doi: 10.1002/jso.25632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutfi W, Zenati MS, Zureikat AH, Zeh HJ, Hogg ME. Health Disparities Impact Expected Treatment of Pancreatic Ductal Adenocarcinoma Nationally. Ann Surg Oncol. Jul 2018;25(7):1860–1867. doi: 10.1245/s10434-018-6487-5 [DOI] [PubMed] [Google Scholar]
- 4.Shapiro M, Chen Q, Huang Q, et al. Associations of Socioeconomic Variables With Resection, Stage, and Survival in Patients With Early-Stage Pancreatic Cancer. JAMA Surg. Apr 2016;151(4):338–45. doi: 10.1001/jamasurg.2015.4239 [DOI] [PubMed] [Google Scholar]
- 5.Hoehn RS, Rieser CJ, Winters S, et al. A Pancreatic Cancer Multidisciplinary Clinic Eliminates Socioeconomic Disparities in Treatment and Improves Survival. Ann Surg Oncol. Feb 1 2021;doi: 10.1245/s10434-021-09594-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swords DS, Mulvihill SJ, Brooke BS, Firpo MA, Scaife CL. Size and Importance of Socioeconomic Status-Based Disparities in Use of Surgery in Nonadvanced Stage Gastrointestinal Cancers. [DOI] [PubMed] [Google Scholar]
- 7.van Roest MH, van der Aa MA, van der Geest LG, de Jong KP. The Impact of Socioeconomic Status, Surgical Resection and Type of Hospital on Survival in Patients with Pancreatic Cancer. A Population-Based Study in The Netherlands. PLoS One. 2016;11(11):e0166449. doi: 10.1371/journal.pone.0166449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieser CJ, Hoehn RS, Zenati M, et al. Impact of Socioeconomic Status on Presentation and Outcomes in Colorectal Peritoneal Metastases Following Cytoreduction and Chemoperfusion: Persistent Inequalities in Outcomes at a High-Volume Center. Ann Surg Oncol. Mar 9 2021;doi: 10.1245/s10434-021-09627-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miura J, Karakousis G. Improving Access to Specialized Centers is Not Enough to Mitigate Socioeconomic Disparities in Complex Oncologic Surgery. Ann Surg Oncol. Feb 6 2021;doi: 10.1245/s10434-021-09631-6 [DOI] [PubMed] [Google Scholar]
- 10.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. Nov 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 11.Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg. Dec 1999;384(6):576–87. doi: 10.1007/s004230050246 [DOI] [PubMed] [Google Scholar]
- 12.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. Jul 2013;258(1):1–7. doi: 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 13.Health UoWSoMaP. Area Deprivation Index {2.0}. Accessed Downloaded from https://www.neighborhoodatlas.medicine.wisc.edu/ {March/1/2021},
- 14.Mora J, Krepline AN, Aldakkak M, et al. Adjuvant therapy rates and overall survival in patients with localized pancreatic cancer from high Area Deprivation Index neighborhoods. Am J Surg. Dec 3 2020;doi: 10.1016/j.amjsurg.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 15.Arias F, Chen F, Fong TG, et al. Neighborhood-Level Social Disadvantage and Risk of Delirium Following Major Surgery. J Am Geriatr Soc. Dec 2020;68(12):2863–2871. doi: 10.1111/jgs.16782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swords DS, Mulvihill SJ, Brooke BS, Firpo MA, Scaife CL. Size and Importance of Socioeconomic Status-Based Disparities in Use of Surgery in Nonadvanced Stage Gastrointestinal Cancers. Ann Surg Oncol. Feb 2020;27(2):333–341. doi: 10.1245/s10434-019-07922-7 [DOI] [PubMed] [Google Scholar]
- 17.Tohme S, Kaltenmeier C, Bou-Samra P, Varley PR, Tsung A. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Ann Surg Oncol. Nov 2018;25(12):3427–3435. doi: 10.1245/s10434-018-6680-6 [DOI] [PubMed] [Google Scholar]
- 18.Kaltenmeier C, Malik J, Yazdani H, et al. Refusal of cancer-directed treatment by colon cancer patients: Risk factors and survival outcomes. Am J Surg. Dec 2020;220(6):1605–1612. doi: 10.1016/j.amjsurg.2020.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Votanopoulos KI, Bartlett D, Moran B, et al. PCI is Not Predictive of Survival After Complete CRS/HIPEC in Peritoneal Dissemination from High-Grade Appendiceal Primaries. Ann Surg Oncol. Mar 2018;25(3):674–678. doi: 10.1245/s10434-017-6315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levinsky NC, Morris MC, Wima K, et al. Should We Be Doing Cytoreductive Surgery with HIPEC for Signet Ring Cell Appendiceal Adenocarcinoma? A Study from the US HIPEC Collaborative. J Gastrointest Surg. Jan 2020;24(1):155–164. doi: 10.1007/s11605-019-04336-4 [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Zuluaga CA, King MC, Diaz-Sarmiento VS, et al. Defining "Complete Cytoreduction" After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC) for the Histopathologic Spectrum of Appendiceal Carcinomatosis. Ann Surg Oncol. Dec 2020;27(13):5026–5036. doi: 10.1245/s10434-020-08844-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.