Abstract
COVID-19 and malignancy can affect the susceptibility of one another. Clinically recovered COVID-19 individuals display immune abnormalities that persist several months after discharge. The lymphopenia-related immunosuppression, functional exhaustion of cytotoxic lymphocytes (such as CD8+ cytotoxic T-cells and natural killer cells), hyperinflammatory responses, oxidative stress, downregulation of interferon response, development of the myeloid-derived suppressor cells, downregulation of tumor suppressor proteins and perhaps reactivation of the latent oncogenic viruses may directly and/or indirectly play a role in the cancer development and recurrence in severe COVID-19 patients. SARS-CoV-2-infected malignant patients may be at higher risk of death of their cancer than SARS-CoV-2-uninfected patients with the same cancers. On the other side, the patients with some types of cancers may be more vulnerable to SARS-CoV-2 infection compared with the non-cancerous individuals, due to their immunocompromised state resulted from malignancy, chemotherapy, and other concomitant abnormalities as well as perhaps greater expression of angiotensin-converting enzyme 2. SARS-CoV-2-infected cancerous patients are unable to produce an effective anti-virus immune response and may exhibit more severe forms of COVID-19. This review described the possible impacts of SARS-CoV-2 infection on cancer development and recurrence, and the potential cancer impacts on COVID-19 development, while the possible interventions are highlighted.
Key Words: COVID-19, Cancer, SARS-CoV-2, Immunosuppression, Inflammation, Oncology, Malignancy
Introduction
The SARS-CoV-2-mediated coronavirus disease 2019 (COVID-19) pandemic has affected a great number of populations in about 200 countries and territories 1 . SARS-CoV-2 is transmitted predominantly by symptomatic, pre-symptomatic, and asymptomatic carriers, mainly via respiratory droplets during face-to-face exposure such as coughing, talking, and sneezing. Aerosols and to a smaller extent, infected surfaces may also spread the virus 2 .
Patients with underlying disorders, including diabetes, hypertension, cancer, chronic respiratory disease, and cardiovascular diseases are more vulnerable to COVID-19 3 . The elderly individuals and those with impaired immune system are at a greater risk of developing serious and even deadly respiratory disorders 3 . The COVID-19-related symptoms appear after an incubation time with a median of approximately 5 days 4,5. The period from the initiation of COVID-19 signs to possible dying differs from 6 to 41 days with an average of 14 days 4,5. It was estimated that approximately 10.0% of patients with COVID‐19 experience prolonged manifestations (for over 3 weeks) and in 1.5%-2.0% of patients, signs persist for over 90 days 6,7. Some cases have been considered as chronic COVID‐19 when symptoms extending beyond 12 weeks 6 . Long COVID19 can be the result of low grades of the virus being sequestered in certain tissues, making it impossible to be identified by conventional diagnostic tests 8 . In the patients with long COVID-19, the virus can exert profound modulatory effects on the immune system and induce low-grade chronic inflammatory responses8,9. Thus, SARS-CoV-2 infection, especially in its long term, can act as a risk factor in cancer development.
Data reported from Wuhan, China showed that about 1.0-2.0% of COVID-19 patients were diagnosed with malignancy 10,11. However, about 6.0% of hospitalized subjects with COVID-19 from New York City had cancer 12 , and about 8.0% of COVID-19 patients who needed the ICU facilities had an active or early history of malignancy 13 . In another study from Italy, about 20.0% of the COVID-19-related deaths occurred in patients with active malignancy 14 .
The immune system performs an essential role in defending against tumor cells. As a result, the occurrence of malignancy is significantly higher in immune-compromised hosts 15 . Based on immune surveillance concepts, an essential duty of the immune system is to carefully examine the development of malignancy in the body and
eliminate tumor cells as they emerge 16 . In the immune system, the Th1 cells show potent anti-tumorigenic effects via recruitment of the natural killer (NK) cells, M1 type macrophages, and CD8+ cytotoxic T lymphocytes (CTLs)17,18. Conversely, type 2 macrophages (M2), myeloid-derived suppressor cells (MDSCs), Th2 cells, and regulatory T (Treg) cells inhibit the anti-tumor immune responses 18,19. Tumor cells also escape immune-mediated recognition/elimination by a number of mechanisms, particularly down-regulating of anti-tumoral immune responses 20 . Clinically recovered COVID-19 individuals display profound immunologic alterations in both innate and adaptive immunity compartments that persist several months after discharge 21-23. Anti-tumor immune responses can be weakened because of these immunologic changes.
The malignant patients exhibit a greater risk of SARS-CoV-2 infection. The results from a study conducted in Spain indicated that 31.4% of the cancer patients were seropositive for specific IgG or IgM against SARS-CoV-2, while seropositivity in the general population was 10.0% 24 . In the general population, the fatality rate of COVID-19 is estimated to range from 2 to 3%. However, cancer patients are more susceptible to COVID-19 and have a 25.0%-39.0% mortality risk if infected with SARS-CoV-2 25 . Thus, COVID-19 and malignancy can influence each other susceptibility. SARS-CoV-2 infection may profoundly attenuate the anti-tumoral immune responses. Therefore, the COVID-19 patients may be immunocompromised to an extent where the anti-tumor response is depleted causing cancer development and cancer recurrence. This review explains the possible SARS-CoV-2-mediated impacts on the development of cancer and cancer recurrence, and the potential cancer impacts on the COVID-19, while the possible interventions are highlighted.
Pathophysiology of COVID-19
Four main structural proteins of coronaviruses include spike (S), nucleocapsid (N), envelope (E), and membrane (M), among which S protein plays a fundamental role in the virus binding to its cell receptor, angiotensin-converting enzyme-2 (ACE2) 1,26. Upon respiratory infection with SARS-CoV-2, an initial proper IFN response (primarily type III IFNs) effectively eliminate the virus without the expression of the clinical symptoms or with the expression of mild signs 27 . If the patient is immunocompromised or if the initial IFN response fails, the virus expands, then enters the bloodstream from the lungs and infects ACE2 expressing organs 27 . The tissues and cells that express ACE2 are considered as the possible target of SARS-CoV-2. The greatest expression of ACE2 has been recorded in the gastrointestinal tract, testis, and kidneys 28 . In the lungs, ACE2 is highly expressed by type II alveolar cells, and in the liver, ACE2 expression was observed in cholangiocytes 1,28. The enterocytes of the small intestine express more ACE2 compared with those from colon regions 28 . ACE2 is also expressed by stratified epithelial cells of the esophagus, proximal tubule cells of kidney, and urothelial cells of the bladder1,26,29. ACE2 expression has also been documented in CNS blood vessels that provide a direct route for SARS-CoV-2 entry into the brain 28,30. Coronaviruses can also attack the peripheral nerves, and then reach the CNS via the synaptic pathway 31 .
Collectively, the massive virus replication, viral-mediated ACE2 downregulation, immune dysfunction, over-inflammatory reactions, cytokine storm, lymphopenia, enormous cell death (in particular endothelial- and epithelial cells) through apoptosis, necrosis and pyroptosis, coagulopathy, vascular leaks, and lung fibrosis can play prominent roles in the COVID-19 pathogenesis32,33. The anti-virus IFN response is impaired in severe COVID-19 patients, however, these patients experience a destructive cytokine storm which is characterized by a dangerous increase in the circulatory quantities of cytokines such as IL-6, TNF-α, IL-1β, IL-2, IL-7, IL-8, MIP-1α, CCL2 and CXCL10 (IP-10) 4,27. Cytokine storm can promote viral sepsis and contributes to coagulopathy, vascular leak, multiple organ failure, in particular, acute respiratory distress syndrome (ARDS) 4,27.
In the lung-related innate immunity, viral-derived RNAs are sensed by toll-like receptors (such as TLR7 and TLR8) and cytoplasmic sensors [such as retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA-5)] leading to the IFN production 27 . However, SARS-CoV-2 can effectively downregulate anti-virus IFN response 34,35. Moreover, a number of SARS-CoV-2-derived molecules activate NLRP3 inflammasome resulting in the pyroptosis and releasing of IL-1β and IL-18 which promote HMGB1 release, Th17 cell activation, neutrophil recruitment, macrophage activation and cytokine storm 36 .
Direct interaction between SARS-CoV-2 with platelets, virus-induced complement activation and microvascular damage over-activate platelets which can lead to thrombosis 37 . Among inflammatory markers, IL-6 and CRP support thrombosis state 33,37 . Diverse cytokines have been implicated in the process of lung fibrosis such as TGF-β and IL-6 38 . During COVID-19, TGF-β is produced by various types of cells such as platelets, macrophages and infected type 2 alveolar cells 33 . TGF-β exerts fibrogenic activity via inducing the proliferation and migration of fibroblasts and stimulating the formation of the extracellular matrix 33,38. Local IL-6 overexpression also exacerbated fibrosis through the promotion of the pro-fibrotic activity of M2 macrophages 33 . IL-6-dependent signaling in the vascular epithelial cells lead to up-regulation of the VEGF and downregulation of the E-cadherin promoting vascular permeability and leakage 33 .
COVID-19 patients, in particular, those with severe forms of illness have lower blood numbers of CD3+ T lymphocytes, B lymphocytes, CD4+ T cells, CD8+ T cells, and NK cells 39 . The results from different studies indicate that approximately 33.0%–96.0% of patients with the severe form of COVID-19 display lymphopenia which has a close relationship with illness progression and fatality rate 39 . Lymphopenia can lead to immune depression and promote cytokine storm, both of which play important roles in virus dissemination and multi-organ failure ,3 33 9. Moreover, total lymphocyte counts are significantly decreased in COVID-19 patients > 50 years old than those < 50 40 .
Additionally, a small subset of monocytes, macrophages and T cells may also be infected by SARS-CoV-2 via ACE2-dependent and ACE2-independent process using L-SIGN, DC-SIGN, CD147, antibody-dependent enhancement (ADE), and perhaps phagocytosis of apoptotic bodies containing the virus 32,41-44. The infection of some types of leukocytes by SARS-CoV-2 can compromise the innate and adaptive immune responses. SARS-CoV-2 can effectively suppress the anti-viral interferon (IFN) response in antigen presenting cells (APCs) such as monocytes and macrophages and dendritic cells (DCs) 4,45.
In convalescent patients, the functional abnormalities of the T and B cells also persist up to 6 months after discharging from hospital 22 . Yang et al. also indicated that the clinically recovered COVID-19 individuals display immune abnormalities such as lower circulatory numbers of Th1-, Th2-, Th17-, Tfh-, memory B-, and central memory T cells within 4-11 weeks after discharge 21 . Kostopoulos et al. also indicated that some immunologic alterations such as reduction of circulatory B cells persist up to eight months after SARS-CoV-2 infection 23 . Therefore, SARS-CoV-2 infection-related immune abnormalities, especially in its long term can disrupt the anti-tumoral immune responses.
Risk of tumor development can be enhanced after SARS-CoV-2 infection
1) Anti-tumoral immune responses are compromised after SARS-CoV-2 infection
In the immune system, NK cells are the first line of protection against tumor- and virus-infected cells 46 . Positivity for the CD16 and CD56 expression is among the most important of NK cell features 47 . The NK cell activity is controlled by a number of activating and inhibitory receptors. The NK cell-mediated elimination of the target cells depends on the balance between activating and inhibitory receptors, causing the NK cell to discriminate normal cells from unwanted cells 48 . In the adaptive immunity, the CD8+ T cells kill cancerous- and viral-infected cells following the identification of the tumor- and viral-related antigenic peptides bound to MHC class I molecules expressed by target cells. CD4+ T cells provide the necessary signals for activating of the CD8+ T cells, which are then differentiated to effector CTLs that kill target cells via release of granules or by FasL‐mediated apoptosis 49 .
The number of CD16+ CD56+ NK cells are markedly diminished in severe COVID-19 than in mild cases and healthy people 50-52. In the severe COVID-19 patients, the total counts of CD4+ T and CD8+ T cells are also reduced compared with other patients, which indicate that COVID-19 can exert profound impacts on lymphocyte 40,50-52.
The reduced numbers of CD56dim NK cells (a subset of NK cells with cytotoxicity activity), and the reduced levels of NK cell-activating cytokines (including IL-12, IL-15, and IL-21) were indicated in the severe COVID-19 patients 53 . The overexpression of an inhibitory receptor, called NK group 2 member A (NKG2A), occurs by CD8+ T- and NK cells from COVID-19 patients in comparison to healthy people 51,54,55. Lower counts of NK and CD8+ T cells expressing CD107a (an activation marker), IFN-γ, and IL-2, were detected in COVID-19 patients in comparison with healthy controls 51 . The intensity of granzyme B expression was also diminished in the NK and CD8+ T cells from COVID-19 patients compared with healthy individuals 51 . The percentages of the TNF-α+ NK-cells were also diminished in COVID-19 patients 51 . The overexpression of NKG2A on CTLs and NK cells from COVID-19 patients is associated with the small expression of IFN-γ, IL-2, TNF-α, CD107a, and granzyme B 51 . Thereby, the overexpression of NKG2A leads to the functional exhaustion of CD8+ T and NK cells in COVID-19 patients compromising immune responses against the cancers and viral pathogens 51,54,55. In convalescent COVID-19 patients, the functional abnormalities of the CD8+ T cells persist up to 6 months after discharge from the hospital 22 .
The NKG2A overexpression and next exhaustion of T lymphocytes and NK cells occur in some types of cancer displaying tumor growth 54,56. The binding of NKG2A to its relevant ligands prevents CD8+ T and NK cell-mediated activities, whereas NKG2A blocking on CTLs and NK cells diminishes tumor growth through improving the CD8+ T- and NK cell-mediated activities 54,57. Therefore, it is important to improve the NK cells and CTL-related activity and avoid their exhaustion to prevent the damping of anti-tumor immunity during SARS-CoV-2 infection. The targeting of NKG2A may repress the NK cell and CTL exhaustion and thus cause virus clearance in the initial step of SRAS-CoV-2 infection 51 . Monalizumab, an anti-NKG2A monoclonal antibody, improves the CD8+ CTL and NK cell-mediated responses in cancers and successfully prevents the progression of tumors with no major harmful impacts in phase 2 clinical trials 57,58. COVID-19 patients also have greater plasma quantities of IL-6 and TNF-α which may impair the NK cell activity, thus the targeting of TNF-α and IL-6 can improve the NK cell activity in COVID-19 patients 28 .
It should also be noted that CD4+ T lymphocytes play a central role in the induction of anti-tumoral and anti-viral immunity. CD4+ T lymphocytes recognize antigenic peptides associated with the MHC class II molecules on the membrane of APCs and provide aid for NK cells and CD8+ CTLs to exert anti-tumoral and anti-viral effects. The marked reduction of the MHC II molecules expression on CD14+ monocytes was also reported in severe cases of COVID-19, which has been associated with profound depletion of CD4+ T- and NK cells 52 . The low expression of MHC II molecules on CD14+ monocytes has been attributed to the IL-6 and in vitro experiments using tocilizumab prevent the reduction of the expression of MHC II molecules by monocytes incubated with plasma samples collected from immunocompromised COVID-19 patients 52 .
Collectively, the results from aforesaid studies clearly demonstrate that the SARS-CoV-2 infection attenuates the immunosurveillance against tumors that may increase the tumor incidence or tumor recurrence in COVID-19 patients.
2) SARS-CoV-2 infection promotes pro-tumoral inflammatory responses
Tumor-promoting inflammation is one of the main characteristics of cancers. Both chronic and acute inflammation potently affect cancer development 59 . The inflammatory-related mediators can aid tumor development through inducing cell proliferation, angiogenesis, DNA damage, cytoskeleton remodeling, and degradation of the extra-cellular matrix 59-61. Inflammation may promote metastasis by producing mediators that enhance vascular permeability 62 . Some cytokines present in the tumor microenvironment, such as IL-6 and TNF-α, and epiregulin can enhance the survival of the metastatic cancer cells 63 .
Hyperinflammatory responses and elevated quantities of inflammatory cytokines are hallmarks of the severe form of COVID-19 4,28. Cytokine storm is a harmful systemic inflammatory reaction emerging from the releasing of the vast amounts of pro-inflammatory cytokines and chemokines, which operate a crucial role in the occurrence of some main SARS-CoV-2-related complications, such as ARDS 4 . TNF-α and IL-6 are the main players of cytokine storm that were correlated with COVID-19 severity 64-66. The IL-6 and TNF-α concentrations are inversely associated with lymphocyte count, which means that the cytokine syndrome may dampen the specific immune response against SARS-CoV-2 infection 64,65. TNF-α and IL-6 promote carcinogenesis and contribute to metastasis, tumor invasion, and angiogenesis 59,67. Hospitalized subjects with cancer who have elevated IL-6 and TNF-α levels are at greater risk of death 68 . Both cytokines may play key roles in the tumor progression in malignant patients infected with SARS-CoV-2. Reducing the development of tumors in COVID-19 patients by targeting IL-6 and TNF-α needs to be clarified in future studies.
Elevated levels of the chemokines including CCL2, CCL4, CXCL8, CXCL9, and CXCL10 have been also demonstrated in patients with COVID-19 patients 28,69. The mentioned chemokines could contribute in cancer development via several mechanisms such as tumor cell expansion, cancer stem cell proliferation, metastasis, angiogenesis, tumor invasion, epithelial-mesenchymal transition (EMT) induction, MDSC attraction, and fibroblast recruitment 70,71. The appearance of various subsets of MDSCs was indicated in COVID-19 patients correlating with viral load and disease severity 72-74. Hence, the elevated expression of the chemokines can support tumor progression in malignant patients with COVID-19. Thus, targeting tumor-promoting chemokines or blocking their receptors could reduce tumor progression.
ACE2 exerts anti-inflammatory and anti-oxidative effects during infection 4 . The SARS-CoV-2-dependent depletion of ACE2 potentiates the pro-inflammatory and oxidative impacts of angiotensin II4. Oxidative stress acts as a starter and enhancer of carcinogenesis as this phenomenon promotes cell proliferation, tumor invasion, angiogenesis, tumor cell survival, chemo-resistance, and radio-resistance75.
3) SARS-CoV-2 can exert direct oncogenic impacts
A direct relation has been postulated between coronavirus infection and dysregulation of the cell cycle leading to the cellular transformation. The Nsp3 and Nsp15 of SARS‐CoV have been implicated in the degradation of the tumor suppressor proteins P53 and retinoblastoma (pRb), respectively76,77. Similarly, Nsp3 and Nsp15 of SARS‐CoV-2 can affect tumor suppressor proteins. The results from an in silico analysis indicated that the S2 subunit of SARS‐CoV‐2 potently interacts with tumor suppressors P53 and BRCA1/2 78.
SARS-CoV-2-induced alteration in the activity of E2F transcription factors and RB1 can also promote malignancies. Rb controls the movement from the G1 phase to the S phase in the cell cycle by modulating the E2F activity79. The RB1 activity was remarkably reduced, whereas the activity of E2F was enhanced in COVID-19 patients indicating that SARS-CoV-2 may inactivate tumor suppressor Rb resulting in the elevated E2F activity that promotes cell proliferation same as some other oncogenic viruses79.
The MAPK, JAK-STAT and NF-κB-mediated signaling pathways may also contribute to the tumorigenesis in SARS-CoV-2-infected individuals. The SARS-CoV-2-derived molecules, such as S protein can be identified by some TLRs such as TLR2, which activates the transcription factors NF-κB and MAPKs through recruitment of MyD88 80,81. Furthermore, JAK-STAT signaling pathway is activated following the binding some cytokines to their relevant receptors 45,65. The involvements of the MAPK and JAK-STAT-mediated signaling pathways in the development of numerous cancers have been indicated 82. The targeting of MAPK and JAK-STAT-mediated signaling pathways may interfere with tumorigenesis 82,83.
4) Possible reactivation of oncogenic viruses may lead to cancer development after SARS-CoV-2 infection
Infections play a key role in the development of human malignancies, as about 15.0% of cancers are attributed to oncogenic infectious agents 84 . Some infectious agents such as HTLV‐1, HPV, EBV, and KSHV cause latent infection, where most infected persons are asymptomatic 85 . The viral oncoproteins exclusively produced in HTLV‐1-, HPV-, EBV-, and KSHV-infected cells support cell proliferation 84,85. However, HIV itself may have not a direct oncogenic impact, but it increases the risk of cancers by enabling oncogenic agents to expand in co-infected individuals 85 . Similarly, SARS-CoV-2-mediated immune impairment may allow oncogenic pathogens to transform normal cells into malignant cells. It has been reported that some SARS-CoV-2-related proteins and some therapeutic agents can reactivate KSHV which may enhance the risk of cancer development, even in fully recovered COVID-19 patients 86 . Furthermore, the possible presence of SARS-CoV-2-derived oncoproteins that can transform the normal cells into malignant cells needs to be clarified in future studies.
Malignancy promotes the COVID-19 severity
As mentioned above, in innate immunity, IFNs including type I and type III IFNs, serve as the first line of protection against viruses such as coronaviruses 45,87. An early proper IFN response can effectively control SARS-CoV-2 infection28,45. Following the initial innate response, induction of an appropriate adaptive immune response is also necessary to clear SARS-CoV-2 and prevent disease progression to severe phases 28,88. Both innate and adaptive immune responses are impaired in malignant patients 89 . Lymphopenia (an independent poor predictor in COVID-19 patients) is common in cancer patients who are under active or even watchful treatment 90 . Thus, the immunocompromised state in cancer patients makes these patients more susceptible to COVID-19, and SARS-CoV-2-infected cancerous patients exhibit more severe complications of COVID-19 91 .
The infection rate of SARS-COV-2 in cancer patients is greater than the general population, and cancer patients with COVID-19 showed worsening symptoms and bad outcomes 92 . Malignant patients with COVID-19 display a greater risk of developing serious and even deadly respiratory disorders 93 . It has also been observed that patients with hematological cancers exhibit more serious COVID-19-related complications and more deaths compared with non-malignant care professionals with COVID-19 94 . The patients with lung cancer also
develop severe COVID-19-related lung complications and therefore are at higher risk of dying from SARS-CoV-2 infection 95 . The ACE2 upregulation was commonly found in patients suffering from lung cancer. As a result, the increased ACE2 expression in lung cancer could likely promote the susceptibility of lung cancer patients to SARS-CoV-2 infection 96 .
Conclusions
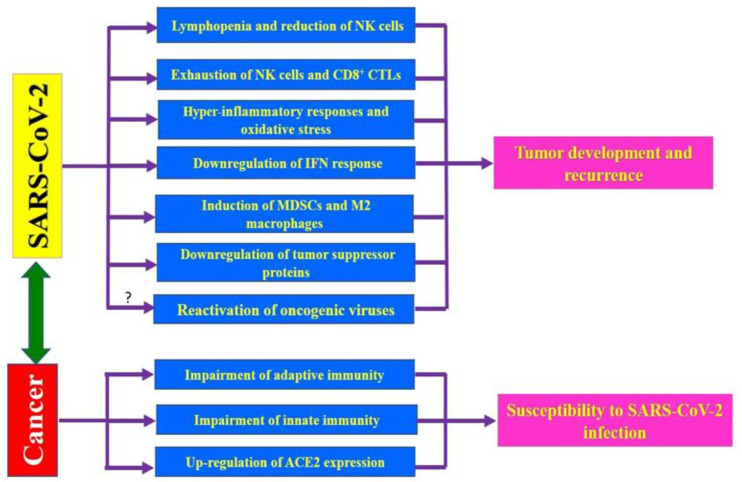
SARS-CoV-2-mediated immunosuppression, hyper-inflammation, and oxidative stress may direct and/or indirectly promote the development of some cancers in predisposed individuals who are infected with SARS-CoV-2 (Figure 1). Furthermore, downregulation of the tumor suppressor proteins and possible reactivation of latent oncogenic viruses in COVID-19 patients can enhance the risk of certain cancers after SARS-CoV-2 infection. The possible presence of the oncogenes in the SARS-CoV-2 needs to be evaluated in future studies. Some COVID‐19 patients experience prolonged manifestations which may persist for several months after infection 6,7. Moreover, clinically recovered COVID-19 individuals display profound immunologic alterations that extend several months after discharge 21-23. Thus, SARS-CoV-2-infected individuals, especially chronic cases, may need post-infection monitoring for cancer development. On the other hand, patients with malignancy are more vulnerable to SARS-CoV-2 and are at higher risk of developing severe symptoms of COVID-19 (Figure 1). Therefore, SARS-CoV-2-infected cancerous patients may be at greater risk of death from COVID-19-related complications. It should also be noted that COVID-19 and malignancy may have some similarities regarding their pathogenicity. Therefore, the identification and the targeting of common pathways between COVID-19 and cancers need more attention in an attempt to treat both diseases using similar therapeutic agents. Ultimately, even during a pandemic, cancer patients need a timely diagnosis, treatment, and monitoring. Moreover, the major risk for cancerous patients in the COVID-19 outbreak may be due to the inability to earn sufficient healthcare facilities 97 .
Figure 1.
Impacts of COVID-19 and cancer on each other. The SARS-CoV-2 infection may enhance the risk of cancer development and recurrence through induction of exhaustion of cytotoxic lymphocytes, stimulation of hyper-inflammatory responses, induction of oxidative stress, reduction of the number of lymphocytes and NK cells, down-regulation of IFN response, induction of the pro-tumoral cells such as MDSCs and M2 macrophages, downregulation of tumor suppressor proteins, and perhaps through re-activation of the oncogenic viruses. On the other side, the malignancy can enhance the susceptibility to SARS-CoV-2 infection and increases the severity of COVID-19-related complications through impairing both innate- and adaptive immune responses to virus as well as upregulation of angiotensin-converting enzyme 2 (ACE2) in certain cancers such as lung cancer.
CONFLICTS OF INTEREST
The authors declare no conflict of interests.
Abbreviations
ARDS: Acute respiratory distress syndrome
COVID-19: Novel coronavirus disease 2019
EBV: Epstein-Barr virus
HIV: Human immunodeficiency virus
HPV: Human papillomavirus
HTLV‐1: Human T-cell lymphotropic virus type 1
ICU: Intensive care unit
IFN: Interferon
JAK–STAT: Janus kinase-signal transducer and activator of transcription
KSHV: Kaposi sarcoma-associated herpesvirus
MAPK: Mitogen-activated protein kinase
MHC: Major histocompatibility complex
MyD88: Myeloid differentiation factor 88
NF-κB: Nuclear factor kappa B
SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2
TNF: Tumor necrosis factor
References
- 1.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Fisher D, Heymann D. Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18(1):57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafarzadeh A, Chauhan P, Saha B, etc Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257:118102. doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jafarzadeh A, Nemati M, Saha B, etc Protective Potentials of Type III Interferons (IFN-λ) in COVID-19 Patients: Lessons from Different Basically and Clinically Properties Attributed to the Type I- and III Interferons. Viral Immunol. 2021;34(5):307–320. doi: 10.1089/vim.2020.0076. [DOI] [PubMed] [Google Scholar]
- 6.Greenhalgh T, Knight M, A'Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 7.Honigsbaum M, Krishnan L. Taking pandemic sequelae seriously: from the Russian influenza to COVID-19 long-haulers. Lancet. 2020;396(10260):1389–1391. doi: 10.1016/S0140-6736(20)32134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saini G, Aneja R. Cancer as a prospective sequela of long COVID-19. BioEssays. 2021;43(6):e2000331. doi: 10.1002/bies.202000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585(7825):339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 10.Desai A, Sachdeva S, Parekh T, et al. COVID-19 and Cancer: Lessons From a Pooled Meta-Analysis. JCO Glob Oncol. 2020;6:557–559. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 15.Casey SC, Li Y, Felsher DW. An essential role for the immune system in the mechanism of tumor regression following targeted oncogene inactivation. Immunol Res. 2014;58(2-3):282–291. doi: 10.1007/s12026-014-8503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monzavi-Karbassi B, Pashov A, Kieber-Emmons T. Tumor-Associated Glycans and Immune Surveillance. Vaccines. 2013;1(2):174–203. doi: 10.3390/vaccines1020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheikhi A, Jafarzadeh A, Kokhaei P, et al. Whole Tumor Cell Vaccine Adjuvants: Comparing IL-12 to IL-2 and IL-15. Iran J Immunol. 2016;13(3):148–166. [PubMed] [Google Scholar]
- 18.Jafarzadeh A, Minaee K, Farsinejad AR, et al. Evaluation of the circulating levels of IL-12 and IL-33 in patients with breast cancer: influences of the tumor stages and cytokine gene polymorphisms. Iran J Basic Med Sci. 2015;18(12):1189–1198. [PMC free article] [PubMed] [Google Scholar]
- 19.Golubovskaya V, Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel) 2016;8(3):36. doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28(8):383–91. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Zhong M, Zhang E, et al. Broad phenotypic alterations and potential dysfunction of lymphocytes in individuals clinically recovered from COVID-19. J Mol Cell Biol. 2021;13(3):197–209. doi: 10.1093/jmcb/mjab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuwa HA, Shaw TN, Knight SB, et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med (N Y) . 2021;2(6):720–735. doi: 10.1016/j.medj.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kostopoulos IV, Orologas-Stavrou N, Rousakis P, et al. Recovery of Innate Immune Cells and Persisting Alterations in Adaptive Immunity in the Peripheral Blood of Convalescent Plasma Donors at Eight Months Post SARS-CoV-2 Infection. Microorganisms. 2021;9(3):546. doi: 10.3390/microorganisms9030546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palka-Kotlowska M, Custodio-Cabello S, Oliveros-Acebes E, et al. Review of risk of COVID-19 in cancer patients and their cohabitants. Int J Infect Dis. 2021;105:15–20. doi: 10.1016/j.ijid.2021.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdihamid O, Cai C, Kapesa L, et al. The Landscape of COVID-19 in Cancer Patients: Prevalence, Impacts, and Recommendations. Cancer Manag Res. 2020;12:8923–8933. doi: 10.2147/CMAR.S272008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jafarzadeh A, Nemati M, Saha B, et al. Protective Potentials of Type III Interferons in COVID-19 Patients: Lessons from Differential Properties of Type I- and III Interferons. Viral Immunol. 2021;34(5):307–320. doi: 10.1089/vim.2020.0076. [DOI] [PubMed] [Google Scholar]
- 28.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. A ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 31.Vellingiri B, Jayaramayya K, Iyer M, et al. COVID-19: A promising cure for the global panic. Sci Total Environ. 2020;725:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jafarzadeh A, Nemati M, Jafarzadeh S. Contribution of STAT3 to the pathogenesis of COVID-19. Microb Pathog. 2021;154:104836. doi: 10.1016/j.micpath.2021.104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen K, Xiao F, Hu D, et al. SARS-CoV-2 Nucleocapsid Protein Interacts with RIG-I and Represses RIG-Mediated IFN-β Production. Viruses. 2020;13(1):47. doi: 10.3390/v13010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, Zhuang MW, Han L, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct Target Ther. 2020;5(1):299. doi: 10.1038/s41392-020-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Berg DF, te Velde AA. Severe COVID-19: NLRP3 Inflammasome Dysregulated. Front Immunol. 2020;11:1580. doi: 10.3389/fimmu.2020.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page EM, Ariëns RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. 2021;200:1–8. doi: 10.1016/j.thromres.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojo AS, Balogun SA, Williams OT, et al. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm Med. 2020;2020:6175964. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jafarzadeh A, Jafarzadeh S, Nozari P, et al. Lymphopenia an important immunological abnormality in patients with COVID-19: Possible mechanisms. Scand J Immunol. 2021;93(2):e12967. doi: 10.1111/sji.12967. [DOI] [PubMed] [Google Scholar]
- 40.Yao Z, Zheng Z, Wu K, et al. Immune environment modulation in pneumonia patients caused by coronavirus: SARS-CoV, MERS-CoV and SARS-CoV-2. Aging (Albany NY) 2020;12(9):7639–7651. doi: 10.18632/aging.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeffers SA, Tusell SM, Gillim-Ross L, et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U S A. 2004;101(44):15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang ZY, Huang Y, Ganesh L, et al. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J Virol. 2004;78(11):5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koch C, Staffler G, Huttinger R, et al. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int Immunol. 1999;11(5):777–786. doi: 10.1093/intimm/11.5.777. [DOI] [PubMed] [Google Scholar]
- 44.Wang K, Chen W, Zhou YS, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv. 2020:988345. [Google Scholar]
- 45.Park A, Iwasaki A. Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020;27(6):870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Hu J, Li R, et al. Enhanced NK cell adoptive antitumor effects against breast cancer in vitro via blockade of the transforming growth factor-beta signaling pathway. Onco Targets Ther. 2015;8:1553–9. doi: 10.2147/OTT.S82616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rezvani K, Rouce RH. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillgrass AE, Chew MV, Krneta T, et al. Overexpression of IL-15 promotes tumor destruction via NK1.1+ cells in a spontaneous breast cancer model. BMC Cancer. 2015;15:293. doi: 10.1186/s12885-015-1264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. 2019;234(6):8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 50.He R, Lu Z, Zhang L, et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol. 2020;127:104361. doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osman M, Faridi RM, Sligl W, et al. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv. 2020;4(20):5035–5039. doi: 10.1182/bloodadvances.2020002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andre P, Denis C, Soulas C, et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell. 2018;175(7):1731–1743. doi: 10.1016/j.cell.2018.10.014. e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barathan M, Mohamed R, Yong YK, et al. Viral Persistence and Chronicity in Hepatitis C Virus Infection: Role of T-Cell Apoptosis, Senescence and Exhaustion. Cells. 2018;7(10):165. doi: 10.3390/cells7100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mamessier E, Sylvain A, Thibult ML, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121(9):3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Hall T, Andre P, Horowitz A, et al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J Immunother Cancer. 2019;7(1):263. doi: 10.1186/s40425-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaqinuddin A, Kashir J. Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents. Med Hypotheses. 2020;140:109777. doi: 10.1016/j.mehy.2020.109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piotrowski I, Kulcenty K, Suchorska W. Interplay between inflammation and cancer. Reports of practical oncology and radiotherapy. Rep Pract Oncol Radiother. 2020;25(3):422–427. doi: 10.1016/j.rpor.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zappavigna S, Cossu AM, Grimaldi A, et al. Anti-Inflammatory Drugs as Anticancer Agents. Int J Mol Sci. 2020;21(7):2605. doi: 10.3390/ijms21072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cata JP, Wang H, Gottumukkala V, et al. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110(5):690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perricone C, Triggianese P, Bartoloni E, et al. The anti-viral facet of anti-rheumatic drugs: Lessons from COVID-19. J Autoimmun. 2020;111:102468. doi: 10.1016/j.jaut.2020.102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 66.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate forms of Coronavirus Disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taher MY, Davies DM, Maher J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem Soc Trans. 2018;46(6):1449–1462. doi: 10.1042/BST20180136. [DOI] [PubMed] [Google Scholar]
- 68.Stoll JR, Vaidya TS, Mori S, et al. Association of IL-6 and TNF-α with mortality in hospitalized cancer patients. JAAD. 2021;84(2):273–282. doi: 10.1016/j.jaad.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong Y, Liu Y, Cao L, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jafarzadeh A, Nemati M, Jafarzadeh S. The important role played by chemokines influence the clinical outcome of Helicobacter pylori infection. Life Sci. 2019;231:116688. doi: 10.1016/j.lfs.2019.116688. [DOI] [PubMed] [Google Scholar]
- 71.Jafarzadeh A, Fooladseresht H, Nemati M, et al. Higher circulating levels of chemokine CXCL10 in patients with breast cancer: Evaluation of the influences of tumor stage and chemokine gene polymorphism. Cancer Biomark. 2016;16(4):545–554. doi: 10.3233/CBM-160596. [DOI] [PubMed] [Google Scholar]
- 72.Xue G, Jiang M, Zhao R, et al. Elevated frequencies of CD14(+)HLA-DR(lo/neg) MDSCs in COVID-19 patients. Aging. 2021;13(5):6236–6246. doi: 10.18632/aging.202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dean MJ, Ochoa JB, Sanchez-Pino M, et al. Transcriptome and Functions of Granulocytic Myeloid-Derived Suppressor Cells Determine their Association with Disease Severity of COVID-19. medRxiv. 2021;2021:03.26.21254441. [Google Scholar]
- 74.Takano T, Matsumura T, Adachi Y, et al. Myeloid cell dynamics correlating with clinical outcomes of severe COVID-19 in Japan. Int Immunol . 2021;33(4):241–247. doi: 10.1093/intimm/dxab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma-Lauer Y, Carbajo-Lozoya J, Hein MY, et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc Natl Acad Sci U S A. 2016;113(35):E5192–5201. doi: 10.1073/pnas.1603435113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhardwaj K, Liu P, Leibowitz JL, et al. The coronavirus endoribonuclease Nsp15 interacts with retinoblastoma tumor suppressor protein. J Virol. 2012;86(8):4294–304. doi: 10.1128/JVI.07012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh N, Bharara Singh A. S2 subunit of SARS-nCoV-2 interacts with tumor suppressor protein p53 and BRCA: an in silico study. Transl Oncol. 2020;13(10):100814. doi: 10.1016/j.tranon.2020.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Policard M, Jain S, Rego S, Dakshanamurthy S. Immune characterization and profiles of SARS-CoV-2 infected patients reveals potential host therapeutic targets and SARS-CoV-2 oncogenesis mechanism. Virus Res. 2021;301:198464. doi: 10.1016/j.virusres.2021.198464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dosch SF, Mahajan SD, Collins AR. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142(1-2):19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jafarzadeh A, Nemati M, Khorramdelazad H, et al. The Toll-like Receptor 2 (TLR2)-related Immunopathological Responses in the Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Iran J Allergy Asthma Immunol. 2019;18(3):230–250. doi: 10.18502/ijaai.v18i3.1117. [DOI] [PubMed] [Google Scholar]
- 82.Braicu C, Buse M, Busuioc C, et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers (Basel) 2019;11(10):1618. doi: 10.3390/cancers11101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pencik J, Pham HT, Schmoellerl J, et al. JAK-STAT signaling in cancer: From cytokines to non-coding genome. Cytokine. 2016;87:26–36. doi: 10.1016/j.cyto.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Howley PM. Gordon Wilson Lecture: Infectious Disease Causes of Cancer: Opportunities for Prevention and Treatment. Trans Am Clin Climatol Assoc. 2015;126:117–132. [PMC free article] [PubMed] [Google Scholar]
- 85.Yasunaga JI, Matsuoka M. Oncogenic spiral by infectious pathogens: Cooperation of multiple factors in cancer development. Cancer Sci. 2018;109(1):24–32. doi: 10.1111/cas.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Dai L, Barrett L, et al. SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus. Commun Biol. 2021;4(1):682. doi: 10.1038/s42003-021-02220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 88.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pandya PH, Murray ME, Pollok KE, et al. The Immune System in Cancer Pathogenesis: Potential Therapeutic Approaches. J Immunol Res. 2016;2016:4273943. doi: 10.1155/2016/4273943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gosain R, Abdou Y, Singh A, et al. COVID-19 and Cancer: a Comprehensive Review. Curr Oncol Rep. 2020;22(5):53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Quteimat OM, Amer AM. The Impact of the COVID-19 Pandemic on Cancer Patients. Am J Clin Oncol. 2020;43(6):452–455. doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang F, Shi S, Zhu J, et al. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020;92(10):2067–2073. doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 93.Russell B, Moss C, George G, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Addeo A, Obeid M, Friedlaender A. COVID-19 and lung cancer: risks, mechanisms and treatment interactions. J Immunother Cancer. 2020;8(1):e000892. doi: 10.1136/jitc-2020-000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gottschalk G, Knox K, Roy A. ACE2: At the crossroad of COVID-19 and lung cancer. Gene Rep. 2021;23:101077. doi: 10.1016/j.genrep.2021.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21(4):e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]