Abstract
Synapses are specialized sites where neurons connect and communicate with each other. Activity-dependent modification of synaptic structure and function provides a mechanism for learning and memory. The advent of high-resolution time-lapse imaging in conjunction with fluorescent biosensors and actuators enables researchers to monitor and manipulate the structure and function of synapses both in vitro and in vivo. This review focuses on recent imaging studies on the synaptic modification underlying learning and memory.
Keywords: learning, memory, synapse, dendritic spine, LTP, LTD
1. Introduction
Learning and memory, one of the most complex and perplexing functions of the brain, are essential for the survival of animals. From a neural circuit point of view, learning is a process to transform a neural network to adapt to the environment, and memory is the state of maintaining such a network. The neural network is made up by the constituent neurons with their synaptic connections. Taking advantage of calcium (Ca) imaging and molecular signals associated with neuronal activities (e.g., activation of immediate early genes [IEG]), elegant studies have led to the identification of neuronal populations involved in memory coding [1–7]. However, the mechanisms by which memory is allocated at the level of synapses remain a major puzzle, largely due to their small size and the lack of techniques (molecular, anatomical, or physiological) to identify learning-related synapses in behaving animals.
Most excitatory synapses reside on dendritic spines, small protrusions from dendrites. The appearance and disappearance of spines are good indicators of the formation and loss of synaptic connections, and morphological changes of spines are associated with changes in the efficacy of synaptic transmission [8–10]. Importantly, spine formation, elimination, and morphological changes have been observed in the context of various learning paradigms, and spine stabilization is associated with long-lasting memory storage [11–13]. As many excellent review articles have discussed the structure, function, and molecular signaling of spines under physiological and pathological conditions [14–21], we will focus on in vitro and in vivo studies on the dynamics and morphological changes of spines in response to learning and memory.
2. Structural changes in dendritic spines in vitro
2.1. Morphological changes of dendritic spines
Various forms of synaptic plasticity, the persistent change in synaptic efficacy, are widely believed to be the cellular substrate underlying learning and memory. Among them, long-term potentiation (LTP) and long-term depression (LTD), two opposite forms of synaptic plasticity, have been studied most extensively. LTP and LTD were initially discovered by electrophysiological recording [22], but subsequent research has revealed accompanying morphological changes in dendritic spines. In hippocampal slices, localized electrical stimulation (i.e., high frequency stimulation [HFS] or theta burst stimulation [TBS]) or two-photon (2P) glutamate uncaging at individual spines leads to robust enlargement of the heads of targeted spines [23–26]. Simultaneous electrophysiological recording of the same postsynaptic neuron shows that spine head enlargement is accompanied by increased current mediated by AMPA-type glutamate receptors (AMPAR). Moreover, glutamate uncaging-induced spine head enlargement is transient in large spines but persistent in small spines [23]. While activation of NMDA-type glutamate receptors (NMDAR) as well as signaling mediated by molecules such as calmodulin and CaMKII are required for LTP-induced spine enlargement [23, 26], protein synthesis is also important for the maintenance of the enlarged spine head [25]. Furthermore, LTP induction increases the stability of nascent spines, and their survival rate positively correlates with spine head enlargement [26].
On the other hand, localized low frequency stimulation (LFS, an LTD-induction protocol) leads to persistent spine head shrinkage, which is coupled with the reduction of excitatory postsynaptic potential (EPSP) slope recorded from the same neuron. While NMDAR blocker and calcineurin inhibitor prevent both LTD induction and spine shrinkage, pretreatment with an inhibitor of PP1/2A (a downstream effector of calcineurin) blocks LTD but not LFS-induced spine shrinkage. These results suggest that LTD and morphological changes of spines share some molecular signals, but the pathways later diverge [24]. Interestingly, LTD-induced spine shrinkage is reversed by HFS-induced LTP, and LTP-induced spine enlargement is reversed by LFS-induced LTD [24, 25], suggesting shared mechanisms underlying synaptic LTP and LTD. It is noteworthy that an induction protocol that can elicit LTD in vitro may not be effective in vivo [27, 28]. Such sensitivity to stimulation parameters highlights the need to exercise caution when applying in vitro results to explain the synaptic mechanisms underlying learning and memory in vivo.
2.2. Spine formation and spine loss
In The Organization of Behavior, Donald Hebb presented his famous postulate on the activity-dependent plasticity of neuronal connections. It is noteworthy that his original formulation of the postulate is not limited to changes in the efficacy of existing synapses (as in LTP or LTD), but also encompasses the formation of new connections: ‘The most obvious and I believe much the most probable suggestion concerning the way in which one cell could become more capable of firing another is that synaptic knobs develop and increase the area of contact between the afferent axon and efferent soma. (“Soma” refers to dendrites and body, or all of the cell except its axon.)’ [29]. Indeed, in addition to morphological changes that accompany LTP or LTD, spine formation and loss also occur in vitro. Combining localized electric stimulation with 2P imaging, researchers have found that new dendritic spines or filopodia (thin dendritic protrusions without enlarged heads) emerge on the manipulated dendritic segement after the induction of long-lasting functional enhancement [30–32], while spine loss follows LFS [31]. Taking advantage of the spatial precision of 2P glutamate uncaging, Kwon et al. further induced de novo spine growth from dendritic shafts of layer (L) 2/3 pyramidal neurons (PNs) in cultured cortical slices. Both AMPAR- and NMDAR-mediated currents, as well as Ca influx, are detected in these new spines, suggesting that they are functional. They also found that the success rate to induce a new spine depends on both the frequency and the duration of the uncaging pulse, and some of the newly formed spines persist [33].
2.3. Spatial rules
Excitatory synaptic transmission is accompanied by the influx of Ca2+ ions into the postsynaptic neuron. The sub-micron spatial resolution of 2P imaging in combination with Ca indicators (molecules that drastically change their fluorescence upon binding to Ca2+ ions) enables researchers to monitor the activities of neighboring synapses simultaneously. Earlier studies have shown that in hippocampal slices, nearby synapses are more likely to be coactive than synapses located far away, and the prevalence of co-activation decreases as the inter-synaptic distance increases [34, 35]. Similar phenomena have been observed in L2/3 PNs in the sensory cortices of developing and adult mice in vivo [35, 36]. Moreover, the plasticity of an individual synapse influences its neighbors. Using glutamate uncaging to induce LTP in hippocampal slices, Harvey and Svoboda showed that LTP induction at one spine reduces the threshold of LTP induction of neighboring spines (within 10 μm) in a short time window (10 min) [37].
Obviously not all adjacent synapses are synchronously active. What happens to the synapses “out-of-sync”? In the developing visual cortex, Winnubst et al. showed that spines that are not coactive with their neighbors exhibit reduced synaptic activities over time, and the proBDNF/p75NTR signaling pathway is involved in this process. Intriguingly, the time window that gives the optimal desynchronization-induced depression (i.e., 1.6 s) nicely matches the duration of spontaneous waves in the retina and their correlated activity in the visual cortex, suggesting that such mechanisms may be used to shape the developing network in vivo. In addition, a synapse’s transmission reliability decreases when its activity is artificially desynchronized with that of its neighbors [36]. In cultured hippocampal slices, another study shows that LTP induction at several spines along a dendritic segment results in the shrinkage and loss of functional AMPAR of unstimulated spines within the same region. Such spine shrinkage is likely not due to a depletion of local resources by the adjacent spine enlargement, as it cannot be prevented by blocking LTP-induced spine enlargement; rather it is an active process involving calcineurin, IP3Rs, and group I mGluR-mediated signaling [38].
3. In vivo studies of spine dynamics
The capability to learn a new skill or association is crucial for the animal’s survival. It is generally believed that learning shapes neural circuits through modifications of synapses. Transgenic animals or viral labeling approaches allow selectively labeling of a subset of neurons in the living brain [39, 40]. Furthermore, 2P laser scanning microscopy offers deep penetration through thick opaque preparations, making it possible for live imaging in the intact brain [41]. In comparison with earlier imaging studies in cultured systems, imaging the living brain preserves the synapses’ natural environment and allows researchers to investigate synaptic plasticity that is directly associated with a particular behavior such as learning.
3.1. Motor skill learning
The motor cortex is indispensable for the acquisition of new motor skills in adult mice. Synaptic remodeling has been found in many experiments using different motor learning paradigms. Xu et al. trained the mouse with a single pellet reaching task, in which the mouse learns to reach its forepaw through a narrow slit to grasp and retrieve a food pellet [42]. Using in vivo 2P imaging, the researchers showed that significantly more new spines formed along the apical dendrites of L5 PNs in the primary motor cortex (M1) of thy1-YFP-H line mice. Such new spines formed rapidly (within an hour from the onset of the first training session), and the rate of spine formation (the number of newly formed spines divided by the total number of spines measured pre-training) during this training session positively correlated with the number of successful reaches. Moreover, no additional spine formed in mice that failed to learn the task, or in those tricked to do a lot of non-specific forelimb movement (Fig. 1A, B) [42]. Studies using different forelimb-specific tasks (i.e., pasta matrix or lever-press) have reported similarly elevated spine formation associated with the improvement of motor performance [43, 44]. In all these studies, the initial increase in spine formation was followed by enhanced spine elimination, so that the spine density only increased transiently [42–44], suggesting a remodeling of the existing circuit rather than simple addition of new connections (Fig. 1C). The transient synaptogenesis after learning has also been observed in the hippocampus in earlier EM studies [45, 46]. Besides forelimb tasks, Yang et al. trained the mouse to run on an accelerating rotarod. As the mouse gradually adapted to higher rotating speed, new spines formed along apical dendrites of L5 PNs. In contrast, no additional spine formed in association with running on the rotarod with steady speed [47].
Figure 1.
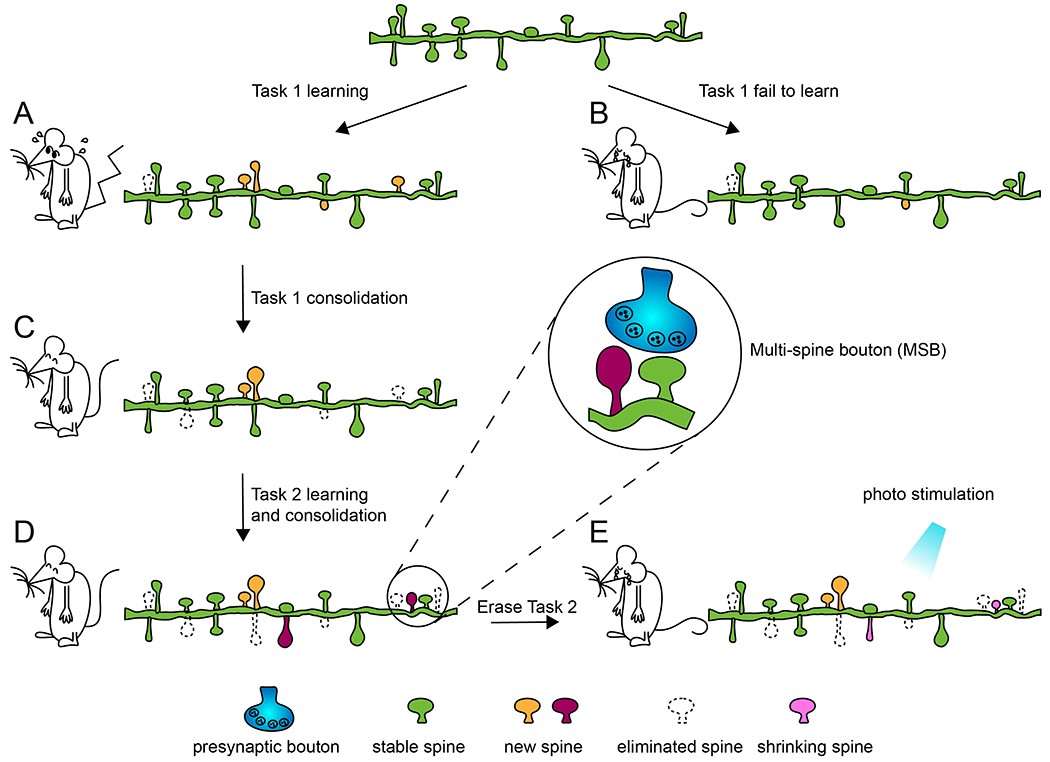
Illustration of learning-related spine dynamics.
The animal is trained on a learning task (Task 1). (A) Learning is accompanied by elevated spine formation beyond the basal level. (B) If the animal does not learn, basal level spine dynamics persist. (C) Consolidation of learned Task 1 preferentially stabilizes learning-related new spines and eliminates some pre-existing spines at an elevated rate. Thus, learning only transiently increases spine density, but rewires the neural circuit. Furthermore, new spines tend to emerge in clusters, which likely promotes their subsequent survival. (D) Learning and consolidation of a different task (Task 2) induces the formation and stabilization of a new set of spines. (E) Selectively shrinking Task 2-related spines by optogenetic actuators disrupts Task 2 performance. Inset: a new spine shares the same presynaptic bouton with an existing spine, resulting in a multispine bouton (MSB).
Challenging mice with different motor tasks sequentially, researchers have shown that new spines formed during motor training are task specific. For example, when a mouse that had mastered the single pellet reaching task was trained later with either the single pellet reaching task again or the pasta handling task (i.e., another forelimb-specific motor task), only the latter enhanced spine formation [42]. Similarly, after the mouse had mastered rotarod running, reversing the rotation direction could further induce spine formation [47]. These results suggest that different motor skills engage different neural circuits and require the modification of distinct sets of synaptic connections (Fig. 1D).
Successful acquisition of a new motor skill requires practice and repetition. While elevated spine formation is associated with the improvement of task performance, it is not observed in the late phase of training when performance plateaus [42]. Furthermore, compared to pre-existing spines, newly-formed spines are vulnerable to elimination [42], as they may lack functional synapses [48] and may be eliminated in the selective stabilization process. On the other hand, repetition of the newly-acquired motor task selectively stabilizes the learning-induced new spines (Fig. 1C) [42], and a greater portion of learning-associated new spines were eliminated if motor training was terminated prematurely [44]. Furthermore, the survival of learning-associated new spines correlates with the later performance of the motor skill [42, 44, 47]. It is worth mentioning that spine stabilization is not limited to newly formed spines. Using the songbird zebra finches as a model system, another study also showed that hearing a tutor song led to the rapid stabilization, accumulation, and enlargement of dendritic spines in the hyperstriatum ventrale pars caudalis (HVC), a forebrain nucleus necessary for sensorimotor integration and song learning, of juvenile birds [49]. Thus, besides learning-induced synapse formation, learning-associated synapses also need to be strengthened and stabilized for long-lasting neural circuit changes. Together they provide a structural underpinning of motor memory. The formation and stabilization of learning-associated new spines are accompanied by destabilization and loss of other spines existing before learning starts (Fig. 1C). However, what determines spines to be eliminated (e.g., local resource competition vs. global homeostatic regulation) and the functional roles of eliminated spines remain unknown.
Perhaps not surprising, learning-associated spine remodeling is region- and cell type-specific. However, the specificity varies among motor tasks. In the single pellet reaching task, spine dynamics increase within 300 μm of the functionally mapped forelimb M1 region contralateral to the trained limb, but remain unperturbed in the ipsilateral forelimb M1, contralateral hindlimb M1 and contralateral sensory cortex [42]. Moreover, the same group examined the dynamics of spines on apical dendrites of L2/3 PNs in the same region labelled by in utero electroporation, and found that, while spine density was significantly higher than that of L5 PNs, motor training did not further enhance their dynamics [50]. As most YFP+ neurons in thy1-YFP-H mice are corticospinal neurons [51], its selective synapse modification during fine skill learning may reflect the circuit rewiring to orchestrate a complicate motor skill [52]. The lever-press task changes spine dynamics in a compartmentalized manner: training elevates spine turnover on the distal dendrites of L2/3 PNs, but not on the perisomatic dendrites [43]. On the other hand, rotarod running seems to be less selective, as elevated spine dynamics are found in both L2/3 and L5 neurons, with comparable dynamics [53].
While spine imaging dominates in vivo studies for synapse remodeling during motor learning, less is known about the presynaptic partners of formed or eliminated spines. A recent study follows the dynamics of presynaptic axonal boutons in superficial M1 when the mouse is trained on an accelerating rotarod. They found that the formation of boutons on axons projecting from secondary motor areas (M2) increases during mid-phase training (day 2-4), while the elimination of boutons on axons from the motor thalamus decreases in late phase learning (day 4-7) [54]. Another study followed the presynaptic axonal varicosities from parallel fibers in the cerebellum, whose synapses with Purkinje cells play crucial roles in cerebellum-dependent learning, during acrobatic motor skill learning. The authors found that the fraction of dynamic parallel fiber varicosities significantly decreases during motor training, largely due to decrease addition of new varicosities [55]. Given the complex circuit organization, it is difficult to directly compare the dynamics of dendritic spines and axonal boutons from specific neuronal types. Thus, to understand circuit-specific modification by motor learning, it is crucial to co-imaging of pre- and post-synaptic structures of a specific neuronal connection. It can be achieved by co-imaging sparsely labeled pre- and post-synaptic neurons and search for their putative connection, followed by post hoc electron microscope (EM) confirmation of imaged synapses. Unfortunately, such data are still missing in the motor learning studies.
3.2. Fear learning and extinction
The associative fear conditioning is another widely used paradigm to study learning and memory. In auditory-cued fear conditioning, a specific tone is paired with an aversive stimulus (i.e., foot shock) to elicit a conditioned response (i.e., freezing). While paired conditioning increases spine formation, unpaired conditioning elevates spine elimination in the auditory cortex (ACx) [56–58]. Such fear conditioning-elevated spine formation transiently increases spine density (i.e., within 2h), which returns to the control level after 7 days [57]. Moreover, the amount of new spines formed during fear conditioning correlates with the percentage of time the animal freezes in the chamber [58]. Compared to controls, a significantly higher fraction of spines formed during conditioning persist over time. Memory recall does not reduce spine formation, nor does it affect the survival of spines formed during earlier conditioning [57], suggesting that reactivation of a fear memory doesn’t need to modify the existing neural network at the level of synaptic structures.
While it is difficult to unlearn a motor skill, repetitive exposure to the conditioning cue without foot-shock gradually diminishes the conditioned response, a process called fear extinction. Opposite to the elevated spine formation during fear conditioning, spine elimination increases in ACx during fear extinction. Extinction preferentially eliminates the new spines induced by conditioning, suggesting that, rather than forming a new circuit, extinction weakens the circuit that has been modified or strengthened by conditioning. Furthermore, both conditioning-induced spine formation and extinction-induced spine elimination are cue-specific. When the mouse is conditioned to freeze with two different auditory tones (i.e., 4 kHz and 12 kHz), extinction with the 4 kHz tone preferentially eliminates the spines formed during the 4 kHz conditioning [58].
Leveraging spectral variants of fluorescent proteins and post hoc spectral un-mixing to coimage presynaptic axonal boutons and postsynaptic dendritic spines, Yang and colleagues further demonstrated a selective remodeling of lateral amygdala (LA) to the ACx pathway during auditory fear conditioning. They found that both spine formation on L5 PNs and bouton formation on LA axons increased in ACx L1. In contrast, there is no change in the bouton dynamics of axons projecting from the medial geniculate body (MG) or the anterior cingulate cortex (ACC) to L1 of ACx. Intriguingly, they found that nearly all new synaptic contacts were made by adding new partners to existing synaptic elements (i.e., new boutons onto existing spines or new spines onto existing boutons) (Fig. 1 insert). This suggests that fear learning likely modify existing circuits rather than forming new connections between previously unconnected pairs of neurons through de novo formation of both pre- and post-synaptic components [56].
Interestingly, fear conditioning-induced spine remodeling is not limited to the auditory cortex. In both M1 and the frontal association cortex (FrA), fear learning remodels spines in a way opposite to that in ACx, i.e., increased spine elimination during conditioning and increased spine formation during extinction [59, 60]. Moreover, the percentage of spine elimination positively correlates with the percentage of freezing after fear conditioning, and the percentage of spine formation negatively correlates with the percentage of freezing after fear extinction [59]. In another fear conditioning paradigm (a tactile variant of trace eyeblink conditioning, TTEBC), 60 Hz whisker stimulation is paired with an air puff to the eye to elicit eyeblink. In the barrel cortex, TTEBC leads to elevated spine elimination on the apical dendrites of L5 PNs, leading to a progressive decrease in spine density [61]. Together, these data suggest broad impact of fear learning on the brain network, with simultaneous strengthening and weakening of distinct circuits.
3.3. Spatial rules underlying synapse remodeling
Cortical neurons have elaborated dendritic branches, on which most synapses reside. Rather than passively funneling synaptic information, dendrites are believed to be the basic organizational unit for integrating synaptic inputs [62]. Co-activation of synapses over a short stretch of dendrite elicits dendritic spikes [63], which leads to stronger and longer-lasting responses compared to ordinary EPSPs. Dendritic spikes may propagate into the soma to influence the neuronal output; they may also act locally to induce synaptic plasticity [64, 65]. Thus, synaptic inputs from distal dendrites can be non-linearly integrated, dramatically increasing the information processing capacities of neurons [66, 67].
Indeed, structural dynamics of synapses during learning have been found to cluster along dendrites. During accelerated rotarod training, sibling branches of L5 PNs in M1 exhibit different rates of spine formation [68]. And forward and backward running activates different dendritic branches of M1 L5 PNs [69]. More evidence suggests that clustered synaptic changes occur over a shorter length scale. Training mice with the single pellet reaching task, Fu et al. found that one third of the newly formed spines emerged in clusters (i.e., within 10 μm along the dendritic segment). Moreover, the clustered new spines are more likely to survive over time [70]. Interestingly, new spines in the same cluster are usually formed during sequential training sessions, and formation of the second spine in the cluster is accompanied by the head enlargement of the first spine [70]. As spine head enlargement indicates synaptic strengthening [10], this finding suggests that clustered new spines participate in the same neuronal circuit, which is activated by the specific motor task. Indeed, following surface AMPARs during the same forelimb task, Roth et al. further showed that training leads to an increase in AMPAR levels at a subset of spatially clustered spines in M1 [71].
Simulation further shows that under control conditions new spines appear to avoid existing stable spines, rather than being uniformly added along dendrites. However, succedent new spines in clusters overcome this spatial constraint and form in close vicinity to neighboring stable spines [70]. Recently, Frank et al. also found that in the retrosplenial cortex learning-related clustered spines were more likely to form at “hotspots”, i.e., dendritic segments with relatively high baseline spine turnover [72]. What is the spatial restraint of new spine formation and why new spines can overcome it to be clustered? On one hand, clustered spine formation may be due to the diffusion limit of synaptic signaling molecules. 2P fluorescence lifetime imaging (FLIM) studies have characterized the spread of many synaptic molecules, such as Rho GTPase, in response to the stimulation of a single dendritic spine, and shown that they modulate homo-synaptic and heterosynaptic plasticity [73, 74]. For example, after single spine LTP induction, the activity of Ras spreads ~10 μm along the dendrite and invades neighboring spines by diffusion, regulating local LTP threshold [37]. Alternatively, new spines may compete for existing pre-synaptic partners. Combining in vivo 2P imaging and EM of new spines induced by fear conditioning, Yang et al. [56] found that new spines are mostly formed onto existing axonal boutons, resulting in multisynapse boutons (MSBs). This result corroborates the earlier finding of significantly increased MSBs in rabbit hippocampal CA1 region in response to trace eyeblink conditioning, a form of associative learning [75]. A related phenomenon is the learning-associated generation of multisynapse spines (MSSs), namely, a spine forming synapses with two or more distinct axonal boutons [46, 56, 76, 77]. Thus, in addition to de novo synapse formation, learning also induces the formation of synapses with pre-existing synaptic components and creates a fan-in or fan-out connectivity.
The advantage of clustered spine formation remains to be explored. New spines in clusters appear more stable, hence with the potential to provide a structural basis for lasting memory [70]. Position functionally-related synapses close to each other may also increase the computational power of the neural network, as co-activation of neighboring synapses may evoke dendritic spikes and allow non-linear integration of synaptic inputs [67, 78].
4. Summary and open questions
In this review, we summarized in vitro and in vivo studies investigating the synaptic mechanisms underlying learning and memory, with a focus on dendritic spines. While in vitro studies nicely demonstrate the correlation between addition, loss, and morphological changes of dendritic spines with persist alterations in excitatory synaptic transmission, in vivo studies depict a much complex story: learning-induced spine formation and elimination are spatiotemporally regulated, as well as circuit- and task-specific.
Despite the significant progress in understanding the synaptic rules of learning, many questions remain. Below we pick three interesting questions as a starter, with a discussion of potential technical approaches to tackle them.
What are the functional roles of spines formed or lost during learning?
Almost all studies on learning-induced spine changes focus on spine formation and elimination; little is known about their functional roles. In particular, how are their synaptic activities associated with learning-related behaviors? It is now possible to characterize the Ca activities of individual spines in awake, behaving animals [79–81]. However, learning-induced spine formation and elimination occur with a relatively low frequency at unpredictable locations along the dendritic arbor. It implies that in order to capture such dynamic spines, one needs to monitor a sufficiently large population of spines simultaneously throughout the learning process. This calls for continuous volumetric imaging with single spine resolution over at least many minutes, which would be achievable with the steady improvement in volumetric 2P imaging techniques [82–84] and the development of more photo-stable fluorescent biosensors of synaptic activities [85–88].
Does learning one task leave a synaptic trace that influences later learning?
Previous studies have revealed that the structural alterations induced by a sensory manipulation impact the neural circuit’s response to subsequent manipulations. For example, in the mouse visual cortex, spines formed during the first monocular deprivation (MD) enlarge during the second MD, facilitating a rapid shift in the eye representation in the binocular zone [89, 90]. As memory traces of distinct, but related, tasks are believed to engage different but overlapping sets of synapses, how does learning one task affect later learning at the synaptic level? Addressing this question requires the identification of synaptic populations activated during different tasks, either by functional imaging or molecular markers that specifically label active synapses (discussed below). When sequentially trained on different tasks (e.g., different motor skills or associations), it is also interesting to see if learning the second task destabilizes the synaptic connections formed during the first task.
Can we manipulate memory at the synaptic level?
Taking advantage of IEGs, researchers have devised creative ways to synthesize or erase memories in live animals [91–94]. Pushing this line of ideas to the synaptic level, Hayashi-Takagi et al. engineered a protein AS-PaRac1 (activated synapse targeting photoactivatable Rac1) for light-induced manipulation of potentiated spines. Photo-stimulation of AS-PaRac1 shrinks the learning-potentiated spines, causing the mouse to lose the learnt skill (Fig. 1E) [95]. It is conceivable that a biomarker with high specificity to active synapses and precise temporal control of expression may allow us to identify all synapses activated in a learning process. Combination with optogenetics or pharmacogenetics will further enable us to consolidate or erase a memory trace through direct manipulation at the synaptic level.
Highlights.
Morphological changes of dendritic spines are associated with LTP and LTD in vitro
Learning induces spine formation and stabilization in a circuit-specific manner
Clustered synaptic modifications facilitate dendritic computation
Acknowledgements
YZ acknowledge the support from National Institutes of Health (grants R01MH109475, R01NS104950, and R21HD101266) and Max Planck Fellowship. The authors would like to thank Dr. Ju Lu, Dr. Kacper Lukasiewicz and Dr. Wenjie Bian for constructive criticism of the manuscript.
Footnotes
Competing interests
The authors declare that no competing interests exist.
References
- [1].Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, Wei B, Veshkini M, La-Vu M, Lou J, Flores SE, Kim I, Sano Y, Zhou M, Baumgaertel K, Lavi A, Kamata M, Tuszynski M, Mayford M, Golshani P, Silva AJ, A shared neural ensemble links distinct contextual memories encoded close in time, Nature 534(7605) (2016) 115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jercog P, Rogerson T, Schnitzer MJ, Large-scale fluorescence calcium-imaging methods for studies of long-term memory in behaving mammals, Cold Spring Harb Perspect Biol 8(5) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA, Neuronal competition and selection during memory formation, Science 316(5823) (2007) 457–60. [DOI] [PubMed] [Google Scholar]
- [4].Kee N, Teixeira CM, Wang AH, Frankland PW, Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus, Nat Neurosci 10(3) (2007) 355–62. [DOI] [PubMed] [Google Scholar]
- [5].Kim WB, Cho JH, Encoding of contextual fear memory in hippocampal-amygdala circuit, Nat Commun 11(1) (2020) 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].DeNardo LA, Liu CD, Allen WE, Adams EL, Friedmann D, Fu L, Guenthner CJ, Tessier-Lavigne M, Luo L, Temporal evolution of cortical ensembles promoting remote memory retrieval, Nat Neurosci 22(3) (2019) 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roy DS, Park Y-G, Ogawa SK, Cho JH, Choi H, Kamensky L, Martin J, Chung K, Tonegawa S, Brain-wide mapping of contextual fear memory engram ensembles supports the dispersed engram complex hypothesis, bioRxiv (2019) 668483. [Google Scholar]
- [8].Araya R, Vogels TP, Yuste R, Activity-dependent dendritic spine neck changes are correlated with synaptic strength, Proc Natl Acad Sci U S A 111(28) (2014) E2895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tonnesen J, Nagerl UV, Dendritic spines as tunable regulators of synaptic signals, Front Psychiatry 7 (2016) 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yuste R, Bonhoeffer T, Morphological changes in dendritic spines associated with long-term synaptic plasticity, Annu Rev Neurosci 24 (2001) 1071–89. [DOI] [PubMed] [Google Scholar]
- [11].Segal M, Dendritic spines: Morphological building blocks of memory, Neurobiol Leam Mem 138 (2017) 3–9. [DOI] [PubMed] [Google Scholar]
- [12].Lu J, Zuo Y, Shedding light on learning and memory: optical interrogation of the synaptic circuitry, Curr Opin Neurobiol 67 (2020) 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bailey CH, Kandel ER, Structural changes accompanying memory storage, Annu Rev Physiol 55 (1993) 397–426. [DOI] [PubMed] [Google Scholar]
- [14].Sala C, Segal M, Dendritic spines: the locus of structural and functional plasticity, Physiol Rev 94(1) (2014) 141–88. [DOI] [PubMed] [Google Scholar]
- [15].Nimchinsky EA, Sabatini BL, Svoboda K, Structure and function of dendritic spines, Annu Rev Physiol 64 (2002) 313–53. [DOI] [PubMed] [Google Scholar]
- [16].Ebrahimi S, Okabe S, Structural dynamics of dendritic spines: molecular composition, geometry and functional regulation, Biochim Biophys Acta 1838(10) (2014) 2391–8. [DOI] [PubMed] [Google Scholar]
- [17].Rochefort NL, Konnerth A, Dendritic spines: from structure to in vivo function, EMBO Rep 13(8) (2012) 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maiti P, Manna J, Ilavazhagan G, Rossignol J, Dunbar GL, Molecular regulation of dendritic spine dynamics and their potential impact on synaptic plasticity and neurological diseases, Neurosci Biobehav Rev 59 (2015) 208–37. [DOI] [PubMed] [Google Scholar]
- [19].Chidambaram SB, Rathipriya AG, Bolla SR, Bhat A, Ray B, Mahalakshmi AM, Manivasagam T, Thenmozhi AJ, Essa MM, Guillemin GJ, Chandra R, Sakharkar MK, Dendritic spines: revisiting the physiological role, Prog Neuropsychopharmacol Biol Psychiatry 92 (2019) 161–193. [DOI] [PubMed] [Google Scholar]
- [20].Fiala JC, Spacek J, Harris KM, Dendritic spine pathology: cause or consequence of neurological disorders?, Brain Res Brain Res Rev 39(1) (2002) 29–54. [DOI] [PubMed] [Google Scholar]
- [21].Hering H, Sheng M, Dendritic spines: structure, dynamics and regulation, Nat Rev Neurosci 2(12) (2001) 880–8. [DOI] [PubMed] [Google Scholar]
- [22].Nicoll RA, A Brief History of Long-Term Potentiation, Neuron 93(2) (2017) 281–290. [DOI] [PubMed] [Google Scholar]
- [23].Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H, Structural basis of long-term potentiation in single dendritic spines, Nature 429(6993) (2004) 761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou Q, Homma KJ, Poo MM, Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses, Neuron 44(5) (2004) 749–57. [DOI] [PubMed] [Google Scholar]
- [25].Yang Y, Wang XB, Frerking M, Zhou Q, Spine expansion and stabilization associated with long-term potentiation, J Neurosci 28(22) (2008) 5740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hill TC, Zito K, LTP-induced long-term stabilization of individual nascent dendritic spines, J Neurosci 33(2) (2013) 678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goh JJ, Manahan-Vaughan D, Synaptic depression in the CA1 region of freely behaving mice is highly dependent on afferent stimulation parameters, Front Integr Neurosci 7 (2013) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gruart A, Leal-Campanario R, Lopez-Ramos JC, Delgado-Garcia JM, Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: Lessons from studies in behaving mammals, Neurobiol Learn Mem 124 (2015) 3–18. [DOI] [PubMed] [Google Scholar]
- [29].Hebb DO, The organization of behaviour : a neuropsychological theory, Wiley, New York, 1949. [Google Scholar]
- [30].Engert F, Bonhoeffer T, Dendritic spine changes associated with hippocampal long-term synaptic plasticity, Nature 399(6731) (1999) 66–70. [DOI] [PubMed] [Google Scholar]
- [31].Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T, Bidirectional activity-dependent morphological plasticity in hippocampal neurons, Neuron 44(5) (2004) 759–67. [DOI] [PubMed] [Google Scholar]
- [32].Maletic-Savatic M, Malinow R, Svoboda K, Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity, Science 283(5409) (1999) 1923–7. [DOI] [PubMed] [Google Scholar]
- [33].Kwon HB, Sabatini BL, Glutamate induces de novo growth of functional spines in developing cortex, Nature 474(7349) (2011) 100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C, Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites, Neuron 72(6) (2011) 1012–24. [DOI] [PubMed] [Google Scholar]
- [35].Takahashi N, Kitamura K, Matsuo N, Mayford M, Kano M, Matsuki N, Ikegaya Y, Locally synchronized synaptic inputs, Science 335(6066) (2012) 353–6. [DOI] [PubMed] [Google Scholar]
- [36].Winnubst J, Cheyne JE, Niculescu D, Lohmann C, Spontaneous activity drives local synaptic plasticity in vivo, Neuron 87(2) (2015) 399–410. [DOI] [PubMed] [Google Scholar]
- [37].Harvey CD, Svoboda K, Locally dynamic synaptic learning rules in pyramidal neuron dendrites, Nature 450(7173) (2007) 1195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oh WC, Parajuli LK, Zito K, Heterosynaptic structural plasticity on local dendritic segments of hippocampal CA1 neurons, Cell Rep 10(2) (2015) 162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR, Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP, Neuron 28(1) (2000) 41–51. [DOI] [PubMed] [Google Scholar]
- [40].Chen BE, Lendvai B, Nimchinsky EA, Burbach B, Fox K, Svoboda K, Imaging high-resolution structure of GFP-expressing neurons in neocortex in vivo, Learn Mem 7(6) (2000) 433–41. [DOI] [PubMed] [Google Scholar]
- [41].Denk W, Strickler JH, Webb WW, Two-photon laser scanning fluorescence microscopy, Science 248(4951) (1990) 73–6. [DOI] [PubMed] [Google Scholar]
- [42].Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y, Rapid formation and selective stabilization of synapses for enduring motor memories, Nature 462(7275) (2009) 915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen SX, Kim AN, Peters AJ, Komiyama T, Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning, Nat Neurosci 18(8) (2015) 1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Clark TA, Fu M, Dunn AK, Zuo Y, Jones TA, Preferential stabilization of newly formed dendritic spines in motor cortex during manual skill learning predicts performance gains, but not memory endurance, Neurobiol Learn Mem 152 (2018) 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].O’Malley A, O’Connell C, Regan CM, Ultrastructural analysis reveals avoidance conditioning to induce a transient increase in hippocampal dentate spine density in the 6 hour post-training period of consolidation, Neuroscience 87(3) (1998) 607–13. [DOI] [PubMed] [Google Scholar]
- [46].Radwanska K, Medvedev NI, Pereira GS, Engmann O, Thiede N, Moraes MF, Villers A, Irvine EE, Maunganidze NS, Pyza EM, Ris L, Szymanska M, Lipinski M, Kaczmarek L, Stewart MG, Giese KP, Mechanism for long-term memory formation when synaptic strengthening is impaired, Proc Natl Acad Sci U S A 108(45) (2011) 18471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang G, Pan F, Gan WB, Stably maintained dendritic spines are associated with lifelong memories, Nature 462(7275) (2009) 920–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K, Spine growth precedes synapse formation in the adult neocortex in vivo, Nat Neurosci 9(9) (2006) 1117–24. [DOI] [PubMed] [Google Scholar]
- [49].Roberts TF, Tschida KA, Klein ME, Mooney R, Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning, Nature 463(7283) (2010) 948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tjia M, Yu X, Jammu LS, Lu J, Zuo Y, Pyramidal neurons in different cortical layers exhibit distinct dynamics and plasticity of apical dendritic spines, Front Neural Circuits 11 (2017) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Porrero C, Rubio-Garrido P, Avendano C, Clasca F, Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice, Brain Res 1345 (2010) 59–72. [DOI] [PubMed] [Google Scholar]
- [52].Wang X, Liu Y, Li X, Zhang Z, Yang H, Zhang Y, Williams PR, Alwahab NSA, Kapur K, Yu B, Chen M, Ding H, Gerfen CR, Wang KH, He Z, Deconstruction of corticospinal circuits for goal-directed motor skills, Cell 171(2) (2017) 440–455 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ma L, Qiao Q, Tsai JW, Yang G, Li W, Gan WB, Experience-dependent plasticity of dendritic spines of layer 2/3 pyramidal neurons in the mouse cortex, Dev Neurobiol 76(3) (2016) 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hasegawa R, Ebina T, Tanaka YR, Kobayashi K, Matsuzaki M, Structural dynamics and stability of corticocortical and thalamocortical axon terminals during motor learning, PLoS One 15(6) (2020) e0234930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Carrillo J, Cheng SY, Ko KW, Jones TA, Nishiyama H, The long-term structural plasticity of cerebellar parallel fiber axons and its modulation by motor learning, J Neurosci 33(19) (2013) 8301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yang Y, Liu DQ, Huang W, Deng J, Sun Y, Zuo Y, Poo MM, Selective synaptic remodeling of amygdalocortical connections associated with fear memory, Nat Neurosci 19(10) (2016) 1348–55. [DOI] [PubMed] [Google Scholar]
- [57].Moczulska KE, Tinter-Thiede J, Peter M, Ushakova L, Wernle T, Bathellier B, Rumpel S, Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall, Proc Natl Acad Sci U S A 110(45) (2013) 18315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lai CSW, Adler A, Gan WB, Fear extinction reverses dendritic spine formation induced by fear conditioning in the mouse auditory cortex, Proc Natl Acad Sci U S A 115(37) (2018) 9306–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lai CS, Franke TF, Gan WB, Opposite effects of fear conditioning and extinction on dendritic spine remodelling, Nature 483(7387) (2012) 87–91. [DOI] [PubMed] [Google Scholar]
- [60].Xu Z, Adler A, Li H, Perez-Cuesta LM, Lai B, Li W, Gan WB, Fear conditioning and extinction induce opposing changes in dendritic spine remodeling and somatic activity of layer 5 pyramidal neurons in the mouse motor cortex, Sci Rep 9(1) (2019) 4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Joachimsthaler B, Brugger D, Skodras A, Schwarz C, Spine loss in primary somatosensory cortex during trace eyeblink conditioning, J Neurosci 35(9) (2015) 3772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Branco T, Hausser M, The single dendritic branch as a fundamental functional unit in the nervous system, Curr Opin Neurobiol 20(4) (2010) 494–502. [DOI] [PubMed] [Google Scholar]
- [63].Losonczy A, Magee JC, Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons, Neuron 50(2) (2006) 291–307. [DOI] [PubMed] [Google Scholar]
- [64].Spruston N, Pyramidal neurons: dendritic structure and synaptic integration, Nat Rev Neurosci 9(3) (2008) 206–21. [DOI] [PubMed] [Google Scholar]
- [65].Hardie J, Spruston N, Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons, J Neurosci 29(10) (2009) 3233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Poirazi P, Mel BW, Impact of active dendrites and structural plasticity on the memory capacity of neural tissue, Neuron 29(3) (2001) 779–96. [DOI] [PubMed] [Google Scholar]
- [67].Kastellakis G, Poirazi P, Synaptic clustering and memory formation, Front Mol Neurosci 12 (2019) 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB, Sleep promotes branch-specific formation of dendritic spines after learning, Science 344(6188) (2014) 1173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cichon J, Gan WB, Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity, Nature 520(7546) (2015) 180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fu M, Yu X, Lu J, Zuo Y, Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo, Nature 483(7387) (2012) 92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Roth RH, Cudmore RH, Tan HL, Hong I, Zhang Y, Huganir RL, Cortical synaptic AMPA receptor plasticity during motor learning, Neuron 105(5) (2020) 895–908 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Frank AC, Huang S, Zhou M, Gdalyahu A, Kastellakis G, Silva TK, Lu E, Wen X, Poirazi P, Trachtenberg JT, Silva AJ, Hotspots of dendritic spine turnover facilitate clustered spine addition and learning and memory, Nat Commun 9(1) (2018) 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hedrick NG, Yasuda R, Regulation of Rho GTPase proteins during spine structural plasticity for the control of local dendritic plasticity, Curr Opin Neurobiol 45 (2017) 193–201. [DOI] [PubMed] [Google Scholar]
- [74].Yasuda R, Biophysics of biochemical signaling in dendritic spines: implications in synaptic plasticity, Biophys J 113(10) (2017)2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA, Associative learning elicits the formation of multiple-synapse boutons, J Neurosci 21(15) (2001) 5568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jasinska M, Siucinska E, Cybulska-Klosowicz A, Pyza E, Furness DN, Kossut M, Glazewski S, Rapid, learning-induced inhibitory synaptogenesis in murine barrel field, J Neurosci 30(3) (2010) 1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Aziz W, Kraev I, Mizuno K, Kirby A, Fang T, Rupawala H, Kasbi K, Rothe S, Jozsa F, Rosenblum K, Stewart MG, Giese KP, Multi-input Synapses, but Not LTP-Strengthened Synapses, Correlate with Hippocampal Memory Storage in Aged Mice, Curr Biol 29(21) (2019) 3600–3610 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kastellakis G, Cai DJ, Mednick SC, Silva AJ, Poirazi P, Synaptic clustering within dendrites: an emerging theory of memory formation, Prog Neurobiol 126 (2015) 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Scholl B, Thomas CI, Ryan MA, Kamasawa N, Fitzpatrick D, Cortical response selectivity derives from strength in numbers of synapses, Nature (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wilson DE, Whitney DE, Scholl B, Fitzpatrick D, Orientation selectivity and the functional clustering of synaptic inputs in primary visual cortex, Nat Neurosci 19(8) (2016) 1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A, Functional mapping of single spines in cortical neurons in vivo, Nature 475(7357) (2011) 501–5. [DOI] [PubMed] [Google Scholar]
- [82].Lu R, Sun W, Liang Y, Kerlin A, Bierfeld J, Seelig JD, Wilson DE, Scholl B, Mohar B, Tanimoto M, Koyama M, Fitzpatrick D, Orger MB, Ji N, Video-rate volumetric functional imaging of the brain at synaptic resolution, Nat Neurosci 20(4) (2017) 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kazemipour A, Novak O, Flickinger D, Marvin JS, Abdelfattah AS, King J, Borden PM, Kim JJ, Al-Abdullatif SH, Deal PE, Miller EW, Schreiter ER, Druckmann S, Svoboda K, Looger LL, Podgorski K, Kilohertz frame-rate two-photon tomography, Nat Methods 16(8) (2019) 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Szalay G, Judak L, Katona G, Ocsai K, Juhasz G, Veress M, Szadai Z, Feher A, Tompa T, Chiovini B, Maak P, Rozsa B, Fast 3D imaging of spine, dendritic, and neuronal assemblies in behaving animals, Neuron 92(4) (2016) 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Marvin JS, Scholl B, Wilson DE, Podgorski K, Kazemipour A, Muller JA, Schoch S, Quiroz FJU, Rebola N, Bao H, Little JP, Tkachuk AN, Cai E, Hantman AW, Wang SS, DePiero VJ, Borghuis BG, Chapman ER, Dietrich D, DiGregorio DA, Fitzpatrick D, Looger LL, Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR, Nat Methods 15(11) (2018) 936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP, Tsegaye G, Tsang A, Wong A, Patel R, Macklin JJ, Chen Y, Konnerth A, Jayaraman V, Looger LL, Schreiter ER, Svoboda K, Kim DS, High-performance calcium sensors for imaging activity in neuronal populations and microcompartments, Nat Methods 16(7) (2019) 649–657. [DOI] [PubMed] [Google Scholar]
- [87].Inoue M, Takeuchi A, Manita S, Horigane SI, Sakamoto M, Kawakami R, Yamaguchi K, Otomo K, Yokoyama H, Kim R, Yokoyama T, Takemoto-Kimura S, Abe M, Okamura M, Kondo Y, Quirin S, Ramakrishnan C, Imamura T, Sakimura K, Nemoto T, Kano M, Fujii H, Deisseroth K, Kitamura K, Bito H, Rational engineering of XCaMPs, a multicolor GECI suite for in vivo imaging of complex brain circuit dynamics, Cell 177(5) (2019) 1346–1360 e24. [DOI] [PubMed] [Google Scholar]
- [88].Mohr MA, Bushey D, Aggarwal A, Marvin JS, Kim JJ, Marquez EJ, Liang Y, Patel R, Macklin JJ, Lee CY, Tsang A, Tsegaye G, Ahrens AM, Chen JL, Kim DS, Wong AM, Looger LL, Schreiter ER, Podgorski K, jYCaMP: an optimized calcium indicator for two-photon imaging at fiber laser wavelengths, Nat Methods 17(7) (2020) 694–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M, Experience leaves a lasting structural trace in cortical circuits, Nature 457(7227) (2009) 313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M, Prior experience enhances plasticity in adult visual cortex, Nat Neurosci 9(1) (2006) 127–32. [DOI] [PubMed] [Google Scholar]
- [91].Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA, Selective erasure of a fear memory, Science 323(5920) (2009) 1492–6. [DOI] [PubMed] [Google Scholar]
- [92].Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S, Creating a false memory in the hippocampus, Science 341(6144) (2013) 387–91. [DOI] [PubMed] [Google Scholar]
- [93].Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, Lim SC, Santos SL, Denny CA, Drew MR, Distinct hippocampal engrams control extinction and relapse of fear memory, Nat Neurosci 22(5) (2019) 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S, Optogenetic stimulation of a hippocampal engram activates fear memory recall, Nature 484(7394) (2012) 381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H, Labelling and optical erasure of synaptic memory traces in the motor cortex, Nature 525(7569) (2015) 333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


