Glioneuronal tumors (GNTs) are a diverse group of central nervous system (CNS) neoplasms that primarily affects children and young adults [6]. Their histopathological diagnosis can be extremely challenging due to overlapping morphological features among the different (sub-)types. In recent years, the use of next-generation sequencing and DNA methylation arrays revealed a large spectrum of different types of GNTs that are often characterized by a unique (epi-)genetic profile [2–5, 12, 13]. However, the molecular landscape of GNT is far from being exhaustively described. Interestingly, the vast majority of GNTs are driven by one of a variety of aberrations in the mitogen-activated protein kinase (MAPK) signaling pathway, including mutations, fusions or structural rearrangements in BRAF, NF1, FGFR1 or NTRK1/2/3, and other rarer alterations [1, 3, 8, 11, 12]. Aberrant activation of the MAPK pathway is not only important from a diagnostic perspective, it also offers therapeutic opportunities since inhibitors are frequently available [9].
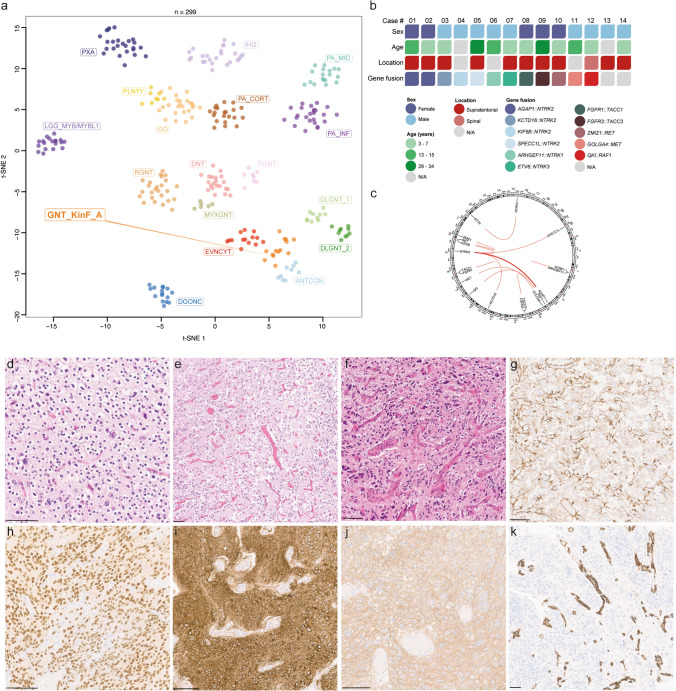
To identify novel epigenetic subgroups of GNTs, we used an unsupervised visualization approach with a comprehensive dataset of DNA methylation profiles covering the entire spectrum of existing molecular CNS tumor classes [2]. These analyses revealed a specific cluster of tumors (n = 14) with varying histological features of different GNT types (Fig. 1a). Clinicopathological characteristics are summarized in Fig. 1b and supplementary table 1 (online resource). Analysis of copy-number variations derived from DNA methylation array data indicated structural aberrations affecting the gene locus of different targetable kinases (Fig. 1b, c). Subsequent transcriptome and DNA sequencing [10, 14] in 12/14 of the cases confirmed oncogenic gene fusions involving several kinases including the NTRK1/2/3, FGFR1/3, MET, RET and RAF1 genes. Of note, seven of the cases harbored rearrangements involving the NTRK gene family. For the most common partner (n = 5), NTRK2 was fused downstream of either AGAP1 (n = 2), KCTD16 (n = 1), SPECC1L (n = 1) or KIF5B (n = 1). Single cases showed an ARHGEF11::NTRK1 fusion or ETV6::NTRK3 fusion. Genetic alterations within the FGFR signaling pathway were seen in two of the cases, with one case showing an FGFR1::TACC1 fusion and another an FGFR3::TACC3 fusion, both rearrangements reported in particular in extraventricular neurocytoma [7, 12]. In addition, oncogenic gene fusions of ZMIZ1::RET, GOLGA4::MET and QKI::RAF1 were observed. Apart from a homozygous deletion of CDKN2A/B observed in one of the cases (Supplementary Table 1, online resource), no other relevant aberration was detected. These data suggest a remarkably wide range of different gene fusions that drive tumors within this epigenetic group and in parallel highlights attractive therapeutic targets in particular for patients with incomplete surgical resection or tumor progressions.
Fig. 1.
Unsupervised, nonlinear t-distributed stochastic neighbor embedding (t-SNE) projection of DNA methylation array profiles from 299 tumors. DNA methylation profiling reveals a molecular distinct group of glioneuronal tumors (GNT_KinF_A; a). Reference DNA methylation classes: dysembryoplastic neuroepithelial tumor (DNT), rosette-forming glioneuronal tumor (RGNT), diffuse leptomeningeal glioneuronal tumor subtype 1 (DLGNT_1), diffuse leptomeningeal glioneuronal tumor subtype 2 (DLGNT_2), extraventricular neurocytoma (EVNCYT), papillary glioneuronal tumor (PGNT), ganglioglioma (GG), polymorphous low-grade neuroepithelial tumor of the young (PLNTY), myxoid glioneuronal tumor, PDGFRA-mutant (MYXGNT), diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC), anaplastic neuroepithelial tumor with condensed nuclei (ANTCON), angiocentric glioma MYB/MYBL1-altered (LGG_MYB/MYBL1), pilocytic astrocytoma hemsipheric (PA_CORT), pilocytic astrocytoma infratentorial (PA_INF), pilocytic astrocytoma midline (PA_MID), pleomorphic xanthoastrocytoma (PXA) and infant-type hemispheric glioma (IHG). Summary of clinical characteristics and key molecular findings in the 14 tumors investigated (b). Circos plot of the different gene fusions detected in the series (lines link fusion gene partners according to chromosomal location; c). Histologically, tumors show a moderate to high increase in cellular density of largely monomorphic (d–e) or slightly pleomorphic neoplastic cells (f). An oligodendroglial morphology with perinuclear halos is focally present in most of the tumors (d). Immunohistochemistry for GFAP is largely restricted to reactive astrocytes or a minor proportion of neoplastic cells (g). Tumor cells show immunoreactivity of OLIG2 (h), MAP2 (i), and synaptophysin (j). CD34 expression is restricted to the vessels (k). Scale bars 100 µm
The nine male and five female patients ranged in age at time of initial diagnosis from 3 to 34 years (n = 12; mean age 11.2 years). Tumors were located supratentorially (n = 10), with the exception of one case located in the spinal cord (Fig. 1b and Supplementary Table 1, online resource). Due to the diverse origins and the retrospective nature of the series, availability of clinical data (in particular in terms of patient outcome) was restricted for some of the cases and did not allow a reliable assessment of the malignancy of the tumors. Histologically (n = 10), the tumors shared a moderate to high increase in cellular density of largely monomorphic or slightly pleomorphic neoplastic cells (Fig. 1d–f). Only one of the tumors was characterized by a more pronounced cellular pleomorphism (Fig. 1f). The tumor cells typically had round to oval, partly hyperchromatic nuclei with prominent nucleoli (Fig. 1d–e). An oligodendroglial morphology with perinuclear halos was seen in the majority of the tumors (n = 7; Fig. 1d). In one case, spindle-shaped cells were observed focally. About half of the tumors (n = 6) focally showed perivascular rosettes, mostly together with small neuropil islands. Calcifications were seen in a small number of tumors (n = 2). Focal reactive vascular proliferation was detected in only two of the cases (Fig. 1f). Necrosis was not observed. Mitotic activity was absent or low, with the exception of two cases exhibiting a slightly higher rate of up to 0.8 and 1.7 mitosis per mm2. Immunoreactivity for GFAP was largely restricted to reactive astrocytes or a minor proportion of neoplastic cells (Fig. 1g). Tumor cells showed immunoreactivity of OLIG2, MAP2 and synaptophysin (Fig. 1h–j). Several tumors showed focal positivity for NeuN. CD34 expression was restricted to the vessels (Fig. 1k). The proliferation index (Ki-67) ranged from 1 to 20%. A summary of the morphological and immunohistochemical features of the tumors are given in Supplementary Table 2 (online resource).
Together, these findings suggest a molecularly distinct group of pediatric-type GNT characterized by oncogenic activation of different kinases. Although enriched for gene fusions involving the NTRK gene family, tumors within this epigenetic group show a remarkable spectrum of different rearrangements including very rare events in primary CNS tumors such as RAF1 and RET fusions. Given their morphological overlap with other GNTs and the lack of a pathognomonic alteration, we provisionally suggest the term ‘glioneuronal tumor kinase-fused’ (GNT_KinF_A) to describe this novel group of tumors. In addition, our findings emphasize the potential benefit of molecular profiling to identify targetable alterations in GNTs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank L. Hofmann and L. Dörner for skillful technical assistance and the microarray unit of the DKFZ Genomics and Proteomics Core Facility for providing Illumina DNA methylation array-related services. This study was supported by the Hertie Network of Excellence in Clinical Neuroscience and the CRC 1389 of the Deutsche Forschungsgemeinschaft. P. Sievers is supported by the Else Kröner Fresenius Foundation and a fellow of the Hertie Academy of Excellence in Clinical Neuroscience. D.T.W. Jones and F. Sahm gratefully acknowledge support for the Everest Centre for Low-Grade Paediatric Brain Tumour Research (The Brain Tumour Charity (UK), GN-000707). T.S. Jacques is grateful for funding from the Brain Tumour Charity, Children with Cancer UK, Great Ormond Street Hospital Children’s Charity, Olivia Hodson Cancer Fund, Cancer Research UK and the National Institute of Health Research. All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
David T. W. Jones and Felix Sahm share senior authorship.
Contributor Information
David T. W. Jones, Email: david.jones@dkfz.de
Felix Sahm, Email: felix.sahm@med.uni-heidelberg.de.
References
- 1.Alvarez-Breckenridge C, Miller JJ, Nayyar N, Gill CM, Kaneb A, D'Andrea M, et al. Clinical and radiographic response following targeting of BCAN-NTRK1 fusion in glioneuronal tumor. NPJ Precis Oncol. 2017;1:5. doi: 10.1038/s41698-017-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng MY, Sill M, Chiang J, Schittenhelm J, Ebinger M, Schuhmann MU, et al. Molecularly defined diffuse leptomeningeal glioneuronal tumor (DLGNT) comprises two subgroups with distinct clinical and genetic features. Acta Neuropathol. 2018;136:239–253. doi: 10.1007/s00401-018-1865-4. [DOI] [PubMed] [Google Scholar]
- 4.Hou Y, Pinheiro J, Sahm F, Reuss DE, Schrimpf D, Stichel D, et al. Papillary glioneuronal tumor (PGNT) exhibits a characteristic methylation profile and fusions involving PRKCA. Acta Neuropathol. 2019;137:837–846. doi: 10.1007/s00401-019-01969-2. [DOI] [PubMed] [Google Scholar]
- 5.Huse JT, Snuderl M, Jones DT, Brathwaite CD, Altman N, Lavi E, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133:417–429. doi: 10.1007/s00401-016-1639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas CG, Gupta R, Doo P, Lee JC, Cadwell CR, Ramani B, et al. Comprehensive analysis of diverse low-grade neuroepithelial tumors with FGFR1 alterations reveals a distinct molecular signature of rosette-forming glioneuronal tumor. Acta Neuropathol Commun. 2020;8:151. doi: 10.1186/s40478-020-01027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131:833–845. doi: 10.1007/s00401-016-1539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryall S, Tabori U, Hawkins C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun. 2020;8:30. doi: 10.1186/s40478-020-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahm F, Schrimpf D, Jones DT, Meyer J, Kratz A, Reuss D, et al. Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol. 2016;131:903–910. doi: 10.1007/s00401-015-1519-8. [DOI] [PubMed] [Google Scholar]
- 11.Sievers P, Appay R, Schrimpf D, Stichel D, Reuss DE, Wefers AK, et al. Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol. 2019 doi: 10.1007/s00401-019-02038-4. [DOI] [PubMed] [Google Scholar]
- 12.Sievers P, Stichel D, Schrimpf D, Sahm F, Koelsche C, Reuss DE, et al. FGFR1:TACC1 fusion is a frequent event in molecularly defined extraventricular neurocytoma. Acta Neuropathol. 2018;136:293–302. doi: 10.1007/s00401-018-1882-3. [DOI] [PubMed] [Google Scholar]
- 13.Solomon DA, Korshunov A, Sill M, Jones DTW, Kool M, Pfister SM, et al. Myxoid glioneuronal tumor of the septum pellucidum and lateral ventricle is defined by a recurrent PDGFRA p. K385 mutation and DNT-like methylation profile. Acta Neuropathol. 2018;136:339–343. doi: 10.1007/s00401-018-1883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stichel D, Schrimpf D, Casalini B, Meyer J, Wefers AK, Sievers P, et al. Routine RNA sequencing of formalin-fixed paraffin-embedded specimens in neuropathology diagnostics identifies diagnostically and therapeutically relevant gene fusions. Acta Neuropathol. 2019;138:827–835. doi: 10.1007/s00401-019-02039-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.