Abstract
Background:
Prospective genetic evaluation of patients at our referral research hospital presents clinical research challenges.
Objective:
This study sought not only a single-gene explanation for participants’ immune-related presentations, but viewed each participant holistically, with the potential to have multiple genetic contributions to their immune-phenotype and other heritable comorbidities relevant to their presentation and health.
Methods:
We developed a program integrating exome sequencing, chromosomal microarray, phenotyping, results return with genetic counseling, and reanalysis in 1505 individuals from 1000 families with suspected or known inborn errors of immunity.
Results:
Probands were 50.8% female, 71.5% ≥18 years, and had diverse immune presentations. Overall, 327/1000 probands (32.7%) received 361 molecular diagnoses. These included 17 probands with diagnostic copy number variants, 32 probands with secondary findings, and 31 probands with multiple molecular diagnoses. Reanalysis added 22 molecular diagnoses, predominantly due to new disease-gene associations (9/22, 40.9%). One-quarter of the molecular diagnoses (92/361) did not involve immune-associated genes. Molecular diagnosis was correlated with younger age, male sex, and a higher number of organ systems involved. This program also facilitated the discovery of new gene-disease associations such as SASH3-related immunodeficiency. A review of treatment options and ClinGen actionability curations suggest that at least 251/361 (69.5%) of these molecular diagnoses could translate into ≥1 management option.
Conclusion:
This program contributes to our understanding of the diagnostic and clinical utility whole exome analysis on a large scale.
Keywords: genomics, exome sequencing, chromosomal microarray analysis, copy number variation, Mendelian disorder, secondary findings, immunology, immune system, genetics, inborn errors of immunity
CAPSULE SUMMARY:
Comprehensive exome analysis has diagnostic and clinical utility: one-quarter of the molecular diagnoses in this study were found in genes not associated with inborn errors of immunity.
INTRODUCTION
The use of genomics in clinical research has evolved substantially over the past decade. For patients with immune defects, like other rare diseases, a timely molecular diagnosis reduces unnecessary testing, guides medical management, and facilitates recurrence risk assessment (1).
Indeed, researchers have successfully leveraged genomics to define hundreds of Mendelian disorders of the immune system, (2) often illuminating basic biology in the process (3,4). New mechanisms underlying specific forms of inborn errors of immunity (IEI) have facilitated precision medicine through novel targeted treatments for rare diseases (5) and key advances for common diseases (6). And yet, interpretation of next-generation sequencing (NGS) data from panel, exome, genome, and structural variant assays remains a challenge. Specifically for patients with IEI, the role of multiple molecular diagnoses (i.e., potentially leading to ‘blended phenotypes’), integration of copy number variant (CNV) analysis, and the utility of re-analysis remains largely unexplored.
A recent review of the application of NGS in IEI examined the molecular diagnostic yield across fourteen studies using gene panels and/or exome sequencing. Diagnostic yields were highly variable (15%−79%), depending upon cohort characteristics, gene panel size (ranging from 12–571) and sequencing approaches. CNV assessment increased diagnostic yield by 4.2% on average. However, the heterogeneity and lack of methodological detail regarding assessment of variant pathogenicity highlights the need for a more standardized analytical approach (7). Limited analysis of exome data through phenotype-directed ‘virtual IEI panels’ reduces diagnostic yield. In another study of 61 unrelated participants with IEI, the authors performed an extended analysis of 4,813 disease-associated genes after first screening 260 IEI-associated genes. Findings unrelated to the immune system comprised 36.8% of the molecular diagnoses from this study (n = 7/19) (8), underscoring the diagnostic utility of analyses not limited by organ systems.
For this study, we comprehensively evaluated participants’ exomes, returned results to participants with a written report, and provided counseling. Clinically relevant findings were defined as primary findings related to a participant’s phenotype, secondary findings (SF) as recommended by the American College of Medical Genetics and Genomics (ACMG) (9), and incidental findings, those which were unsuspected clinically but had strong evidence supporting pathogenicity. While single gene, Mendelian models of heritable disease have been highly successful, we recognize that these models are simplistic and cannot explain the complete genetic architecture of IEI. Recent genomic innovations such as polygenic models of disease, susceptibility, and modifiers must be studied and then translated into clinical care. Clinicians and researchers are beginning to consider a more comprehensive view of their patients’ genomic data, considering not only single-gene etiologies but also the possibility of multiple other molecular diagnoses, low-penetrance variants, modifiers, and the use of predictive genomic medicine such as actionable secondary findings and pharmacogenomics. This clinical sequencing program is a first step in that direction.
We present the results of the first 1,505 participants from 1,000 unrelated families. Our program encompassed clinical exome sequencing with chromosomal microarray analysis (CMA), a standardized analytic approach to the entire data set (not only IEI genes) based on the ACMG variant interpretation guidelines(10), detailed standardized phenotyping using the Human Phenotype Ontology (HPO) (11), reanalysis, and return of results with genetic counseling.
METHODS
Population
A total of 1,505 individuals from 1,000 unrelated families were recruited from the National Institute of Allergy and Infectious Diseases (NIAID) Division of Intramural Research (DIR) between 2017–2019 to enroll onto a centralized sequencing protocol. The study was approved by the NIAID Institutional Review Board (NCT03206099). All participants provided written informed consent.
The primary inclusion criterion was co-enrollment of the proband in another NIAID research protocol. Collaborating NIAID investigators performed detailed clinical evaluations of probands, which were subsequently coded using the HPO (details in online repository). Individuals with molecularly confirmed or clinically diagnosed genetic disorders prior to enrollment were included. In fact, many participants had extensive prior evaluations, including genetic testing. The status of prior research-based genetic testing, particularly when inconclusive, could not always be ascertained. Standardizing the baseline genetic evaluation across this rich cohort is a goal of our program.
Sequencing and CMA Methods
Research-based exome sequencing was performed on all study participants (details in online repository). Relevant findings were confirmed by Sanger sequencing or other appropriate methods meeting Clinical Laboratory Improvement Amendments/College of American Pathologists (CLIA/CAP) requirements. CNVs were evaluated with an Agilent 180K custom oligonucleotide chromosomal microarray on a subset of participants (details in online repository). Cases were prioritized for CMA based on clinical judgment regarding syndromic presentation, early age of onset, or a single high-priority variant identified by sequencing for a recessive condition.
Ancestry Inference
We generated genomic ancestry principal components from germline variation using peddy version 0.4.6 (12) and the 1000 Genomes phase 3 reference panel of 2,504 individuals (13), and principal components 1, 2, and 3 were plotted using the ggplot2 package in R (14). Self-reported race and ethnicity were also collected from all participants.
Data Interpretation
Clinical interpretation of variants was performed according to the ACMG guidelines(10) using the Genomic Research Integration System (GRIS) developed by the NIAID Bioinformatics and Computational Biosciences Branch (https://www.niaid.nih.gov/research/bioinformatics-computational-biosciences-branch). GRIS is a custom tool that integrates seqr [https://seqr.broadinstitute.org/] and PhenoTips [https://phenotips.com/] in a secure web-based portal. Functional data was used to inform the classification of variants in select cases, particularly when performed as part of a clinical assay (e.g., dihydrorhodamine testing for chronic granulomatous disease). Research-based functional testing, which is highly informative in establishing pathogenicity, was also performed for select cases, although not pursued systematically. For further detail on data interpretation, quality control, and illustrative examples for detailed genetic variant assessments, see online repository.
For the purposes of this summary, a case was classified as having a molecular diagnosis in the following scenarios: (1.) hemizygous or heterozygous pathogenic or likely pathogenic variant(s) for disorders that include X-linked inheritance in males or autosomal dominant inheritance in either sex; (2.) two pathogenic or likely pathogenic variants in the same gene for disorders with autosomal recessive inheritance where segregation data were consistent with a trans configuration or phase was unknown; (3.) one pathogenic or likely pathogenic variant plus a variant of uncertain significance (VUS) in the same gene for disorders with autosomal recessive inheritance where segregation data was consistent with a trans configuration or phase was unknown.
Reanalysis was performed by using variant- and case-level approaches, consistent with ACMG guidance (15):
The cohort received a targeted variant-level reanalysis in the spring of 2021, focusing on recently published disease-gene associations and recently published variants within the Human Gene Mutation Database that were also present in our database.
When additional relatives were added to a case, or a referring team requested it (typically in cases of evolving phenotypes), case-level reanalysis was performed.
All variants implicated in a molecular diagnosis are submitted to ClinVar under submission abbreviation: NIAID_CSP.
Statistical Methods
Cohort demographics were assessed using descriptive statistics. Multiple logistic regression was performed to determine the correlates of molecular diagnosis and receipt of a variant of uncertain significant with the following model: ~Sex + Age + Inferred ancestry + Top-level HPO Term Count + Absence of heterozygosity (AOH) >10 Mb over multiple chromosomes + (Age × Sex). All analyses were done in R version 4.0.2 (16). Scripts are included in the online supplement.
RESULTS
Participants
We performed genetic analyses on 1505 participants (1000 probands and 505 relatives). All participants received exome sequencing and 430 received CMA (374 probands and 56 relatives). Probands were predominantly adult (71.5% ≥ 18 years; range 0–90 years) and 50.8% female. Four probands (0.4%) were evaluated posthumously. Participants’ self-identified race and ethnicity is presented in Table 1. Principal component analysis predicted 796, 70, 66, 51, and 17 out of 1000 probands had genomic ancestry derived from European, admixed American, African, East Asian, and South Asian superpopulations (17,18), respectively (see Figure E1 in the Online Repository).
Table 1.
Demographic characteristics of cohort.
| DEMOGRAPHICS | N | % |
|---|---|---|
|
| ||
| AGE AT ENROLLMENT | ||
| 0–17 | 285 | 28.5 |
| 18–44 | 336 | 33.6 |
| 45–64 | 255 | 25.5 |
| >65 | 124 | 12.4 |
| SEX (% FEMALE) | 508 | 50.8 |
| RACE | ||
| AMERICAN INDIAN, ALASKAN NATIVE | 8 | 0.8 |
| ASIAN | 54 | 5.4 |
| BLACK OR AFRICAN AMERICAN | 54 | 5.4 |
| NATIVE HAWAIIAN, OTHER PACIFIC ISLANDER | 4 | 0.4 |
| WHITE | 790 | 79 |
| OTHER | 90 | 9 |
| ETHNICITY | ||
| HISPANIC | 71 | 7.1 |
| NON-HISPANIC | 883 | 88.3 |
| UNKNOWN | 46 | 4.6 |
| LIVING vs. DECEASED STATUS | ||
| POSTHUMOUS ENROLLMENT | 4 | 0.4 |
| RELATIVES ENROLLED | ||
| PROBAND ONLY | 741 | 74.1 |
| TRIO | 110 | 11 |
| NON-TRIO RELATIVES | 149 | 14.9 |
The cohort was predominantly non-consanguineous. Eighteen probands (1.8%) had AOH suggestive of recent identity by descent, defined as mean AOH length across multiple chromosomes of >10 Mb(19).
One quarter of probands had at least one previous molecular diagnosis (256 with 260 prior molecular diagnoses) from prior clinical or research genetic testing.
Probands had a wide range of immune abnormalities ranging from disseminated or recurrent infections to multi-organ autoimmune, inflammatory, or atopic conditions, either in isolation or in combination (See Figure E2 and Table E5 in the Online Repository). Approximately one quarter of the probands (249/1,000) had phenotypes involving more than 11 top-level HPO categories, which roughly correspond to organ systems. The most common top-level HPO categories in order of decreasing frequency were the immune system, respiratory system, digestive system, integument, and cardiovascular system.
Molecular Diagnoses and Other Findings
Findings in the Full Cohort
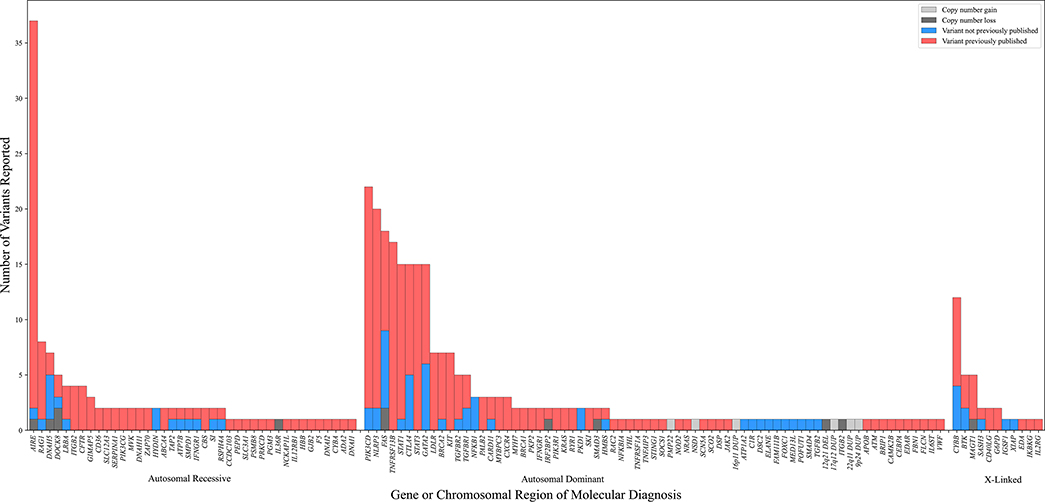
Approximately one-third of probands 327/1,000 probands (32.7%) received at least one molecular diagnosis (361 total molecular diagnoses) (Figure 1). Specifically, 296/1,000 (29.6%) probands received a single molecular diagnosis and 31/1,000 (3.1%) received multiple molecular diagnoses; 28/1,000 (2.8%) probands had two diagnoses and 3/1,000 (0.3%) probands had three diagnoses (See Table E1 in the Online Repository). The multiple molecular diagnoses more often affected distinct (n = 26) rather than overlapping organ systems (n = 5) for a given participant. An example of distinct organ systems would be P0002940, who received the molecular diagnoses of FAS-related autoimmune lymphoproliferative syndrome and SLC12A3-related hypokalemic metabolic alkalosis; an overlapping organ system example would be P0002231, who received molecular diagnoses related to defects in STAT1 and TNFRSF13B.
Figure 1.
Figure 1 shows the distribution of molecular diagnoses by mode of inheritance. Blue shading indicates the subset of variants which had not been previously reported in the Human Gene Mutation Database Pro or reported in the literature in association with disease. Copy number variation is indicated by dark and light gray, indicating loss and gain, respectively.
Of the molecular diagnoses, 249/361 (69.0%) had autosomal dominant inheritance; the remaining were either autosomal recessive (79/361, 21.9%) or X-linked (33/361, 9.1%). Of note, 77/403 (19.1%) of the variants contributing to these 361 molecular diagnoses had not, to our knowledge, been previously reported in the literature (Figure 1).
Additionally, 196 variants not constituting a molecular diagnosis (e.g., VUS, single pathogenic or likely pathogenic allele in recessive condition) were reported in 157 probands (15.7%) (See Table E4 in the Online Repository).
Molecular diagnoses in genes associated with IEI
Three quarters (270/361, 74.8%) of molecular diagnoses reflected specific patient populations that are investigated within the NIAID DIR (e.g., GATA2, FAS, PIK3CD, AIRE, STAT3) or disorder included in the 2021 update of the IUIS gene list (2). The most common molecular diagnoses in this category included variants in AIRE (n = 25), PIK3CD (n = 22), NLRP3 (n = 20), TNFRSF13B (n = 15), and FAS (n = 14) (Figure 1).
Molecular diagnoses in genes not implicated in IEI at the time of analysis
For 83/1,000 probands (8.3%), exome sequencing, CMA, and subsequent analyses resulted in 91 molecular diagnoses across 64 genes or genomic regions not included in the 2021 IUIS list(2) (See Table E2 in the Online Repository). These were comprised of four types of findings:
Findings in genes related to immunology or infectious disease research at our institution but not on the IUIS list. These included primary ciliary dyskinesia (n = 10) and KIT-related mastocytosis (n = 7).
Findings in novel genes related to IEI. Specifically, these included individuals with pathogenic variants in GIMAP5 (n = 2) and SASH3 (n = 2).
SFs: 32 probands received 33 molecular diagnoses across 15 genes based on the ACMG recommendations for SFs v2.0 (9) (See Table E2 in the Online Repository) that were apparently consistent with the proband’s personal or family history in 18/33 (54.5%) cases. The most common secondary molecular diagnoses were LDLR-associated familial hypercholesterolemia (n = 7), BRCA2-associated hereditary breast and ovarian cancer syndrome (n = 7), and MYBPC3-associated hypertrophic cardiomyopathy (n = 3). Of note, the occurrence of molecular diagnoses related to Loeys-Dietz syndrome were enriched in this cohort due to an active Loeys-Dietz syndrome research program at NIAID; participants with molecular diagnoses consistent with Loeys-Dietz syndrome and recruited from that research program were excluded from this discussion of SFs (n = 12).
Other findings: the remaining 39 probands received 41 molecular diagnoses outside of the IUIS or ACMG gene lists. These molecular diagnoses included relatively common genetic disorders such as, multi-gene deletion/duplication syndromes (n = 6), polycystic kidney disease (n = 2), Charcot-Marie-Tooth disease (n = 1), GJB2-related deafness (n = 1); and exceedingly rare syndromes, such as FAM111B-related hereditary fibrosing poikiloderma, with tendon contractures, myopathy, and pulmonary fibrosis (n = 1).
Participants with prior molecular diagnoses
Given the extensive prior evaluations performed on this cohort of participants, 256/1,000 (25.6%) probands were referred to our program with 260 apparent reported prior molecular diagnoses; all were re-sequenced. Most of these probands (223/256, 87.1%) had molecular diagnoses from the IUIS gene list (224/260, prior molecular diagnoses, 86.1%). Among these probands with prior molecular diagnoses, 3/256 (1.2%, excluding the 12 patients with Loeys-Dietz syndrome) had a prior molecular diagnosis involving a gene on the ACMG SF list. Prior molecular diagnoses were confirmed in 98.5% of the cases (256/260); the four exceptions were related to a gene in which variants cannot be reliably detected by NGS or Sanger sequencing due to a pseudogene (NCF1, n = 4). In one case with a prior molecular diagnosis, biallelic LRBA variants were analytically confirmed. However, the pathogenicity classification was downgraded as being inconsistent with a molecular diagnosis, based upon updated population-based variant frequency data.
Importantly, in multiple cases (25/256, 9.7%), our exome analyses on participants with prior molecular diagnoses demonstrated additional, previously unrecognized genetic contributions to their disease, comprising a total of 28 additional molecular diagnoses. These additional molecular diagnoses included SFs (n = 11 probands with 12 SFs), likely pathogenic variants in TNFRSF13B (n = 3 probands), molecular diagnoses from CMA (n = 3 probands), and other findings (n = 8 probands).
Participants without prior molecular diagnoses
Most of the referred participants (744/1,000, 74.4%) did not have an apparent molecular diagnosis at the time of enrollment; an unknown proportion of these participants had prior non-diagnostic genetic evaluations. In 75/744 (10.1%) we identified at least one molecular diagnosis; of these, two cases (2/75, 2.7%) received two molecular diagnoses. More than a third of these new molecular diagnoses (30/77, 39.0%) were identified in genes from the IUIS list. The remaining 47/77 (61.0%) of new diagnoses were not part of the 2021 IUIS list.
CMA contribution to molecular diagnoses
Among probands where CMA was performed (n = 374), 17 had 18 CNVs that contributed to a molecular diagnosis (17/1,000 = 1.7% of total probands, 17/374 = 4.5% of probands with CMAs) (See Table E4 in the Online Repository). In 16/18 (88.9%) of these CNVs, CMA findings led directly to a molecular diagnosis through a heterozygous or homozygous defect, inherited in an autosomal dominant or recessive pattern, respectively. In 2/17 probands (11.8%), one allele was identified through exome sequencing and one allele was identified through CMA (DOCK8-related immunodeficiency and DNAH5-related primary ciliary dyskinesia).
In total, 9/18 (50.0%) of the CMA-based molecular diagnoses were from genes on the IUIS gene list, including FAS (n = 2), DOCK8 (n = 2, one of which was caused by segmental uniparental isodisomy), ITGB2 (n = 1), IRF2BP2 (n = 1), MAGT1 (n = 1), IL36R (n = 1) and AIRE (n = 1). One of 18 (5.5%) was from a gene on the ACMG SF list, although this proband was recruited through the Loeys-Dietz syndrome research program. Nine of 18 (50.0%) were related to other findings such as PMP22-related Charcot-Marie-Tooth disease (n = 1), DNAH5-related primary ciliary dyskinesia (n = 1), copy number gain consistent with tetrasomy 9p (n = 1), or multi-gene deletion/duplication syndromes (n = 6).
New gene identification (See Table E3 in the Online Repository)
Our program’s infrastructure has been key in establishing novel causes of IEI, even within the first 1000 families enrolled. Employing a systematic approach to data sharing across NIAID research groups, this program facilitated identification of pathogenic defects in SASH3 associated with combined immunodeficiency with immune dysregulation (20). Another participant from this cohort was identified as having GIMAP5-related immunodeficiency, which to date, has only been described in four kindreds (21), [BioRxiv https://doi.org/10.1101/2021.02.22.432146]. Lastly, participants originally recruited into other NIAID research protocols and subsequently referred to our program were included in novel gene-disease associations for NCKAP1L (22) and PIK3CG (23) since the start of this program.
Reanalyses
Using targeted reanalysis, we identified 22 new molecular diagnoses, which were included in the above descriptions (22/361 molecular diagnoses were identified by reanalysis, 6.1%). Approximately 15.1 to 34.0 months elapsed between the initial analysis and reanalysis (median = 23.9 months). Newly described disease-gene associations accounted for 9/22 (40.9%) molecular diagnoses, including the genes GIMAP5 (n = 2), SASH3 (n = 2), IL6ST (n = 1), NCKAP1L (n = 1), SOCS1 (n = 1), PIK3CG (n = 1), and PPP3CA (n = 1). Upward re-classification of a variant based on all the current evidence was also a major driver of new molecular diagnoses, contributing 5/21 (23.8%) cases and involving the genes STAT3 (n = 1), DOCK8 (n = 1), CYBB (n =1), BRIP1 (n = 1), and TNFAIP3 (n = 1). Two additional cases (2/21 or 9.5%) were identified by careful re-evaluation of the regions implicated by CNV detected via CMA, including an association of 22q duplication (n = 1)(24) and 1q42 deletion including the gene IRF2BP2 (n = 1). As well, the addition of new segregation data yielded diagnoses involving the genes VWF (n = 1) and HMBS (n = 1), and additional clinical data yielded diagnoses involving the genes ATP1A1 (n = 1) and MYH7 (n = 1). Finally, a partial update to the ACMG SF list that included PALB2 accounted for new molecular diagnoses (n = 2) upon reanalysis. In total, 42.9% (9/21) of these new molecular diagnoses were represented in the current IUIS list (See Table E4 in the Online Repository).
Correlates of Molecular Diagnosis
Younger age, higher number of top-level HPO terms, and male sex were significantly associated with a molecular diagnosis, with odds ratios 0.97 [95% confidence interval (CI): 0.96–0.98; p = 8.63e-10] for each year increase in age; 1.11 [95% CI: 1.06–1.16; p = 2.86e-05] for each unit increase in top-level HPO term count, and 1.95 [95% CI: 1.15–3.32, p = 0.014] for male sex). Consistent with this finding, the effect of male sex appeared to be driven by X-linked molecular diagnoses, as it was no longer significant after removing participants with X-linked molecular diagnoses from the model (p = 0.085). The median age of those receiving a molecular diagnosis was 22.0 years versus 43.0 years for those with an inconclusive analysis. Inferred ancestry and mean AOH were not significantly associated with a molecular diagnosis or the likelihood of receiving a variant of uncertain significance, nor was there evidence of an interaction of age and sex.
DISCUSSION
To our knowledge, this is the largest systematic study of clinical molecular genetic analysis in families with immune-related phenotypes reported to date from a single center. We performed exome sequencing on 1,505 participants from 1,000 unrelated families, and applied CMA in a targeted subset. This approach sought to fully integrate genomics into the clinical care of our participants, tracking each exome sequencing or CMA request with CLIA-validated results documented in the electronic medical record and providing genetic counseling. This transparency, uniformity, and clear delivery of clinical genetic data is distinguished from sequencing predominantly for research, which tends to be more fluid or ad hoc to maximize discovery. Throughout this program we sought not only a single gene or Mendelian explanation for a participant’s suspected IEI, but viewed each participant holistically, as an individual with the potential to have multiple genetic contributions to their IEI and other heritable comorbidities relevant to their clinical presentation and future health.
The wide range in diagnostic yield from exome sequencing reported in the literature is highly dependent upon cohort characteristics, sequencing technology, and analyses (7,25). We identified a molecular diagnosis in a third of our probands, with young age, male sex, and multi-system involvement being associated with a greater likelihood of receiving a molecular diagnosis. This is consistent with prior reports across various clinical settings (1,26,27). Among the molecular diagnoses, 4.4% were due to CNV, consistent with prior publications (7). To date, the IEI literature on exome sequencing has largely focused on molecular diagnostic yield of gene lists summarized by IUIS (2,28,29). A quarter of our molecular diagnoses fell outside of this category, demonstrating the diagnostic advantage of analyzing the entire exome. This is consistent with limited prior reports in the IEI field but is yet to be reported at this scale (1,8).
Many non-IUIS molecular diagnoses were expected given the parameters of the IUIS gene list. For example, the IUIS list excludes genes causing primary ciliary dyskinesia, which creates a susceptibility to respiratory infection due to poorly functioning cilia rather than an IEI (30). Other non-IUIS molecular diagnoses presented opportunities to consider non-immune genes that that might cause phenotypes overlapping with IEI. For example, we identified a pathogenic variant in FAM111B in a proband with mucocutaneous candidiasis, hypoparathyroidism, hepatitis, pneumonitis, alopecia, vitiligo, and Sjogren’s syndrome -- features sometimes observed in AIRE deficiency (31–33). The FAM111B defect explained several of these symptoms, as well as myopathy, skin abnormalities, and hemorrhagic diathesis.
Other non-IUIS molecular diagnoses delineated complex phenotypes (e.g., CAMK2B for epilepsy; PMP22 duplication for peripheral neuropathy; GJB2 for deafness; PKD1 for polycystic kidney disease). It is possible that these complex-phenotype phenomena were enriched among our referrals; however, our findings underscore the possibility of extra-immune molecular diagnoses in individuals referred for IEI and are important considerations when investigating phenotypic outliers among rare disease cohorts. These observations demonstrate the utility and power of exome-wide analysis to comprehensively diagnose complex patients.
Consistent with prior publications,(34–36) 3% of our probands were found to have a SF (9). The fact that fewer than half of the families with SFs had a presentation consistent with the molecular diagnosis underscores the limitations of using clinical history as the sole means of identifying individuals at risk for actionable disorders. Not only does the return of such results create an opportunity for the affected families to proactively manage disease risk, but it also enhances rare disease research. Accounting for co-occurrent, highly penetrant genetic disorders is critical when characterizing IEI natural history.
Critically, molecular diagnoses are most medically valuable when they translate into management options. A recent review of IEI treatment options (37) and the ClinGen Actionability Expert Workgroup curations (clinicalgenome.org/curation-activities/clinical-actionability), combined with these findings, suggest that 251/361 (69.5%) of the reported molecular diagnoses could translate into at least one management option including supportive therapy (n = 139), preventive therapy (n = 176), allogenic hematopoietic stem cell or other transplant (n = 107), targeted therapy (n = 73), gene therapy (n = 1), or other evidenced-based management options (referencing diagnoses from the SF list or diagnoses in genes that have received favorable actionability curations by ClinGen, n = 47 and n = 16, respectively). Genomically-informed management of patients with suspected IEI has the potential to dramatically impact clinical care, considering that 1 in 5 of the molecular diagnoses described here has a targeted or gene therapy option available. This finding is consistent with limited prior publications within IEI, none of which also consider the clinical utility of molecular diagnoses unrelated to IEI(38–40). This topic is yet to be reported on at this scale to our knowledge.
Scalable reanalysis is a formidable challenge for clinical genetics, and, to our knowledge, this is the first publication of a systematically implemented reanalysis project across an immune cohort and one of the largest reports to date. Although is it likely that genomic re-analysis is conducted systematically on private cohorts among experts in IEI, the details and results of these activities are yet to be published. Even in the relatively short time frame of this study, we identified 22 new molecular diagnoses upon reanalysis, mostly from newly recognized gene-disease associations. A similar yield from reanalysis using new disease-gene discoveries has been reported in other disease populations (41). It should also be noted that two of these diagnoses could only be accomplished through concomitant NGS and CMA, highlighting how implementation of multiple platforms can provide value in select cases. Overall, these findings demonstrate the feasibility and yield of a targeted approach to reanalysis.
The molecular diagnoses summarized above have sufficient evidence to indicate pathogenicity. Beyond these variants, there were numerous VUS, only a fraction of which have been reported back to participants. There is considerable work needed to investigate these variants. Many may have importance as new, more effective methods for variant prioritization emerge (42). Close collaborations between basic and clinical scientists are crucial, particularly considering that most IEIs provide uniquely accessible tissue for functional validation. Furthermore, the possibility of identifying additional rare cases by genotype underscores the importance of genomic data sharing.
This study should be interpreted in the context of several limitations. The patients with suspected IEIs referred the NIH Clinical Center are likely not representative of clinical populations elsewhere. More granular ascertainment and documentation of prior clinical and research evaluations at baseline would have provided additional detail for analysis in this study. Importantly, this study did not assess participant perspectives on the outcomes of sequencing, nor did it capture longitudinal changes in clinical care concurrent with the return of exome sequencing results. It is also important to acknowledge that a randomized study design would provide clearer data on the relative impact of re-analysis on molecular diagnostic yield. Additionally, although the prospective structure of the program allows for longitudinal follow-up, technologies, including sequencing platforms, evolved over time. Genomic contributions to IEI may be further elucidated through other approaches and future technologies, such as long-read sequencing and de novo genome assembly, complementary technologies such as RNA-seq (44–46), superior CNV assessment, and/or deep sequencing for more sensitive detection of mosaicism (47). Additionally, evidence from genome-wide association studies for autoimmune diseases (48) and other rare disease investigations suggest that more complex genetic architecture underlies some presentations (49,50). It is likely that some of the currently unsolved cases from this cohort have polygenic contributions to their phenotype (51).
We have developed a program of clinical molecular genetic analysis in a large cohort of participants referred for diverse immunologic phenotypes. This study aids our understanding of the utility of a broad-based genomic approach to complex phenotypes – all genes considered for all patients and contributes to a growing literature supporting the use of broad genetic testing as a front-line test. These genomic data will enrich our understanding of basic immunity, molecular diagnostics, and clinical care both for the 1000 families included here, as well as the many families who will be evaluated in the coming years.
Supplementary Material
Clinical Implication:
Comprehensive analysis of exome data has diagnostic and clinical utility for patients with suspected inborn errors of immunity.
ACKNOWLEDGEMENTS
The authors would like to acknowledge sequencing services and professional partnership from the Center for Inherited Disease Research, Human Genome Sequencing Center. Special thanks to Amy Breman, PhD, Pawel Stankiewicz, MD, PhD, and Benjamin Berkman, JD, MPH, for their expertise and counsel. This research was supported by the Intramural Research Program of the NIH, primarily NIAID. Contributing investigators were also supported by the intramural research primarily NIAID. Contributing investigators were also supported by the intramural research programs at NIAMS (LMF), NHLBI (KO and KK), and NHGRI (LGB and JJJ), as well as the NIH Clinical Center (Department of Laboratory Medicine).
FUNDING:
This research was supported by the Intramural Research Program of the NIH, including investigators from the intramural research programs at NIAID, NIAMS, NHLBI, and NHGRI, as well as the NIH Clinical Center.
ABBREVIATIONS
- AOH
Absence of heterozygosity
- ACMG
American College of Medical Genetics and Genomics
- CMA
Chromosomal microarray analysis
- CLIA/CAP
Clinical Laboratory Improvement Amendments/College of American Pathologists
- CNV
Copy number variant
- DIR
Division of Intramural Research
- GRIS
Genomic Research Integration System
- HPO
Human phenotype ontology
- IEI
Inborn errors of immunity
- NIAID
National Institute of Allergy and Infectious Diseases
- NGS
Next-generation sequencing
- SF
Secondary findings
- VUS
Variant of uncertain significance
Footnotes
CONFLICT OF INTEREST: LGB is a member of the Illumina Medical Ethics Board and receives honoraria from Cold Spring Harbor Press. All other authors disclose no other conflicts of interest.
DISCLOSURE OF CONFLICTS OF INTEREST
LGB is a member of the Illumina Medical Ethics Board and receives honoraria from Cold Spring Harbor Press.
TRIAL REGISTRATION: ClinicalTrials.gov identifier NCT03206099
dbGaP STUDY ACCESSION: phs001899.v2.p1
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Morgan N. Similuk, Centralized Sequencing Program | DIR | NIAID.
Jia Yan, Centralized Sequencing Program | DIR | NIAID.
Rajarshi Ghosh, Centralized Sequencing Program | DIR | NIAID.
Andrew J. Oler, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology.
Luis M. Franco, Functional Immunogenomics Unit | Systemic Autoimmunity Branch | National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Michael Setzer, Centralized Sequencing Program | DIR | NIAID.
Michael Kamen, Centralized Sequencing Program | DIR | NIAID.
Colleen Jodarski, Centralized Sequencing Program | DIR | NIAID.
Thomas DiMaggio, Fungal Pathogenesis Section | Laboratory of Clinical Immunology and Microbiology.
Joie Davis, Immunopathogenesis Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Rachel Gore, Centralized Sequencing Program | DIR | NIAID.
Leila Jamal, Johns Hopkins/NIH Genetic Counseling Training Program; Genetics Branch, Center for Cancer Research, National Cancer Institute; NIH Clinical Center Department of Bioethics.
Adrienne Borges, Centralized Sequencing Program | DIR | NIAID.
Nicole Gentile, Centralized Sequencing Program | DIR | NIAID.
Julie Niemela, Centralized Sequencing Program | DIR | NIAID.
Chenery Lowe, Health, Behavior, and Society | Johns Hopkins Bloomberg School of Public Health.
Kathleen Jevtich, School of Medicine | Uniformed Services University of Health Sciences
Yunting Yu, College of Medicine | Penn State
Haley Hullfish, School of Medicine | University of Miami
Amy P. Hsu, Immunopathogenesis Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Celine Hong, Center for Precision Health Research | NHGRI.
Patricia Littel, Genetic Immunotherapy Section | Laboratory of Clinical Immunology and Microbiology.
Bryce A. Seifert, Centralized Sequencing Program | DIR | NIAID.
Joshua Milner, Institute of Genomic Medicine | Columbia University.
Jennifer J. Johnston, Center for Precision Health Research | NHGRI.
Xi Cheng, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Zhiwen Li, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Daniel Veltri, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Ke Huang, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Krishnaveni Kaladi, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Jason Barnett, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Lingwen Zhang, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Nikita Vlasenko, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Yongjie Fan, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Eric Karlins, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Satishkumar Ranganathan Ganakammal, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Robert Gilmore, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Emily Tran, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Alvin Yun, Operations and Engineering Branch | Office of Cyber Infrastructure and Computational Biology | NIAID.
Joseph Mackey, Operations and Engineering Branch | Office of Cyber Infrastructure and Computational Biology | NIAID.
Svetlana Yazhuk, Operations and Engineering Branch | Office of Cyber Infrastructure and Computational Biology | NIAID.
Justin Lack, NIAID Collaborative Bioinformatics Resource | Leidos Biomedical Research, Inc..
Vasu Kuram, NIAID Collaborative Bioinformatics Resource | Leidos Biomedical Research, Inc.
Wen Cao, NIAID Collaborative Bioinformatics Resource | Leidos Biomedical Research, Inc.
Susan Huse, NIAID Collaborative Bioinformatics Resource | Leidos Biomedical Research, Inc..
Karen Frank, Laboratory Medicine | Laboratory Medicine | NIH.
Gary Fahle, Microbiology | Laboratory Medicine | NIH.
Sergio Rosenzweig, Immunology Service | Laboratory Medicine | NIH.
Yan Su, Immunology Service | Laboratory Medicine | NIH.
SuJin Hwang, Tumor Vaccines and Biotechnology Branch, Division of Cellular and Gene Therapies, Center for Biologics Evaluation and Research, Food and Drug Administration.
Weimin Bi, Department of Molecular and Human Genetics | Baylor Genetics.
John Bennett, Clinical Mycology | Laboratory of Clinical Immunology and Microbiology | NIAID.
Ian A. Myles, Epithelial Therapeutics Unit | Laboratory of Clinical Immunology and Microbiology | NIAID.
Suk See De Ravin, Laboratory of Host Defenses | Laboratory of Clinical Immunology and Microbiology | NIAID.
Ivan Fuss, Mucosal Immunity Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Warren Strober, Mucosal Immunity Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Bibiana Bielekova, Neuroimmunological Diseases Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Adriana Almeida de Jesus, Translational Autoinflammatory Disease Studies Unit | Laboratory of Clinical Immunology and Microbiology | NIAID.
Raphaela Goldbach-Mansky, Translational Autoinflammatory Disease Studies Unit | Laboratory of Clinical Immunology and Microbiology | NIAID.
Peter Williamson, Translational Mycology Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Kelly Kumar, Cardiovascular and Pulmonary Branch | NHLBI.
Caeden Dempsy, Food Allergy Research Unit | Laboratory of Allergic Diseases | NIAID.
Pamela Frischmeyer-Guerrerio, Food Allergy Research Unit | Laboratory of Allergic Diseases | NIAID.
Robin Eisch, Mast Cell Biology Section | Laboratory of Allergic Diseases | NIAID.
Hyejeong Bolan, Mast Cell Biology Section | Laboratory of Allergic Diseases | NIAID.
Dean D. Metcalfe, Mast Cell Biology Section | Laboratory of Allergic Diseases | NIAID.
Hirsh Komarow, Mast Cell Biology Section | Laboratory of Allergic Diseases | NIAID.
Melody Carter, Mast Cell Biology Section | Laboratory of Allergic Diseases | NIAID.
Kirk M. Druey, Lung and Vascular Inflammation Section | Laboratory of Allergic Diseases | NIAID.
Irini Sereti, HIV Pathogenesis Section | Laboratory of Immunoregulation | NIAID.
Lesia Dropulic, Medical Virology Section | Laboratory of Immunoregulation | NIAID.
Amy D. Klion, Human Eosinophil Section | Laboratory of Parasitic Diseases | NIAID.
Paneez Khoury, Human Eosinophil Section | Laboratory of Parasitic Diseases | NIAID
Elise M. O’ Connell, Helminth Immunology Section | Laboratory of Parasitic Diseases | NIAID.
Nicole C. Holland-Thomas, Human Eosinophil Section | Laboratory of Parasitic Diseases | NIAID.
Thomas Brown, Human Eosinophil Section | Laboratory of Parasitic Diseases | NIAID.
David H. McDermott, Molecular Signaling Section | Laboratory of Molecular Immunology | NIAID.
Philip M. Murphy, Molecular Signaling Section | Laboratory of Molecular Immunology | NIAID.
Vanessa Bundy, Division of Allergy and Immunology | Children’s National Health System.
Michael D. Keller, Division of Allergy and Immunology | Children’s National Health System.
Christine Peng, Division of Allergy and Immunology | Children’s National Health System.
Helen Kim, Division of Allergy and Immunology | Children’s National Health System.
Stephanie Norman, Division of Allergy and Immunology | Children’s National Health System.
Ottavia M. Delmonte, Immune Deficiency Genetics Diseases Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Elizabeth Kang, Genetic Immunotherapy Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Helen C. Su, Human Immunological Diseases Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Harry Malech, Genetic Immunotherapy Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Alexandra Freeman, Immunopathogenesis Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Christa Zerbe, Immunopathogenesis Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Gulbu Uzel, Immunopathogenesis Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Jenna R.E. Bergerson, Primary Immune Deficiency Clinic | Laboratory of Clinical Immunology and Microbiology | NIAID.
V. Koneti Rao, Primary Immune Deficiency Clinic (ALPS Clinic) | Laboratory of Clinical Immunology and Microbiology | NIAID.
Kenneth N. Olivier, Cardiovascular and Pulmonary Branch | NHLBI.
Jonathan J. Lyons, Translational Allergic Immunopathology Unit | Laboratory of Allergic Diseases | NIAID.
Andrea Lisco, HIV Pathogenesis Section | Laboratory of Immunoregulation | NIAID.
Jeffrey I. Cohen, Medical Virology Section | Laboratory of Infectious Diseases | NIAID.
Michail S. Lionakis, Fungal Pathogenesis Section | Laboratory of Clinical Immunology and Microbiology | NIAID
Leslie G. Biesecker, Center for Precision Health Research | NHGRI.
Sandhya Xirasagar, Bioinformatics and Computational Biosciences | Office of Cyber Infrastructure and Computational Biology | NIAID.
Luigi Notarangelo, Immune Deficiency Genetics Diseases Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Steven M. Holland, Immunopathogenesis Section | Laboratory of Clinical Immunology and Microbiology | NIAID.
Magdalena A. Walkiewicz, Centralized Sequencing Program | DIR | NIAID.
REFERENCES.
- 1.Stray-Pedersen A, Sorte HS, Samarakoon P, Gambin T, Chinn IK, Coban Akdemir ZH, et al. Primary immunodeficiency diseases: Genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017. Jan;139(1):232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. The Ever-Increasing Array of Novel Inborn Errors of Immunity: an Interim Update by the IUIS Committee. J Clin Immunol. 2021. Apr;41(3):666–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma CA, Stinson JR, Zhang Y, Abbott JK, Weinreich MA, Hauk PJ, et al. Germline hypomorphic CARD11 mutations in severe atopic disease. Nat Genet. 2017. Aug;49(8):1192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotlarz D, Marquardt B, Barøy T, Lee WS, Konnikova L, Hollizeck S, et al. Human TGF-β1 deficiency causes severe inflammatory bowel disease and encephalopathy. Nat Genet. 2018. Mar;50(3):344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leiding JW, Forbes LR. Mechanism-Based Precision Therapy for the Treatment of Primary Immunodeficiency and Primary Immunodysregulatory Diseases. J Allergy Clin Immunol Pract. 2019. Mar;7(3):761–73. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Frange P, Blanche S, Casanova JL. Pathogenesis of infections in HIV-infected individuals: insights from primary immunodeficiencies. Curr Opin Immunol. 2017. Oct;48:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yska HAF, Elsink K, Kuijpers TW, Frederix GWJ, van Gijn ME, van Montfrans JM. Diagnostic Yield of Next Generation Sequencing in Genetically Undiagnosed Patients with Primary Immunodeficiencies: a Systematic Review. J Clin Immunol. 2019. Aug;39(6):577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudilla F, Franco-Jarava C, Martínez-Gallo M, Garcia-Prat M, Martín-Nalda A, Rivière J, et al. Expanding the Clinical and Genetic Spectra of Primary Immunodeficiency-Related Disorders With Clinical Exome Sequencing: Expected and Unexpected Findings. Front Immunol. 2019;10:2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med Off J Am Coll Med Genet. 2017. Feb;19(2):249–55. [DOI] [PubMed] [Google Scholar]
- 10.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med Off J Am Coll Med Genet. 2015. May;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köhler S, Carmody L, Vasilevsky N, Jacobsen JOB, Danis D, Gourdine JP, et al. Expansion of the Human Phenotype Ontology (HPO) knowledge base and resources. Nucleic Acids Res. 2019. Jan 8;47(D1):D1018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen BS, Quinlan AR. Who’s Who? Detecting and Resolving Sample Anomalies in Human DNA Sequencing Studies with Peddy. Am J Hum Genet. 2017. Mar 2;100(3):406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015. Oct 1;526(7571):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickham H ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 15.Deignan JL, Chung WK, Kearney HM, Monaghan KG, Rehder CW, Chao EC, et al. Points to consider in the reevaluation and reanalysis of genomic test results: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med Off J Am Coll Med Genet. 2019. Jun;21(6):1267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/ [Google Scholar]
- 17.Siva N 1000 Genomes project. Nat Biotechnol. 2008. Mar;26(3):256. [DOI] [PubMed] [Google Scholar]
- 18.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020. May;581(7809):434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiszniewska J, Bi W, Shaw C, Stankiewicz P, Kang SHL, Pursley AN, et al. Combined array CGH plus SNP genome analyses in a single assay for optimized clinical testing. Eur J Hum Genet EJHG. 2014. Jan;22(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delmonte OM, Bergerson JRE, Kawai T, Kuehn HS, McDermott DH, Cortese I, et al. SASH3 variants cause a novel form of X-linked combined immunodeficiency with immune dysregulation. Blood. 2021. Apr 19; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drzewiecki K, Choi J, Brancale J, Leney-Greene MA, Sari S, Dalgiç B, et al. GIMAP5 maintains liver endothelial cell homeostasis and prevents portal hypertension. J Exp Med. 2021. Jul 5;218(7):e20201745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook SA, Comrie WA, Poli MC, Similuk M, Oler AJ, Faruqi AJ, et al. HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science. 2020. Jul 10;369(6500):202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda AJ, Maher TJ, Zhang Y, Lanahan SM, Bucklin ML, Compton SR, et al. Human PI3Kγ deficiency and its microbiota-dependent mouse model reveal immunodeficiency and tissue immunopathology. Nat Commun. 2019. Sep 25;10(1):4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun D, Lee J, Heimall J, Jyonouchi S. Immunodeficiency in 22q11.2 duplication syndrome. J Allergy Clin Immunol Pract. 2021. Feb;9(2):996–998.e3. [DOI] [PubMed] [Google Scholar]
- 25.Thaventhiran JED, Lango Allen H, Burren OS, Rae W, Greene D, Staples E, et al. Whole-genome sequencing of a sporadic primary immunodeficiency cohort. Nature. 2020. Jul;583(7814):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Retterer K, Juusola J, Cho MT, Vitazka P, Millan F, Gibellini F, et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med Off J Am Coll Med Genet. 2016. Jul;18(7):696–704. [DOI] [PubMed] [Google Scholar]
- 27.Snoeijen-Schouwenaars FM, van Ool JS, Verhoeven JS, van Mierlo P, Braakman HMH, Smeets EE, et al. Diagnostic exome sequencing in 100 consecutive patients with both epilepsy and intellectual disability. Epilepsia. 2019. Jan;60(1):155–64. [DOI] [PubMed] [Google Scholar]
- 28.Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J Clin Immunol. 2020. Feb 11; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousfiha A, Jeddane L, Picard C, Ailal F, Bobby Gaspar H, Al-Herz W, et al. The 2017 IUIS Phenotypic Classification for Primary Immunodeficiencies. J Clin Immunol. 2018. Jan;38(1):129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas JS, Davis SD, Omran H, Shoemark A. Primary ciliary dyskinesia in the genomics age. Lancet Respir Med. 2020. Feb;8(2):202–16. [DOI] [PubMed] [Google Scholar]
- 31.Ferre EMN, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight. 2016. Aug 18;1(13):e88782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercier S, Küry S, Barbarot S. Hereditary Fibrosing Poikiloderma with Tendon Contractures, Myopathy, and Pulmonary Fibrosis. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Mirzaa G, et al. , editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [cited 2021 Oct 6]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK390610/ [PubMed] [Google Scholar]
- 33.Ferré EMN, Yu Y, Scherban A, Lee CCR, Rosen LB, Burbelo PD, et al. A Case of Mistaken Identity: Discovery of a de novo FAM111B mutation in a patient with an APECED-like phenotype. Prep.
- 34.Hart MR, Biesecker BB, Blout CL, Christensen KD, Amendola LM, Bergstrom KL, et al. Secondary findings from clinical genomic sequencing: prevalence, patient perspectives, family history assessment, and health-care costs from a multisite study. Genet Med Off J Am Coll Med Genet. 2019. May;21(5):1100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Luo W, Wang M, Wegman-Ostrosky T, Frone MN, Johnston JJ, et al. Prevalence of pathogenic/likely pathogenic variants in the 24 cancer genes of the ACMG Secondary Findings v2.0 list in a large cancer cohort and ethnicity-matched controls. Genome Med. 2018. Dec 24;10(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewey FE, Murray MF, Overton JD, Habegger L, Leader JB, Fetterolf SN, et al. Distribution and clinical impact of functional variants in 50,726 whole-exome sequences from the DiscovEHR study. Science. 2016. Dec 23;354(6319):aaf6814. [DOI] [PubMed] [Google Scholar]
- 37.King JR, Notarangelo LD, Hammarström L. An appraisal of the Wilson & Jungner criteria in the context of genomic-based newborn screening for inborn errors of immunity. J Allergy Clin Immunol. 2021. Feb;147(2):428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arts P, Simons A, AlZahrani MS, Yilmaz E, AlIdrissi E, van Aerde KJ, et al. Exome sequencing in routine diagnostics: a generic test for 254 patients with primary immunodeficiencies. Genome Med. 2019. Jun 17;11(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon AJ, Golan AC, Lev A, Stauber T, Barel O, Somekh I, et al. Whole exome sequencing (WES) approach for diagnosing primary immunodeficiencies (PIDs) in a highly consanguineous community. Clin Immunol Orlando Fla. 2020. May;214:108376. [DOI] [PubMed] [Google Scholar]
- 40.Engelbrecht C, Urban M, Schoeman M, Paarwater B, van Coller A, Abraham DR, et al. Clinical Utility of Whole Exome Sequencing and Targeted Panels for the Identification of Inborn Errors of Immunity in a Resource-Constrained Setting. Front Immunol. 2021;12:665621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu P, Meng L, Normand EA, Xia F, Song X, Ghazi A, et al. Reanalysis of Clinical Exome Sequencing Data. N Engl J Med. 2019. Jun 20;380(25):2478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manolio TA, Fowler DM, Starita LM, Haendel MA, MacArthur DG, Biesecker LG, et al. Bedside Back to Bench: Building Bridges between Basic and Clinical Genomic Research. Cell. 2017. Mar 23;169(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark MM, Stark Z, Farnaes L, Tan TY, White SM, Dimmock D, et al. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genomic Med. 2018;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonorazky HD, Naumenko S, Ramani AK, Nelakuditi V, Mashouri P, Wang P, et al. Expanding the Boundaries of RNA Sequencing as a Diagnostic Tool for Rare Mendelian Disease. Am J Hum Genet. 2019. Mar 7;104(3):466–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kremer LS, Bader DM, Mertes C, Kopajtich R, Pichler G, Iuso A, et al. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat Commun. 2017. Jun 12;8:15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murdock DR, Dai H, Burrage LC, Rosenfeld JA, Ketkar S, Müller MF, et al. Transcriptome-directed analysis for Mendelian disease diagnosis overcomes limitations of conventional genomic testing. J Clin Invest. 2021. Jan 4;131(1):141500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aluri J, Cooper MA. Genetic Mosaicism as a Cause of Inborn Errors of Immunity. J Clin Immunol. 2021. May;41(4):718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caliskan M, Brown CD, Maranville JC. A catalog of GWAS fine-mapping efforts in autoimmune disease. Am J Hum Genet. 2021. Apr 1;108(4):549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Timpson NJ, Greenwood CMT, Soranzo N, Lawson DJ, Richards JB. Genetic architecture: the shape of the genetic contribution to human traits and disease. Nat Rev Genet. 2018. Feb;19(2):110–24. [DOI] [PubMed] [Google Scholar]
- 50.Bogaert DJA, Dullaers M, Lambrecht BN, Vermaelen KY, De Baere E, Haerynck F. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016. Sep;53(9):575–90. [DOI] [PubMed] [Google Scholar]
- 51.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018. Sep;19(9):581–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.