Abstract
Background:
Obesity-related complications including visceral fat, metabolic abnormalities, nutrient deficiencies, and immune perturbations are interdependent but have been individually associated with childhood asthma.
Objective:
To endotype childhood obesity-related asthma by quantifying contributions of obesity-related complications to symptoms and pulmonary function.
Methods:
Multi-omics analysis using Similarity Network Fusion followed by mediation analysis were performed to quantify prediction of obese asthma phenotype by different combinations of anthropometric, metabolic, nutrient, and Th cell transcriptome and DNA methylome datasets.
Results:
Two clusters (n=28 and 26) distinct in their anthropometric (neck and midarm circumference, waist to hip ratio (WHR) and BMI z-score), metabolic, nutrient, and Th cell transcriptome and DNA methylome footprint predicted 5 or more pulmonary function indices across 7 different dataset combinations. Metabolic measures attenuated the association of neck, WHR and BMI z-score with FEV1/FVC ratio and ERV, of neck, midarm, and BMI z-score with FRC, but only of WHR with IC. Nutrient levels attenuated the association of neck, midarm circumference, and BMI z-score with FRC, and of WHR with FEV1/FVC ratio, ERV and IC. Th cell transcriptome attenuated the association of all four anthropometric measures with FEV1/FVC ratio, but only of WHR with ERV and IC. The DNA methylome attenuated the association of all four anthropometric measures with FEV1/FVC ratio and ERV, but only of WHR with IC.
Conclusion:
Anthropometric, metabolic, nutrient, and immune perturbations have individual but interdependent contributions to obese asthma phenotype, with the most consistent effect of WHR, highlighting the role of truncal adiposity in endotyping childhood obesity-related asthma.
Keywords: Obesity, asthma, fat distribution, metabolic abnormalities, nutrients, Th lymphocytes
Graphical Abstract
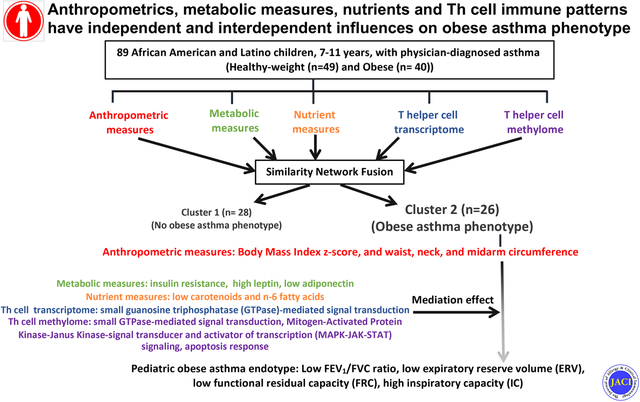
Capsule summary
Obesity-related complications including visceral fat, metabolic abnormalities, nutrient deficiencies, and immune perturbations have individual as well as interdependent influences on lung function deficits contributing to the pediatric obese asthma endotype
Introduction
Childhood obesity and asthma are highly prevalent conditions that are inter-related and disproportionately affect minority populations, including those of African American and Hispanic descent.1–3 Compared to healthy-weight children with asthma, obese children with asthma have more severe disease with worse pulmonary function deficits,3 and are less responsive to currently available treatments for asthma.4
Early investigations into contribution of obesity-related complications to asthma emphasized the role of excess adipose tissue with a predominant truncal distribution.5–7 Subsequent studies highlighted the interactive relationship between truncal fat and metabolic abnormalities including insulin resistance and dyslipidemia.6,8,9 Recognizing the key role of diet in obesity, macro- and micronutrients have also been linked with obesity-related asthma. Higher levels of pro-inflammatory saturated fatty acids (SFA) and n-6/n-3 polyunsaturated fatty acid (PUFA) ratio,10,11 and lower levels of carotenoids, the anti-oxidants found in fruits and vegetables,12 as well as vitamin D, have been associated with asthma and obesity,13 including pulmonary function deficits.11,14 In keeping with their interactive relationship with truncal fat, insulin resistance and dyslipidemia also correlate with fatty acid and micronutrient levels.11
In addition, obesity is associated with adipose tissue-mediated systemic non-atopic inflammation.6,15 In keeping with this observation, obese children with asthma have non-atopic T helper 1 (Th1) polarized systemic inflammation that correlates with pulmonary function deficits.3,15,16 Exploration of Th cell transcriptome to identify biological pathways underlying non-atopic responses revealed upregulation of genes in the Cell Division Cycle 42 (CDC42) pathway, a Rho-GTPase pathway, in Th cells from obese children with asthma as compared to healthy-weight children with asthma.17 CDC42 expression was associated with expression of interferon gamma (IFNγ) and tumor necrosis factor (TNF), two cytokines linked with Th1 cells. The DNA methylome in obese asthmatic Th cells was also enriched for Rho-GTPase pathways.18 Th cell transcriptome and methylome were both enriched in their association with pulmonary function deficits found in obesity-related asthma.18
Although these obesity-related complications are inter-related, the interdependence of their influences on the obese asthma phenotype are not well understood. To address this gap in knowledge, we used multi-omics analytic tools to quantify the independent and interdependent associations of anthropometric, metabolic, and nutrition measurements and Th cell immune patterns, defined by the Th cell transcriptome and DNA methylome, with the obese asthma phenotype, defined by symptom burden-based classification of asthma severity and control, and pulmonary function indices. We hypothesized that effects of obesity on anthropometrics, metabolic profile, nutrition, and Th cell immune patterns have individual as well as interdependent influences on childhood asthma.
Methods
Study Participants
The data included in this analysis was collected from 120 African American and Hispanic children ages 7 and 11 years with a physician diagnosis of asthma, as previously reported and detailed in the online repository.18 Children were classified as healthy-weight (body mass index (BMI) 5th to <85th percentile) (n=61) or obese (BMI>95th percentile for sex and age) (n=59) at recruitment using the CDC definition.19 The study was approved by the Albert Einstein College of Medicine IRB where the study was conducted and by the Children’s National Medical Center IRB for the current analysis of the de-identified data sets.
Study Measures (details for all measures are included in the online repository)
Asthma severity, asthma control and pulmonary function testing
Participant-reported data was used to calculate the Composite Asthma Severity Index (CASI) score,20 a measure of severity, and Asthma Control Test (ACT) score,21 a measure of control. Pulmonary function testing (spirometry and lung volume quantification using nitrogen washout) was performed on all participants according to the American Thoracic Society guidelines.22 Percent predicted values of pulmonary function indices23,24 were included in the analysis.
Anthropometric Measurements
Participants underwent measurement of weight in kilograms, and of height, neck, midarm, waist, and hip circumference in centimeters. These measures, along with BMI z-score and waist to hip ratio (WHR), comprised the anthropometric dataset.
Metabolic Measures
Fasting serum from all participants was used to quantify measures of glucose and lipid metabolism and adipokines. The metabolic dataset included total cholesterol, HDL, LDL, triglyceride, insulin, glucose, leptin, and adiponectin levels, as well as the homeostatic measurement of insulin resistance (HOMA-IR) values, calculated as (glucose (mg/dl) × Insulin (uU/ml) /405).25
Nutrient Measures
Carotenoids, 25-hydroxy vitamin D, α-tocopherol, and SFA, MUFA, PUFA levels and n6/n3 PUFA ratio, also quantified in fasting serum, comprised the nutrition dataset.
Th-cell Transcriptome
RNA from unstimulated Th cells underwent directional RNA-sequencing (RNA-seq).17 103 of the 120 RNA-seq libraries passed quality control, including 48 from obese asthmatic and 55 from healthy-weight Th cells. Using DESeq2, differentially expressed genes between the two groups at false discovery rate (FDR (q value)) <0.05) were retained as the transcriptome dataset.
Quantification of the Th-cell DNA Methylome
The enzyme digestion-based HELP (Hpall Tiny Fragment Enrichment by Ligation-mediated PCR)-tagging assay was used to quantify the Th-cell DNA methylome.26 99 of the 120 libraries passed quality control criteria, including 45 from obese asthmatic and 54 from healthy-weight asthmatic Th cells. Multivariate analysis was performed to account for effects of technical covariates (batch of library preparation and of sequencing), and biological covariates. CpG sites where the model was statistically significant at an FDR (q value) <0.05, and between-group difference in methylation was significant (p value<0.05), were retained as the DNA methylome dataset.
Statistical Analysis (additional details in the online repository)
The analytic approach is summarized in Figure 1. Based on data availability on all variables in all datasets, the study cohort narrowed to 89 participants, including 49 healthy-weight and 40 obese children with asthma. Variables in the lower dimension predictive datasets (anthropometric, metabolics and nutrition) were compared between the study groups using Welch’s two sample t-test and reported as means with standard deviation. Statistical significance was set a priori at p<0.05. Th cell transcriptome and DNA methylome subsets for the 89 samples were analyzed as detailed above.
Figure 1. Summary of statistical analysis plan.
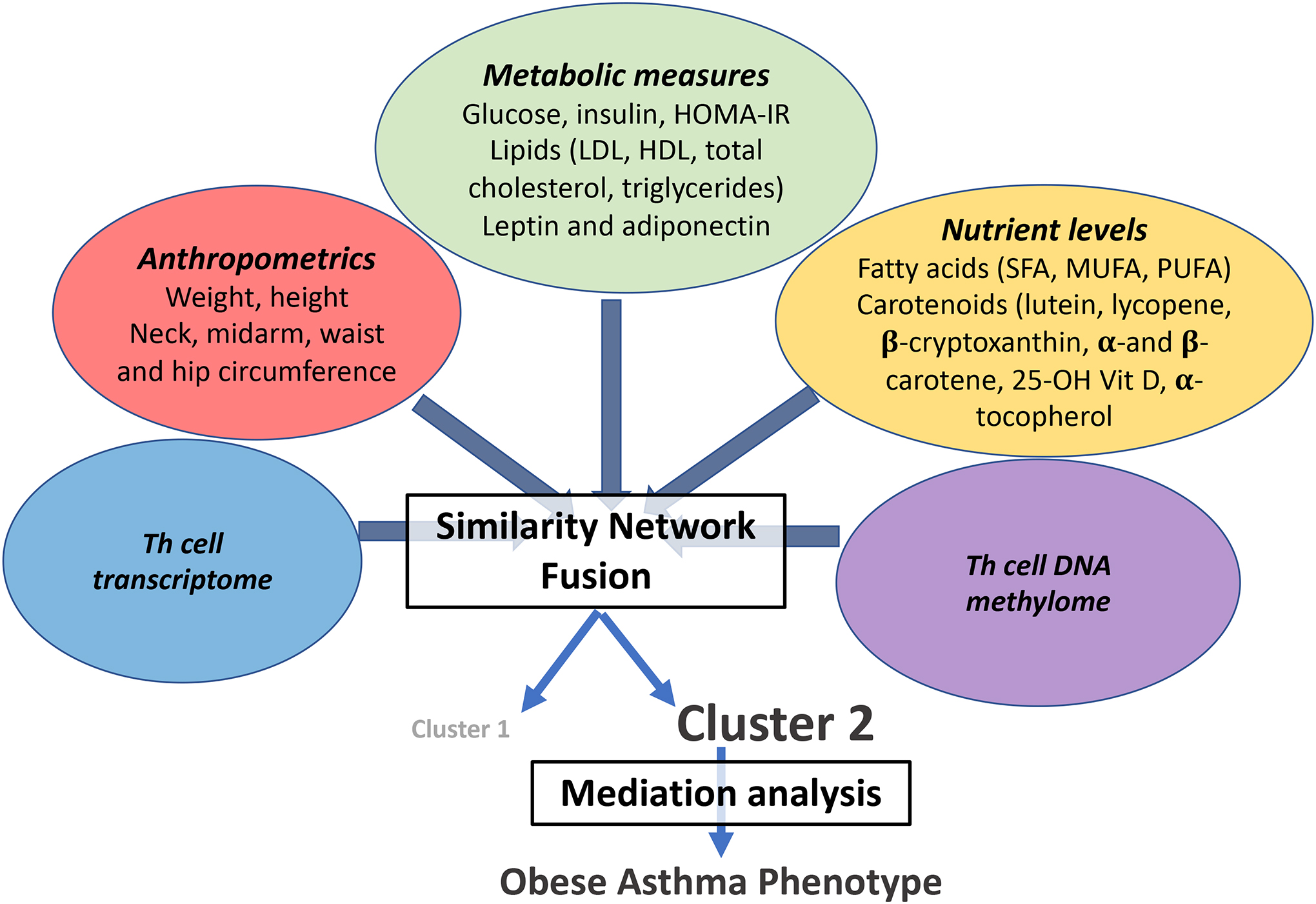
Similarity network fusion was performed on predictive datasets, including anthropometric and metabolic measures, nutrient levels, and Th cell transcriptome and DNA methylome to identify their association with the obese asthma phenotype. The individual contribution of each predictive dataset to the obese asthma phenotype was quantified by mediation analysis.
Multi-omic Analysis through Similarity Network Fusion (SNF)
We performed multi-omic analysis using the SNFtool package27 on R statistical software v.4.1.0 to quantify the individual and composite contribution of the predictive datasets to obese asthma phenotype (CASI and ACT scores, and pulmonary function). A total of twenty-one fusions were performed for the twenty-one different combinations of the five predictive datasets. Samples were clustered using spectral clustering for each of these combinations and Welch’s two sample t-tests were conducted to quantify the discriminating ability of the clusters within each combination for CASI and ACT scores and pulmonary function indices.
Downstream analysis on clusters identified by SNF tool
To verify the SNF findings, univariate analyses were conducted on the predictive datasets comparing their distribution between the two clusters from SNF analysis and their association with CASI, ACT, and pulmonary function. Given that more than one predictive dataset correlated with CASI, ACT, and pulmonary function, we conducted mediation analyses28,29 to identify the independent contribution of anthropometric, nutrition and metabolic datasets and that of the Th cell transcriptome and methylome to outcomes of interest. Biological relevance of Th cell transcriptome and DNA methylome mediating the associations was derived using NetworkAnalyst software and reported as Gene Ontology (GO) pathways.30 To elucidate the clinical relevance of predictor datasets, we quantified variance of FEV1/FVC ratio, ERV, FRC and IC explained by neck circumference, midarm circumference, WHR and BMI z-score in the anthropometric dataset, leptin, adiponectin and HOMA-IR in the metabolic dataset, and total carotenoids and n-6 PUFA, that were significantly different (p<0.05) between clusters. Analysis was limited to these three datasets since they can be more readily quantified in a clinical setting at this time as compared to the Th cell transcriptome and DNA methylome.
Th cell transcriptome, DNA methylome, and patient characteristics are available at dbGAP study ID 33254.
Results
Characteristics of the study cohort
The 89 study participants were similar in their demographics but differed in several pulmonary function indices, including FVC, FEV1/FVC ratio, RV/TLC ratio, ERV, FRC and IC [Table 1]. As expected, anthropometric measures were higher in obese children with asthma than healthy-weight children with asthma, except for height, which did not differ between the groups [Table 2]. Among the metabolic measures, HOMA-IR and leptin levels were higher, and adiponectin levels were lower, in obese children as compared to healthy-weight children with asthma [Table 2]. Fatty acid levels did not differ substantially between the two groups, while β-carotene and total carotenoid levels were lower in obese children relative to healthy-weight children with asthma [Table 2]. Gene expression of 365 genes and methylation at 497 CG sites also differed between these two groups [Table E1a and E1b].
Table 1.
Demographic and clinical characteristics of the study groups
| Healthy-Weight (n=49) | Obese (n=40) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 8.79 ± 1.27 | 8.93 ± 1.37 | NS |
| Male (n (%)) | 27 (55.10) | 24 (60.00) | NS |
| Hispanic (n (%)) | 29 (59.18) | 27 (67.50) | NS |
| Pulmonary function indices * | |||
| FVC | 95.02 ± 13.01 | 103.23 ± 12.00 | <0.01 |
| FEV 1 | 91.41 ± 15.57 | 94.18 ± 16.55 | 0.42 |
| FEV 1 /FVC ratio | 84.24 ± 7.46 | 79.75 ± 8.33 | 0.01 |
| FEF25–75% | 79.27 ± 29.38 | 74.51 ± 29.05 | 0.48 |
| TLC | 93.36 ± 11.78 | 95.85 ± 13.99 | 0.38 |
| RV | 113.49 ± 31.49 | 100.43 ± 38.14 | 0.09 |
| RV/TLC ratio | 29.38 ± 5.90 | 24.80 ± 6.77 | <0.01 |
| ERV | 88.36 ± 26.54 | 71.13 ± 19.15 | <0.01 |
| FRC | 106.41 ± 22.91 | 90.38 ± 22.83 | <0.01 |
| IC | 81.36 ± 13.14 | 98.73 ± 14.91 | <0.01 |
| Measures of asthma severity and control | |||
| CASI | 7.95 ± 3.47 | 8.58 ± 3.20 | 0.38 |
| ACT | 19.58 ± 4.60 | 19.62 ± 4.53 | 0.97 |
All variables are reported as mean ± SD other than sex and ethnicity which are reported as proportions (n(%).
Pulmonary function indices are reported as percent-predicted values other than the ratios which are reported as percentages. A CASI score of greater than 3 is suggestive of higher asthma severity and an ACT score of 19 and higher is suggestive of good asthma control.
Table 2.
Comparison of anthropometric, metabolic and micronutrient measures between healthy-weight and obese children with asthma
| Healthy-Weight (n=49) | Obese (n=40) | P value | |
|---|---|---|---|
| Anthropometric measures | |||
| Neck (cms) | 27.52 ± 2.20 | 30.89 ± 2.50 | <0.001 |
| Midarm (cms) | 20.12 ± 2.80 | 25.80 ± 3.90 | <0.001 |
| Waist (cms) | 61.20 ± 6.10 | 77.90 ± 11.07 | <0.001 |
| Hip (cms) | 74.14 ± 7.20 | 87.34 ± 13.98 | <0.001 |
| Waist/Hip Ratio | 0.83 ± 0.07 | 0.90 ± 0.08 | <0.001 |
| Height (cms) | 136.65 ± 9.43 | 139.43 ± 9.61 | 0.17 |
| Weight (kgs) | 32.05 ± 7.17 | 47.11 ± 12.44 | <0.001 |
| BMI (kg/m2) | 16.95 ± 1.87 | 23.86 ± 3.95 | <0.001 |
| BMI z-score | 0.10 ± 0.87 | 1.84 ± 0.46 | <0.001 |
| Metabolic measures | |||
| Glucose (mg/dl) | 88.03 ± 7.71 | 91.24 ± 9.03 | 0.10 |
| Cholesterol (mg/dl) | 154.46 ± 29.73 | 155.55 ± 31.85 | 0.87 |
| HDL (mg/dl) | 52.23 ± 12.76 | 50.25 ± 9.01 | 0.39 |
| LDL (mg/dl) | 92.45 ± 25.75 | 97.13 ± 30.65 | 0.44 |
| Triglycerides (mg/dl) | 71.97 ± 38.74 | 70.98 ± 30.05 | 0.89 |
| Insulin (mU/ml) | 11.88 ± 6.93 | 16.11 ± 12.02 | 0.06 |
| HOMA-IR* | 2.56 ± 1.49 | 3.71 ± 3.03 | 0.03 |
| Leptin (ng/ml) | 7.67 ± 5.98 | 18.99 ± 9.67 | <0.001 |
| Adiponectin (μg/ml) | 17.90 ± 7.84 | 14.84 ± 6.16 | 0.04 |
| Nutrient measures | |||
| Total FA (mg/dl) | 3372.39 ± 654.38 | 3274.27 ± 625.57 | 0.48 |
| SFA (mg/dl) | 1165.83 ± 340.15 | 1130.76 ± 280.53 | 0.60 |
| MUFA (mg/dl) | 673.99 ± 166.59 | 635.31 ± 165.33 | 0.28 |
| PUFA (mg/dl) | 1528.59 ± 247.81 | 1505.16 ± 266.03 | 0.67 |
| n6 PUFA (mg/dl) | 1445.18 ± 227.39 | 1418.71 ± 241.73 | 0.59 |
| n3 PUFA (mg/dl) | 83.41 ± 27.42 | 87.45 ± 38.34 | 0.58 |
| n6/n3 Ratio | 18.87 ± 5.40 | 17.73 ± 5.68 | 0.34 |
| Lutein (μg/ml) | 0.20 ± 0.05 | 0.19 ± 0.07 | 0.60 |
| β-Cryptoxanthin (μg/ml) | 0.11 ± 0.03 | 0.10 ± 0.05 | 0.41 |
| Lycopene (μg/ml) | 0.52 ± 0.18 | 0.48 ± 0.16 | 0.30 |
| α-Carotene (μg/ml) | 0.04 ± 0.03 | 0.04 ± 0.02 | 0.48 |
| β-Carotene (μg/ml) | 0.28 ± 0.17 | 0.20 ± 0.09 | <0.001 |
| Total Carotenoids (μg/ml) | 1.13 ± 0.34 | 0.99 ± 0.26 | 0.03 |
| Vitamin D (ng/ml) | 1.42 ± 0.13 | 1.38 ± 0.12 | 0.11 |
| α-Tocopherol (μg/ml) | 8.83 ± 2.15 | 8.28 ± 2.21 | 0.25 |
Calculated as (glucose (mg/dl) × insulin (uU/ml) /405
Similarity Network Fusion analysis of predictive datasets and their combined association with the obese asthma phenotype
Figure 2 summarizes the predictive ability for CASI and ACT score, and pulmonary function indices [Figure 2, x-axis], of the sample clusters, identified by the SNF tool for each of the 21 combinations of the predictive datasets [Figure 2, y-axis]. Sample clusters in 7 of the 21 combinations predicted 5 or more pulmonary function indices, of which FEV1/FVC ratio, ERV, IC, and to a lesser degree, FRC, were most frequently predicted. Few clusters predicted CASI or ACT score. Among the 7 combinations, samples in the 2 clusters were identical for 6 combinations and included 54 of the 89 samples; 28 were in ‘Cluster 1’ and 26 in ‘Cluster 2’. The 6 combinations included the transcriptome and anthropometric (TxA), transcriptome, anthropometric and nutrition (TxAxN), transcriptome, methylome, anthropometric and nutrition (TxMxAxN), anthropometric and nutrition (NxA), anthropometric and metabolic (AxMb), and methylome, anthropometric, and metabolic (MxAxMb) combinations.
Figure 2. Summary of SNF analysis.
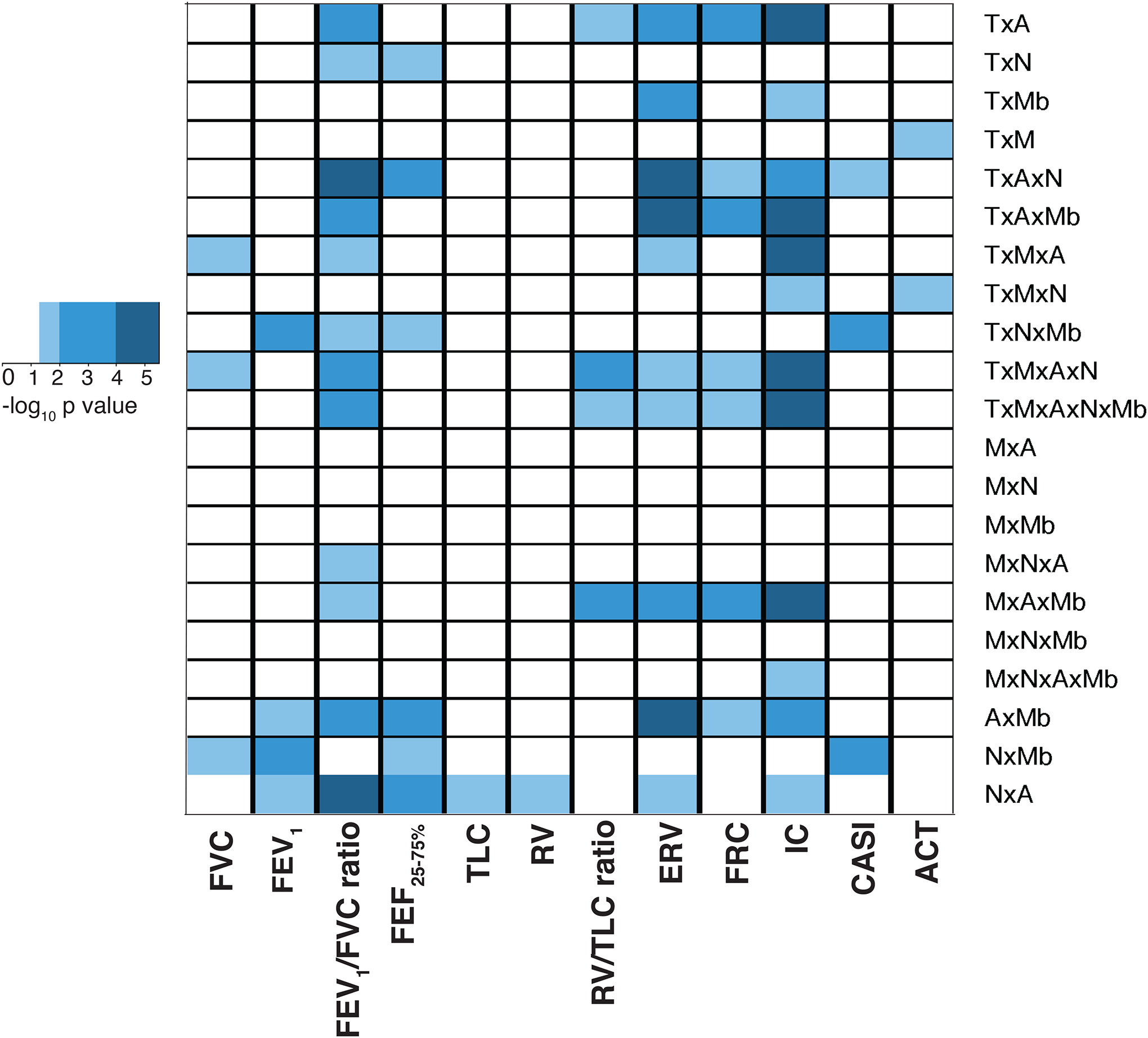
The heatmap reports the signficance of prediction of CASI, ACT and pulmonary function indices, plotted on the x-axis, by the sample clusters, determined by SNF tool, for each of the 21 different combinations of the 5 predictive datasets, including anthropometric measures (A), metabolic measures (Mb), nutrient levels (N), and Th cell transcriptome (T) and DNA methylome (M), plotted on y-axis. The color key summarizes the significance of the predictive ability of the sample clusters within each combination, for CASI, ACT or pulmonary function index. White denotes an association that did not reach statistical significance (p=>0.05); light blue denotes associations with p<0.05 and ≥0.01, intermediate shade of blue denotes associations with p<0.01 and ≥0.0001, and dark blue denotes associations with p<0.0001.
Association of clusters with the obese asthma phenotype and variables in predictive datasets
To confirm the prediction ability of SNFtool-based clustering, we compared CASI, ACT and pulmonary function between the two clusters and found that the clusters were predictive of pulmonary function but not of CASI or ACT score. Percent predicted FEF25–75%, ERV, FRC, and percent FEV1/FVC ratio were lower, and IC was higher in Cluster 2 relative to Cluster 1 [Figure E1a]. Between-cluster comparison of individual variables within each predictive dataset revealed that all variables in the anthropometrics dataset were higher in Cluster 2 as compared to Cluster 1 [Figure E1b]. In the metabolic dataset, leptin, insulin and HOMA-IR were higher and adiponectin was lower, with no difference in lipids, in Cluster 2 as compared to Cluster 1 [Figure E1c]. In the nutrition dataset, n-6 PUFA, lutein, β-cryptoxanthin, lycopene, β-carotene, and total carotenoids were lower in Cluster 2 relative to Cluster 1 [Figure E1d]. In addition, 195 genes were differentially expressed and 286 CGs were differentially methylated between Cluster 1 and Cluster 2 [Table E2a and E2b]. Distribution of variables in lower dimension datasets in the samples that clustered and those that did not cluster are summarized in Table E3. Furthermore, variables within the anthropometric, nutrient, metabolic, Th cell transcriptome, and Th cell methylome datasets that distinguished the clusters correlated with ERV, FRC and IC, and percent FEV1/FVC ratio [Figure E2]. These findings verify those of SNF analysis regarding the contribution of each predictive dataset to clustering of samples associated with the obese asthma phenotype.
Mediation analysis to identify independent effects of predictive datasets on obese asthma phenotype
Since the anthropometric dataset was present in each of the 6 combinations with identical sample clusters, we quantified the effect of metabolic, nutrition, Th cell transcriptome and methylome datasets on the association of the anthropometric measures (neck, midarm, WHR and BMI z-score) with the 4 pulmonary function indices (FEV1/FVC ratio, ERV, IC, and FRC) that were discriminated by the 2 clusters. The association of FEV1/FVC ratio with neck, WHR, and BMI z-score was rendered non-significant by the metabolic dataset [Figure 3a], that with WHR was rendered non-significant by the nutrition dataset [Figure 3a], and the association with neck, midarm, WHR and BMI z-score were attenuated by the Th cell transcriptome and methylome [Figure 3b]. The association of ERV with neck, WHR, and BMI z-score was rendered non-significant by the metabolic dataset [Figure 3c], and that with WHR, and BMI z-score, was rendered non-significant by the nutrition dataset [Figure 3c]. Although the Th cell transcriptome only attenuated the association of ERV with WHR [Figure 3d], the methylome attenuated the association with neck, midarm, WHR, and BMI z-score [Figure 3d]. FRC was associated with neck, midarm and BMI z-score but not with WHR; all these associations were rendered non-significant by both metabolic and nutrition datasets [Figure 3e]. The transcriptome only attenuated the association with neck and WHR [Figure 3f] while the methylome attenuated the association of FRC with WHR [Figure 3f]. IC correlated with neck, midarm, WHR and BMI z-score, but its association with WHR was the only one rendered non-significant by both metabolic and nutrition datasets [Figure 3g] as well as by the Th transcriptome and methylome [Figure 3h].
Figure 3. Mediation effects of metabolic and nutrient datasets, and Th cell transcriptome and DNA methylome, on the association of anthropometric measures with pulmonary function.
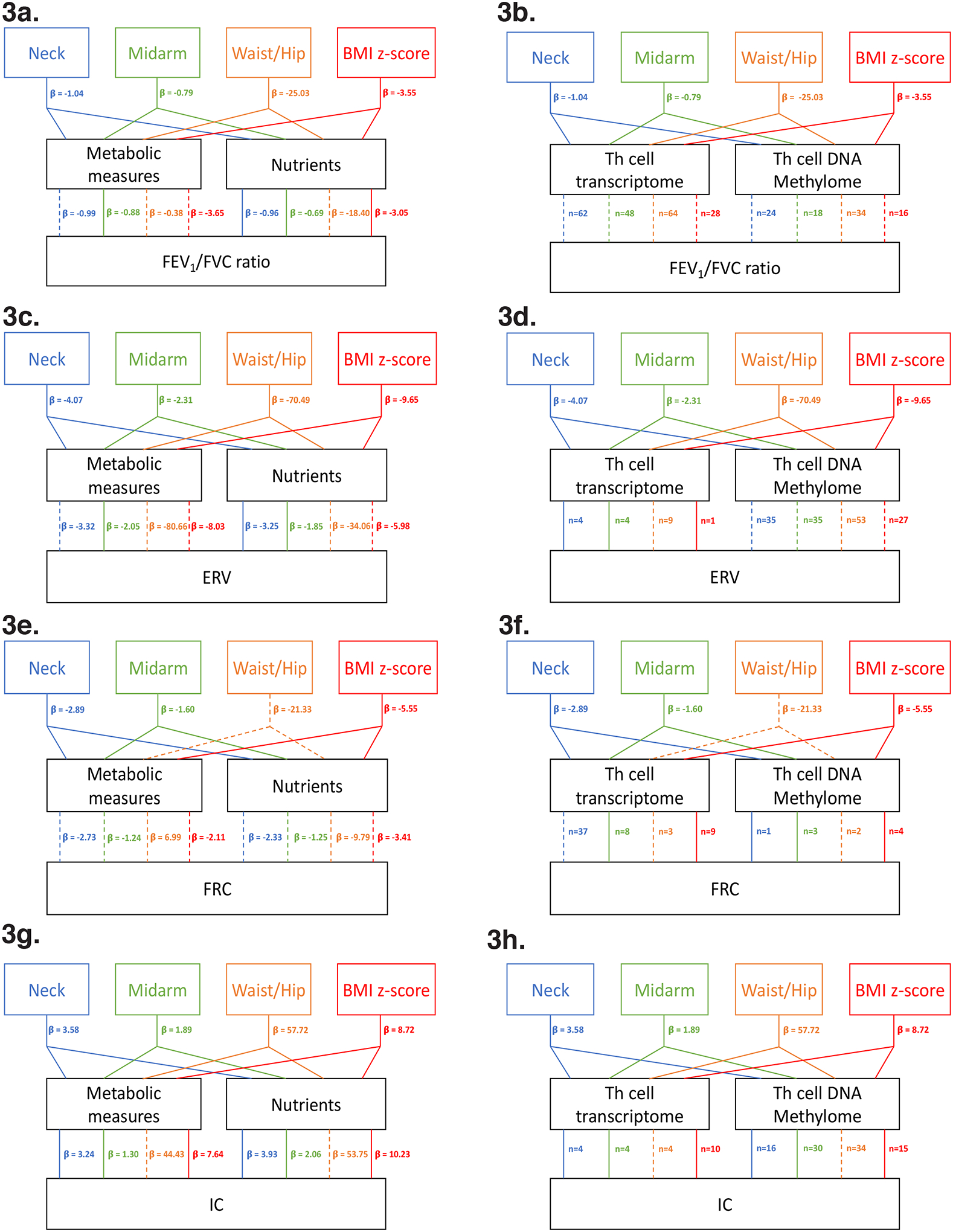
The mediation effects of metabolic and nutrient datasets are reported as change in beta (β) value of the association while that of the transcriptome and DNA methylome are reported as the number of genes mediating the effect, since the large number of genes precluded biologically meaningful interpretation of change in β value of the association. Statistically significant associations between anthropometric measures (neck, midarm, WHR, and BMI z-score), and pulmonary function indices (FEV1/FVC ratio, ERV, FRC and IC) are depicted with solid lines, while significant associations of anthropometric measures with the pulmonary function index that were rendered non-significant by the predictor dataset, or were not significant even prior to mediation analysis (e.g. WHR with FRC), are shown as dashed lines. Statistical significance was set a priori at p<0.05. a. summarizes the mediation effect of metabolic and nutrition datasets, and b. the mediation effect of Th cell transcriptome and methylome, on the association of FEV1/FVC ratio with neck, midarm, WHR, and BMI z-score. c. summarizes the mediation effect of metabolic and nutrition datasets, and d. the mediation effect of Th cell transcriptome and methylome, on the association of ERV with neck, midarm, WHR, and BMI z-score. e. summarizes the mediation effect of metabolic and nutrition datasets, and f. the mediation effect of Th cell transcriptome and methylome, on the association of FRC with neck, midarm, WHR, and BMI z-score. g. summarizes the mediation effect of metabolic and nutrition datasets, and h. the mediation effect of Th cell transcriptome and methylome, on the association of IC with neck, midarm, WHR, and BMI z-score.
Given the findings of mediation analysis, to define the clinical relevance of variables within predictor datasets that were different between clusters, we quantified the predictive ability of neck, midarm, WHR, BMI z-score from the anthropometric dataset, HOMA-IR, leptin and adiponectin from the metabolic dataset, and total carotenoids and n-6 PUFA from the nutrient dataset for FEV1/FVC ratio, ERV, FRC, and IC. These variables together predicted 20.2% variance of FEV1/FVC ratio, 41.7% variance of ERV, 22.6% variance of FRC, and 51.2% variance of IC.
Pathway analysis of differentially expressed and methylated genes that mediated the association of anthropometric measures with pulmonary function
The highest number of differentially expressed genes (n=64) mediated the association of anthropometrics with FEV1/FVC ratio with a 93% overlap between those mediating the association of neck, WHR and BMI-z-score [Table E4a]. Although fewer genes mediated the association of anthropometrics with ERV and FRC, they overlapped by 63% to 90% with those mediating the association with FEV1/FVC ratio [Table E4b]. The overlapping differentially expressed genes were enriched for coagulation pathways, small GTPase mediated signal transduction, transmembrane receptor protein tyrosine kinase signaling, epidermal growth factor receptor signaling, and cell migration [Figure 4a, b].
Figure 4. Pathway analysis of Th cell transcriptome and DNA methylome that mediated the association of anthropometrics with pulmonary function.
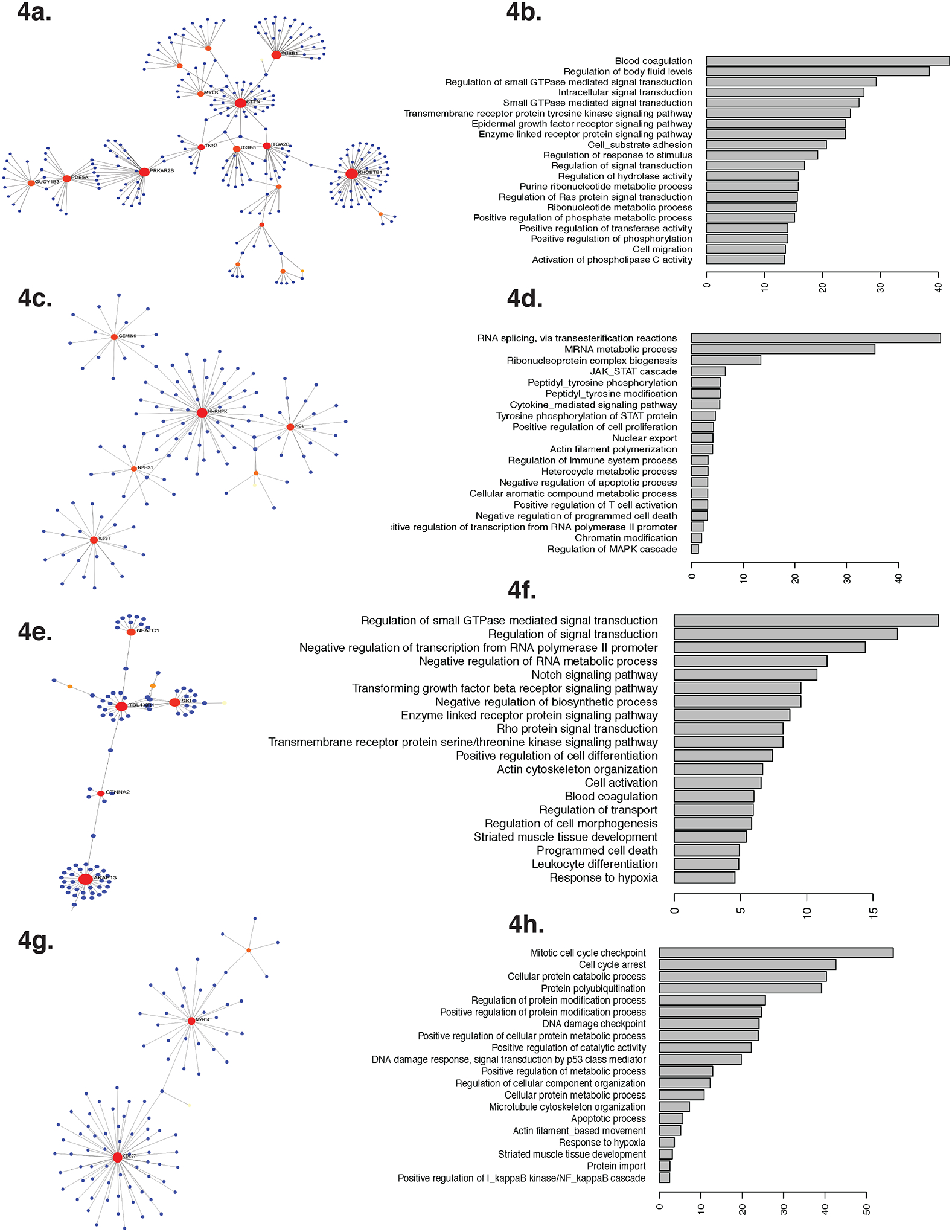
There was over 63% overlap between differentially expressed genes that mediated the association of anthropometric measures with FEV1/FVC ratio, ERV and IC. Functional interpretation of these differentially expressed genes are visualized as a. Pathway analysis, and b. top 20 Gene Ontology pathways for biological process in which the differentially expressed genes were enriched. Unlike the transcriptome, there was minimal overlap among the differentially methylated genes that mediated the association of anthropometric measures with different pulmonary function indices. Functional interpretation of the differentially methylated genes that mediated the association of anthropometric measures are reported as pathway analyses and their top 20 Gene Ontology pathways enriched for biological processes for ERV [Fig. c, d], for FEV1/FVC ratio [Fig. e, f] and for IC [Fig. g, h].
Unlike the transcriptome, a higher number of differentially methylated genes mediated the association of neck, BMI z-score and WHR, with ERV, with 73% overlap between them. Furthermore, only 20% of CG sites mediating the association of anthropometrics with ERV overlapped with those mediating the association with FEV1/FVC ratio or IC. The enrichment of differentially methylated genes also differed; those mediating the association of anthropometrics with ERV were enriched for RNA processing, Janus kinase (JAK)-signal transducer and activator of transcription (STAT) (JAK-STAT) and mitogen-activated protein kinase (MAPK) signaling, actin polymerization, and negative regulation of apoptosis [Figure 4c, d], while those mediating the association with FEV1/FVC ratio were enriched for small GTPase-mediated signal transduction, transforming growth factor (TGF) receptor signaling, and RNA processing [Figure 4e, f], and those mediating the association with IC were enriched for cell cycle regulation, DNA damage/ apoptosis response, protein modification, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) regulation [Figure 4g, h].
Discussion
Our -omics approach including anthropometrics, metabolic measures, nutrient levels, Th cell transcriptome, and Th cell DNA methylome, identified a subset of participants that clustered into two distinct groups that were predictive of pulmonary function deficits consistently associated with the pediatric obese asthma phenotype.15,16 As compared to Cluster 1, participants in Cluster 2 had lower pulmonary function indices including FEV1/FVC ratio, ERV, FRC and higher IC. Participants in Cluster 2 had higher truncal and peripheral body fat, metabolic abnormalities, including insulin resistance, high leptin and low adiponectin levels, lower carotenoid and n6 PUFA levels, and differential Th cell gene expression and DNA methylation. These obesity-related complications, including the mechanical load of fat, metabolic abnormalities, nutrient deficiencies, and immune perturbations, were predictive of obese asthma phenotype independently as well as in an interdependent manner, but not predictive of symptoms-based classification of asthma severity and control. Although pulmonary function indices are inter-related, we identify differences in the predictive abilities of obesity-related complications for individual pulmonary function indices. While FEV1/FVC ratio was influenced by all four obesity-related complications, IC correlated with anthropometrics with minimal influence of metabolic abnormalities, nutrient levels, and immune perturbations.
Our observed association of FEV1/FVC ratio with neck and WHR, measures of truncal fat distribution, mid-arm circumference, a measure of peripheral fat distribution, and with BMI z-score, a measure of generalized fat distribution, validates several prior studies that examined the association of one or more of these anthropometric measures with FEV1/FVC ratio as a measure of airflow obstruction in obese children with asthma.3,31 We build on this literature by demonstrating the contribution of the interactive relationship of body fat with metabolic abnormalities, nutrient levels, and altered Th cell immune profiles, on its links with FEV1/FVC ratio. The attenuating effect of each of these obesity-related complications on the association of FEV1/FVC ratio with neck and mid-arm circumference, WHR, and BMI z-score, identified their individual contribution to airflow obstruction in pediatric obesity-related asthma. While each variable within each obesity-related complication can serve as a potential biomarker, our study highlights their co-existence and cumulative contribution to the disease phenotype.
ERV is another pulmonary function index that is reduced in the context of obesity and obesity-related asthma.15,16,32 However, unlike the attenuating effect of metabolic measures, nutrient levels, and Th cell transcriptome and methylome on all associations of FEV1/FVC ratio with anthropometric measures, they only attenuated the association of ERV with WHR. The association of ERV with BMI z-score was attenuated by metabolic measures, nutrient levels and Th cell methylome, but not the Th cell transcriptome, while Th cell methylome was the only predictive dataset that influenced the association of ERV with mid-arm circumference. These observations suggest that metabolic and nutrient levels influence the association of truncal and generalized body fat distribution but not that of peripheral body fat with the obese asthma phenotype. These findings also highlight the discriminating association of obesity-related complications with the obese asthma phenotype.
Intriguingly, FRC, another pulmonary function index influenced in obesity and obesity-related asthma, was predicted by neck and BMI z-score but not by WHR. Similar to their discriminating effects on ERV, metabolic measures and nutrient levels attenuated the association of neck and BMI z-score with FRC, with minimal contribution of Th cell profiles. Lastly, IC, one of the least well described aspects of the obese asthma phenotype, was associated with neck and WHR, mid-arm, and BMI z-score. Metabolic measures, nutrient levels and Th cell immune profiles only attenuated the association of WHR with IC and did not influence any other association, identifying IC as the one pulmonary function index that was most independently associated with body fat distribution.
The association of WHR with pulmonary function indices was most consistently influenced by metabolic abnormalities, nutritional deficiencies, and Th cell immune profiles. These observations are highly clinically relevant and novel for pediatric pulmonology because they identify the association of WHR, metabolic syndrome, and pulmonary disease, reported in the adult population,33 as early as pre-adolescent years. Given the high prevalence and increasing incidence of pediatric obesity,1 these findings highlight the need for routine inclusion of WHR as a measure of truncal adiposity during clinic visits. Given existent pediatric predictive cutoffs,34 and the changing visceral fat deposition for the same body weight in US children,35 partly driven by racial influences on fat distribution,36 inclusion of truncal adiposity as an additional vital sign will facilitate early identification of children at-risk to develop obesity-related asthma. Although dietary carotenoid and fatty acids are not measured routinely, each of the carotenoids included in the analysis have potent anti-oxidant properties, including β-carotene, which comprises 20% of total carotenoids. Given that leptin, adiponectin, HOMA-IR, and carotenoids and n6-PUFA, along with anthropometric measures including WHR, explain up to 50% of the variance of pulmonary function indices, their quantification in obese children or healthy-weight children with truncal adiposity will directly inform endotyping as well as risk stratification, bringing precision medicine into the realm of childhood obesity-related asthma.37 Our findings of the association between metabolic abnormalities with the obese asthma phenotype support their quantification, which is more routine, particularly among children with truncal adiposity, followed by consideration of metformin and lipid lowering agents in addition to nutrient management, that have been effective in adult disease management,38–42 as novel therapeutic options for pediatric obese asthma endotype. Studies are needed to investigate the effectiveness of these interventions in pediatric obesity-related asthma.
There are few known therapeutic targets for the immune perturbations found in pediatric obesity-related asthma. We found a substantial effect of Th cell transcriptome and DNA methylome on the association of anthropometric measures with pulmonary function indices, with marked overlap between differentially expressed genes but not between differentially methylated genes that mediated the association. The overlapping differentially expressed genes were enriched for small GTPase mediated signaling pathways and cell migration, while the Th cell DNA methylome was enriched for JAK-STAT, MAPK, NFkB, and TGF receptor signaling, as well as apoptosis response, cell cycle regulation, actin polymerization and cell migration. We have previously linked upregulation of small GTPases with non-allergic Th cell responses,18 which are also known to influence cell migration, apoptosis and MAPK-NFkB signaling.43–45 These observations suggest that small GTPase upregulation in Th cells may influence the obese asthma phenotype through increased Th cell viability, migration, and activation of JAK-STAT-MAPK-NfkB signaling. These novel biological mechanisms, once confirmed in functional studies, will identify small GTPase pathways and their corresponding proteins as biomarkers as well as novel therapeutic targets for the pediatric obese asthma endotype.
We recognize that our study cohort did not include obese and healthy-weight participants without asthma which precludes our ability to quantity the distinct as well as overlapping effects of obesity-related complications on pulmonary function deficits among those with obesity-related asthma as compared to those with obesity alone. Our findings provide the foundation to further investigate the discriminating roles of these obesity-related complications between obese children with and without asthma. Furthermore, our sample size was small and was additionally limited by the quantification of all variables in the five predictive datasets. Yet, our identification of a subset of samples that clustered together and were similar for several variables in each of the predictive datasets, highlights that discriminating features of obesity tend to co-occur. These associations also suggest that samples that did not cluster either did not have the requisite number of obesity-related complications or these features were not abnormal enough to meet the threshold. Clinically, this suggests that the obese asthma endotype is associated with either higher intensity or number of obesity-related complications. Comparative analyses, like those conducted in this study, between obese children with and without asthma, are needed to address this question. Since these variables were quantified in a cross-sectional manner among children with clinically stable disease at recruitment, their relationship during disease exacerbation could not be ascertained. Having included only pre-adolescent children, and those of African American and Hispanic ethnicities, further influences the generalizability of our findings. However, these findings are highly relevant because of the racial disparities that exist in the burden of pediatric asthma and obesity.1,2 We are also unable to validate our findings in another cohort since there is none other where all these obesity-related complications along with measures that define the obese asthma phenotype have been quantified together.
Conclusion
In summary, this is the first study to conduct a multi-omics analysis and identify the independent and inter-dependent roles of body fat distribution, metabolic, and nutrition abnormalities, and Th cell immune profiles in airflow obstruction and lung volume deficits in obesity that are distinct measures of obese asthma phenotype, narrowing down on the contribution of waist to hip ratio, insulin resistance and leptin/adiponectin levels, perturbations in micronutrients, including carotenoids and dietary fatty acids, as well as a subset of genes in Th cells, to pediatric obesity-related asthma. Confirmation of these findings and distinguishing the contribution of these obesity-mediated complications to lung function in obesity without asthma will define these variables as biomarkers of the obese asthma endotype. Inclusion of these variables in routine evaluation of obese children would identify those at-risk for obesity-related asthma and suggest potential therapeutic strategies.
Supplementary Material
Key messages.
Obesity-related complications including visceral fat, metabolic abnormalities, nutrient deficiencies, and immune perturbations have interdependent influences on lung function contributing to the pediatric obese asthma endotype
Metabolic abnormalities, nutrient deficiencies, and immune perturbations most consistently attenuate the association of waist to hip ratio, a measure of visceral fat, with pulmonary function deficits.
Evaluation of waist to hip ratio in obese children may identify those at-risk for obesity-related asthma
Acknowledgements
We would like to acknowledge the National Institutes of Health (Grants HL118733 and HL141849) for funding this study.
This research was funded by the National Institutes of Health (HL118733 and HL141849)(D.R.).
Abbreviations
- SFA
Saturated fatty acids
- PUFA
Polyunsaturated fatty acids
- MUFA
Monounsaturated fatty acids
- Th
T helper
- CDC42
Cell division cycle 42
- BMI
Body Mass Index
- CASI
Composite Asthma Severity Index
- ACT
Asthma Control Test
- WHR
Waist to hip ratio
- FVC
Forced vital capacity
- FEV1
Forced expiratory volume in 1st second
- FEF25–75%
Forced expiratory flow at 25–75% of FVC
- TLC
Total lung capacity
- RV
Residual volume
- ERV
Expiratory reserve volume
- FRC
Functional residual capacity
- IC
Inspiratory capacity
- FDR
False discovery rate
- JAK-STAT
Janus kinase (JAK)-signal transducer and activator of transcription (STAT)
- MAPK
Mitogen-activated protein kinase
- TGF
Transforming growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflict of interest.
References
- 1.Childhood Obesity Facts | Overweight & Obesity | CDC. 2018.
- 2. https://www.cdc.gov/vitalsigns/childhood-asthma/index.html.
- 3.De A, Rastogi D. Association of pediatric obesity and asthma, pulmonary physiology, metabolic dysregulation, and atopy; and the role of weight management. Expert Rev Endocrinol Metab. 2019;14(5):335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147(6):1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YC, Tu YK, Huang KC, Chen PC, Chu DC, Lee YL. Pathway from central obesity to childhood asthma. Physical fitness and sedentary time are leading factors. Am J Respir Crit Care Med. 2014;189(10):1194–1203. [DOI] [PubMed] [Google Scholar]
- 6.Goossens GH. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts. 2017;10(3):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musaad SM, Patterson T, Ericksen M, Lindsey M, Dietrich K, Succop P, et al. Comparison of anthropometric measures of obesity in childhood allergic asthma: Central obesity is most relevant. J Allergy Clin Immunol. 2009;123(6):1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastogi D, Bhalani K, Hall CB, Isasi CR. Association of pulmonary function with adiposity and metabolic abnormalities in urban minority adolescents. Ann Amer Thor Soc. 2014;11(5):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rastogi D, Jung M, Strizich G, Shaw PA, Davis SM, Klein OL, et al. Association of systemic inflammation, adiposity, and metabolic dysregulation with asthma burden among Hispanic adults. Respir Med. 2017;125:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oddy WH, de Klerk NH, Kendall GE, Mihrshahi S, Peat JK. Ratio of omega-6 to omega-3 fatty acids and childhood asthma. J Asthma. 2004;41(3):319–326. [DOI] [PubMed] [Google Scholar]
- 11.Tobias TAM, Wood LG, Rastogi D. Carotenoids, fatty acids and disease burden in obese minority adolescents with asthma. Clin Exp Allergy. 2019;49(6):838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55(3):207–216. [DOI] [PubMed] [Google Scholar]
- 13.Wood LG. Diet, Obesity, and Asthma. Ann Am Thorac Soc. 2017;14(Supplement_5):S332–s338. [DOI] [PubMed] [Google Scholar]
- 14.Lautenbacher LA, Jariwala SP, Markowitz ME, Rastogi D. Vitamin D and pulmonary function in obese asthmatic children. Pediatr Pulmonol. 2016;51(12):1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rastogi D, Canfield S, Andrade A, Isasi CR, Hall CB, Rubinstein A et al. Obesity-associated asthma in children: A distinct entity. Chest. 2012;141(4):895–905. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, BHagtani RH et al. Inflammation, Metabolic Dysregulation and Pulmonary Function Among Obese Asthmatic Urban Adolescents. Am J Resp Crit Care Med. 2015;191(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rastogi D, Nico J, Johnston AD, Tobias TAM, Jorge Y, Macian F, et al. CDC42-related genes are upregulated in T helper cells from obese asthmatic children. J Allergy Clin Immunol. 2018;141(2):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rastogi D, Johnston AD, Nico J, Loh LN, Jorge Y, Suzuki M, et al. Functional Genomics of the Pediatric Obese Asthma Phenotype Reveal Enrichment of Rho-GTPase Pathways. Am J Respir Crit Care Med. 2020;202(2):259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Overweight & obesity; 2018.
- 20.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index- An outcome measure for use in children and adolescents. J Allergy Clin Immmunol. 2012;129(3):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. [DOI] [PubMed] [Google Scholar]
- 24.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8(3):492–506. [DOI] [PubMed] [Google Scholar]
- 25.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. [DOI] [PubMed] [Google Scholar]
- 26.Rastogi D, Suzuki M, Greally JM. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Sci Rep. 2013;3:2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Mezlini AM, Demir F, Fiume M, Tu Z, Brudno M, et al. Similarity network fusion for aggregating data types on a genomic scale. Nat Methods. 2014;11(3):333–337. [DOI] [PubMed] [Google Scholar]
- 28.Tingley D, Yamamoto T, Hirose K, Imai K, Keele L. mediation: R package for Causal Mediation Analysis. Journal of Statistical Software. 2014;59(5):1–38.26917999 [Google Scholar]
- 29.Yu Q, Wu X, Li B, Scribner RA. Multiple mediation analysis with survival outcomes: With an application to explore racial disparity in breast cancer survival. Stat Med. 2019;38(3):398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia J, Gill EE, Hancock RE. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc. 2015;10(6):823–844. [DOI] [PubMed] [Google Scholar]
- 31.Forno E, Han Y-Y, Mullen J, Celedón JC. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. The Journal of Allergy and Clinical Immunology In Practice. 2018;6(2):570–581.e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baffi CW, Wood L, Winnica D, Strollo PJ, Gladwin MT, Que LG, et al. Metabolic Syndrome and the Lung. Chest. 2016;149(6):1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xi B, Zong X, Kelishadi R, Litwin M, Hong YM, Poh BK, et al. International Waist Circumference Percentile Cutoffs for Central Obesity in Children and Adolescents Aged 6 to 18 Years. J Clin Endocrinol Metab. 2020;105(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez JR, Bohan Brown M, Lopez-Alarcon M, Dawson JA, Guo F, Redden DT, et al. Changes in pediatric waist circumference percentiles despite reported pediatric weight stabilization in the United States. Pediatr Obes. 2017;12(5):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martos-Moreno GA, Martinez-Villanueva J, Gonzalez-Leal R, Barrios V, Sirvent S, Hawkins F, et al. Ethnicity Strongly Influences Body Fat Distribution Determining Serum Adipokine Profile and Metabolic Derangement in Childhood Obesity. Front Pediatr. 2020;8:551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conrad LA, Cabana MD, Rastogi D. Defining pediatric asthma: phenotypes to endotypes and beyond. Pediatr Res. 2021;90(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu TD, Keet CA, Fawzy A, Segal JB, Brigham EP, McCormack MC. Association of Metformin Initiation and Risk of Asthma Exacerbation: A Claims-Based Cohort Study. Ann Am Thorac Soc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haro C, Montes-Borrego M, Rangel-Zuniga OA, Alcalá-Diáz JF, Gomez-Delgado F Perez-Martinez P et al. Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J Clin Endocrinol Metab. 2016;101(1):233–242. [DOI] [PubMed] [Google Scholar]
- 40.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R,et al. Determinants of weight loss success utilizing a meal replacement plan and/or exercise, in overweight and obese adults with asthma. Respirology. 2015;20(2):243–250. [DOI] [PubMed] [Google Scholar]
- 41.Alexeeff SE, Litonjua AA, Sparrow D, Vokonas PS, Schwartz J. Statin Use Reduces Decline in Lung Function: VA Normative Aging Study. Am J Resp Crit Care Med. 2007;176(8):742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tse SM, Li L, Butler MG, Fung V, Kharbanda EO, Larkin EK, et al. Statin exposure is associated with decreased asthma-related emergency department visits and oral corticosteroid use. Am J Resp Crit Care Med. 2013;188(9):1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rougerie P, Delon J. Rho GTPases: masters of T lymphocyte migration and activation. Immunol Lett. 2012;142(1–2):1–13. [DOI] [PubMed] [Google Scholar]
- 44.Tong L, Tergaonkar V. Rho protein GTPases and their interactions with NFkappaB: crossroads of inflammation and matrix biology. Biosci Rep. 2014;34(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas A, Giesler T, White E. p53 mediates bcl-2 phosphorylation and apoptosis via activation of the Cdc42/JNK1 pathway. Oncogene. 2000;19(46):5259–5269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


