Abstract
Oral cancer is one of the most common malignant tumors of the head and neck, not only affects the appearance, but also affects eating and even endangers life. The clinical treatments of oral cancer mainly include surgery, radiotherapy, and chemotherapy. However, unsatisfactory therapeutic effect and toxic side effects are still the main problems in clinical treatment. Tumor microenvironment (TME) is not only closely related to the occurrence, growth, and metastasis of tumor but also works in the diagnosis, prevention, and treatment of tumor and prognosis. Future studies should continue to investigate the relationship of TME and oral cancer therapy. This purpose of this review was to analyze the characteristics of oral cancer microenvironment, summarize the traditional oral cancer therapy and immunotherapy strategies, and finally prospect the development prospects of oral cancer immunotherapy. Immunotherapy targeting tumor microenvironment is expected to provide a new strategy for clinical treatment of oral cancer.
Keywords: Oral cancer, Immunotherapy, Tumor microenvironment, Immune checkpoint blockade, Immune tolerance
Introduction
Oral cancer is one of the most common malignant tumors of the head and neck [1]. Most of them are squamous cell carcinoma (OSCC), which is associated with mucosal variation [2]. Oral cancer usually occurs at the bottom of the mouth, lips, tongue, gums, and the inside of the cheek, including soft or hard palate cancer, gingival cancer, oropharyngeal cancer, lip cancer, tongue cancer, jaw bone cancer, salivary gland cancer, oral base cancer, facial skin mucosa, and maxillary sinus cancer [3]. Oral cancer can be caused by many factors, including long-term alcoholism, poor oral hygiene, excessive exposure to sunlight, betel nut, long-term foreign body stimulation, malnutrition, mucosal leukoplakia or erythema, and oral ulcers. Oral cancer not only affects the appearance but also affects eating and even endangers life. There are many treatment methods for oral cancer [4, 5]. Currently, the conventional treatment methods are surgery therapy, radiotherapy, and chemotherapy [6]. Surgery is generally based on the size of the tumor to choose the appropriate range of resection; some tongue cancer patients may be seriously affected in eating and speaking after surgery [7]. Radiation therapy belongs to local treatment. During radiotherapy, local skin and mucous membrane may be damaged, including radioactive skin injury, radioactive mucous membrane injury, and radioactive stomatitis [8]. Chemotherapy belongs to systemic treatment, which may lead to pancytopenia and severe nausea gastrointestinal reaction [9]. Traditional Chinese Medicine treatment is mainly used for the treatment of oral cancer complications caused by other strategies [10]. Nevertheless, there are few safe and efficient clinical treatment strategies for oral cancer, which is mainly due the complex tumor microenvironment (TME) [11]. TME is closely related to the occurrence, metastasis, and recurrence of malignant tumors and is composed of non-cellular components in the extracellular matrix (ECM) and cellular components, such as fibroblasts and immune cells [12]. TME performs signal transmission through autocrine–paracrine signaling pathway, thus controlling the proliferation and metastasis of tumor cells [13]. In 1863, Virchow et al. first proposed the concept of tumor microenvironment, pointing out the relationship between inflammation and cancer [14]. With the rapid development of tumor cytology and other disciplines, studies on the mechanism of tumor genesis, development, and metastasis have been continuously deepened [15, 16]. Abnormal TME could promote the invasion and metastasis of tumors, as well as hindering effective drug diffusion and immune cell invasion [17, 18]. In the early stage, tumor microenvironment was able to restrain tumor growth and then the TME gradually transformed into the "soil" to assist the survival and development of malignant tumor [19]. Under its influence, tumor microenvironment is characterized by slight acidity, hypoxia, high reactive oxygen species (ROS), high osmotic pressure, and abnormal vasculature [20, 21]. In addition, tumor microenvironment inhibited the proliferation and activity of tumor-specific T cells (CTLs) and promoted regulatory T cells (Tregs), resulting in a decrease in the secretion of immune-activating cytokines, such as tumor necrosis factor (TNF-α) and interferon γ (IFN-γ), and an increase in the secretion of immunosuppressive cytokines, such as transforming growth factor (TGF-β) and interleukin-10 (IL-10). TME could also assist tumor cells to escape immune surveillance, hinder dendritic cells (DCs) from presenting antigens, inhibit the activity of CTLs, and ultimately achieve immune tolerance and immune escape of tumor cells [22]. In recent years, with the deepening understanding of the role of TME in tumor development, immunotherapy based on TME shows an important research value and clinical significance.
There are complex network relationships among the components of TME, and a deep understanding of the composition and characteristics of TME is the premise of developing immunotherapy based on the tumor microenvironment. In this review, we highlighted the tumor microenvironment and immunotherapy strategies of oral cancer. First, the compositions and functions of TME in oral cancer were presented and reviewed. More importantly, the recent development of treatment strategies for oral cancer was summarized, especially for immunotherapy. Finally, the promising development directions of immunotherapy for oral cancer in the future were prospected.
Microenvironment of oral cancer
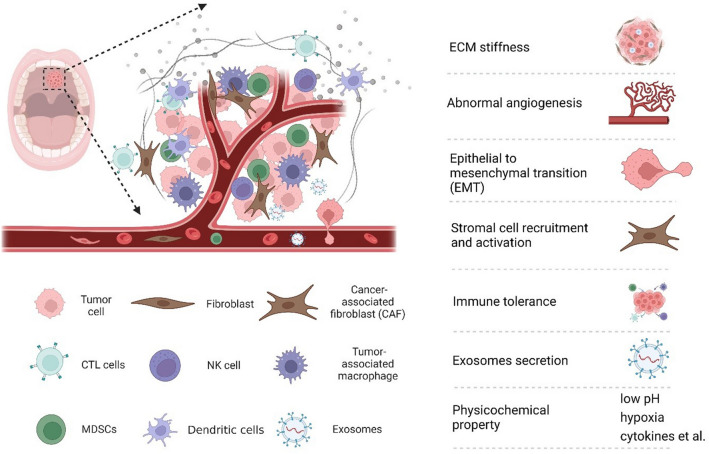
The occurrence, development, and metastasis of tumors are closely related to external environment of oral cancer cells, including the structure, function, and metabolism of tumor tissue, as well as the internal environment of cancer cells (Fig. 1). Tumors and their microenvironment are interdependent and antagonistic [23].
Fig. 1.
Schematic diagram of the microenvironment of oral cancer
Tumor extracellular matrix
ECM is a complex network structure composed of macromolecules secreted by tumor cells into the extracellular stroma [24]. The regulatory abnormalities of ECM are a prominent feature of TME. In the process of tumor genesis and development, tumor cells promote the formation of ECM; in turn, ECM can also regulate the related proliferation of tumor cells. The interaction between tumor cells and ECM can activate multiple specific signaling pathways [25, 26]. Therefore, an adequate understanding of ECM dysregulation is beneficial to identify potential tumor therapeutic targets. The structure and function of collagen, fibronectin, elastin, and other ECM components will be discussed.
Collagens accounts for about 90% of human external matrix and 30% of total protein [27]. Collagen forms macromolecules through cross-linking between molecules and enhances the strength and toughness of tissues [28]. In addition, non-collagenous domains present in collagen can self-assemble or assemble with other ECM proteins, providing the complexity of the supramolecular structure. Thus, collagen can form fibers, beaded fibers, anchored fibers, and even collagen networks. Collagen is a protein with a high glycosylation level and a long half-life, and its degradation is crucial in the formation and development of tumor tissues. Matrix metalloproteinases (MMPs) are involved in the physiological and pathological degradation of collagen. In the process of collagen degradation by MMPs, several signal molecules are released from collagen, subsequent altering the mechanical properties and signal transduction in TME [29]. Collagen is a major component of tumor ECM, which affects tumor cell proliferation and intercellular signaling.
Fibronectin, although low expression in tumors, shows multiple functions in ECM [30, 31]. Fibronectin works in cell adhesion, migration, proliferation, blood coagulation, and vascularization [32]. In ECM, fibronectin connects the structural proteins to construct a complete matrix [33]. In addition to binding multiple structural proteins to enhance ECM, fibronectin can also interact directly with other proteins to perform regulatory functions [34]. First, fibronectin is rich in arginine-glycine-asparagine (RGD) sequences to recognize and bind integrins on cell membranes. Therefore, fibronectin works in intracellular signal transduction through induction of integrin attachment. In addition, fibronectin can also interact directly with many growth factors, such as insulin-like growth factor (IGF), fibroblast growth factor (FGF), TGF-β, hepatocyte growth factor (HGF), and platelet-derived growth factor (PDGF) [35]. Therefore, despite the low levels of fibronectin, it still plays a key role in tumor malignant transformation.
Elastin is an important component of elastic fibers, which mainly exists in ligament and vascular wall. Together with collagen, elastin maintains the strength and strength of tissue by resisting it from deforming or breaking. Elastin is highly elastic compared to collagen due to its amino composition and dynamic three-dimensional structure. Laminin proteins, combined with collagen, form the components of the basement membrane and involves in the vascularization during vascular maturation [36]. Laminin is upregulated during epithelialization, providing an interface for epithelial cell adhesion, allowing it to adhere and stretch, therefore alerting tumor metastasis.
Hyaluronic acid (HA) is another major component of ECM, which has important functions, such as regulating vascular wall permeability, promoting wound healing, and material diffusion and transportation [37, 38]. The number of disaccharide repeat units in HA molecule reaches more than 10,000 Da. The long polymer chain forms random entanglement in the solution, and a large number of hydroxyl groups capture water molecules by forming hydrogen bonds, thus increasing the elastic viscosity of ECM [39]. In addition, HA can serve as an important "reservoir" for water, buffered ion exchange, and water and osmotic balance in ECM. Some substances and biomacromolecules have selective permeability due to their charged surfaces and selective domains. Furthermore, HA can be recognized by tumor cells through intracellular signal transduction molecules, such as membrane receptor CD44. This specific recognition plays a crucial role in the migration and invasion of tumor cells [40, 41].
In addition, ECM is an important "catalyst" for the realization of a variety of growth factors. First, ECM can act as a repository for growth factors and signaling molecules for malignant transformation of tumor cells. PDGF can effectively accumulate in ECM after combining with collagen [42]. Heparin binding growth factor 1 (HBGF-1), a growth factor associated with angiogenesis, also binds type I and type IV collagen [43]. Paralkar et al. illustrated that TGF-β could bind with type IV collagen in the basement membrane [44]. Furthermore, ECM can promote the interaction between growth factors and their receptors. Heparin sulfate proteoglycan could promote the interaction between Wnts and Frizzled, thereby stimulating the proliferation of HCC cells [45]. In addition, degradation of ECM contributes to the release of growth factors and cytokines. MMP is overexpressed in tumors, and a large amount of vascular endothelial growth factor (VEGF) will be released after ECM cutting, which promotes tumor development [46].
Vascular microenvironment
TME is closely related to the generation of tumor angiogenesis; the diameter of the tumor is usually no more than 2 mm in the absence of tumor angiogenesis [47]. When the diameter of solid tumor exceeds 2 mm, it is difficult to obtain enough oxygen and nutrients from the surrounding environment, and tumor cells will secrete angiogenic factors into TME to induce the formation of new blood vessels [48]. Tumor cells directly or indirectly participate in the formation of tumor blood vessels. In hypoxic environment, hypoxia-inducible factor (HIF) directly upregulates the gene expression of VEGF and other angiogenic factors [49]. Marjon et al. found that VEGF mRNA expression level was increased by 10–50 times when the oxygen concentration of tumor cells was reduced from 21 to 0.3% [50]. The mRNA expression of VEGF was relatively high in the necrotic and vascularless areas of tumor tissue. Tumor cells polarize the recruited cells to form tumor-associated stromal cells, promote the expression of vascular growth factor, and indirectly induce the formation of tumor angiogenesis. VEGF can bind to vascular endothelial growth factor receptor (VEGFR) in tumor tissues to activate downstream signaling pathways and change vascular permeability, thereby promoting tumor angiogenesis [51]. Tumor angiogenesis is closely related to tumor growth and metastasis. Inhibition/destruction of tumor angiogenesis microenvironment will be one of the potential tumor treatment strategies.
Stromal cells
Stromal cells mainly include cancer-associated fibroblasts (CAFs), endothelial cells, and adipocytes. They can perform signal transmission and secrete cytokines to regulate tumor cell proliferation, metastasis, and escape from immune system attack through direct or indirect interaction with tumor cells. It is greatly significant for the diagnosis and treatment of tumors by exploiting the role of stromal cells in tumor genesis, development, and metastasis.
CAFs are derived from fibroblasts and mesenchymal stem cells inherent to local tissues of bone marrow and fat. CAFs exist in all stages of solid malignant tumors. CAFs can differentiate into static fibroblasts and bone marrow-derived mesenchymal stem cells, as well as epithelial cells, smooth muscle cells, pericytes, and adipocytes [52]. Tumor cells can recruit CAFs precursor cells and induce their activation into CAFs. In addition, CAFs can secrete a variety of cytokines and ECM to promote tumor proliferation and metastasis. CAFs also involve in vascular generation, ECM remodeling, immunosuppression, and other exogenous pathways conducive to tumor genesis and development [53]. Generally, CAFs inhibit tumors initially, but as tumors further deteriorate and develop, they transform into tumorigenic cells [54]. Cytokines and chemokines secreted by CAFs have immunosuppressive and activation effects on a variety of lymphocytes, including CD8+ T cells, DCs, macrophages, and Tregs. However, CAFs have an immunosuppressive effect against the immune system. The secretion of IL-6, CXCL-9, and TGF-β by CAFs plays a significant inhibitory role in anti-tumor T cell response [55]. CAFs recruit and retain T lymphocytes in tumor tissues through different mechanisms, such as chemokines, cell adhesion molecules, inhibition of immune checkpoint activation, and CD8+ T cell exhaustion [56]. In addition, CAFs are the sources of a variety of growth factors, including TGF-β, VEGF, chemokines, and interleukins [57]. Secretion of these factors can promote the transformation of tumor cells, enhance the dryness of existing tumor stem cells, and promote epithelial mesenchymal transformation (EMT) of tumor cells [58].
Macrophages are another kind of stromal cells in tumor tissue with remarkable heterogeneity and variability. Macrophages can transform their functions and phenotypes according to the surrounding environment [59]. Generally, macrophages can be divided into M1 and M2 subtypes [60]. The M1 subtype consists of classically activated pro-inflammatory macrophages that have bactericidal, tumor-suppressive, and antiangiogenic functions. They can be activated through their pattern recognition receptors when recognizing molecular patterns associated with damaged tissues or pathogens, and then produce inflammatory cytokines, including macrophage colony-stimulating factor 1 (M-CSF1) and granulocyte–macrophage–CSF [61]. M2 subtype macrophages can produce anti-inflammatory, immunosuppressive chemokines, and cytokines, but have no cytotoxic activity to tumor cells [62]. Tumor-associated macrophages (TAMs) are induced to differentiate by growth factors and cytokines in tumor tissues [63]. Recruitment and differentiation of TAM macrophages at tumor sites are mainly induced by granulocyte–macrophage colony-stimulating factor (GM-CSF), CCL2, VEGF, IL-6, and IL-8, which are related to hypoxia, acidity, and inflammation of tumor tissues [64]. TME invasion is determined by CC chemokines produced by local lymphoendothelial cells and stromal cells, which have been confirmed in multiple tumor types [65]. TAM, guided by the increasing gradient of chemotactic molecules, infiltrates the hypoxic/necrotic regions of tumors in large quantities and survives by shifting its metabolism to glycolysis [66]. Besides, TAM can produce high levels of TGF-β, blocking the proliferation and killing effects of cytotoxic T cells, while activating immunosuppressive Treg cells [67].
Another important cell type in tumor tissue is adipocytes. Adipocytes in TME can secrete a variety of tumor-related adipocytokines, which works in the occurrence and development of tumors [68]. Due to the influence of hypoxia and high pressure in tumor microenvironment, pathological metabolic disorder occurs in adipocytes, which changes the secretion of adipokines and lipid metabolites around tumor cells, activates signal transducer and activator of transcription 3 (STAT3) and other tumor-related signaling pathways, and accelerates the further development and deterioration of tumor [69].
Immune cells
Tumor-infiltrating lymphocytes (TILs) are the collective term of all lymphocytes in tumor tissue [70]. TILs are produced by lymphoid organs and circulated to tumor tissues. They are the main cellular components of the host in response to anti-tumor immune response. TILs can be divided into T lymphocytes, B lymphocytes, and natural killer (NK) cells according to its origin and surface markers.
T lymphocytes play a central role in anti-tumor immune response and are the dominant element in TME. Tumor-specific antigen (TSA) can activate highly specific CD8+ T cells CD4+ T cells and tumor-specific antibodies [71]. CD8+ T cells inhibit tumor proliferation by direct lysis of tumor cells or release of IFN-γ and TNF-α [72]. Prolonged exposure to antigens of tumor-specific T cells can lead to their exhaustion. Programmed cell death-1 (PD-1) and T cell immunoglobulin-3 (TIM-3) are considered as immune markers of T cell exhaustion [73]. CD8+ T cells may play a functional state of immune activation or immunosuppression based on the balance between costimulatory and coinhibitory signals in the microenvironment [74]. CD4+ T cells are mainly including Th1, Th2, Th17, and Tregs. Th1 stimulates the tumor-killing effect of CD8+ T cells through cytokines, such as IFN-γ, TGF-β, and IL-2 [75]. Furthermore, it can also promote the activation of macrophages and the maturation of DC cells, while Th2 can assist eosinophils to achieve killing effect [76]. Th17 cells can be converted to Th1 properties and play an anti-tumor role in specific cytokine environments. On the contrary, they are capable to be converted to achieve the properties of Tregs and promote tumor progression in some special conditions [77]. Tregs are a subtype of CD4+ T cells to prevent autoimmune diseases. They are recruited to TME by chemokines secreted by tumor cells and macrophages, further inhibiting the anti-tumor immune response [78]. Tregs are activated after recognizing the tumor-associated antigen (TAA) released by damaged tumor cells, effectively inhibiting the activation of TAA-specific effector T cells, releasing a variety of cytokines, and ultimately inhibiting the anti-tumor immune-killing effect [79]. In addition, Tregs can inhibit the action of a variety of other immune cells [80]. The high infiltration rate of Tregs in tumors is closely associated with local recurrence, rapid tumor progression, and high sentinel lymph node metastasis rate [81]. The ratio between different T cell subsets is also an important indicator of tumor occurrence and development. CD8+/FoxP3+ and CD8+/CD4+ ratios are the most commonly used indicators, indicating the strength of anti-tumor immune activity. In order to escape immune attack, tumor cells inhibit the role of tumor-specific T cells and induce the activation of immunosuppressed Tregs, thus reducing the ratio of CD8+ T cells/Tregs [82].
NK cells, derived from bone marrow lymphoid stem cells, differentiate and mature in bone marrow and thymus. They can kill tumor cells nonspecifically without dependence on major histocompatibility complex (MHC) [83, 84]. NK cells participate in the regulation of adaptive immune response through interaction with DC cells and arise a strong cytotoxic immune response against tumor cells [85]. Tumor cells resist NK cell killing by releasing TGF-β, downregulating antigenic expression and increasing MHC I [86]. In addition, Tregs can also compete with NK cells for IL-2, thereby inhibiting NK cell activity. Tumor-infiltrating NK cells can limit blood metastasis of tumor cells [87].
B lymphocytes are derived from bone marrow pluripotent stem cells, accounting for 15–20% of all infiltrating lymphocytes [88]. B lymphocytes can be differentiated into plasma cells under antigen stimulation, which can secrete antibodies and activate humoral immune response of the body. The infiltration of B lymphocytes is associated with primary melanoma proliferation, reduced risk of metastasis, and prolonged survival [89]. The mechanism of the role of tumor-infiltrating B lymphocytes in anti-tumor immune response is still unclear and needs to be further studied.
Dendritic cells (DCs) are specialized antigen presenting cells that can absorb, process, and present antigens and initiate specific immune responses mediated by T lymphocytes. DCs can recognize tumor antigens to regulate the innate and adaptive immunity [90]. DCs cross-presents antigens to CD8+ T lymphocytes through MHC I molecules after obtaining tumor-associated antigen, thus inducing the hosts’ specific anti-tumor response. DCs can also directly participate in cytotoxic immune responses by activating NK cells [85]. In TME, IL-10, TGF-β1, VEGF, and other factors secreted by tumor cells or TAMs can inhibit the maturation of DCs, thus avoiding the host's anti-tumor immune response [91]. Immature/tolerant DCs can regulate tumor angiogenesis and contribute to tumor proliferation [92]. Mature DCs are mainly distributed around the tumor and are related to the activation state of T lymphocytes, tumor size, and patient survival. The number and distribution of mature DCs can be used as an indicator of the efficacy of immunotherapy for tumor patients [93].
Immune checkpoints
Immune checkpoint (IC) refers to programmed death receptors and their ligands. Immune checkpoint blockade (ICB) therapy based on programmed death receptor (PD-1) and its ligand (PD-L1) can improve the host immune system's aggression against tumor cells by inhibiting the binding of PD-1 and PD-L1 [94]. The basic principle of ICB therapy is based on the activation mechanism of immune cells called T cells. Programmed death receptors are expressed on the surface of T cells and their ligands are expressed on tumor cells and myeloid suppressor cells. The combination of programmed death receptor and its ligand can cause T cells to fail to kill tumor cells, so that tumor cells can escape the immune surveillance of the host. Therefore, ICB therapy based on PD-1/PD-L1 can improve the host immune system's aggression against tumor cells by inhibiting the combination of programmed death receptor and its ligand [95]. Cytotoxic T lymphocyte antigen-4 (CTLA-4) is another classical immune checkpoint and can be phosphorylated to activate phosphoinositide 3-kinase (PI3K) pathways to achieve dephosphorylation of the CD3ζ chain, which limits the signaling potential of the TCR. There are also other important ICs, such as lymphocyte activation gene 3 (LAG-3), TIM-3, Exostosin-like glycosyltransferase 3 (EXTL3), V-domain Ig suppressor of T cell activation (VISTA), and indoleamine 2,3-dioxygenase (IDO) [96–98]. In 2011, the US Food and Drug Administration (FDA) approved the first ICB antibody Ipilimumab for advanced melanoma, and tumor immunotherapy has received unprecedented attention and development since then [99]. In 2018, the Nobel Prize in Physiology or Medicine was awarded to James Allison and Tasuku Honjo for their outstanding contributions to ICB therapy of cytotoxic T lymphocyte antigen-4 (CTLA-4) and PD-1/PD-L1 signaling pathways, respectively [100]. Together, ICB therapies open the possibility of including TME-based markers for selecting patients who are likely to respond to these specific therapies and pave the way to personalized medicine in oncology [101].
Exosomes
In TME, cell-to-cell communication is one of the important factors affecting tumor progression. Exosomes, as important signal carriers of cell-to-cell communication in TME, have attracted much attention in recent years [102]. Exosome refers to the extracellular sac with lipid bilayer membrane structure formed by cells under physiological and pathological conditions through “endocytosis-fusion-efflux” and other regulatory processes [102], mainly containing proteins, mRNA, micro-RNA, and cytokines. Exosomes are involved in many important pathophysiological processes, including remodeling TME, mediating specific cellular communication, regulating angiogenesis, immune escape, and distal metastasis in TME, thereby regulating tumor genesis and development [103]. There are conflicting mechanisms between immune promotion and immunosuppression of exosomes. Tumor-derived exosomes can both stimulate and inhibit specific and non-specific immune responses [104]. Exosomes containing tumor-associated antigens can be effectively absorbed by antigen-presenting cells, such as DCs, resulting in anti-tumor effects [105]. In addition, exosomes produced by premetastatic tumors trigger a broad innate immune response through immune monitoring, leading to the elimination of cancer cells in the premetastatic niche [106]. Exosomes are involved in immune suppression. Exosomes isolated from plasma of head and neck cancer patients can effectively induce apoptosis of CD8+ T cells, inhibit proliferation of CD4+ T cells, and enhance immunosuppression of regulatory T cells. Razzo et al. [107] confirmed that a single intravenous injection of tumor-derived exosomes was sufficient to accelerate tumor progression in mice with OSCC precancerous lesions and reduced the migration of immune cells to tumors.
Physicochemical microenvironment
Due to the rapid proliferation of tumor cells, glucose and energy are consumed seriously, and oxygen is exhausted, which leads to the increase of lactic acid level, interstitial pressure, and acidification of microenvironment in tumor tissues [108].
In normal tissues, extracellular pH is tightly regulated, while dysregulation of pH often occurs in tumor microenvironment (pH 6.7 ~ 7.1). It is mainly due to anaerobic glycolysis and lactic acid produced by tumor cells. The pKa value of lactic acid is approximately 3.9, which is always lower than the pH value of tumor microenvironment, so it usually exists in the form of lactate and H+ ions. In addition, CO2, produced by respiration synthesizes, can be metabolized into H2CO3 under the action of carbonic anhydrase, which can be dissociated into HCO3− and H+ ions [109]. Moreover, abnormal blood perfusion and the absence of functional lymphatic vessels further limit acid excretion from the TME. TME can promote degradation of ECM, leading to local invasion of tumor cells [110]. Acidity is a common characteristic in tumor microenvironment, while delivery systems targeting low pH of tumor tissues can be developed to selectively deliver drugs to tumor cells, so as to achieve specific killing of tumors [111].
Hypoxia is another important feature of TME, which can accelerate the survival and invasion of tumor cells [112]. Hypoxic is often considered as a major adverse driver of immunotherapy resistance, resulting in gene mutations, transformation of signal transduction, and activation of effector molecules [113, 114]. In a hypoxic environment, HIF-1α loses its degradation properties and binds to HIF-1β to form transcriptionally active dimers [115]. These dimers can interact with transcriptional coactivators to induce transcription regulation of target genes associated with immune escape. Hypoxia regulates tumor immunomodulatory process: (1) Hypoxia can recruit various immunosuppressive cells, mainly affecting Tregs, TAM, and marrow-derived suppressor cells (MDSCs), therefore promoting the immune tolerance of tumor cells; (2) Hypoxia can upregulate the expression of inhibitory immune checkpoint on the surface of tumors, thus inhibiting the specific function of T cells and evading host immune surveillance [116]; (3) Autophagy can be induced in a hypoxic environment. Autophagy is a key regulator of cell viability, clearing dysfunctional organelles and preventing the progression of cancer [117]. Nevertheless, advanced tumors can degrade aggregates of harmful proteins [118], remove ROS [119], and promote recruitment of Treg cells [120] by autophagy, ultimately achieving immune escape; (4) Hypoxic-mediated immune effector cell inhibition. Immune effector cells, such as T cells, DCs, and NK cells, were inhibited in hypoxic environment. Hypoxia not only inhibits the ability of CTL to melt tumor cells but also greatly affects the proliferation and activity of CTL and ultimately fails to provide enough CTLs to eliminate tumor cells [121]. Downregulated expression of costimulatory molecules of DCs in hypoxic environment failed to effectively induce T cell activation [122].
In summary, the occurrence, development, metastasis, and recurrence of oral cancers are inseparable from specific tumor immune microenvironment. With the deep understanding of TME, the specific cancer immunotherapy will dramatically alter the landscape in fundamental researches and clinical application of oral cancers.
Immunotherapy for oral cancer
The clinical treatment of tumor is mainly surgery, radiotherapy, and chemotherapy [123]. Surgical resection is currently the preferred treatment for many early and intermediate stage cancers, and can be treated by partial or total removal of the lesion site and surrounding tissue. However, surgical resection is traumatic and limited. It is generally applicable to the primary lesion, unable to eliminate the metastatic cancer cells and unable to touch the cancer cells in the blood circulation. In addition, surgical treatment of patients with higher physical requirements; otherwise it will accelerate the spread and metastasis of cancer cells due to reduced immunity [124]. Radiation therapy is to use radiation to irradiate cancerous tissue and destroy the DNA chain of cancer cells to achieve local treatment of cancer. Radiotherapy usually has a long treatment cycle, high cost, and will produce a variety of complications, damage the body function, and weaken the body immunity [125]. Chemical treatment usually adopts systemic drug treatment, the lack of selectivity, will affect normal cells, side effects, although the molecular targeted drugs and new preparations of nanometer drug has made significant progress, but the treatment effect is not satisfactory, and the cancer is easy to produce resistance and resistance to chemotherapy drugs, tends to increase treatment difficulty [126]. On the other hand, the high osmotic pressure of tumor tissue makes it difficult for chemotherapy drugs to penetrate into the tumor site, which further limits the efficacy of chemotherapy. Generally, traditional Chinese Medicine plays an auxiliary role in reducing complications from chemotherapy or other treatments.
Although the above-mentioned traditional tumor treatment methods can temporarily curb the development of tumor, they cannot fundamentally solve the problems of tumor recurrence and metastasis. Moreover, oral cancer shows a high mutation rate associated with DNA damage caused by smoking and alcohol [127]. Therefore, it is urgent to develop new cancer treatment strategies to achieve efficient tumor treatment while reducing toxicity and improving the quality of life of patients.
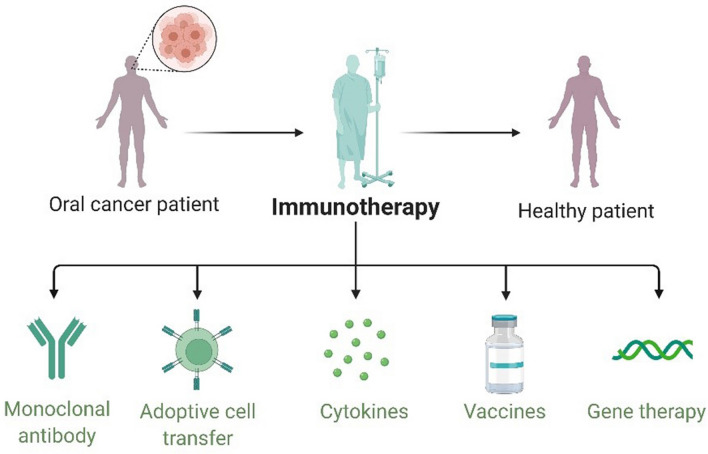
Immunotherapy is a new strategy for tumor therapy, which applies biotechnology and immunological methods to improve the specific immune response to tumor [128, 129]. Tumor immunotherapy was awarded as the most important scientific breakthrough by Science in 2013 due to its excellent efficacy and innovation [17]. In 2020, the Nobel Prize in Chemistry was awarded to French microbiologist Emmanuelle Charpentier and American biologist Jennifer Doudna for their "development of genome editing methods." Immunotherapy has outstanding application value in the field of tumor therapy, including adoptive cell immunotherapy, antibody-based therapy, cytokine therapy, tumor vaccines therapy, and gene therapy (Fig. 2).
Fig. 2.
Immunotherapy strategies for oral caners
Monoclonal antibody-based therapies
Monoclonal antibodies (mAbs) are highly homogeneous antibodies produced by a single B cell clone that bind to a specific epitope. In 1975, the mAbs technology were first developed by Köhler and Milstein by using B cell hybridomas, with unlimited reproductive ability as well as secreting specific antibodies [130]. In 1997, Rituximab was the first mAb approved by FDA for the immunotherapy of B cell non-Hodgkin’s lymphomas by targeting CD20 [131]. Benefiting from the rapid development of immunology and protein engineering, mAbs immunotherapy is currently the fastest growing immunotherapy. These drugs can recruit T cells to the tumor site, directly target the tumor cells, change the host's response to the tumor, and thus inhibit or even eliminate the tumor [132, 133]. Furthermore, mAbs are capable to achieve anti-tumor angiogenesis by inhibiting the oxygen supply and nutrient transport of tumor cells [134]. After decades of research and development, mAbs have shown unique advantages and made great progress in the field of cancer and other major diseases, and they are also the fastest growing and most promising development direction in the field of medicine. There are several ongoing studies with mAbs in oral cancers related to the key words (“oropharyngeal cancer” OR “oral cancer” OR “oral cavity cancer”) and (“Monoclonal antibodies” OR “Monoclonal antibody” OR mAbs OR mAb) from ClinicalTrials. Gov. As shown in Table 1, there are 9 clinical trials, which include 4 anti-angiogenesis mAbs and 5 immune checkpoint inhibitors. In addition, there are several other targets were also important for monoclonal antibody-based oral cancer clinical treatment, including receptor tyrosine-protein kinase erbB-3 [135], nucleophosmin [136], and c-Met protein with tyrosine kinase activity [137]. Moreover, monoclonal antibodies have shown great potential in the prevention of oral cancer. Oral proliferative verrucous leukoplakia (OPVL) is a rare refractory leukoplakia, which is a precancerous lesion of oral cancer. It is likely to develop into oral squamous cell carcinoma or verrucous carcinoma. Nivolumab shows potential to shrink the white lesions in the participant's mouth and reduce cancer risk and is undergoing clinical trial for prevention of OPVL (NCT03692325).
Table 1.
Monoclonal antibody-based trials for oral cancers
| Product name | Sponsor | Target | Estimated date of completion | Phase | Status | Identifier |
|---|---|---|---|---|---|---|
| Cemiplimab | ISA Pharmaceuticals | PD-1 | November 2024 | II | Recruiting | NCT04398524 |
| Bevacizumab | National Cancer Institute (NCI) | VEGF-A | March 2010 | I | Completed | NCT02002182 |
| Anti-EGFR monoclonal antibody | Fudan University | EGFR | December 2020 | II | Recruiting | NCT04508829 |
| Nivolumab | Medical University of South Carolina | PD-1 | November 2021 | I/II | Completed | NCT03021993 |
| Cetuximab | Eastern Cooperative Oncology Group | EGFR | April 2004 | III | Completed | NCT00003809 |
| Sintilimab | Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University | PD-1 | October 2027 | II | Not yet recruiting | NCT05000892 |
| Toripalimab | Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University | PD-1 | June 2027 | II | Not yet recruiting | NCT04825938 |
| Anti-OX40 antibody | Providence Health & Services | OX40 | October 2022 | I | Active, not recruiting | NCT02274155 |
| Bevacizumab | M.D. Anderson Cancer Center | VEGF | March 2022 | I | Active, not recruiting | NCT01552434 |
PD-1 Programmed cell death-1; VEGF-A Vascular endothelial growth factor A; EGFR Endothelial growth factor receptor; OX40 (CD134) A member of the tumor necrosis factor family of receptors
Tumor microenvironment can induce immune tolerance of tumor-specific T cells, weaken T cell function, and lead to immune escape [138]. Among them, the main strategy of tumor cells to achieve immune escape is overexpression of immune checkpoint molecules. Immune checkpoint inhibitors (ICIs) are one of the most promising immune-based interventions. Blockade of immune checkpoint signaling pathway is capable to activate anti-tumor immune response and shows extraordinary clinical application value. The PD-1/PD-L1 blockade has been identified as a potential therapeutic pathway to inhibit the activation of T cells and cytokine production in tumor cells [139, 140]. So far, four mAbs have been approved against PD-1/PD-L1 blockade in clinical studies for oral cancer treatment, including cemiplimab (NCT04398524), nivolumab (NCT03021993), sintilimab (NCT05000892), and toripalimab (NCT04825938). OX40 is a costimulatory molecule that can enhance T cell immunity. MEDI6469, an anti-human OX40 neoadjuvant antibody, was performed in a phase I clinical trial (NCT02274155) prior to surgery. The results demonstrated that anti-OX40 mAb was safe and could induce the activation and proliferation of T cells in hosts, suggesting a potential clinical strategy [141]. Furthermore, mAbs blocking other immune checkpoint receptors, particularly CTLA-4, TIM-3, LAG-3, and IDO, have attracted worldwide attentions due to their impressive results [142]. Nevertheless, the overall response rate of mAbs in the treatment of oral cancers should be further improved.
Tumor angiogenesis is a complex process including vascular endothelial matrix degradation, endothelial cell migration and proliferation, vascular branching, and formation of new basement membrane [143]. Due to abnormal structure and imperfect vascular matrix of tumor vessels, tumor cells can directly penetrate into blood vessels to form metastasis in distant sites without a complex invasion process [144]. Benign tumors have little angiogenesis and slow blood vessel growth, while malignant tumors demonstrate dense angiogenesis and rapid growth. Therefore, angiogenesis plays an important role in the development and metastasis of tumor, and inhibition of this process can significantly prevent the development and metastasis of tumor tissue [145]. As an anti-VEGF mAb, Bevacizumab was first approved by FDA for metastatic colorectal cancer treatment in 2004 and spread to non-small cell lung cancer, glioblastoma, metastatic renal cell carcinoma, advanced cervical cancer, drug-resistant ovarian cancer, metastatic hepatocellular carcinoma, and oral cavity carcinoma et al. [143]. There are 2 clinical trials of Bevacizumab for oral cancer. National Cancer Institute (NCI) has completed its primary outcome measure on March 2010 in Phase I clinical trial (NCT00023959). M.D. Anderson Cancer Center is ongoing its Phase I clinical trial (NCT01552434). This study will evaluate the side effects and best dose of Bevacizumab alone or in combination with other drugs. Based on EGFR-targeted therapy, two clinical trials were performed in the treatment of oropharyngeal carcinoma. Eastern Cooperative Oncology Group evaluated the effectiveness of cetuximab with cisplatin for metastatic or recurrent head and neck cancer patients by the randomized double-blinded phase III trial (NCT00003809). Wang et al. carried out a prospective phase II trial to evaluate the efficacy and safety of anti-EGFR monoclonal antibody combined with intensity-modulated radiation therapy in locally advanced oropharyngeal carcinoma (NCT04508829).
Adoptive cell transfer
Adoptive cellular immunotherapy (ACI) is an important method in tumor biotherapy. ACI refers to the transfer of tumor patients with anti-tumor activity of immune cells, directly killing tumor or stimulate the body’s immune response to kill tumor cells, so as to achieve the purpose of tumor treatment [146]. ACI can be used in clinical treatment of cancer patients alone, more importantly, as a supplement to surgery, radiotherapy, and chemotherapy. Currently, the clinical research and application of ACI therapy cells mainly include lymphokine-activated killer (LAK) cells, chimeric antigen receptor T (CAR-T) cells, CD3AcAb-activatived killer (CD3AK) cells, cytokine-induced killer (CIK) cells, and DC cells [147].
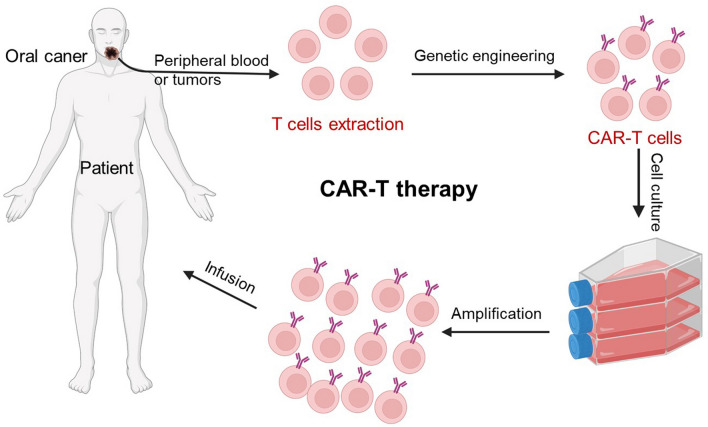
CAR-T cell therapy, one of the most important ACI, has obtained obvious success against hematological tumors by targeting tumor antigens [148]. To obtain CAR-T cells, T cells are isolated from patient’s peripheral blood, then the CARs are transduced by genetic engineering technique to achieve the targeted recognition of tumor cells. After cell amplification, the autologous CAR-T cells are infused back into patients [149]. In 1993, Zelig Eshhar et al. first proposed CAR-T treatment [150]. Over the next 3 decades, this technology has gone through five generations and gradually matures. The latest generation of CAR-T cells contains CD3ζ chain for signal transduction, a co-stimulatory molecule for activation and proliferation, and intracellular domains of cytokine receptors [143, 151]. Mei et al. constructed MUC1-targeting CAR-MUC1-IL22 T cells and validated the cytotoxic function in human tongue squamous carcinoma cells. These cells showed an effective cytotoxic function against tumor cells [152]. Chan and co-workers screened out CD70 CAR-T cells among nine potential targets and evaluated their anti-tumor effect in human oral squamous cell carcinomas. The results demonstrated the specific recognition and efficient elimination of CD70-positive cancer cells and provided a potential CAR-T target [153]. Recently, Yang et al. constructed an all-in-one CAR-T cells by coupling anti-human epidermal growth factor receptor 2 (anti-HER2) CAR signaling and clustered regularly interspaced short palindromic repeats interference (CRISPRi)-mediated PD-1 gene suppression. These symphysis CAR-T cells reversed PD-1/PD-L1 immune checkpoint inhibition and promoted the persistence and effectiveness against HER2-expressing human head and neck squamous cell carcinoma [154].
Although CAR-T therapy has obtained amazing achievements (Fig. 3), the potential severe toxicities still limit their widespread application, mostly cytokine storms, which can induce high fevers and even multi-organ dysfunction [155]. In addition, the infiltration of solid tumors is another major obstacle for CAR-T therapy. Therefore, degradation of tumor extracellular matrix or regulation of tumor microenvironment are expected to be the effective ways to improve the infiltration of CAR-T cells [156]. Significantly, the immunosuppressive microenvironment of tumors further hinders the effectiveness of CAR-T therapy. Combining immunosuppressive microenvironmental regulation strategies with CAR-T cells may provide a synergic anti-tumor therapeutic effect [157].
Fig. 3.
Schematic diagram of CAR-T therapy
Cytokine therapy
Cytokines are generally produced by stimulated immune cells and shows high efficiency. They can directly stimulate immune effector cells and stromal cells at the tumor site and enhance the killing effect of immune cells [158]. Recently, there are several cytokine drugs approved by FDA, such as high-dose IL-2 for the treatment of melanoma and kidney cancer [159], and IFN-α for the adjuvant treatment of stage III melanoma [160]. IL-12 works on activated T and NK cells and has a wide range of biological activities, acting on lymphocytes by regulating activators of the transcriptional protein STAT4. IL-12 is required for T cell independent induction of IFN-γ and plays an important role in the differentiation of Th1 and Th2 cells [161]. Yang and colleagues illustrated that the receptor of IL-12 family member (IL-23) contributed for the tumor lymph node metastasis of oral cancer [162]. IFN-γ belongs to type II interferon, which is mainly produced by NK and NKT cells and has anti-proliferative effect on transformed cells, and can strengthen the antiviral and anti-tumor effects of type I interferon [163]. Ma et al. detected the decreased expression of IFN-γ mRNA and protein in oral cancer tissues, which was negatively correlative with IFN-γ methylation rate, involving in the process of tumorigenesis of oral cancer [164]. GM-CSF is the earliest found cytokine that has effects on DC cells. GM-CSF induces the differentiation of bone marrow dendritic cells (BMDCs), promotes the immune response of Th1 cells, induces angiogenesis, and influences the development of allergic inflammation and autoimmune diseases [165]. TNF-α belongs to the TNF superfamily of cytokines, which is a multifunctional molecule regulating biological processes. TNF-α can not only kill tumor cells directly but also induce immature DC to differentiate into mature DC [166]. There are several ongoing studies with mAbs in oral cancers related to the key words (“oropharyngeal cancer” OR “oral cancer” OR “oral cavity cancer”) and (“cytokine therapy” OR cytokines OR IFN-α OR IL-2 OR TNF-α) from ClinicalTrials. Gov. As shown in Table 2, there are 8 clinical trials, which include 4 IFN-α, 3 interleukin, and 1 salivary cytokine. With the further study of cytokines, more factors will be applied to tumor immunotherapy.
Table 2.
Cytokine therapy clinical trials for oral cancers
| Cytokines | Sponsor | Country | Estimated date of completion | Phase | Status | Identifier |
|---|---|---|---|---|---|---|
| IL-12 | NYU Langone Health | United States | May 2009 | Completed | NCT00899821 | |
| IL-12 or GM-CSF | National Cancer Institute (NCI) | United States | May 2007 | II | Completed | NCT00019331 |
| PEG-IFN-α-2b | M.D. Anderson Cancer Center | United States | March 2006 | II | Completed | NCT00276523 |
| IL-2 gene | H. Lee Moffitt Cancer Center and Research Institute | United States | June 2004 | II | Completed | NCT00006033 |
| IFN-α | Eastern Cooperative Oncology Group | United States | May 2009 | III | Completed | NCT00054561 |
| IFN-α | Hoag Memorial Hospital Presbyterian | United States | February 1999 | II | Completed | NCT00002506 |
| Salivary cytokines | University Hospital, Basel, Switzerland | Switzerland | January 202 | Completed | NCT02807519 | |
| PEG-IFN-α-2b | Dartmouth-Hitchcock Medical Center | United States | November 2002 | I | Completed | NCT00014261 |
IL-12 interleukin-12; IFN-α interferon alfa
Tumor vaccines
Tumor vaccine is a specific, safe, and tolerable cancer treatment strategy. Currently, FDA has approved two preventive vaccines for the treatment of human papillomavirus (HPV) and hepatitis B virus, which can cause liver cancer [167]. HPV is etiologically involved in cervical cancers mainly through inactivation of tumor suppressor proteins, such as p53 and pRB. HPV is consistently and more frequently detected in cancers of the oropharynx and tonsil than at other head and neck sites [168]. In 2010, PROVENGE (Sipuleucel-T), an immune-based vaccine, was approved by FDA as the first therapeutic oncology vaccine for prostate cancer [169]. In addition, multiple tumor vaccines combined with checkpoint blocking modulators or cytokine therapies are evaluated in clinical trials with potential clinical application in solid tumors or metastatic tumors [170].
The anti-tumor process of tumor vaccines as follows: (1) Tumor-specific antigens are absorbed by antigen-presenting cells and further processed by DCs into short peptides that facilitate the presentation of MHC molecules, which are presented to initial CD8+ and CD4+ T cells by MHC I or MHC II, respectively. Antigenic peptides are recognized by TCR on the surface of T cells and provide the first signal of activation to T cells. (2) DCs are further activated and the expression of costimulatory molecules (B7 molecules) on cell surface is upregulated. The B7 molecules can bind to the CD28 molecule on T cells, providing a second signal for T cells to activate. (3) After the initial activation of CD8+ and CD4+ T cells, CD8+ T cells further proliferate and differentiate into effector T cells with tumor-killing ability, while CD4+ T cells mainly differentiate into Th1 and Th2 cells under the stimulation of different cytokines to assist cellular and humoral immunity of the host, respectively. (4) Effector T cells circulate and infiltrate into tumor tissues, recognize, and kill tumor cells. The killed tumor cells can further provide tumor antigens, amplifying the immune response [171, 172].
There are mainly three types of tumor vaccines, including inactivated vaccines, protein/polypeptide vaccines, and nucleic acid-based vaccines [173]. Live tumor cells can produce immunosuppressive cytokines and may form new tumors in vivo [174]. However, inactivated vaccines use TAAs from tumor cells excised from patients (autologous tumor cells) or cultured tumor cells (allogeneic tumor cells) as antigen sources, which are physically or chemically inactivated and added with adjuvants as tumor vaccines [175, 176]. A major advantage of using tumor cells as an antigen source is that there are a series of mutated tumor antigens on tumor cells that are more immunogenic than generic tumor antigens and can synergistically enhance the body to produce specific immune response [174]. Protein/polypeptide vaccines are mainly derived from protein fragments or intact proteins specifically expressed by tumor cells. Polypeptide vaccines are generally obtained by chemical synthesis, saving time, and cost [177]. Protein vaccines are achieved by recombinant protein technology [178]. Many preclinical and clinical trials have confirmed that protein/polypeptide vaccines are safe and have high clinical application value [177, 179]. However, protein/polypeptide vaccines usually target only one or more TAAs, so a combination of antigens is required to induce stronger tumor-specific T cell responses [37, 180, 181]. More than 70 percent of oropharyngeal cancers are associated with human papillomavirus infection. Therefore, HPV vaccine can prevent and treat oropharyngeal cancer [182]. In 2020, FDA approved an expanded indication of Gardasil 9 for the prevention of oropharyngeal and other cancer caused by HPV [183]. These vaccines were mainly developed against E6 and E7 proteins in clinical trials. Vaccines based on nucleic acids (DNA or RNA) are a promising vaccine platform. In the 1990s, scientists discovered that plasmid DNA can induce a strong antibody response against the antigens it encodes [184]. Nucleic acid vaccines have many advantages. First, nucleic acid vaccines allow simultaneous delivery of multiple antigens covering various TAAs or tumor mutations, while simultaneously activating the body's humoral and cellular immune responses, increasing the possibility of overcoming vaccine resistance. Secondly, unlike many peptide-based vaccines, DNA vaccine, encoding full-length tumor antigen, allows the APC cells present at the same time or cross-presented with class I and class II specific human leukocyte antigen (HLA) in patients with multiple epitopes, therefore stimulating a wider range of T cell response [185]. More importantly, nucleic acid vaccines are non-infectious and do not contain contamination from protein or viral sources during production and are therefore considered to be well tolerated for both prevention and treatment [173, 186]. Messenger RNA (mRNA) vaccines are an attractive alternative to DNA vaccines that have emerged in recent years for infectious disease prevention and anticancer therapy. On 18 November 2020, Pfizer announced the results of a Phase III clinical trial of BNT162b2 mRNA vaccine jointly developed with BioNTech in Germany, which showed a 95% protective efficiency. On December 31 2020, the World Health Organization (WHO) designated Pfizer/BioNTech's mRNA vaccine as available for emergency use, the first COVID-19 vaccine authorized by WHO. In addition to helping the world fight an urgent pandemic, the mRNA vaccine has greatly advanced the field of vaccine development. In a sense, the novel Coronavirus pandemic has hastened the development of mRNA vaccine, ushering in a new era of vaccine development. It is a predictable event that such vaccines, which can be produced quickly and cheaply, will soon appear in the field of cancer treatment, bringing benefits to human health [186, 187]. However, the problems of mRNA instability, low in vivo delivery efficiency and high innate immunogenicity still need to be further solved [188].
There are several ongoing studies with cancer vaccination in oral cancers related to the key words (“oropharyngeal cancer” OR “oral cancer” OR “oral cavity cancer”) and vaccine from ClinicalTrials. Gov. As shown in Table 3, there are 17 clinical trials, which include 10 HPV antigen-related vaccine trials, 4 protein/polypeptide-based vaccine trials, 2 vaccine-combined monoclonal antibody therapy, and 1 DC-based vaccine. In general, HPV antigen-related vaccines and combined therapies with other strategies are the primary strategies for oral cancers. In addition, the development of neoantigen and nucleic acid vaccine will be the potential prospects for oral cancer clinical treatment.
Table 3.
Tumor vaccination clinical trials for oral cancers
| Vaccines | Sponsor | Nationality | Estimated date of completion | Phase | Status | Identifier |
|---|---|---|---|---|---|---|
| HPV Vaccination | Oslo University Hospital | Norway | February 2017 | Completed | NCT02934724 | |
| ADXS-HPV | Andrew Sikora | United States | August 2023 | II | Active | NCT02002182 |
| Utomilumab and ISA101b | M.D. Anderson Cancer Center | United States | June 2022 | II | Active | NCT03258008 |
| HPV Vaccine PRGN-2009 Alone or in Combination with Anti-PD-L1/TGF-Beta Trap (M7824) | National Cancer Institute (NCI) | United States | October 2023 | I/II | Recruiting | NCT04432597 |
| HPV Vaccination | Regenstrief Institute, Inc | United States | June 2015 | Completed | NCT02551887 | |
| HPV Vaccination | Regenstrief Institute, Inc | United States | May 2016 | Completed | NCT02558803 | |
| HPV Vaccination | Boston Medical Center | United States | April 2019 | Completed | NCT03346915 | |
| HPV Vaccination | National Cancer Institute (NCI) | United States | July 2022 | Active | NCT00867464 | |
| Recombinant Fowl Pox Vaccine rF-CEA (6D)/TRICOM With GM-CSF | National Cancer Institute (NCI) | United States | January 2013 | I | Completed | NCT00028496 |
| TheraT® Vectors combined with chemotherapy | University of Chicago | United States | January 2026 | I/II | Not yet recruiting | NCT05108870 |
| PDS0101 + NHS-IL12 + M7824 | National Cancer Institute (NCI) | United States | January 2023 | I/II | Recruiting | NCT04287868 |
| Tumor-specific mutated Ras peptides and IL-2 or GM-CSF | National Cancer Institute (NCI) | United States | May 2007 | II | Completed | NCT00019331 |
| HPV Vaccination | National Cancer Institute (NCI) | United States | April 2015 | I | Completed | NCT00019110 |
| HPV Vaccination | University of Birmingham | United Kingdom | September 2015 | Completed | NCT01330147 | |
| Recombinant fowlpox-TRICOM | National Institute on Deafness and Other Communication Disorders (NIDCD) | United States | April 2015 | I | Completed | NCT00021424 |
| HPV16-E711-19 Nanomer | Dana-Farber Cancer Institute | United States | May 2023 | I/II | Active | NCT02865135 |
| DCs loaded with wild-type p53 peptides with T-helper peptide epitope | Robert Ferris | United States | March 2014 | I | Completed | NCT00404339 |
CEA Carcino-embryonic antigen; GM-CSF granulocyte/macrophage colony-stimulating factor; HPV human papillomavirus; IL-12 interleukin-12; PD-L1 programmed cell death-ligand 1; Ras Rat sarcoma; TGF-β transforming growth factor-β; TRICOM Triad of costimulatory molecules
Gene therapy
Gene therapy can be regulated at the nucleic acid level to achieve the purpose of tumor treatment, including therapeutic genes, nucleic acid vaccine, RNA interference, and gene probe technology [189–192]. Gene therapy can also target the genes those are involved in the immune system interactions. Oncolytic virus is a natural or genetically engineered virus. It belongs to a special gene therapy method that can selectively replicate in tumor cells to cause tumor cell lysis and activate the hosts’ immune system. Oncolytic virus mediates anti-tumor activity mainly through the following ways: selectively replicating in tumor cells, leading to tumor lysis. The tumor-associated antigen released by lysis activates the immune response of the host, thereby removing tumor cells. Viral infection also causes tumor cells to release cytokines, which in turn clear metastatic tumors [193]. In addition, gene therapy strategies targeting immune-related genes of oral cancer are also an important direction for future research. Currently, there are several ongoing studies based on gene-based immunotherapy in oral cancers. As shown in Table 4, there are 5 clinical trials, involving cytokines, Car-T, and gene vaccines. With the continuous innovation of molecular biology, cell biology, and clinical medical technology, gene therapy will play an important role in the process of overcoming the intractable tumor and is expected to become a routine strategy for tumor treatment in the future [194].
Table 4.
Gene therapy-based clinical trials for oral cancers
| Product name | Sponsor | Nationality | Estimated date of completion | Phase | Status | Identifier |
|---|---|---|---|---|---|---|
| IL-12 gene medicine | Dana-Farber Cancer Institute | United States | November 2000 | I/II | Completed | NCT00004070 |
| Allovectin-7® | Vical | United States | June 2002 | III | Completed | NCT00050388 |
| E6 TCR gene therapy | National Cancer Institute (NCI) | United States | June 2016 | I/II | Completed | NCT02280811 |
| TheraT® expressing HPV-specific antigens | Hookipa Biotech GmbH | United States | June 2022 | I/II | Recruiting | NCT04180215 |
| HPV-Specific T Cells | Baylor College of Medicine | United States | October 2022 | I | Active, not recruiting | NCT02379520 |
IL-12 interleukin-12; Allovectin-7® HLA-B7/β-2 microglobulin plasmid DNA/lipid complex; E6 TCR E6 T cell receptor; HPV Human papilloma virus
Challenges and perspective
The occurrence of malignant tumors, including oral cancers, results from the accumulation of genetic mutations and epigenetic modifications that lead to tumor-related phenotypes, including unlimited proliferation, apoptosis resistance, angiogenesis, invasion, and metastasis [195]. Moreover, in the process of cancer development, the tumor and the surrounding microenvironment constantly interact and evolve, forming characteristics conducive to tumor growth and invasion, leading to tumor development and metastasis [196]. For most patients, metastatic tumors remain an incurable disease that cannot be cured with traditional treatments. The development of tumor immunotherapy has brought hope for the cure of tumors, especially advanced tumors. Different from traditional therapeutic strategies, tumor immunotherapy mainly activates the host immune system and then mobilizes specific immune cells to recognize and kill tumor cells [197]. The immunotherapy can achieve a persistent anti-tumor response due to the host's immune memory effect. Therefore, immunotherapy can not only effectively inhibit the primary tumor but also further prevent tumor recurrence and inhibit metastasis, making it the only emerging therapy that is expected to completely cure tumors.
For decades, tumor immunotherapy has changed the treatment pattern of multiple solid tumors and hematological malignancies, bringing good news to tumor patients, especially patients with advanced malignant tumors or multidrug resistance. However, tumor immunotherapy is still faced with many difficulties, such as low immune response rate and lack of effective and reliable efficacy prediction markers, which is one of the biggest challenges to achieve accurate personalized immunotherapy. In addition, TME is a complex heterogeneous ecosystem, and the occurrence, development, and metastasis of tumor are closely related to the internal and external environment of tumor cells. It not only affects the growth and metabolism of tumor cells but also affects the structure, function, and metabolism of tumor tissues. The various microenvironmental characteristics, including hypoxic microenvironment, metabolic microenvironment, acidic microenvironment, and mechanical microenvironment, have attracted the attentions of scientists and clinicians. Combining drugs that modulate TME with standard therapies, such as traditional chemotherapy regimens with drugs that improve the acidic environment of tumors, promises exciting therapeutic results.
With the continuous emergence and innovation of new technologies and new methods, and the increasingly close interdisciplinary integration, tumor immunotherapy ushers in the rapid development, showing great potential to cure tumors. However, due to the heterogeneity of tumor and the difference of individual immune environment, immunotherapy cannot show good therapeutic effect in all individuals. The selection of specific targets, screening of suitable tumor patients, and the combined application of multiple therapies can partially solve the current problems of tumor immunotherapy. The development of immunotherapy drugs and related clinical trials based on multiple targets have provided a new direction for clinical work and are expected to greatly improve the current plight of malignant tumor treatment. We look forward to exciting clinical studies that are underway or will soon be conducted, but we clearly recognize that there are still some limitations and need to be improved in the current approach to immunotherapy drugs and rational application. The development of tumor immunotherapy drugs should be based on solid and scientific theories and guided by the real clinical needs. In the future, the development of basic medicine, tumor immunology, pharmacy, bioinformatics, and other disciplines will further promote the development of tumor immunotherapy. Expanding benefit groups and improving efficiency are the direction of future research. Tumor immunotherapy designed and developed based on new theories, new technologies, and new methods can safely and effectively enhance the immune system, destroy tumor cells, and finally achieve the goal of cure with limited toxicity. Immunotherapy has an important development prospect and is a potential method for future clinical treatment of malignant tumors.
Conclusions
Oral cancer severely affects the appearance, eating and even endangers life. There are few safe and efficient clinical treatment strategies for oral cancer, which is mainly due the complex tumor immune microenvironment. Tumor immunotherapy is the specific killing of tumor cells by activating the host's own immune system, which is not only suitable for the treatment of primary tumor but also has a specific killing effect on metastatic tumor. Therefore, tumor immunotherapy based on TME demonstrates a potential prospect in clinical anti-tumor therapy. Most importantly, a healthy attitude and lifestyle remain the first element in the prevention and treatment of oral cancer.
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFC2400405), National Natural Science Foundation of China (51973217), Jilin Province Science and Technology Development Program (20200201075JC), and Science and Technology Research Program of Education Department of Jilin Province (JJKH20221097KJ).
Abbreviations
- ACI
Adoptive cellular immunotherapy
- anti-HER2
Anti-human epidermal growth factor receptor 2
- BMDCs
Bone marrow dendritic cells
- CAFs
Cancer-associated fibroblasts
- CTLs
Cancer-specific T cells
- CEA
Carcinoembryonic antigen
- Car-T
Chimeric antigen receptor T
- CD3AK
CD3AcAb-activatived killer
- CRISPRi
Clustered regularly interspaced short palindromic repeats interference
- CIK
Cytokine-induced killer
- CTLA-4
Cytotoxic T lymphocyte antigen-4
- DCs
Dendritic cells
- EGFR
Endothelial growth factor receptor
- EMT
Epithelial mesenchymal transformation
- ECM
Extracellular matrix
- EXTL3
Exostosin-like glycosyltransferase 3
- FGF
Fibroblast growth factor
- FDA
Food and Drug Administration
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HBGF-1
Heparin binding growth factor-1
- HGF
Hepatocyte growth factor
- HLA
Human leukocyte antigen
- HPV
Human papillomavirus
- HA
Hyaluronic acid
- HIF
Hypoxia-inducible factor
- IC
Immune checkpoint
- ICB
Immune checkpoint blockade
- ICIs
Immune checkpoint inhibitors
- IDO
Indoleamine-2,3-dioxygenase
- IFN-α
Interferon alfa
- IFN-γ
Interferon γ
- IGF
Insulin-like growth factor
- IL-10
Interleukin-10
- IL-12
Interleukin-12
- LAG-3
Lymphocyte activation gene 3
- LAK
Lymphokine-activated killer
- mRNA
Messenger RNA
- MHC
Major histocompatibility complex
- MMPs
Matrix metalloproteinases
- M-CSF1
Macrophage colony-stimulating factor 1
- mAbs
Monoclonal antibodies
- NCI
National Cancer Institute
- OPVL
Oral proliferative verrucous leukoplakia
- OSCC
Oral squamous cell carcinoma
- PI3k
Phosphoinositide 3-kinase
- PDGF
Platelet-derived growth factor
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-ligand 1
- Ras
Rat sarcoma
- RGD
Arginine-glycine-asparagine
- STAT3
Signal transducer and activator of transcription 3
- TIM-3
T cell immunoglobulin-3
- TRICOM
Triad of costimulatory molecules
- TAA
Tumor-associated antigen
- TAM
Tumor-associated macrophages
- TGF-β
Transforming growth factor
- Tregs
Regulatory T cells
- TILs
Tumor-infiltrating lymphocytes
- TNF-α
Tumor necrosis factor
- TSA
Tumor-specific antigen
- ROS
Reactive oxygen species
- TME
Tumor microenvironment
- VISTA
V-domain Ig suppressor of T cell activation
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- WHO
World Health Organization
Author contributions
SZ, HL, and JC contributed to conception; CL, MW, TZ, and HZ were involved in interpretation of data; CL, MW, HZ, CL, and HL prepared original draft; CL and JC contributed to review structure; HL, SZ, and JC were involved in review and editing. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (2021YFC2400405), National Natural Science Foundation of China (51973217), Jilin Province Science and Technology Development Program (20200201075JC), and Science and Technology Research Program of Education Department of Jilin Province (JJKH20221097KJ).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Song Zhu, Email: zhusong@jlu.edu.cn.
Jie Chen, Email: chenjie@ciac.ac.cn.
References
- 1.Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol. 2010;46:414–417. doi: 10.1016/j.oraloncology.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Rhodus NL, Kerr AR, Patel K. Oral cancer: leukoplakia, premalignancy, and squamous cell carcinoma. Dent Clin North Am. 2014;58:315–340. doi: 10.1016/j.cden.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Speight PM, Farthing PM. The pathology of oral cancer. Br Dent J. 2018;225:841–847. doi: 10.1038/sj.bdj.2018.926. [DOI] [PubMed] [Google Scholar]
- 4.Mignogna MD, Fedele S, Lo Russo L. The world cancer report and the burden of oral cancer. Eur J Cancer Prev. 2004;13:139–142. doi: 10.1097/00008469-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 5.De Virgilio A, Costantino A, Mercante G, et al. Present and future of de-intensification strategies in the treatment of oropharyngeal carcinoma. Curr Oncol Rep. 2020 doi: 10.1007/s11912-020-00948-1. [DOI] [PubMed] [Google Scholar]
- 6.Dhanuthai K, Rojanawatsirivej S, Thosaporn W, et al. Oral cancer: a multicenter study. Med Oral Patol Oral Cir Bucal. 2018;23:e23–e29. doi: 10.4317/medoral.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn D, Zittel S, Moratin J, et al. Prospective feasibility analysis of salvage surgery in recurrent oral cancer in terms of quality of life. Oral Oncol. 2020;102:104580. doi: 10.1016/j.oraloncology.2020.104580. [DOI] [PubMed] [Google Scholar]
- 8.Huang SH, O'Sullivan B. Oral cancer: current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal. 2013;18:e233–e240. doi: 10.4317/medoral.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohmae M, Kato I, Fujita Y, Meshii N, Motoki A, Nakazawa M, Uzawa N. A novel method of intra-arterial chemotherapy for oral cancer. J Clin Oncol. 2018;36:e18086. doi: 10.1200/JCO.2018.36.15_suppl.e18086. [DOI] [Google Scholar]
- 10.Wang D, Duan XJ, Zhang YH, Meng Z, Wang J. Traditional Chinese medicine for oral squamous cell carcinoma a Bayesian network meta-analysis protocol. Medicine. 2020;99:e22955. doi: 10.1097/MD.0000000000022955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilborough AE, Lambert DW, Khurram SA. Extranodal extension in oral cancer: a role for the nodal microenvironment? J Oral Pathol Med. 2019;48:863–870. doi: 10.1111/jop.12870. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Chen J, Feng Y, et al. An immune cocktail therapy to realize multiple boosting of the cancer-immunity cycle by combination of drug/gene delivery nanoparticles. Sci Adv. 2020 doi: 10.1126/sciadv.abc7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo ZY, Wang Q, Lau WB, et al. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett. 2016;377:174–182. doi: 10.1016/j.canlet.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 14.Virchow R. Cellular pathology as based upon physiological and pathological histology. JB Lippincott. 1863 doi: 10.5962/bhl.title.32770. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNutt M. Cancer immunotherapy. Science. 2013;342:1417. doi: 10.1126/science.1249481. [DOI] [PubMed] [Google Scholar]
- 18.Guan X, Lin L, Chen J, Hu Y, Sun P, Tian H, Maruyama A, Chen X. Efficient PD-L1 gene silence promoted by hyaluronidase for cancer immunotherapy. J Control Release. 2019;293:104–112. doi: 10.1016/j.jconrel.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Gong H, Chao Y, Xiang J, et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016;16:2512–2521. doi: 10.1021/acs.nanolett.6b00068. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JM, Yang YJ, Kawazoe N, Chen GP. Encapsulation of individual living cells with enzyme responsive polymer nanoshell. Biomaterials. 2019;197:317–326. doi: 10.1016/j.biomaterials.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Musetti S, Huang L. Nanoparticle-mediated remodeling of the tumor microenvironment to enhance immunotherapy. ACS Nano. 2018;12:11740–11755. doi: 10.1021/acsnano.8b05893. [DOI] [PubMed] [Google Scholar]
- 23.Elmusrati A, Wang J, Wang CY. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int J Oral Sci. 2021 doi: 10.1038/s41368-021-00131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouw JK, Ou GQ, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Bio. 2014;15:771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marozzi M, Parnigoni A, Negri A, Viola M, Vigetti D, Passi A, Karousou E, Rizzi F. Inflammation, extracellular matrix remodeling, and proteostasis in tumor microenvironment. Int J Mol Sci. 2021;22:8102. doi: 10.3390/ijms22158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco-Fernandez B, Gaspar VM, Engel E, Mano JF. Proteinaceous hydrogels for bioengineering advanced 3D tumor models. Adv Sci. 2021;8:2003129. doi: 10.1002/advs.202003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderrest M, Garrone R. Collagen family of proteins. Faseb J. 1991;5:2814–2823. doi: 10.1096/fasebj.5.13.1916105. [DOI] [PubMed] [Google Scholar]
- 28.Eyre DR, Weis MA, Wu JJ. Maturation of collagen ketoimine cross-links by an alternative mechanism to pyridinoline formation in cartilage. J Biol Chem. 2010;285:16675–16682. doi: 10.1074/jbc.M110.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamano Y, Zeisberg M, Sugimoto H, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha 3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alpha V beta 3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/S1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer LM, Ma WJ, Mosher DF. Dynamic structure of plasma fibronectin. Crit Rev Biochem Mol. 2016;51:213–227. doi: 10.1080/10409238.2016.1184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi Y, Mio T, Takigawa K, Striz I, Romberger DJ, Spurzem JR, Rennard SI. Fibronectin production by cultured human lung fibroblasts in three-dimensional collagen gel culture. In Vitro Cell Dev-An. 1998;34:203–210. doi: 10.1007/s11626-998-0125-7. [DOI] [PubMed] [Google Scholar]
- 32.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabatier L, Chen DL, Fagotto-Kaufmann C, Hubmacher D, Mckee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.e08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Clark RAF. Fibronectin at select sites binds multiple growth factors and enhances their activity: expansion of the collaborative ECM-GF paradigm. J Invest Dermatol. 2014;134:895–901. doi: 10.1038/jid.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin FB, Ren XD, Pan Z, Macri L, Zong WX, Tonnesen MG, Rafailovich M, Bar-Sagi D, Clark RAF. Fibronectin growth factor-binding domains are required for fibroblast survival. J Invest Dermatol. 2011;131:84–98. doi: 10.1038/jid.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 37.Hu YY, Lin L, Chen J, Maruyama A, Tian HY, Chen XS. Synergistic tumor immunological strategy by combining tumor nanovaccine with gene-mediated extracellular matrix scavenger. Biomaterials. 2020;252:120114. doi: 10.1016/j.biomaterials.2020.120114. [DOI] [PubMed] [Google Scholar]
- 38.Guan XW, Chen J, Hu YY, Lin L, Sun PJ, Tian H, Chen XS. Highly enhanced cancer immunotherapy by combining nanovaccine with hyaluronidase. Biomaterials. 2018;171:198–206. doi: 10.1016/j.biomaterials.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 39.Balazs EA, Laurent TC, Jeanloz RW. Nomenclature of hyaluronic-acid. Biochem J. 1986;235:903. doi: 10.1042/bj2350903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang JC, Zhang LL, Wan DL, Zhou L, Zheng SS, Lin SZ, Qiao YT. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Tar. 2021;6:1–24. doi: 10.1038/s41392-021-00544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Fang HP, Hu YY, Wu JY, Zhang SJ, Feng YJ, Lin L, Tian HY, Chen XS. Combining mannose receptor mediated nanovaccines and gene regulated PD-L1 blockade for boosting cancer immunotherapy. Bioact Mater. 2022;7:167–180. doi: 10.1016/j.bioactmat.2021.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somasundaram R, Schuppan D. Type I, II, III, IV, V, and VI collagens serve as extracellular ligands for the isoforms of platelet-derived growth factor (AA, BB, and AB) J Biol Chem. 1996;271:26884–26891. doi: 10.1074/jbc.271.43.26884. [DOI] [PubMed] [Google Scholar]
- 43.Thompson JA, Anderson KD, DiPietro JM, Zwiebel JA, Zametta M, Anderson WF, Maciag T. Site-directed neovessel formation in vivo. Science. 1988;241:1349–1352. doi: 10.1126/science.2457952. [DOI] [PubMed] [Google Scholar]
- 44.Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-D. [DOI] [PubMed] [Google Scholar]
- 45.Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–6254. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 46.Hawinkels LJ, Zuidwijk K, Verspaget HW, et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. 2008;44:1904–1913. doi: 10.1016/j.ejca.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Jin KT, Yao JY, Fang XL, Di H, Ma YY. Roles of lncRNAs in cancer: focusing on angiogenesis. Life Sci. 2020;252:117647. doi: 10.1016/j.lfs.2020.117647. [DOI] [PubMed] [Google Scholar]
- 48.Wong PP, Bodrug N, Hodivala-Dilke KM. Exploring novel methods for modulating tumor blood vessels in cancer treatment. Curr Biol. 2016;26:R1161–R1166. doi: 10.1016/j.cub.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 49.El Shorbagy S, abuTaleb F, Labib HA, Ebian H, Harb OA, Mohammed MS, Rashied HA, Elbana KA, Haggag R, Prognostic significance of VEGF and HIF-1 alpha in hepatocellular carcinoma patients receiving sorafenib versus metformin sorafenib combination. J Gastrointest Cancer. 2021;52:269–279. doi: 10.1007/s12029-020-00389-w. [DOI] [PubMed] [Google Scholar]
- 50.Marjon PL, Bobrovnikova-Marjon EV, Abcouwer SF. Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol Cancer. 2004;3:1–12. doi: 10.1186/1476-4598-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang MP, Huang B, Li GW, Zeng SN. Apatinib affect VEGF-mediated cell proliferation, migration, invasion via blocking VEGFR2/RAF/MEK/ERK and PI3K/AKT pathways in cholangiocarcinoma cell. Bmc Gastroenterol. 2018;18:1–10. doi: 10.1186/s12876-018-0870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu TY, Han CC, Wang SW, Fang PQ, Ma ZF, Xu L, Yin R. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12:1–15. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gieniec KA, Butler LM, Worthley DL, Woods SL. Cancer-associated fibroblasts-heroes or villains? Brit J Cancer. 2019;121:293–302. doi: 10.1038/s41416-019-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costa A, Kieffer Y, Scholer-Dahirel A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–479. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Cazet AS, Hui MN, Elsworth BL, et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun. 2018;9:1–18. doi: 10.1038/s41467-018-05220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia CC, Wang GY, Wang TT, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition via the transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in hepatocellular carcinoma. Int J Biol Sci. 2020;16:2542–2558. doi: 10.7150/ijbs.45446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22:6995. doi: 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belgiovine C, Frapolli R, Liguori M, Digifico E, Colombo FS, Meroni M, Allavena P, D'Incalci M. Inhibition of tumor-associated macrophages by trabectedin improves the antitumor adaptive immunity in response to anti-PD-1 therapy. Eur J Immunol. 2021;51:2677–2686. doi: 10.1002/eji.202149379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 63.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho H, Seo Y, Loke KM, et al. Cancer-stimulated CAFs enhance monocyte differentiation and protumoral TAM activation via IL6 and GM-CSF secretion. Clin Cancer Res. 2018;24:5407–5421. doi: 10.1158/1078-0432.CCR-18-0125. [DOI] [PubMed] [Google Scholar]
- 65.Marcuzzi E, Angioni R, Molon B, Cali B. Chemokines and chemokine receptors: orchestrating tumor metastasization. Int J Mol Sci. 2019;20:96. doi: 10.3390/ijms20010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126:3672–3679. doi: 10.1172/JCI84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Utomo L, Bastiaansen-Jenniskens YM, Verhaar JAN, van Osch GJVM. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthr Cartilage. 2016;24:2162–2170. doi: 10.1016/j.joca.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 68.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 70.Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. 2021;18:842–859. doi: 10.1038/s41423-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muenst S, Laubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. 2016;279:541–562. doi: 10.1111/joim.12470. [DOI] [PubMed] [Google Scholar]
- 72.Gerber AL, Munst A, Schlapbach C, Shafighi M, Kiermeir D, Husler R, Hunger RE. High expression of FOXP3 in primary melanoma is associated with tumour progression. Brit J Dermatol. 2014;170:103–109. doi: 10.1111/bjd.12641. [DOI] [PubMed] [Google Scholar]
- 73.Mukherji B. Immunology of melanoma. Clin Dermatol. 2013;31:156–165. doi: 10.1016/j.clindermatol.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Giraldo NA, Becht E, Remark R, Damotte D, Sautes-Fridman C, Fridman WH. The immune contexture of primary and metastatic human tumours. Curr Opin Immunol. 2014;27:8–15. doi: 10.1016/j.coi.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in T(H)1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 76.Spencer LA, Weller PF. Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol. 2010;88:250–256. doi: 10.1038/icb.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hus I, Maciag E, Rolinski J. The role of Th17 cells in anti-cancer immunity. Postep Hig Med Dosw. 2010;64:244–250. [PubMed] [Google Scholar]
- 78.Fehervari Z, Sakaguchi S. CD4(+) Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI200423395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang XG, Shapiro DJ. The immune system and inflammation in breast cancer. Mol Cell Endocrinol. 2014;382:673–682. doi: 10.1016/j.mce.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma MW, Medicherla RC, Qian M, et al. Immune response in melanoma: an in-depth analysis of the primary tumor and corresponding sentinel lymph node. Modern Pathol. 2012;25:1000–1010. doi: 10.1038/modpathol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruno A, Ferlazzo G, Albini A, Noonan DM. A think tank of TINK/TANKs: tumor-infiltrating/tumor-associated natural killer cells in tumor progression and angiogenesis. Jnci-J Natl Cancer I. 2014;106:1–13. doi: 10.1093/jnci/dju200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahmani S, Yazdanpanah N, Rezaei N. Natural killer cells and acute myeloid leukemia: promises and challenges. Cancer Immunol Immunother. 2022 doi: 10.1007/s00262-022-03217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tel J, Anguille S, Waterborg CEJ, Smits EL, Figdor CG, de Vries IJM. Tumoricidal activity of human dendritic cells. Trends Immunol. 2014;35:38–46. doi: 10.1016/j.it.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balsamo M, Vermi W, Parodi M, et al. Melanoma cells become resistant to NK-cell-mediated killing when exposed to NK-cell numbers compatible with NK-cell infiltration in the tumor. Eur J Immunol. 2012;42:1833–1842. doi: 10.1002/eji.201142179. [DOI] [PubMed] [Google Scholar]
- 87.Ladanyi A. Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma. Pigm Cell Melanoma R. 2015;28:490–500. doi: 10.1111/pcmr.12371. [DOI] [PubMed] [Google Scholar]
- 88.Hussein MR, Elsers DAH, Fadel SA, Omar AEM. Immunohistological characterisation of tumour infiltrating lymphocytes in melanocytic skin lesions. J Clin Pathol. 2006;59:316–324. doi: 10.1136/jcp.2005.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ladanyi A, Kiss J, Mohos A, et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immun. 2011;60:1729–1738. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, Zhong H, Han BH, Ferris RL. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer Metast Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 91.Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-beta receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018;14:1423–1431. doi: 10.1080/21645515.2018.1431598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fainaru O, Almog N, Yung CW, Nakai K, Montoya-Zavala M, Abdollahi A, D'Amato R, Ingber DE. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. Faseb J. 2010;24:1411–1418. doi: 10.1096/fj.09-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kobayashi M, Suzuki K, Yashi M, Yuzawa M, Takayashiki N, Morita T. Tumor infiltrating dendritic cells predict treatment response to immmunotherapy in patients with metastatic renal cell carcinoma. Anticancer Res. 2007;27:1137–1141. [PubMed] [Google Scholar]
- 94.Hu Y, Lin L, Chen J, Hao K, Zhang S, Guo X, Guo Z, Tian H, Chen X. Highly enhanced antitumor immunity by a three-barreled strategy of the l-arginine-promoted nanovaccine and gene-mediated PD-L1 blockade. ACS Appl Mater Interfaces. 2020;12:41127–41137. doi: 10.1021/acsami.0c12734. [DOI] [PubMed] [Google Scholar]
- 95.Wang K, Chen J, Lin L, Yan N, Yang WH, Cai KY, Tian HY, Chen XS. Anion receptor-mediated multicomponent synergistic self-assembly of porphyrin for efficient phototherapy to elicit tumor immunotherapy. Nano Today. 2022;46:101579. doi: 10.1016/j.nantod.2022.101579. [DOI] [Google Scholar]
- 96.Toor SM, Nair VS, Decock J, Elkord E. Immune checkpoints in the tumor microenvironment. Semin Cancer Biol. 2020;65:1–12. doi: 10.1016/j.semcancer.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 97.Feng YJ, Wu JY, Chen J, et al. Targeting dual gene delivery nanoparticles overcomes immune checkpoint blockade induced adaptive resistance and regulates tumor microenvironment for improved tumor immunotherapy. Nano Today. 2021;38:101194. doi: 10.1016/j.nantod.2021.101194. [DOI] [Google Scholar]
- 98.Chang P, Chen S, Chang X, Zhu J, Tang Q, Ma L. EXTL3 could serve as a potential biomarker of prognosis and immunotherapy for prostate cancer and its potential mechanisms. Eur J Med Res. 2022;27:115. doi: 10.1186/s40001-022-00740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mullard A. 2011 FDA drug approvals The US FDA approved 30 new therapeutics last year, including 11 first-in-class agents. Nat Rev Drug Discov. 2012;11:91–94. doi: 10.1038/nrd3657. [DOI] [PubMed] [Google Scholar]
- 100.Ledford H, Else H, Warren M. Cancer immunologists scoop medicine nobel prize. Nature. 2018;562:20–21. doi: 10.1038/d41586-018-06751-0. [DOI] [PubMed] [Google Scholar]
- 101.Petitprez F, Meylan M, de Reynies A, Sautes-Fridman C, Fridman WH. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front Immunol. 2020;11:784. doi: 10.3389/fimmu.2020.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rak J. Extracellular vesicles - biomarkers and effectors of the cellular interactome in cancer. Front Pharmacol. 2013;4:21. doi: 10.3389/fphar.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li I, Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. 2019;18:1–10. doi: 10.1186/s12943-019-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 106.Plebanek MP, Angeloni NL, Vinokour E, et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun. 2017;8:1–12. doi: 10.1038/s41467-017-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Razzo BM, Ludwig N, Hong CS, Sharma P, Fabian KP, Fecek RJ, Storkus WJ, Whiteside TL. Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma. Carcinogenesis. 2020;41:625–633. doi: 10.1093/carcin/bgz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 109.Dharmaratne NU, Kaplan AR, Glazer PM. Targeting the hypoxic and acidic tumor microenvironment with ph-sensitive peptides. Cells-Basel. 2021;10:541. doi: 10.3390/cells10030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Estrella V, Chen TA, Lloyd M, et al. Acidity generated by the tumor microenvironment drives local invasion. Can Res. 2013;73:1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen J, Guo P, Jiao ZX, Lin L, Xu CN, Tian HY, Chen XS. Poly(L-glutamic acid)-based zwitterionic polymer in a charge conversional shielding system for gene therapy of malignant tumors. Acs Appl Mater Inter. 2020;12:19295–19306. doi: 10.1021/acsami.0c02769. [DOI] [PubMed] [Google Scholar]
- 112.Sinevici N, O'sullivan J. Oral cancer: deregulated molecular events and their use as biomarkers. Oral Oncol. 2016;61:12–18. doi: 10.1016/j.oraloncology.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 113.Yuan CS, Deng ZW, Qin D, Mu YZ, Chen XG, Liu Y. Hypoxia-modulatory nanomaterials to relieve tumor hypoxic microenvironment and enhance immunotherapy: where do we stand? Acta Biomater. 2021;125:1–28. doi: 10.1016/j.actbio.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 114.Barsoum IB, Koti M, Siemens DR, Graham CH. Mechanisms of hypoxia-mediated immune escape in cancer. Can Res. 2014;74:7185–7190. doi: 10.1158/0008-5472.CAN-14-2598. [DOI] [PubMed] [Google Scholar]
- 115.Lu YJ, Li YR, Wang ZS, Xie SL, Wang Q, Lei XY, Ruan Y, Li JS. Downregulation of RGMA by HIF-1A/miR-210-3p axis promotes cell proliferation in oral squamous cell carcinoma. Biomed Pharmacother. 2019;112:108608. doi: 10.1016/j.biopha.2019.108608. [DOI] [PubMed] [Google Scholar]
- 116.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abd El-Aziz YS, Leck LYW, Jansson PJ, Sahni S. Emerging role of autophagy in the development and progression of oral squamous cell carcinoma. Cancers. 2021;13:6152. doi: 10.3390/cancers13246152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rouschop KMA, van den Beucken T, Dubois L, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang HF, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakagawa Y, Negishi Y, Shimizu M, Takahashi M, Ichikawa M, Takahashi H. Effects of extracellular pH and hypoxia on the function and development of antigen-specific cytotoxic T lymphocytes. Immunol Lett. 2015;167:72–86. doi: 10.1016/j.imlet.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 122.Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol. 2015;42:378–386. doi: 10.1053/j.seminoncol.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 123.Rwigema JCM, Langendijk JA, van der Laan HP, Lukens JN, Swisher-McClure SD, Lin A. A model-based approach to predict short-term toxicity benefits with proton therapy for oropharyngeal cancer. Int J Radiat Oncol. 2019;104:553–562. doi: 10.1016/j.ijrobp.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 124.Byars LT. Surgical management of mandible invaded by oral cancer. Surg Gynecol Obstet. 1954;98:564–570. [PubMed] [Google Scholar]
- 125.Carpenter DJ, Mowery YV, Broadwater G, Rodrigues A, Wisdom AJ, Dorth JA. The risk of carotid stenosis in head and neck cancer patients after radiation therapy. J Vasc Surg. 2018;68:935. doi: 10.1016/j.jvs.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hartner L. Chemotherapy for oral cancer. Dent Clin North Am. 2018;62:87–97. doi: 10.1016/j.cden.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 127.Philips R, Han CH, Swendseid B, Curry J, Argiris A, Luginbuhl A, Johnson J. Preoperative immunotherapy in the multidisciplinary management of oral cavity cancer. Front Oncol. 2021;11:682075. doi: 10.3389/fonc.2021.682075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mohan SP, Bhaskaran MK, George AL, Thirutheri A, Somasundaran M, Pavithran A. Immunotherapy in oral cancer. J Pharm Bioallied Sci. 2019;11:S107–S111. doi: 10.4103/JPBS.JPBS_31_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mei Z, Huang JW, Qiao B, Lam AKY. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int J Oral Sci. 2020 doi: 10.1038/s41368-020-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 131.Maloney DG, GrilloLopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, Varns C, Rosenberg J, Levy R. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 132.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15:361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taylor RP. Of mice and mechanisms: identifying the role of complement in monoclonal antibody-based immunotherapy. Haematol-Hematol J. 2006;91:146–147. [PubMed] [Google Scholar]
- 134.Lima AB, Macedo LT, Sasse AD. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis. J Clin Oncol. 2010;28:e18087. doi: 10.1200/jco.2010.28.15_suppl.e18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brand TM, Hartmann SF, Bhola NE, et al. Human Papillomavirus regulates HER3 expression in head and neck cancer: implications for targeted HER3 Therapy in HPV+ patients. Clin Cancer Res. 2017;23:3072–3083. doi: 10.1158/1078-0432.CCR-16-2203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 136.Shandilya J, Swaminathan V, Gadad SS, Choudhari R, Kodaganur GS, Kundu TK. Acetylated NPM1 localizes in the nucleoplasm and regulates transcriptional activation of genes implicated in oral cancer manifestation. Mol Cell Biol. 2009;29:5115–5127. doi: 10.1128/MCB.01969-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohnishi Y, Sakamoto T, Li ZG, Yasui H, Hamada H, Kubo H, Nakajima M. Curcumin inhibits epithelial-mesenchymal transition in oral cancer cells via c-met blockade. Oncol Lett. 2020;19:4177–4182. doi: 10.3892/ol.2020.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 139.Qiao XW, Jiang J, Pang X, Huang MC, Tang YJ, Liang XH, Tang YL. The evolving landscape of PD-1/PD-L1 pathway in head and neck cancer. Front Immunol. 2020;11:1721. doi: 10.3389/fimmu.2020.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Di Molfetta S, Feola T, Fanciulli G, Florio T, Colao A, Faggiano A, Grp N. Immune checkpoint blockade in lung carcinoids with aggressive behaviour: one more arrow in our quiver? J Clin Med. 2022 doi: 10.3390/jcm11041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Duhen R, Ballesteros-Merino C, Frye AK, et al. Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat Commun. 2021;12:1–14. doi: 10.1038/s41467-021-21383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang H, Mao L, Zhang T, Zhang LM, Wu YT, Guo W, Hu JZ, Ju HY, Ren GX. Altered expression of TIM-3, LAG-3, IDO, PD-L1, and CTLA-4 during nimotuzumab therapy correlates with responses and prognosis of oral squamous cell carcinoma patients. J Oral Pathol Med. 2019;48:669–676. doi: 10.1111/jop.12883. [DOI] [PubMed] [Google Scholar]
- 143.Cheng G, Dong H, Yang C, Liu Y, Wu Y, Zhu L, Tong X, Wang S. A review on the advances and challenges of immunotherapy for head and neck cancer. Cancer Cell Int. 2021;21:406. doi: 10.1186/s12935-021-02024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goradel NH, Asghari MH, Moloudizargari M, Negandari B, Haghi-Aminjan H, Abdollahi M. Melatonin as an angiogenesis inhibitor to combat cancer: mechanistic evidence. Toxicol Appl Pharm. 2017;335:56–63. doi: 10.1016/j.taap.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 145.van Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptor and angiogenesis: opportunities for combined anticancer strategies. Int J Cancer. 2005;117:883–888. doi: 10.1002/ijc.21479. [DOI] [PubMed] [Google Scholar]
- 146.Lacerna LV, Hester J, Pineda AA, Burgstaler E, Klein HG. Clinical/technical challenges in adoptive cellular immunotherapy (ACI): the role of cytapheresis. Immunol Ser. 1989;48:175–189. [PubMed] [Google Scholar]
- 147.Rohaan MW, Wilgenhof S, Haanen J. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019;474:449–461. doi: 10.1007/s00428-018-2484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ying Z, Huang XF, Xiang X, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25:947–953. doi: 10.1038/s41591-019-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gorchakov AA, Kulemzin SV, Kochneva GV, Taranin AV. Challenges and prospects of chimeric antigen receptor T-cell therapy for metastatic prostate cancer. Eur Urol. 2020;77:299–308. doi: 10.1016/j.eururo.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 150.Eshhar Z, Waks T, Gross G, Schindler DG. Specific Activation and targeting of cytotoxic lymphocytes through Chimeric single chains consisting of antibody-binding domains and the gamma-subunit or zeta-subunit of the immunoglobulin and T-cell receptors. P Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer. 2019;120:26–37. doi: 10.1038/s41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mei Z, Zhang K, Lam AKY, Huang JW, Qiu F, Qiao B, Zhang Y. MUC1 as a target for CAR-T therapy in head and neck squamous cell carinoma. Cancer Med. 2020;9:640–652. doi: 10.1002/cam4.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park YP, Jin LC, Bennett KB, Wang DR, Fredenburg KM, Tseng JE, Chang LJ, Huang JP, Chan EKL. CD70 as a target for chimeric antigen receptor T cells in head and neck squamous cell carcinoma. Oral Oncol. 2018;78:145–150. doi: 10.1016/j.oraloncology.2018.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang ZF, Li LY, Turkoz A, et al. Contextual reprogramming of CAR-T cells for treatment of HER2(+) cancers. J Transl Med. 2021;19:1–18. doi: 10.1186/s12967-021-03132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang LCS, Lo A, Scholler J, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Di S, Zhou M, Pan Z, Sun R, Chen M, Jiang H, Shi B, Luo H, Li Z. Combined adjuvant of poly I: C improves antitumor effects of CAR-T cells. Front Oncol. 2019;9:241. doi: 10.3389/fonc.2019.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 159.Jiang T, Zhou CC, Ren SX. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462. doi: 10.1080/2162402X.2016.1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tarhini AA, Gogas H, Kirkwood JM. IFN-alpha in the treatment of Melanoma. J Immunol. 2012;189:3789–3793. doi: 10.4049/jimmunol.1290060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth F R. 2014;25:377–390. doi: 10.1016/j.cytogfr.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 162.Chien MH, Hsin CH, Chou LSS, Chung TT, Lin CH, Weng MS, Chou MY, Chen MK, Yang SF. Interleukin-23 receptor polymorphism as a risk factor for oral cancer susceptibility. Head Neck-J Sci Spec. 2012;34:551–556. doi: 10.1002/hed.21779. [DOI] [PubMed] [Google Scholar]
- 163.Dunn GP, Bruce AT, Sheehan KCF, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 164.Tian SB, Jiang CY, Liu XQ, Xu S, Zhang ZY, Chen HZ, Zhang YH, Liu YP, Ma D. Hypermethylation of IFN-gamma in oral cancer tissues. Clin Oral Invest. 2017;21:2535–2542. doi: 10.1007/s00784-017-2052-z. [DOI] [PubMed] [Google Scholar]
- 165.Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. 2017;9:347–360. doi: 10.2217/imt-2016-0141. [DOI] [PubMed] [Google Scholar]
- 166.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metast Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 167.Guo CQ, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the international agency for research on cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 169.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 170.Papachristofilou A, Hipp MM, Klinkhardt U, et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer. 2019;7:1–14. doi: 10.1186/s40425-019-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nobuoka D, Yoshikawa T, Takahashi M, et al. Intratumoral peptide injection enhances tumor cell antigenicity recognized by cytotoxic T lymphocytes: a potential option for improvement in antigen-specific cancer immunotherapy. Cancer Immunol Immun. 2013;62:639–652. doi: 10.1007/s00262-012-1366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Faghfuri E, Pourfarzi F, Faghfouri AH, Shadbad MA, Hajiasgharzadeh K, Baradaran B. Recent developments of RNA-based vaccines in cancer immunotherapy. Expert Opin Biol Th. 2021;21:201–218. doi: 10.1080/14712598.2020.1815704. [DOI] [PubMed] [Google Scholar]
- 174.Chiang CLL, Benencia F, Coukos G. Whole tumor antigen vaccines. Semin Immunol. 2010;22:132–143. doi: 10.1016/j.smim.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chiang CL, Coukos G, Kandalaft LE. Whole tumor antigen vaccines: where are we? Vaccines. 2015;3:344–372. doi: 10.3390/vaccines3020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Srivatsan S, Patel JM, Bozeman EN, Imasuen IE, He S, Daniels D, Selvaraj P. Allogeneic tumor cell vaccines: the promise and limitations in clinical trials. Hum Vaccin Immunother. 2014;10:52–63. doi: 10.4161/hv.26568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Malonis RJ, Lai JR, Vergnolle O. Peptide-based vaccines: current progress and future challenges. Chem Rev. 2020;120:3210–3229. doi: 10.1021/acs.chemrev.9b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cox MM. Recombinant protein vaccines produced in insect cells. Vaccine. 2012;30:1759–1766. doi: 10.1016/j.vaccine.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nascimento IP, Leite LC. Recombinant vaccines and the development of new vaccine strategies. Braz J Med Biol Res. 2012;45:1102–1111. doi: 10.1590/S0100-879X2012007500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Slingluff CL., Jr The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 2011;17:343–350. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li AW, Sobral MC, Badrinath S, et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater. 2018;17:528–534. doi: 10.1038/s41563-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tseng SH, Liu L, Peng S, Kim J, Ferrall L, Hung CF, Wu T. Control of spontaneous HPV16 E6/E7 expressing oral cancer in HLA-A2 (AAD) transgenic mice with therapeutic HPV DNA vaccine. J Biomed Sci. 2021;28:63. doi: 10.1186/s12929-021-00759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cheng L, Wang Y, Du J. Human papillomavirus vaccines: an updated review. Vaccines. 2020;8:391. doi: 10.3390/vaccines8030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 185.Van Nuffel AM, Wilgenhof S, Thielemans K, Bonehill A. Overcoming HLA restriction in clinical trials: immune monitoring of mRNA-loaded DC therapy. Oncoimmunology. 2012;1:1392–1394. doi: 10.4161/onci.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang H, Xia X. RNA cancer vaccines: developing mRNA nanovaccine with self-adjuvant property for cancer immunotherapy. Hum Vaccin Immunother. 2021;17:2995–2998. doi: 10.1080/21645515.2021.1921524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Son S, Nam J, Zenkov I, Ochyl LJ, Xu Y, Scheetz L, Shi J, Farokhzad OC, Moon JJ. Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Lett. 2020;20:1499–1509. doi: 10.1021/acs.nanolett.9b03483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Neufeld EF, Sweeley CC. Gene therapy for human genetic disease. Science. 1972 doi: 10.1126/science.178.4061.648.a. [DOI] [Google Scholar]
- 190.Chen J, Guo ZP, Tian HY, Chen XS. Production and clinical development of nanoparticles for gene delivery. Mol Ther-Meth Clin D. 2016;3:16023. doi: 10.1038/mtm.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hu YY, Lin L, Guo ZP, Chen J, Maruyama A, Tian HY, Chen XS. In situ vaccination and gene-medeiated PD-L1 blockade for enhanced tumor immunotherapy. Chin Chem Lett. 2021;32:1770–1774. doi: 10.1016/j.cclet.2020.12.055. [DOI] [Google Scholar]
- 192.Chen J, Jiao ZX, Lin L, Guo ZP, Xu CN, Li YH, Tian HY, Chen XS. Polylysine-modified polyethylenimines as siRNA carriers for effective tumor treatment. Chin J Polym Sci. 2015;33:830–837. doi: 10.1007/s10118-015-1632-0. [DOI] [Google Scholar]
- 193.Chaurasiya S, Fong YM, Warner SG. Oncolytic virotherapy for cancer: clinical experience. Biomedicines. 2021;9:419. doi: 10.3390/biomedicines9040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 195.Chen HB, Gu ZJ, An HW, et al. Precise nanomedicine for intelligent therapy of cancer. Sci China Chem. 2018;61:1503–1552. doi: 10.1007/s11426-018-9397-5. [DOI] [Google Scholar]
- 196.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.