Abstract
Hematopoietic cell transplantation (HCT) is a potentially curative treatment for many hematologic and non-hematologic disorders. Graft-versus-host-disease (GVHD) in its acute or chronic forms remains the most important non-relapse post-HCT complication. Biomarkers offer objective, unbiased information on systemic disorders, and significant focus has been placed on the discovery of biomarkers for GVHD. Ideally, a GVHD biomarker is actionable, utilizing the results of biomarker testing to guide clinical management of disease and clinical trial design. While many GVHD biomarkers have been identified, none have been properly qualified for clinical use. The National Institutes of Health (NIH) and Food and Drug Administration (FDA) provided biomarker subtype definitions; however, confusion remains about the proper definition and application of these subtypes in the HCT field. The 2014 NIH Consensus development project provided a framework for the development of biomarkers into clinical practice. This review aims to clarify the biomarker subtype definitions and re-emphasize the developmental framework. Armed with this knowledge, clinicians can properly translate GVHD biomarkers for clinical use.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) offers a curative option for many malignant and nonmalignant conditions. Graft-versus-host disease (GVHD) is a complex immunologic process that occurs on a pathobiological spectrum and manifests clinically as acute and chronic GVHD. Acute graft-versus-host disease (aGVHD) remains a major source of morbidity and mortality and chronic graft-versus-host disease (cGVHD) is the most common long-term complication of allo-HCT 1, 2. For malignancies, allo-HCT is a successful immunotherapy in large part due to the graft-versus-tumor (GVT) effect. GVT is however tethered to GVHD and management decisions that balance the benefits of GVT with the risks of GVHD can be a challenge for clinicians.
Biomarkers offer objective, unbiased information on systemic disorders, and the need for actionable biomarkers has led to the discovery of multiple plasma biomarkers for GVHD. Both acute and cGVHD biomarkers have a significant number of potential clinical applications, however, while many biomarkers have been identified, none have been properly qualified for clinical use.
The National Institutes of Health (NIH) and Food and Drug Administration (FDA) provided biomarker subtype definitions 3, however confusion remains about the proper definition and application of these subtypes. For example, a risk biomarker and its defined threshold cannot be used as a predictive biomarker, which based on the current literature continues to occur. The 2014 NIH Consensus development project provided a 4-part framework for biomarkers to be implemented into clinical practice (Figure 1) 4. Prior studies have not completed the proper development framework steps, therefore biomarkers with adequate statistical tests performance to reliably apply clinically are lacking. This review will re-emphasize the recommended workflow for proper translation of GVHD biomarkers. Accurate understanding of the biomarker definitions and appropriate use for specific outcomes would allow for proper biomarker guided-therapeutic trials.
Figure 1.
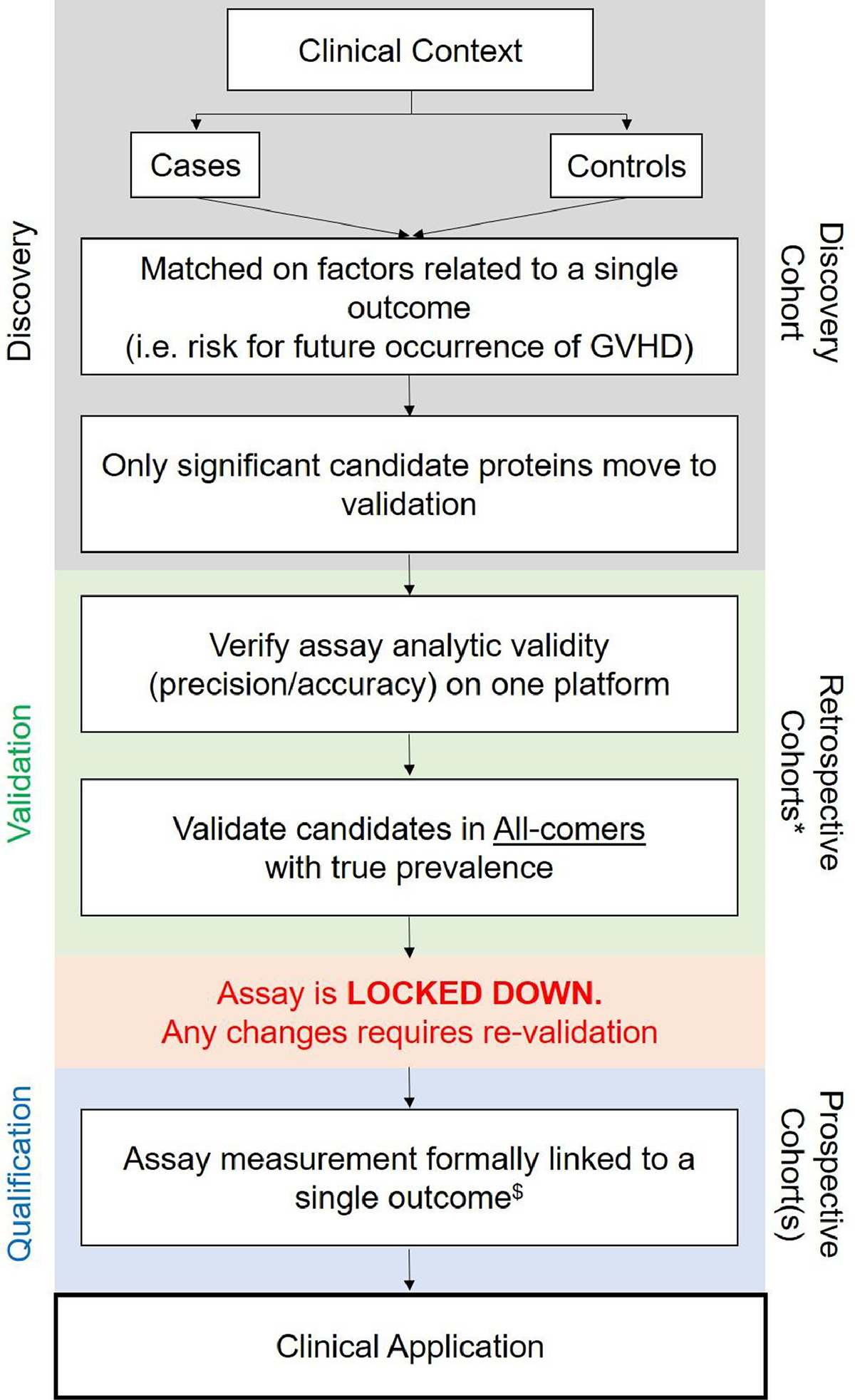
Recommended practices for biomarker development. *Validation requires evaluation in at least 2 independent cohorts.$Once a candidate protein reaches the qualification phase,it is called a biomarker of a specific type (see definitions).
1.0. FDA Biomarkers Definitions
The ‘Biomarkers, EndpointS and other Tools’ (BEST) Resource was established in 2016 by the FDA-NIH joint leadership council to define and outline biomarker roles in biomedical research, clinical practice and medical / pharmaceutical product development, and these guidelines have been regularly updated 3. Five biomarker subtypes are typically used: diagnostic, predictive, response, prognostic, and risk. An overview of the biomarker subtype definitions per the NIH BEST Resource and applied to GVHD is found in Table 1.
Table 1.
Biomarker definitions as per NIH BEST Resource 3
| Biomarker Subtype | Definition |
|---|---|
| Diagnostic | An assay used to confirm the presence of the disease |
| Predictive | An assay used to identify individuals who are more likely than similar individuals without the biomarker to experience a favorable or unfavorable effect from exposure to a specific medical product (before treatment is received) |
| Response | An assay used to show that a biological response has occurred in an individual who has been exposed to a medical product (after treatment is received) |
| Prognostic | An assay used to identify likelihood of a clinical event, disease recurrence or progression in patients who have the disease |
| Risk | An assay that indicates the potential for developing the disease in individuals who do not have clinically apparent disease |
A diagnostic biomarker is used to detect or confirm the presence of the disease 3. For example, cytogenetic Philadelphia (Ph) translocation BCR-ABL1 t(9;22)(q34;q11) is a diagnostic biomarker in chronic myeloid leukemia (CML) 5. Diagnostic biomarkers are most useful in a disease where treatment is indicated.
A predictive biomarker is used to identify a patient’s likelihood of response to or outcome of a particular treatment before the treatment is initiated 3. While imperfect, PD-L1 expression in tumors detected using immunohistochemistry is a predictive biomarker of response to anti-PD-1/PD-L1 therapy 6. Importantly, the FDA-NIH definition of a predictive biomarker requires assessment for each specific therapy, which differentiates prognostic and predictive biomarkers. Predictive biomarkers have become popular in hematology-oncology as they help optimize therapy decisions.
Response biomarkers show biologic response in patients after treatment is initiated 3. These biomarkers are measured prior to therapy and a change represents an impact on a clinical endpoint. International normalized ratio (INR) is used as a response biomarker when evaluating a patient’s response to anticoagulation with warfarin 7.
A prognostic biomarker helps determine the anticipated course for patients with clinically evident disease 3. The T315I mutation in patients with Ph+ CML is a negative prognostic factor 5. Another example is positive minimal residual disease following chemotherapy induction in acute leukemia.
A risk biomarker indicates potential for developing the disease in patients who do not have any clinical symptoms 3. For instance, Factor V Leiden is a risk biomarker for an increased likelihood to develop a deep vein thrombosis 8. The utility of a risk biomarker partially relies on whether interventions to reduce the risk of developing the disease exist and has low toxicity. The main distinction between risk and prognostic biomarkers is that a risk biomarker is obtained in patients who do not have the disease versus those who do.
2.0. Biomarker Development
Figure 1 provides 4 critical steps for the translation of GVHD biomarkers for clinical use, which consists of discovery of candidate proteins, validation of those with a unique assay, lockdown of this assay and finally qualification of the biomarker. The framework provides guidance to avoid previous biomarker development mistakes, including the absence of validation cohorts independent of the discovery or cases/controls cohorts, and strong reliance on retrospective rather than prospective evaluation 4. It is important to highlight that the developmental process must be completed for each biomarker subtype. For example, an aGVHD risk biomarker cannot be used as an aGVHD predictive biomarker without repeating all 4 steps, and may not ultimately prove to be a biomarker for the new outcome. It is also critical that after the discovery step, analytic validity of an assay is established on a unique platform (Table 2). For instance, if an enzyme-linked immunosorbent assay (ELISA) is performed, the ELISA kit and technique must be the same at all steps. No previous biomarker studies have completed all of these steps; thus, no biomarkers qualify for clinical application yet.
Table 2.
Assay analytical parameters
| 1. | Precision: Repeatability and reproducibility of an assay |
| 2. | Accuracy: Proximity of results to true value |
| 3. | Sensitivity: Limit of detection of an assay |
| 4. | Specificity: Interference and cross reactivity of an assay |
| 5. | Robustness: Capacity of an assay to remain unaffected by small but deliberate variations in method parameters, and provides an indication of its reliability during normal usage |
During the developmental phases of GVHD biomarkers, special attention must be paid to statistical tests performance and establishing biomarker cut-points (Table 3). Receiver operating characteristic curve (ROC) is generally used to establish performance of an assay and is a good representation of sensitivity and specificity for all possible biomarker cut-points. Sensitivity indicates true positive rate (TP) while specificity indicates true negative rate (TN) for a specific cut-point. To establish the more granular positive predictive value (PPV) and negative predictive value (NPV), both a cut-point and GVHD incidence must be accounted for. Although prevalence is used for predictive values in the general population, in the case of HCT where all patients start at day of transplant, GVHD incidence, the number of individuals who develop GVHD during a particular period (such as a month), has been used. Indeed, if incidence increases, PPV increases while NPV decreases. Therefore, for rare GVHD subtypes such as bronchiolitis obliterans syndrome (BOS), using a biomarker’s sensitivity and specificity provides a better picture to the clinician. For clinical applications, several cut-point values for each biomarker should be tested to accommodate the best balance between efficacy and toxicity of an intervention. For example, when a drug is safe such as defibrotide for sinusoidal obstruction syndrome, lower cut-points of a high biomarker with more false positives will be acceptable. In contrast, for GVHD treatments such as corticosteroids that impact GVT, higher cut-points will ensure that most patients are truly GVHD positive and will receive the intervention exposing a minimum of false positive patients.
Table 3.
Statistical tests performance
| 1. | Sensitivity: Proportion of subjects who test positive for a specific condition among a group of people who have the condition; how well a test can detect a specific condition in people who actually have the condition |
| 2. | Specificity: Proportion of subjects who test negative for a specific condition among a group of people who do not have the condition |
| 3. | Receiver operator characteristic (ROC) curve: A plot of the true-positive rate versus the false-positive rate for all possible cut points of a biomarker |
| 4. | Incidence: number of individuals who develop a specific disease during a particular time period |
| 5. | *Positive predictive value (PPV): Likelihood that a person who has a positive test result does have the disease |
| 6. | *Negative predictive value (NPV): Likelihood that a person who has a negative test result indeed does not have the disease |
Require biomarker cut-point values and depend on incidence of disease
3.0. Acute GVHD Biomarkers
Acute GVHD remains the main cause of nonrelapse mortality (NRM) and a major hurdle for success of allo-HCT. A summary of plasma aGVHD biomarkers by subtype is found in Table 4.
Table 4.
Plasma Biomarkers for Acute GVHD
| Name | Study | (n) | Associations/Timepoints in aGVHD (D0 = HCT date) | Framework steps completed | Potential clinical implementation | Ref |
|---|---|---|---|---|---|---|
| Diagnostic Biomarkers: Systemic | ||||||
| Biomarker panel: IL-2-R-α, HGF, IL-8, TNFR-1 | Paczesny 2009 | 424 | Identified GVHD at onset of symptoms | Step 1: Discovery Step 2: 2 cohorts |
• Improve diagnostic accuracy • Differentiate GVHD vs other complications |
9 |
| Diagnostic Biomarkers: Organ specific | ||||||
| REG3α | Ferrara 2011 | 1014 | Increased levels at onset of symptoms in GI-GVHD | Step 1: Discovery Step 2: 2 cohorts |
• Improve diagnostic accuracy in specific organs (ie colitis/dermatitis vs GI/skin GVHD) | 11 |
| TIM3 | Hansen 2013 | 149 | Increased levels associated with GI-GVHD | Step 1: Discovery Step 2: 2 cohorts |
13 | |
| Elafin | Paczesny 2010 | 492 | Increased levels at onset of skin GVHD | Step 1: Discovery | 15 | |
| Solán 2021 | 149 | Increased levels in skin GVHD | Step 1: Previous discovery | 16 | ||
| Predictive Biomarkers | ||||||
| ST2 | Vander Lugt 2013 | 673 | Increased levels at onset of treatment with corticosteroids associated with SR-aGVHD | Step 1: Discovery Step 2: 3 cohorts |
• Intensify for high risk group • Reduce immunosuppression for low/standard risk group |
19 |
| Response Biomarkers | ||||||
| ST2, TIM3 | McDonald 2017 | 165 | Increased levels after 14d of steroids predicts treatment failure | Step 1: Hypothesis | • Monitoring treatment response • Guide GVHD management • Future: Clinical efficacy endpoint |
23 |
| Prognostic Biomarkers | ||||||
| REG3α | Ferrara 2011 | 1014 | Increased levels at diagnosis associated with NRM | Step 1: Discovery Step 2: 2 cohorts |
• Anticipate course of disease • Adjust immunosuppression |
11 |
| Rowan 2020 | 415 | Increased levels D+7, D+14 and D+21 associated with NRM | Step 1: Previous discovery Step 2: Multi-center cohorts |
25 | ||
| ST2 | Vander Lugt 2013 | 673 | Increased levels D+14 associated with NRM | Step 1: Discovery Step 2: 3 cohorts |
19 | |
| Ponce 2015 | 113 | Increased levels D+28 associated with TRM in CBT | Step 1: Previous discovery | 27 | ||
| Abu Zaid 2017 | 211 | Increased levels D+28 associated with NRM | Step 1: Previous discovery Step 2: Clinical trial cohorts |
26 | ||
| Kanakry 2017 | 58 | Increased levels D+30 associated with NRM in haplo PTCy | Step 1: Previous discovery | 28 | ||
| Rowan 2020 | 415 | Increased levels D+7, D+14 and D+21 associated with NRM | Step 1: Previous discovery Step 2: Multi-center cohorts |
25 | ||
| Biomarker Algorithm | Levine 2015 | 792 | Score based on ST2 + Reg3a (+TNFR1) at GVHD categorizes into groups for NRM | Step 1: Previous discovery Step 2: 3 cohorts |
21 | |
| Hartwell 2017 | 1287 | Score based on ST2 + Reg3a D+7 categorizes into groups for NRM | Step 1: Previous discovery Step 2: multi-center cohorts |
30 | ||
| Hotta 2021 | 112 | Score based on ST2 + Reg3a D+7 categorizes into groups for NRM | Step 1: Previous discovery Step 2: Multi-center cohorts |
31 | ||
| Major-Monfried 2018 | 507 | Score based on ST2 + Reg3a after 7d of steroids categorizes into groups for NRM in SR-aGVHD | Step 1: Previous discovery Step 2: 2 cohorts |
32 | ||
| Srinagesh 2019 | 615 | Score based on ST2 + Reg3a after 28d of steroids categorizes into groups for NRM | Step 1: Previous discovery | 33 | ||
| TIM3 | Abu Zaid 2017 | 211 | Increased levels D+28 associated with NRM | Step 1: Previous discovery Step 2: Clinical trial cohorts |
26 | |
| Risk Biomarkers | ||||||
| No validated aGVHD risk biomarker exists | • Implement preemptive strategies | |||||
NRM, nonrelapse mortality, aGVHD, acute graft versus host disease, TRM, transplant related mortality, CBT, cord blood transplant, SR, steroid refractory, Haplo, haploidentical, PT Cy, post-transplant cyclophosphamide
3.1. Acute GVHD Biomarkers by Subtype
Diagnostic Biomarkers
Plasma biomarkers have been developed to confirm the presence of systemic or organ specific aGVHD. A biomarker panel of IL-2 Receptor-α (IL-2Rα), tumor necrosis factor receptor-1 (TNFR-1), interleukin-8 (IL-8) and hepatocyte growth factor (HGF) obtained at the onset of the clinical symptoms was able to confirm systemic aGVHD with high diagnostic accuracy 9.
Organ specific biomarkers may serve a more useful role in assisting in aGVHD diagnosis as they often represent proteins related to aGVHD damage from target tissues. Reg3α is a peptide primarily found in Paneth cells of the intestines and is released systemically as aGVHD damage occurs 10. Reg3α has emerged as the most validated GI-aGVHD biomarker and concentrations at symptom onset were able to distinguish GI-aGVHD 11, 12. The soluble form of T-cell immunoglobulin mucin protein-3 (TIM3), which may prevent immune suppression mediated via membrane-TIM3, was also associated with the GI-aGVHD 13. Elafin, a serine protease inhibitor primarily made by keratinocytes in the skin 14, was found to be elevated at onset of skin aGVHD 15. Elafin levels also correlated with higher incidence of stage III-IV skin aGVHD following haplo-Hct with post-transplant cyclophosphamide (PTCy) 16. In a prospective study, plasma elafin was elevated in cutaneous GVHD but levels were unable to distinguish GVHD and other causes of rash 17.
Diagnostic biomarkers can help improve diagnostic accuracy. These biomarkers can also help distinguish aGVHD from other common post-HCT complications with overlapping symptoms, such as diarrhea from infectious colitis. In the BMTCTN 1202 study, diagnostic biopsies were obtained in 40% of suspected GVHD cases, but treatment initiation did not correspond with biopsy results and 10.5% of biopsies were equivocal 18. Although a direct comparison of biopsies and diagnostic biomarkers is unlikely to be pursued, high biomarkers before or at treatment initiation (see predictive biomarkers) could help the decision making when biopsies are ambiguous.
Predictive Biomarkers
Per the FDA-NIH definition, a predictive biomarker must be assessed relative to each treatment. Stimulation-2 (ST2), the interleukin-33 (IL-33) decoy receptor involved in inflammatory signaling, is the most validated biomarker for aGVHD and has been studied in a variety of clinical scenarios. When ST2 was measured at the start of corticosteroid treatment, patients with high ST2 were over twice as likely to have treatment-resistant aGVHD 19. ST2 also emerged as a possible predictive biomarker for ruxolitinib for the treatment of steroid-refractory aGVHD. In the REACH1 clinical trial, significantly elevated ST2 levels were found in non-responders compared to responders 20. Reg3α alone or in combination with ST2 has shown potential as a prognostic biomarker, however requires further evaluation relative to each specific therapy to be validated as a predictive biomarker 11, 21.
Imperfectly qualified predictive biomarkers have been used for aGVHD clinical trials. A biomarker score based on ST2 and Reg3α values created to estimate 6-month NRM was used to preemptively treat patients with alpha-1 antitrypsin (AAT) 22. The study found no reduction of SR-aGVHD in the AAT group when compared to historical cohorts, however using imperfectly qualified biomarkers can skew study results.
Properly qualified predictive biomarkers can be used for more personalized aGVHD management. Predictive biomarkers would allow intensification of treatment for high risk GVHD patients and reduction of therapy for low or standard risk patients. There are multiple ongoing clinical trials for novel treatment agents for aGVHD, and predictive biomarkers could be used as an enrichment factor or trial eligibility for additional aGVHD therapies. Using these biomarkers, a subset of aGVHD patients who might benefit most from novel second-line agents can be enrolled.
Response Biomarkers
According to the FDA-NIH definition, a response biomarker is measured pre- and post-initiation of therapy to evaluate response to the treatment. A few of the aGVHD biomarkers have shown potential as response biomarkers for first-line aGVHD treatment. ST2 levels measured 14 days after starting systemic steroids was able to predict treatment failure by day 56 23. In the same study, the ability to predict therapy failure improved with the addition of TIM3 values 23.
Defining steroid-refractory aGVHD relies on a clinician’s objective assessment and there is no standard of care on when to initiate second-line therapies. If improvement is noted, there is also no standard of care for the duration of therapy or taper rate of steroids. If first-line treatment fails, evidence supports initiating second-line therapy at the early stage may prevent advanced organ injury or development of severe aGVHD 24. In practice, clinicians typically evaluate response at 1 week of treatment to decide on adjusting immunosuppressives. However, early clinical response has a low positive predictive value and does not correlate with long-term outcomes. Once qualified for clinical use, response biomarkers can assist in medication management decisions. For example, an early clinical responder with unchanged ST2 levels is not likely to require escalation of therapy. On the other hand, a patient with no clinical response and increasing ST2 levels will likely require additional therapy. For aGVHD treatment, clinical response at 28 days is currently the primary endpoint of many aGVHD clinical trials. Validated response biomarkers could provide earlier and more complete data for better evaluation of the effectiveness of novel aGVHD therapies.
Prognostic Biomarkers
Prognostic biomarkers for aGVHD are used to evaluate outcomes such as GVHD severity and NRM in patients who have GVHD. Elevated Reg3α at GVHD diagnosis was associated with grade 2–4 GI-GVHD and 1-yr NRM 11 and high levels in the first 21 days post-HCT correlated with NRM in both adult and pediatric patients 25. ST2 values 14 days post-HCT was a better indicator for risk of death than other known risk factors 19, and levels 28 days post-HCT also correlated with 2-year NRM 26. ST2 concentrations have also shown utility in alternative allo-HCT settings including cord blood transplant and haplo-HCT with PTCy 27, 28. Like Reg3α, elevated ST2 was associated with increased NRM in both adult and pediatric cohorts 25. Elafin levels was not prognostic of 6-month NRM in a contemporary cohort 29. At 28 days post-HCT, TIM3 concentrations in addition to ST2, correlated with 2-year NRM 26.
As previously mentioned, the combined values of ST2 and Reg3α were used to create an algorithm as a prognostic biomarker to separate patients into groups with distinctly different 6-month NRM. The algorithm, known as the Magic algorithm probability (MAP), uses biomarker values with increased weight on ST2 at 7 days post-HCT 30. This algorithm was tested and confirmed in a Japanese retrospective cohort of 112 patients 31. The formula has also been applied in two studies at 7 and 28 days after corticosteroid treatment to estimate NRM, and showed patients with higher scores were more likely to die, independent of clinical response 32 33.
A note of caution however when applying an algorithm that utilizes multiple biomarkers and was generated for an alternative purpose. Change may occur in one biomarker while the other biomarker remains the same. This change will impact the algorithm score, so validation of the individual markers or development of a specific algorithm for that outcome is required. Also, it is important to highlight that several of the cited studies incorrectly categorized these biomarkers as risk or response. According to the BEST resource, a biomarker associated with outcomes is more accurately categorized as a prognostic biomarker.
Properly validated prognostic biomarkers can help anticipate the course of disease and assist in clinical management decisions. Acute GVHD grade at diagnosis does not correlate with outcomes. Currently patients receive first-line therapy with high dose corticosteroids which leads to a significant number of undertreated and overtreated patients. Undertreatment can lead to significant morbidity and mortality. Overtreatment can increase a patient’s risk of infection and negatively impact the desirable GVT effect. Patients with GVHD who have lower prognostic biomarker scores may benefit from reduced immunosuppressive treatment.
Risk Biomarkers
According to the NIH-FDA definition, a risk biomarker for aGVHD indicates the risk to develop aGVHD. As discussed in the prognostic section, several studies previously incorrectly labeled biomarkers as risk. Currently, no biomarker that anticipates future aGVHD exists, which represents an important knowledge gap. TIM3 showed potential as a risk biomarker when levels 14 days post-HCT were associated with future grade 3–4 aGVHD with area under the ROC of 0.76, however, the PPV was only 16%, probably due to a grade 3–4 aGVHD incidence of 6.5% 23.
Ideally, an aGVHD risk biomarker would provide information for future clinically significant aGVHD. Once risk biomarkers are discovered and validated, they offer a unique opportunity for aGVHD prevention. These biomarkers could prompt increased surveillance. The data provided by qualified risk biomarkers can also assist in the difficult balance of GVHD and GVT, delaying immunosuppressive weans for patients with high risk of subsequent aGVHD and accelerating weans for patients with high risk of relapse. Risk biomarkers could be utilized as inclusion criteria for preemptive clinical trials.
4.0. Chronic GVHD Biomarkers
Chronic GVHD remains the most important long-term complication of allo-HCT causing significant morbidity, mortality and impact on quality of life. Identification and validation of biomarkers in cGVHD have lagged compared to aGVHD for a variety of reasons including: (a) heterogenous impact on recipient organs, (b) increased time frame of onset and course of the disease and (c) lack of multicenter trials with sufficient number of patients’ samples 34. Additionally, age related differences in the biology of cGVHD may exist 35, 36. A summary of plasma cGVHD biomarkers by subtype is found in Table 5.
Table 5.
Plasma Biomarkers for Chronic GVHD
| Name | Study | (n) | Associations/Timepoints in cGVHD (D0 = HCT date) | Framework steps completed | Potential clinical implementation | Ref |
|---|---|---|---|---|---|---|
| Diagnostic Biomarkers | ||||||
| sBAFF | Sarantopoulos 2007 | 104 | Increased levels in active cGVHD | Step 1: Discovery | • Improve diagnostic accuracy • Differentiate GVHD vs other complications |
39 |
| Ahmed 2015 | 115 | Increased levels at 6 and 12 months | Step 1: Previous discovery | 41 | ||
| Kariminia 2016 | 283 | Increased levels around time of diagnosis | Step 1: Previous discovery Step 2: 2 cohorts |
42 | ||
| Rozmus 2019 | 107 | Increased levels around onset of symptoms | Step 1: Previous discovery | 43 | ||
| CXCL9 | Kitko 2014 | 320 | Increased levels at diagnosis | Step 1: Discovery Step 2: 2 cohorts |
44 | |
| Kariminia 2016 | 85 | Increased levels in 1 replication cohort | Step 1: Previous discovery Step 2: 2 cohorts |
42 | ||
| Hakim 2016 | 95 | Increased levels and upregulation of gene expression | Step 1: Previous discovery | 45 | ||
| CXCL10 | Ahmed 2015 | 115 | Increased levels at 6 and 12 months | Step 1: Hypothesis | 41 | |
| Kariminia 2016 | 283 | Increased levels in both replication cohorts | Step 1: Previous discovery Step 2: 2 cohorts |
43 | ||
| Hakim 2016 | 95 | Increased levels and upregulation of gene expression | Step 1: Hypothesis | 45 | ||
| Biomarker panel: ST2, MMP3, CXCL9, OPN | Yu 2016 | 172 | Increased levels at diagnosis | Step 1: Discovery Step 2: 2 cohorts |
47 | |
| MMP3 | Liu 2016 | 112 | Increased levels in BOS patients | Step 1: Discovery | 48 | |
| DKK3 | Inamoto 2020 | 186 | Increased levels at diagnosis | Step 1: Discovery | 50 | |
| Reg3α | DePriest 2021 | 289 | Increased levels associated with GI-cGVHD | Step 1: Previous discovery Step 2: 2 cohorts |
51 | |
| Predictive Biomarkers | ||||||
| No validated cGVHD predictive biomarker exists | • Intensify for high risk group • Reduce immunosuppression for low/standard risk group |
|||||
| Response Biomarkers | ||||||
| sBAFF | Whittle 2011 | 46 | Increased levels 1 month after ECP predicted response of cutaneous cGVHD | Step 1: Previous discovery | • Monitoring treatment response • Guide GVHD management • Future: Clinical efficacy endpoint |
58 |
| ST2 | Dunavin 2018 | 16 | ST2 levels declined after 2-, 4- and 6-months of ECP | Step 1: Previous discovery | 57 | |
| Prognostic Biomarkers | ||||||
| CXCL9 | Giesen 2020 | 480 | Increased levels at symptom onset associated with severe cGVHD | Step 1: Previous discovery | • Anticipate course of disease • Adjust immunosuppression |
60 |
| DKK3 | Inamoto 2020 | 186 | Increased levels at diagnosis associated with NRM | Step 1: Discovery | 50 | |
| MMP-9 | Inamoto 2021 | 33 | Increased levels at BOS diagnosis associated with OS | Step 1: Discovery | 61 | |
| Reg3α | DePriest 2021 | 289 | Increased levels at GI-cGVHD diagnosis associated with nrm | Step 1: Previous discovery Step 2: 2 cohorts |
51 | |
| Risk Biomarkers | ||||||
| Biomarker panel: ST2, MMP3, CXCL9, OPN | Yu 2016 | 172 | Levels D+100 associated with cGVHD development | Step 1: Discovery Step 2: 2 cohorts |
• Implement preemptive strategies | 47 |
| CXCL9 | Abu Zaid 2017 | 211 | Increased levels D+100 or D+180 associated with cGVHD development | Step 1: Previous discovery Step 2: Clinical trial cohorts |
26 | |
| Dai 2021 | 287 | Increased levels D+28 associated with severe cGVHD development | Step 1: Previous discovery | 64 | ||
| CD163 | Inamoto 2017 | 167 | Increased levels D+80 associated with de-novo cGVHD | Step 1: Previous discovery | 66 | |
4.1. Chronic GVHD Biomarkers by Subtype
Diagnostic Biomarkers
Potential biomarkers to assist in the diagnosis of cGVHD have been discovered. Soluble B-cell activating factor (sBAFF), which plays a role in immune reconstitution and B lymphocyte homeostasis 37, 38, was one of the first biomarkers correlated with cGVHD 39. Multiple studies found sBAFF to be elevated in patients with both early and late onset cGVHD 39–43, and patients who subsequently developed cGVHD had significantly different BAFF/B cell ratios at 3 months post-HCT 38. Of note, studies have shown that treatment with corticosteroids can impact sBAFF levels and total B cell number, questioning the diagnostic utility of sBAFF for some cGVHD patients 39, 43.
Chemokine (C-X-C motif) ligand 9 (CXCL9) and chemokine (C-X-C motif) ligand 10 (CXCL10) are inflammatory chemokines involved in the activation and recruitment of various immune cells. Increased CXCL9 concentrations were found in patients with new onset cGVHD 44 and in cGVHD patients at 6 and 9 months post-HCT 41. A gene expression study also found upregulation of CXCL9 and CXCL10 genes along with elevated plasma levels at the onset of cGVHD 45. A follow-up study showed CXCL9 and CXCL10 significantly correlated with cGVHD in one replication cohort, but only CXCL10 in the second 42. It is important to highlight that a study found viral infections such as with cytomegalovirus could impact levels of pro-inflammatory biomarkers such as CXCL10 46.
A 4-biomarker panel consisting of ST2, CXCL9, matrix metalloproteinase-3 (MMP-3), and osteopontin (OPN) had significant correlation with cGVHD diagnosis 47. MMP3 plasma concentrations were individually analyzed and found to be significantly different in patients with and without BOS 48.
Dickkopf-related protein 3 (DKK3) is a modulator of Wnt signaling pathways and involved in pathologic fibrosis and autoimmunity 49. DKK3 was first identified as a potential cGVHD diagnostic biomarker of sclerotic skin cGVHD by proteomics, but elevated levels were associated with any subcategory of cGVHD, suggesting it might be more of a systemic biomarker 50. Reg3α, the most validated GI-aGVHD biomarker, remains underexplored as a potential GI-cGVHD marker. Reg3α concentrations were found to be correlated with GI-cGVHD 51 and warrants further prospective evaluation.
The NIH cGVHD Consensus project in 2005 and 2014 provided standardization of cGVHD diagnosis, however implementation of the criteria continues to be a challenge 52, 53. There is currently no standard of care for post-transplant visits, so the diagnostic evaluation in some locations may be performed by a clinician with limited transplant and GVHD experience. Additionally, early signs and symptoms may not be diagnostic for cGVHD and a patient could have irreversible organ damage prior to meeting the cGVHD diagnostic criteria 40. Current evaluation tools are also unable to differentiate active cGVHD from cumulative cGVHD damage. Once qualified for clinical use, diagnostic biomarkers could help simplify and standardize cGVHD diagnosis. Diagnostic biomarkers could also lead to earlier diagnosis and treatment, which has been shown to reduce the impact of cGVHD 54.
Predictive Biomarkers
To date, no predictive biomarker to anticipate cGVHD treatment response exists representing a major unmet need. Currently, only about 50% of cGVHD patients respond to steroids and prognosis for steroid-refractory cGVHD (SR-cGVHD) remains very poor 55. Per the FDA-NIH definition, a predictive biomarker is assessed specifically prior to each treatment. Priority should be placed on identifying predictive biomarkers for 1st line therapy. Once validated, these biomarkers will help identify patients at highest risk for SR-cGVHD and help select initially steroid dosing. Thankfully, newer agents for SR-cGVHD such as ruxolitinib and ROCK2 inhibitor belumosudil have been FDA-approved 55, 56, and predictive biomarkers for each of these treatments can be established. This would eventually allow a personalized treatment approach to cGVHD, which may offer an opportunity to reduce the impact of clinically significant cGVHD prior to irreversible organ damage.
Response Biomarkers
Currently, studies to identify biomarkers that show a biologic response to cGVHD are lacking. A limited sample size study measured ST2 levels prior to and at 2-month intervals of extracorporeal photopheresis (ECP), a second-line treatment modality for cGVHD. The study found ST2 levels declined over the course of treatment, however all patients had favorable response to ECP, so the study was unable to correlate ST2 with disease activity or outcomes 57. Another study found sBAFF levels at 1 month after ECP predicted response of cutaneous cGVHD at 3 and 6-months 58.
A biomarker analysis of patients receiving Ibrutinib, a kinase inhibitor, found a reduction in multiple inflammatory biomarkers, including CXCL9 and CXCL10, after initiation of therapy and showed a continued downtrend at subsequent time points 59. Once validated, response biomarkers could also serve as an early indicator of response and clinical efficacy endpoints for novel cGVHD treatment clinical trials.
The NIH cGVHD consensus response criteria require a subjective evaluation and has potential for bias. Response biomarkers could help standardize the disease response evaluation, which would be particularly useful in cGVHD clinical trials that rely on accurate clinical data. Response biomarkers that precede clinical improvement could provide valuable information for management decisions. This would be especially helpful in therapies such as ECP where clinical improvement may not be evident for several weeks. Currently, there is no standard of care on timing to initiate second-line cGVHD treatment. Recognizing treatment failure early could help initiate alternative agents which may prevent progressive cGVHD damage.
Prognostic Biomarkers
Prognostic biomarkers to predict severe cGVHD and long-term outcomes have been identified. Elevated CXCL9 concentrations measured at onset of non-severe cGVHD symptoms were associated with subsequent development of severe cGVHD 60. Increased DKK3 concentrations in newly diagnosed cGVHD patients were associated with increased NRM 50. A study of a small cohort of BOS patients investigated matrix metalloproteinase-9 (MMP-9), a protein shown to contribute to neutrophil migration to the lungs, and found increased concentrations at diagnosis were associated with worse overall survival 61. Another study found high Reg3α levels at time of onset of cGVHD were prognostic of increased NRM 51.
Distinguishing cGVHD patients who will have mild disease from those who will develop severe cGVHD would impact management decisions. Mild forms of cGVHD have been associated with increased overall survival due to an increase GVT effect 62, but severe forms can lead to significant morbidity and mortality 63. Prognostic biomarkers could help identify patients at the highest risk for severe cGVHD, leading to more aggressive therapies upfront. These patients could potentially benefit from starting a second-line cGVHD therapy in addition to systemic steroids. Conversely, patients at low risk for severe disease may benefit from decreased initial treatment, which would limit the impact on the GVT effect 54.
Risk Biomarkers
Unlike for aGVHD, risk biomarkers that predict future cGVHD have been identified. The 4-biomarker panel consisting of ST2, CXCL9, MMP-3, and OPN when measured at 100 days post-HCT, at least 3 months before the clinical diagnosis of cGVHD, was able to stratify patients more likely to develop cGVHD 47. A multicenter phase 3 trial of patients found CXCL9 levels at either 100 or 180 days post-HCT correlated with subsequent cGVHD development 26. An additional study found CXCL9 concentrations and genetic polymorphisms in CXCR3 ligands as early as 28 days post-HCT were correlated with the risk of severe cGVHD 64. CD163 is a scavenger receptor shed by activated monocytes/macrophages during times of oxidative stress 65. CD163 concentrations at 80 days post-HCT were associated with subsequent de novo-onset cGVHD 61. Of note, no association was found with subsequent quiescent-onset cGVHD, suggesting CD163 concentrations may be influenced by aGVHD and its treatment 66.
As with risk biomarkers for aGVHD, qualified risk biomarkers could offer an opportunity for cGVHD prevention. Currently, there is marked heterogeneity in discontinuation of immunosuppressants after HCT 67. Data provided by risk biomarkers could help personalize strategies for adjusting these medications, such as early taper of prophylactic immunosuppression in low risk patients, allowing improved immune reconstitution. Similar to aGVHD, qualified cGVHD risk biomarkers could provide inclusion criteria for preemptive clinical trials, identifying patients would potentially benefit most from these interventions.
5.0. Clinical implementation successes and challenges
An important achievement towards clinical application of GVHD biomarkers is the rapid turn-around for real time biomarker analysis by commercial laboratories for ST2 and REG3α. The next steps include incorporation of biomarkers into randomized clinical trials and finally clinical practice. Currently however, no biomarker for either acute or chronic GVHD have completed the qualification step. With that being said, BMTCTN 1501 (NCT02806947) a randomized phase II multicenter trial, was a positive study evaluating sirolimus and prednisone in patients with Minnesota standard-risk and low biomarker score aGVHD. The study aimed to create risk-adapted GVHD therapy at GVHD onset based on clinical parameters and prognostic biomarker values, and found that patients with ‘standard risk aGVHD’ who received sirolimus had similar outcomes to those who received corticosteroids 68. This study highlights the potential for biomarkers to help accurately identify GVHD patients that can be treated with a steroid-free regimen. Two additional studies attempted to use risk/prognostic biomarkers early post-HCT, however these studies showed no impact on patient outcomes 22, 69. The studies found no difference in the treatment vs control groups, which may be due to the poor sensitivity/specificity of the biomarker or from the treatment itself.
When compared to aGVHD, biomarkers for cGVHD are even less along the path to clinical application, although the NIH consensus criteria have helped provide a framework for a unified approach to cGVHD 4, 40, 54, 63, 70, 71. Chronic GVHD biomarker levels are more difficult to validate due to overlap syndrome, infections, recipient chimerism and outpatient sample processing 4. Unlike aGVHD, no comprehensive biorepository for cGVHD samples allowing for discovery of predictive, response and risk biomarkers exists. Many of the promising cGVHD biomarkers require validation in large independent cohorts.
Conclusion
Although a significant amount of research focus has been dedicated to GVHD biomarkers, no risk biomarker for aGVHD or predictive biomarker for cGVHD have been identified, representing major unmet needs. Both acute and chronic GVHD biomarkers have a significant number of potential clinical applications, however, there is much work left to be done before GVHD biomarkers can be incorporated in routine clinical practice. The FDA-NIH Biomarker Working Group provided updated definitions, however these biomarkers have not always been used in the proper clinical context according to the BEST subtype definitions. Clinical decision making for GVHD prophylaxis, preemption, treatment, and treatment monitoring requires a delicate balance between GVHD and the GVT effect. Once properly qualified, we believe biomarkers can provide an extra tool to assist with these decisions and will prove to positively impact patient outcomes.
Highlights.
Plasma biomarkers represent potentially non-invasive, objective, and cost-efficient risk stratification of patients with GVHD
Use of the correct biomarker NIH-FDA BEST terminology will forward the GVHD biomarker effort as it will enable physicians in our field and other fields and regulatory authorities to speak the same language
Since no GVHD biomarkers have yet completed the FDA 4-step framework, additional studies are required before GVHD biomarkers qualify for clinical use
No risk biomarker for aGVHD or predictive biomarker for cGVHD has been identified
A randomized phase 2 clinical trial successfully used biomarkers to guide steroid-free GVHD treatment
Acknowledgements:
The authors thank Dr. Michelle Hudspeth for critical reading of our manuscript. This work was supported by National Institutes of Health grants R01CA168814 (to S.P.), R21HL139934 (to S.P.), and R01CA264921 (to S.P.).
Footnotes
Disclosure:
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. S.P. holds a patent on “Biomarkers and assays to detect chronic graft versus host disease” (U.S. Patent #10,571,478 B2).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med. 2017;377:2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med. 2017;377:2565–2579. [DOI] [PubMed] [Google Scholar]
- 3.FDA-NIH. BEST (Biomarkers, EndpointS, and other Tools) Resource: Food and Drug Administration (US); 2016. [PubMed] [Google Scholar]
- 4.Paczesny S, Hakim FT, Pidala J, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biol Blood Marrow Transplant. 2015;21:780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95:691–709. [DOI] [PubMed] [Google Scholar]
- 6.Niu M, Yi M, Li N, Luo S, Wu K. Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp Hematol Oncol. 2021;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e152S–e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kujovich JL. Factor V Leiden thrombophilia. Genet Med. 2011;13:1–16. [DOI] [PubMed] [Google Scholar]
- 9.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao D, Kim YH, Jeong S, et al. Survival signal REG3alpha prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest. 2018;128:4970–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara JL, Harris AC, Greenson JK, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris AC, Ferrara JL, Braun TM, et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood. 2012;119:2960–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen JA, Hanash SM, Tabellini L, et al. A novel soluble form of Tim-3 associated with severe graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka N, Fujioka A, Tajima S, Ishibashi A, Hirose S. Elafin is induced in epidermis in skin disorders with dermal neutrophilic infiltration: interleukin-1 beta and tumour necrosis factor-alpha stimulate its secretion in vitro. Br J Dermatol. 2000;143:728–732. [DOI] [PubMed] [Google Scholar]
- 15.Paczesny S, Braun TM, Levine JE, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2:13ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solan L, Carbonell D, Muniz P, et al. Elafin as a Predictive Biomarker of Acute Skin Graft-VersusHost Disease After Haploidentical Stem Cell Transplantation Using Post-Transplant High-Dose Cyclophosphamide. Front Immunol. 2021;12:516078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George L, Mahabal G, Mohanan E, et al. Limited utility of plasma elafin as a biomarker for skin graft-versus-host disease following allogeneic stem cell transplantation. Clin Exp Dermatol. 2021;46:1482–1487. [DOI] [PubMed] [Google Scholar]
- 18.Reshef R, Saber W, Bolanos-Meade J, et al. Acute GVHD Diagnosis and Adjudication in a Multicenter Trial: A Report From the BMT CTN 1202 Biorepository Study. J Clin Oncol. 2021;39:1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vander Lugt MT, Braun TM, Hanash S, et al. ST2 as a marker for risk of therapy-resistant graftversus-host disease and death. N Engl J Med. 2013;369:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine JE, Braun TM, Harris AC, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2:e21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gergoudis SC, DeFilipp Z, Ozbek U, et al. Biomarker-guided preemption of steroid-refractory graft-versus-host disease with alpha-1-antitrypsin. Blood Adv. 2020;4:6098–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald GB, Tabellini L, Storer BE, et al. Predictive Value of Clinical Findings and Plasma Biomarkers after Fourteen Days of Prednisone Treatment for Acute Graft-versus-host Disease. Biol Blood Marrow Transplant. 2017;23:1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagasia M, Greinix H, Robin M, et al. Extracorporeal photopheresis versus anticytokine therapy as a second-line treatment for steroid-refractory acute GVHD: a multicenter comparative analysis. Biol Blood Marrow Transplant. 2013;19:1129–1133. [DOI] [PubMed] [Google Scholar]
- 25.Rowan CM, Pike F, Cooke KR, et al. Assessment of ST2 for risk of death following graft-versus-host disease in pediatric and adult age groups. Blood. 2020;135:1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu Zaid M, Wu J, Wu C, et al. Plasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCT. Blood. 2017;129:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponce DM, Hilden P, Mumaw C, et al. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. 2015;125:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanakry CG, Bakoyannis G, Perkins SM, et al. Plasma-derived proteomic biomarkers in human leukocyte antigen-haploidentical or human leukocyte antigen-matched bone marrow transplantation using post-transplantation cyclophosphamide. Haematologica. 2017;102:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zewde MG, Morales G, Gandhi I, et al. Evaluation of Elafin as a Prognostic Biomarker in Acute Graft-versus-Host Disease. Transplant Cell Ther. 2021;27:988 e981–988 e987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartwell MJ, Ozbek U, Holler E, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2:e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotta M, Satake A, Yoshimura H, et al. Elevation of Early Plasma Biomarkers in Patients with Clinical Risk Factors Predicts Increased Nonrelapse Mortality after Allogeneic Hematopoietic Stem Cell Transplantation. Transplant Cell Ther. 2021;27:660 e661–660 e668. [DOI] [PubMed] [Google Scholar]
- 32.Major-Monfried H, Renteria AS, Pawarode A, et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood. 2018;131:2846–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinagesh HK, Ozbek U, Kapoor U, et al. The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood Adv. 2019;3:4034–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolff D, Greinix H, Lee SJ, et al. Biomarkers in chronic graft-versus-host disease: quo vadis? Bone Marrow Transplant. 2018;53:832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuvelier GDE, Li A, Drissler S, et al. “Age Related Differences in the Biology of Chronic Graft-Versus-Host Disease After Hematopoietic Stem Cell Transplantation”. Front Immunol. 2020;11:571884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz KR, Kariminia A, Ng B, et al. Immune profile differences between chronic GVHD and late acute GVHD: results of the ABLE/PBMTC 1202 studies. Blood. 2020;135:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. Journal of immunology. 2009;183:3561–3567. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson CA, Sun L, Kim HT, et al. Post-transplantation B cell activating factor and B cell recovery before onset of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitko CL, Pidala J, Schoemans HM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transplant Cell Ther. 2021;27:545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed SS, Wang XN, Norden J, et al. Identification and validation of biomarkers associated with acute and chronic graft versus host disease. Bone Marrow Transplant. 2015;50:1563–1571. [DOI] [PubMed] [Google Scholar]
- 42.Kariminia A, Holtan SG, Ivison S, et al. Heterogeneity of chronic graft-versus-host disease biomarkers: association with CXCL10 and CXCR3+ NK cells. Blood. 2016;127:3082–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozmus J, Kariminia A, Abdossamadi S, et al. Comprehensive B Cell Phenotyping Profile for Chronic Graft-versus-Host Disease Diagnosis. Biol Blood Marrow Transplant. 2019;25:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitko CL, Levine JE, Storer BE, et al. Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood. 2014;123:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hakim FT, Memon S, Jin P, et al. Upregulation of IFN-Inducible and Damage-Response Pathways in Chronic Graft-versus-Host Disease. J Immunol. 2016;197:3490–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhlin M, Mattsson J, Maeurer M. Update on viral infections in lung transplantation. Curr Opin Pulm Med. 2012;18:264–270. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Storer BE, Kushekhar K, et al. Biomarker Panel for Chronic Graft-Versus-Host Disease. J Clin Oncol. 2016;34:2583–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Yue Z, Yu J, et al. Proteomic Characterization Reveals That MMP-3 Correlates With Bronchiolitis Obliterans Syndrome Following Allogeneic Hematopoietic Cell and Lung Transplantation. Am J Transplant. 2016;16:2342–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maruotti N, Corrado A, Neve A, Cantatore FP. Systemic effects of Wnt signaling. J Cell Physiol. 2013;228:1428–1432. [DOI] [PubMed] [Google Scholar]
- 50.Inamoto Y, Martin PJ, Lee SJ, et al. Dickkopf-related protein 3 is a novel biomarker for chronic GVHD after allogeneic hematopoietic cell transplantation. Blood Adv. 2020;4:2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DePriest BP, Li H, Bidgoli A, et al. Regenerating Islet-Derived 3-alpha is a Prognostic Biomarker for Gastrointestinal Chronic Graft-Versus-Host Disease. Blood Adv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duarte RF, Greinix H, Rabin B, et al. Uptake and use of recommendations for the diagnosis, severity scoring and management of chronic GVHD: an international survey of the EBMT-NCI Chronic GVHD Task Force. Bone Marrow Transplant. 2014;49:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuvelier GDE, Nemecek ER, Wahlstrom JT, et al. Benefits and challenges with diagnosing chronic and late acute GVHD in children using the NIH consensus criteria. Blood. 2019;134:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pidala J, Kitko C, Lee SJ, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIb. The 2020 Preemptive Therapy Working Group Report. Transplant Cell Ther. 2021;27:632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N Engl J Med. 2021;385:228–238. [DOI] [PubMed] [Google Scholar]
- 56.Blair HA. Belumosudil: First Approval. Drugs. 2021;81:1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunavin N, Braun MW, Li M, Godwin AK, Abhyankar S, Yankee TM. Biomarker profiling of steroid-resistant chronic GvHD patients undergoing extracorporeal photopheresis demonstrates high ST2 levels at treatment onset and decline during therapy. ADVANCES IN CELL AND GENE THERAPY. 2019;2:e32. [Google Scholar]
- 58.Whittle R, Taylor PC. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host disease to extracorporeal photopheresis. Blood. 2011;118:6446–6449. [DOI] [PubMed] [Google Scholar]
- 59.Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giesen N, Schwarzbich MA, Dischinger K, et al. CXCL9 Predicts Severity at the Onset of Chronic Graft-versus-host Disease. Transplantation. 2020;104:2354–2359. [DOI] [PubMed] [Google Scholar]
- 61.Inamoto Y, Martin PJ, Onstad LE, et al. Relevance of Plasma Matrix Metalloproteinase-9 for Bronchiolitis Obliterans Syndrome after Allogeneic Hematopoietic Cell Transplantation. Transplant Cell Ther. 2021;27:759 e751–759 e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inamoto Y, Martin PJ, Storer BE, et al. Association of severity of organ involvement with mortality and recurrent malignancy in patients with chronic graft-versus-host disease. Haematologica. 2014;99:1618–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams KM, Inamoto Y, Im A, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2020 Etiology and Prevention Working Group Report. Transplant Cell Ther. 2021;27:452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai H, Rachakonda SP, Penack O, et al. Polymorphisms in CXCR3 ligands predict early CXCL9 recovery and severe chronic GVHD. Blood Cancer J. 2021;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Timmermann M, Hogger P. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic Biol Med. 2005;39:98–107. [DOI] [PubMed] [Google Scholar]
- 66.Inamoto Y, Martin PJ, Paczesny S, et al. Association of Plasma CD163 Concentration with De Novo-Onset Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pidala J, Lee SJ, Quinn G, Jim H, Kim J, Anasetti C. Variation in management of immune suppression after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pidala J, Hamadani M, Dawson P, et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial. Blood. 2020;135:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weissinger EM, Metzger J, Schleuning M, et al. A multicenter prospective, randomized, placebo-controlled phase II/III trial for preemptive acute graft-versus-host disease therapy. Leukemia. 2021;35:1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeFilipp Z, Couriel DR, Lazaryan A, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2020 Treatment of Chronic GVHD Report. Transplant Cell Ther. 2021;27:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolff D, Radojcic V, Lafyatis R, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 Highly morbid forms report. Transplant Cell Ther. 2021;27:817–835. [DOI] [PMC free article] [PubMed] [Google Scholar]


