Abstract
Background:
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality among cancer survivors. Hypertension, which is common among cancer survivors with a prevalence of greater than 70% by age 50 potentiates the risk for CVD in a more than additive fashion. [1–5] For example, childhood cancer survivors who develop hypertension may have up to a twelve times higher risk for heart failure than survivors who remain normotensive. Studies have shown that mild valvular disease (28% incidence), cardiomyopathy (7.4%), arrhythmias (4.6%), and coronary artery disease (3.8%) are amongst the most common CVD in childhood cancer survivors.[8] Amongst adolescent and young adult cancer survivors the most common reasons for cardiovascular-related hospital admission are venous / lymphatic disease (absolute excess risk 19%), cardiomyopathy and arrhythmia (15%), hypertension (13%), and ischemic heart disease (12%).[6] Additionally, cancer therapies can increase the risk for both hypertension and CVD.[1, 2] Therefore, early detection and treatment of hypertension is essential to reducing cardiovascular morbidity and mortality among survivors.
Methods:
We present a literature review, which identified over 20 clinical trials, systemic reviews, and meta-analyses (13 clinical trials, 8 systemic reviews or meta-analyses) by searching PubMed, Google Scholar, and the Cochrane Library for relevant articles addressing hypertension in cancer survivors.
Results:
While our understanding of the complex relationship between cancer therapies and CVD has grown significantly over the past two decades, there remains several gaps in knowledge when specifically addressing CVD in the survivor population. This review provides an up-to-date survivor-centered approach to the screening and treatment of hypertension, which considers survivor specific cardiovascular risk, applies guideline directed therapies when appropriate, screens for survivor specific factors that may influence antihypertensive medication selection, and lastly considers the prohypertensive mechanisms of antineoplastic agents as a potential target for antihypertensive medications.
Conclusions:
Screening for and treating hypertension among survivors can promote cardiovascular health in this vulnerable population.
Keywords: cancer survivors, hypertension, Cardio-Oncology
Epidemiology/background
Cardiovascular disease (CVD) is common among cancer survivors, and there is an increased risk of cardiovascular-related death in this population.[1–9] There is also evidence that amongst survivors, hypertension may accentuate the burden of certain types of CVD such as heart failure, coronary artery disease, and stroke.[1, 10] Despite these findings, hypertension, which continues to have a high prevalence in this population may be underdiagnosed and undertreated in cancer survivors.[11–14]
Risk of Cardiovascular Disease
The relative risk for CVD including coronary artery disease, heart failure, and stroke are approximately 10 times greater among childhood cancer survivors (age 0-14 at cancer diagnosis) when compared with siblings.[1, 2] In adolescents and young adults (age 15-39 at cancer diagnosis), the risk for CVD is at least two times greater compared with individuals without a history of cancer.[3] The risk for coronary artery disease, cardiomyopathy, stroke, pericarditis, and venous thromboembolism is also elevated for survivors of adult onset cancer.[7, 8]
Cardiovascular Disease Subtypes
A cross-sectional study of childhood cancer survivors that had undergone systemic screening for cardiovascular disease (median age at cancer diagnosis 8 years, median age at evaluation 31 years) found that valvular disease (28% prevalence, mostly mild in severity), cardiomyopathy (7.4%), conduction / rhythm disorders (4.6%), and coronary artery disease (3.8%) were amongst the most common cardiovascular diseases identified in this population. Of note, the majority of these patients were asymptomatic.[9] A Danish cohort study of >43,000 adolescent and young adult survivors aged 15-39 with a mean follow up of 15 years analyzed CVD at first hospitalization. This data found that amongst survivors of cancer diagnosed in adolescence or young adulthood, the most common reasons for cardiovascular-related hospital admission were venous / lymphatic disease (absolute excess risk 19%), cardiomyopathy and arrhythmia (15%), hypertension (13%), and ischemic heart disease (12%).[6]
Risk of Cardiovascular-Related Death and All-Cause Mortality
CVD is a leading cause of death among survivors. Although there has been some variation in the literature the following studies largely define CVD death as death due to coronary artery disease, stroke, or heart failure. Cardiovascular-related death is approximately seven-fold higher in childhood cancer survivors (CCS) than in age-matched peers, and at least three-fold higher among adolescent and young adult survivors (AYAs).[10, 11] For survivors of adult onset cancer, the risk for all-cause mortality is approximately 4 times greater among those with CVD compared with survivors without CVD.[7]
Effect of Hypertension on the Development of Cardiovascular Disease in Cancer Survivors
Cardiovascular risk factors, particularly hypertension, accentuate the burden of CVD among survivors. Among childhood cancer survivors with hypertension, the risk for congestive heart failure may be at least twelve times greater than among those without hypertension.[1] Additionally, in the setting of treatment with potentially cardiotoxic therapies such as chest radiation and anthracycline exposure, hypertension has been shown to potentiate cardiovascular disease. Previous reviews have described the separate adverse cardiovascular effects that hypertension and cardiotoxic therapies have, and how a two hit phenomenon from hypertension and then subsequent exposure to cardiotoxic therapy may increase the risk of cardiovascular disease. [12] In a landmark study, Armstrong et. al. demonstrated a relative excess risk due to interaction (RERI) between hypertension and previous chest radiation for many serious cardiovascular outcomes including coronary artery disease (RERI 24.2) and heart failure (RERI 41.4)[1]. Hypertension also increases the risk for heart failure in a more than additive fashion among CCS with a previous history of anthracycline exposure (RERI 44.5).[1] This effect has also been observed among AYAs, in which the development of hypertension has been shown to increase the risk for coronary artery disease, heart failure, or stroke approximately 4 fold.[3] For adult-age stem cell transplant recipients, the presence of hypertension increases the risk for congestive heart failure by 3.5-fold.[13] In the setting of high-dose anthracycline exposure (≥ 250 mg/m2) the risk is increased 35-fold.[13]
Burden of Hypertension Amongst Cancer Survivors
While cancer treatment history is not a readily modifiable cardiovascular risk factor among survivors, hypertension is common and modifiable. CCS are two times more likely to use anti-hypertensive medications when compared with siblings.[14] In the setting of adult onset cancer, approximately one-third of patients develop hypertension either during or after cancer treatment.[15]
Despite the significant impact of hypertension on cardiovascular health and mortality among survivors, hypertension is often under diagnosed or inadequately treated in this population. Among childhood, adolescent, and young adult survivors, 69% report not receiving follow-up care for late effects.[16] Gibson et. al. found 8% of CCS had previously undiagnosed hypertension, and among those with a diagnosis of hypertension, 22% were uncontrolled.[5]
Screening
Several survivorship guidelines support identifying and treating hypertension. For survivors of childhood cancer, the Children’s Oncology Group recommendations are based on treatment exposure. Annual blood pressure screening is recommended for survivors with a history of exposure to nephrotoxic agents including ifosfamide, cisplatin, and carboplatin. Higher cumulative doses of these agents, a history of nephrectomy, combination with other nephrotoxic agents, and kidney radiation (particularly ≥ 15 Gy) increase the risk for hypertension. Survivors with a history of nephrectomy should also undergo annual screening. In these survivors, bilateral Wilms tumor, exposure to additional nephrotoxic agents, or radiation impacting the kidney increase risk for hypertension. Annual screening is also recommended for survivors treated with head, neck, chest, and spine radiation. Of note, bilateral upper extremity blood pressures should be performed in survivors treated with neck, chest, or spine radiation to screen for subclavian disease. Annual screening should also be performed among survivors with a history of abdominal radiation, total body irradiation, hematopoietic cell transplant, or anthracycline exposure.[17]
The National Comprehensive Cancer Network recommends annual blood pressure screening for AYAs at risk for renovascular hypertension. Risk factors include ≥10 Gy of radiation, and history of exposure to the combination of radiation and nephrotoxic agents (cisplatin, ifosfamide, aminoglycosides, amphotericin, immunosuppressants), or hematopoietic cell transplantation. Blood pressure screening is also recommended for survivors who received ≥ 15 Gy of radiation combined with anthracycline therapy, mediastinal/chest radiation ≥35 Gy alone, abdominal radiation, or total body irradiation.[18]
For survivors of adult-onset cancer, the National Comprehensive Cancer Network recommends screening throughout the continuum of survivorship and counseling on factors that may increase the risk for cardiovascular disease.[19] Similarly, the American Society of Clinical Oncology recommends comprehensive assessment of CVD risk factors, including hypertension.[20] Of note, the United States Preventive Services Task Force recommends blood pressure screening for all individuals over the age of 18, and suggests annual screening for those 40 or older, or at increased risk for developing hypertension.[21]
While measurements of blood pressure in the clinical setting can be helpful in identifying hypertensive survivors, ambulatory or home blood pressure monitoring should also be considered. This out-of-office monitoring can be helpful in identifying white coat hypertension and masked hypertension. Although limited, there is evidence among individuals receiving active treatment to suggest that white coat hypertension and masked hypertension are common.[22] Additionally, current guidelines support screening for masked hypertension among individuals with consistent clinic blood pressures between 120-129/75-70 mmHg.[23] Specific blood pressure targets among cancer survivors have not been established, however given the data to support an adversely synergistic relationship between hypertension and CVD among survivors, more aggressive blood pressure goals than those recommended for the general population may be warranted.
Effects of cancer therapy
Several cancer therapies have been associated with the development of hypertension either during the acute treatment period, or years after the completion of therapy. Here we will discuss proposed mechanisms for the association of hypertension with common cancer therapies. Table 1 lists cancer treatments that may be associated with the development of hypertension.
Table 1.
Antineoplastic treatments that have been associated with hypertension, common examples, and potential mechanisms for the development of hypertension
| Therapeutic Class | Examples | Potential Mechanism Leading to Hypertension |
|---|---|---|
| VEGF Inhibitors | Bevacizumab Lenvatinib Pazopanib Ramucirumab Sorafenib Vandetanib |
Glomerular injury, microvascular rarefaction, increased vascular stiffness, decreased NO production, increased endothelin-1 activation, aberrations of the RAAS system |
| BRAF Inhibitors | Encorafenib Vemurafenib Dabrafenib |
Aberrations of the RAAS system, decreased NO production |
| MEK Inhibitors | Trametinib | Aberrations of the RAAS system, decreased NO production |
| BTK Inhibitors | Acalabrutinib Ibrutinib |
Vascular tissue fibrosis, decreased NO production |
| Androgen Deprivation Therapy | Abiraterone | Increased synthesis of mineralocorticoid precursors |
| Anthracyclines / Anthracenedione | Doxorubicin Daunorubicin Epirubicin Idarubicin Mitoxantrone |
Reduced capillary density, impaired neovascularization, tunica intima hyperplasia, luminal stenosis, smooth-muscle-cell dysfunction, decreased NO production |
| Alkylating agent | Ifosfamide | Increased risk of renal dysfunction |
| Platinum Based Chemotherapy | Carboplatin Cisplatin |
Renal toxicity and chronic endothelial cell activation |
| Anti-microtubule Agents (Taxanes) | Paclitaxel | May alter sympathetic control of blood pressure |
| Anti-microtubule Agents (Vinka Alkaloids) | Vinblastine Vincristine |
Mitosis mediated inhibition of endothelial cell proliferation |
| Antimetabolites | Gemcitabine | Thrombotic microangiopathy and nephrotoxicity |
| Radiation therapy | Abdominal radiation Head and neck radiation |
Renal artery stenosis (rare) Disturbances of the baroreflex |
Acute Hypertension
Vascular Endothelial Growth Factor Inhibition
Vascular endothelial growth factor inhibitors (VEGFi) are commonly used in the treatment of a wide variety of cancers, often in the metastatic setting. Hypertension is the most common cardiovascular toxicity associated with VEGFi, and occurs in 20 to 90% of adult patients, depending on the specific VEGFi used.[24] Elevations in blood pressure can occur as quickly as 24 hours after initiation of VEGFi, typically plateau within days to weeks, and often resolve within weeks of medication withdrawal.[25, 26] The precise mechanisms underlying VEGFi mediated hypertension have not been fully defined, however microvascular rarefaction, increased vascular stiffness, glomerular injury, decreased nitric oxide production, increased endolelin-1 activation, and aberrations of the renin-angiotensin-aldosterone system (RAAS) have all been implicated. [27–31] A recent article published in Hypertension by Mäki-Petäjä, et al. used hemoglobin video imaging to determine the density and diameter of conjunctival and episcleral microvasculature before and after treatment with the VEGFi Pazopanib, which revealed a reduction in scleral microvascular density by −15.5%. This same study also utilized central arterial pressure waveform analysis to assess arterial stiffness and found an increase in arterial stiffness after treatment with Pazopanib.[32] These findings lend increased evidence to microvascular rarefaction and increased arterial stiffness as major driving factors for the development of hypertension in patient’s treated with VEGFi.
BRAF / MEK Inhibition
Similarly, targeted therapy with BRAF or MEK inhibitors may cause acute hypertension. The METRIC trial, which compared the MEK inhibitor Trametinib to chemotherapy for the treatment of BRAF mutated melanoma found a 12% incidence of grade 3 hypertension in the Trametinib group and a 3% incidence of grade 3 hypertension in the chemotherapy group.[33] This effect may be more severe when BRAF and MEK inhibitors are used in combination. For example, the COMBI-d trial, which evaluated the efficacy of combination therapy with the BRAF inhibitor Dabrafenib and the MEK inhibitor Trametinib for melanoma showed an incidence of any grade hypertension of 14% with Dabrafenib, which rose to an incidence of 22% in patients taking a combination of Dabrafenib and Trametinib.[34] The mechanism for BRAF / MEK inhibitor associated hypertension is not well understood. It has been postulated that multi-kinase inhibitors that inhibit BRAF as well as VEGFi may increase the risk of hypertension by reducing the bioavailability of nitric oxide, but it is unclear to what degree this effect is related to concomitant VEGF inhibition.[35]
Bruton tyrosine kinase Inhibition
Bruton tyrosine kinase (BTK) inhibitors have revolutionized the treatment of several B cell malignancies, including chronic lymphocytic leukemia (CLL). Ibrutinib, a first in class BTK inhibitor, has been associated with an almost 3-fold increased risk of grade 3-4 hypertension with a median time of treatment initiation to the development of new onset hypertension of 4-5 months. [36–38] The Alliance study published in 2021 found a cumulative incidence of Common Terminology Criteria for Adverse Events (CTCAE) grade ≥ 3 hypertension of 17.5% at 12 months, and 25.4% at 36 months. [39] Of note, although most new onset high-grade adverse effects observed with Ibrutinib decline over time, the onset of new grade ≥ 3 hypertension remained relatively consistent at 7 year follow up.[40] Additionally, the development of hypertension after treatment with Ibrutinib has been associated with an increased risk of MACE, while subsequent antihypertensive initiation may be associated with a decreased risk of MACE. [38] While the exact mechanism is not clear, there is evidence that P13K inhibition leads to increased vascular fibrosis in the atria, but it is not clear to what degree this may affect other vasculature.[41] There is also evidence that this hypertensive effect may be due to downregulation of nitric oxide production and endothelial dysfunction.[42, 43]
Second generation Bruton tyrosine kinase inhibitors have less off target effects, and consequently have been found to have fewer adverse effects. The ASPEN trial, which compared Ibrutinib with the second generation Bruton tyrosine kinase inhibitor Zanubritinib for the treatment of symptomatic Waldenstrom macroglobulinemia revealed a 5% higher incidence of grade CTCAE ≥ 3 hypertension with Ibrutinib when compared to Zanubritinib.[44] Acalabrutinib is another second generation Bruton tyrosine kinase inhibitor that has been associated with the development of hypertension. Pooled data from 4 different trials in patients receiving Acalabrutinib for CLL showed a 9% incidence of hypertension being reported as an adverse effect. However, of these patients, 69% had pre-existing hypertension. An eagerly anticipated randomized phase III trial comparing Acalabrutinib with Ibrutinib in previously treated CLL, found an 9% incidence of all grade hypertension and 4% incidence of ≥ 3 hypertension in patients receiving Acalabrutinib compared to a 23% incidence of all grade hypertension and a 9% incidence of ≥ 3 hypertension in patients treated with Ibrutinib.[45] This recently published data further supports the lower incidence of hypertension with second generation Bruton tyrosine kinase inhibitors when compared to first generation agents.
Androgen Deprivation Therapy
Patients with prostate cancer are often treated with androgen deprivation therapy via gonadotropin-releasing hormone agonism and antagonism, androgen receptor inhibition, and CYP17 (cytochrome P450 17A1) inhibition. Enzalutamide (an androgen receptor antagonist) and abiraterone (a CYP17 inhibitor) are associated with hypertension.[46, 47] The specific mechanism leading to hypertension in the setting of enzalutamide use is unclear. In the case of abiraterone, CYP17 inhibition resulting in decreased cortisol synthesis and a subsequent increase in synthesis of mineralocorticoid precursors, is thought to result in hypertension, edema, and hypokalemia.[48] Prednisone is often co-administered with abiraterone as glucocorticoid replacement therapy and the incidence of hypertension is as high as 20% in patients prescribed Abiraterone plus prednisone.[49, 50]
Late Hypertension
Anthracyclines
Anthracyclines are a backbone of therapy for a variety of malignancies. There is now mounting evidence that anthracyclines may directly increase a patient’s risk of developing hypertension. Potential mechanisms include reduced capillary density, impaired neovascularization, histologic changes to vasculature including tunica intima hyperplasia, luminal stenosis, and smooth-muscle-cell loss.[51–53] Additionally vasomotor dysfunction resulting from reduced endothelial NO synthase activity, which leads to a reduction of nitric oxide in the endothelial cells potentially contributes to hypertension.[54, 55]
Alkylating and Alkyl-like Agents
The alkylating agent ifosfamide is known to increase the risk for renal dysfunction among long-term survivors and therefore may be a risk factor for the development of hypertension.[56, 57] Cyclophosphamide has been associated with vascular toxicities including cerebrovascular events, myocardial ischemia, pulmonary hypertension, and systemic hypertension, which are thought to be related to endothelial injury.[58, 59] However, a direct link with an increased risk for hypertension has not been established.
Platinums, or alkyl-like agents, are also associated with vascular toxicities, including hypertension. Renal toxicity and chronic endothelial cell activation are thought to be potential mechanisms leading to the development of hypertension.[60–62] Of importance to the survivor population is the fact that hypertension in platinum based chemotherapy recipients tends to be a long-term effect, and can be present years after therapy.[63] In fact, platinum based chemotherapeutic agents have been detected in the blood of survivors >10 years after therapy and hypertension is associated with higher long-term platinum concentrations.[64]
Antimicrotubule agents
Antimicrotubule agents interrupt mitosis thereby blocking cell proliferation. Vincristine and Vinblastine are vinka alkaloid anti-microtubule agents that have been associated with hypertension. Although the mechanism is unclear, mitosis mediated inhibition of endothelial cell proliferation has been proposed as a possible mechanism that may lead to the development of vascular dysfunction in patient’s treated with Vinka alkaloids. [65] Vincristine and Vinblastine are often used in combination with several other therapies, and there may be confounders that lead to their association with the development of hypertension. [66]
Another well-known group of antimicrotubule medications are Taxanes including Paclitaxel and Docetaxel. There is some limited evidence that taxane medications may be associated with hypertension although this is most commonly seen when treatment is combined with a VEGF inhibitor such as Bevacizumab. [67, 68] The mechanism for Taxane induced hypertension is not well understood, but Paclitaxel may alter the sympathetic control of blood of pressure. [69]
Antimetabolites
The antimetabolite Gemcitabine interferes with deoxyribonucleic acid production. There is evidence that Gemcitabine may cause hypertension via thrombotic microangiopathy and nephrotoxicity. [70]
Radiation therapy
Radiation therapy has been associated with the development of hypertension. [14, 71] Potential mechanisms for the development of hypertension may be site specific. For example, abdominal radiation can rarely cause renal artery stenosis, and head and neck radiation can lead to labile hypertension via disturbances in the baroreflex. [72, 73] One retrospective study showed a particularly high incidence of radiation associated hypertension of 47% in patients undergoing prostate irradiation. The majority of these cases of hypertension developed while patients were in the treatment phase of prostate irradiation.[74] The importance of radiation induced hypertension is underlined by the findings of Armstrong, et. al. who demonstrated that survivors who previously received radiation therapy and then subsequently developed two or more risk factors that included hypertension may have a substantially elevated risk of grade ≥ 3 coronary artery disease, heart failure, and valvular heart disease. This risk was greater than would be expected by simply adding the risk of each of these factors together, which suggests an adversely synergistic relationship between previous radiation exposure and the subsequent development of hypertension.[1] Although contemporary radiation techniques have greatly reduced radiation exposure during therapy, it is important to monitor survivors previously treated with radiation therapy to detect and treat hypertension early.
Treatment
Patients with cancer and survivors are poorly represented in most major trials that established the current approach to the treatment of hypertension.[75, 76] Established guidelines have thus far focused on hypertension during the acute phase of treatment and have not directly addressed the survivor population.[77, 78] Future guidelines may directly address hypertension in the survivor population, but until then we advocate for a systematic approach that considers the unique clinical characteristics of cancer survivors (Figure 1).
Figure 1.
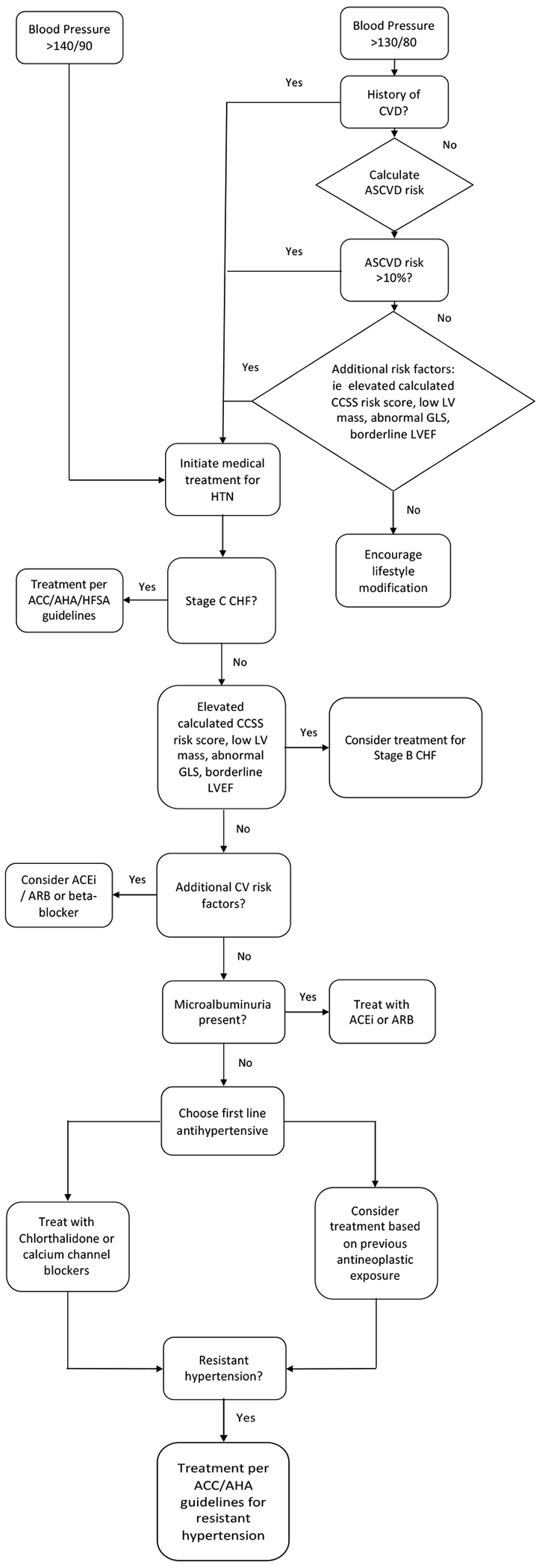
Flow diagram of a proposed strategy for hypertension management among cancer survivors
As previously described, the survivor population is at an increased risk for the development of hypertension and CVD [9,10,11], and hypertension increases the risk for subsequent CVD even more so among survivors than the general population.[1] Therefore, it is important to further delineate each survivor’s individual risk profile. Chen, et. al. have created an online risk calculator to predict the risk of heart failure, ischemic heart disease, and stroke by age 50 in the CCS/AYA survivor population. This calculator has now been validated in several survivor populations (ccss.stjude.org/cvcalc).[79] This risk calculator does have some weaknesses including the fact that <20% of the patients used to construct the model were minorities and it does not include tobacco use or obesity status. However, other more traditional risk calculators such as the Atherosclerotic cardiovascular disease (ASCVD) may underestimate risk in the survivor population since the potential deleterious cardiovascular effects of both cancer and cancer treatment are not accounted for.
Non-pharmacologic therapy including a heart-healthy diet, weight loss for overweight or obese individuals, sodium reduction, potassium supplementation (ideally through dietary means), increased physical activity, and limited alcohol intake should be encouraged among survivors with hypertension as well as survivors with an increased risk for developing hypertension.[23]
It is appropriate to consider pharmacologic therapy among survivors with a blood pressure ≥ 140/90. Additionally, pharmacologic therapy should also be initiated among individuals with a blood pressure ≥ 130/80 mm Hg who either have a history of CVD or an ASCVD risk score ≥ 10%.[23] In the survivor population, we advocate for extending this recommendation to high risk individuals based on the childhood cancer survivor study cardiovascular risk calculator or other cancer diagnosis and treatment related factors. For example, given the strong relationship between low indexed left ventricular cardiovascular mass and risk for adverse cardiovascular events, it may be reasonable to factor LV mass into the decision to initiate antihypertensive therapy.[80]
When selecting antihypertensive agents, consider whether the patient has another indication for a specific antihypertensive class. As discussed above, certain chemotherapeutic agents can cause hypertension due to renal damage. There is also some data suggesting elevated urine albumin to creatinine ratio may be associated with diastolic hypertension in childhood cancer survivors. [81] An ACE inhibitor or ARB may be appropriate among individuals with microalbuminuria. Patients with left ventricular dysfunction should be treated with appropriate medications such as beta-blockers, ACE/ARB, ARNI, spironolactone, etc. as outlined by the ACC/AHA/HFSA guidelines for management of heart failure.[82] During cancer treatment, an absolute reduction in global longitudinal strain has been associated with an increased risk for subsequent heart failure.[83, 84] In this setting, treatment with ACE/ARB or beta blocker reduces the risk for a significant decline in left ventricular ejection fraction.[85] Therefore, it may also be reasonable to consider global longitudinal strain measurements when choosing antihypertensive therapy. Thiazide diuretics, particularly chlorthalidone, are commonly used as a first line agent for the treatment of hypertension, but are often avoided in patients that are actively receiving chemotherapy due to the increased risk of dehydration or electrolyte abnormalities during the acute treatment phase. Long term survivors are often not at the same risk of these adverse events, and thiazide diuretics as well as calcium channel blockers can be considered for the treatment of hypertension if none of the above indications favor a different drug class for initial treatment. Similar to patients without a history of cancer, consideration can be made for the use of mineralocorticoid receptor antagonists and alpha antagonists in survivors with resistant hypertension who are already on multiple antihypertensive medications.[86]
Lastly, particular attention should also be paid to the antineoplastic treatments that the patient has been exposed to as these medications may help to establish a potential mechanism for the development of hypertension, which can be useful when choosing initial antihypertensive therapy if there are no other clear indications for a certain anti-hypertensive class. For example, patients who develop hypertension after receiving antineoplastic agents that have been hypothesized to cause hypertension by mechanisms that lead to vascular dysfunction such as decreased NO bioavailability may benefit from medications that reduce smooth muscle contraction.
Data regarding the use of a particular antihypertensive medication to treat a specific form of antineoplastic induced hypertension is currently limited, but one example includes a retrospective study that showed improved overall survival in patients treated with angiotensin converting enzyme inhibitors or angiotensin receptor blockers who were diagnosed with hypertension while undergoing treatment with tyrosine kinase inhibitors.[87] There is also limited data to suggest that inulin supplementation may reduce systolic blood pressure and prevent diastolic hypertension in women who received neoadjuvant treatment with doxorubicin and cyclophosphamide for breast cancer. This suggestion comes from a randomized control trial, but included a small study population (n=38).[88] Nonetheless, it raises the possibility that other non-pharmacologic treatment may one day prove to be beneficial for patient’s treated with pro-hypertensive antineoplastic agents.
In summary, to optimize the treatment of hypertension in cancer survivors, practitioners must first understand that survivors are at an increased risk for CVD and that traditional risk calculators may underestimate cardiovascular risk in this population. While we advocate for the continued use of traditional risk calculators, we also encourage the supplemental use of survivor specific risk calculators such as the St Jude CCS risk calculator (ccss.stjude.org/cvcalc). Survivors with elevation of any of these risk scores or with other guideline directed indications for more strict blood pressure control would likely benefit from a blood pressure goal of <130/80. When deciding on which antihypertensive medication to initiate we suggest that established guidelines such as those for the treatment of systolic heart failure be applied when appropriate. If there is no guideline directed therapy that applies to the patient, practitioners can consider screening for microalbuminuria and initiating ACEi/ARBs if present or choosing ACEi/ARBs or beta-blockers if there is a reduction in global longitudinal strain. In the absence of any strong indication for a particular antihypertensive medication, survivors should be treated with Class 1 antihypertensive medications such as thiazide diuretics, ACEi, ARBs, or calcium channel blockers if there are no contraindications. It may also be reasonable to review the mechanisms by which a particular antineoplastic agent may induce hypertension and choose antihypertensive medications that may act against this pro-hypertensive mechanism (Figure 1).
Monitoring for Treatment Success
Ambulatory or at home blood pressure monitoring can be helpful not only for the diagnosis of hypertension, but also to ensure that blood pressure goals are being met. Factors that often exacerbate hypertension such as anxiety and chronic pain are common in the survivor population, and sporadic hypertension (such as white coat hypertension) still portends a significant cardiovascular risk.[89–91] Therefore, methods to monitor blood pressure outside of the office may ensure that hypertension is controlled throughout the day when such factors are contributors to blood pressure fluctuation.
Conclusion
Despite efforts to understand how different antineoplastic treatments may increase the risk for hypertension many of these mechanisms are not fully defined, and there is a paucity of clinical trials evaluating the use of specific antihypertensive medications in the survivor population. Survivor populations are heterogeneous with a wide range of prior treatment exposures, and it is likely that diagnosis and treatment for hypertension may need to be tailored to different subsets of the survivor population. Further investigation is clearly required, but this review offers an up-to-date, cancer survivor-centered analysis of the available literature and provides an approach that can be easily adopted by any clinician to treat survivors that develop hypertension.
Acknowledgments
This publication is supported in part by the To-morrow’s Research Fund St. Baldrick’s Scholar Award (Award Number 636214). The content is solely the responsibility of the authors and does not necessarily represent the official views of St. Baldrick’s Foundation.
This publication is supported in part by the American Heart Association (Award Number (19CDA34760181). The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association.
The research reported in this publication was supported by CTSA award No. KL2TR002648 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare no conflicts of interest
Ethical approval: No new data was collected during the writing of this review. IRB approval was not required
References
- 1.Armstrong GT, et al. , Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol, 2013. 31(29): p. 3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oeffinger KC, et al. , Chronic Health Conditions in Adult Survivors of Childhood Cancer. New England Journal of Medicine, 2006. 355(15): p. 1572–1582. [DOI] [PubMed] [Google Scholar]
- 3.Chao C, et al. , Cardiovascular Disease Risk Profiles in Survivors of Adolescent and Young Adult (AYA) Cancer: The Kaiser Permanente AYA Cancer Survivors Study. J Clin Oncol, 2016. 34(14): p. 1626–33. [DOI] [PubMed] [Google Scholar]
- 4.Afifi AM, et al. , Causes of death after breast cancer diagnosis: A US population-based analysis. Cancer, 2020. 126(7): p. 1559–1567. [DOI] [PubMed] [Google Scholar]
- 5.Gibson TM, et al. , Blood Pressure Status in Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev, 2017. 26(12): p. 1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rugbjerg K, et al. , Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943-2009. J Natl Cancer Inst, 2014. 106(6): p. dju110. [DOI] [PubMed] [Google Scholar]
- 7.Armenian SH, et al. , Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. Journal of Clinical Oncology, 2016. 34(10): p. 1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strongman H, et al. , Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet, 2019. 394(10203): p. 1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulrooney DA, et al. , Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-sectional Study. Ann Intern Med, 2016. 164(2): p. 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong GT, et al. , Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol, 2009. 27(14): p. 2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad PK, et al. , Long-term non-cancer mortality in pediatric and young adult cancer survivors in Finland. Pediatr Blood Cancer, 2012. 58(3): p. 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammed T, et al. , Etiology and management of hypertension in patients with cancer. Cardiooncology, 2021. 7(1): p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armenian SH, et al. , Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood, 2011. 118(23): p. 6023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meacham LR, et al. , Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev, 2010. 19(1): p. 170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraeman KH, et al. , Incidence of New-Onset Hypertension in Cancer Patients: A Retrospective Cohort Study. International Journal of Hypertension, 2013. 2013: p. 379252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellblom AV, et al. , Self-reported late effects and long-term follow-up care among 1889 long-term Norwegian Childhood, Adolescent, and Young Adult Cancer Survivors (the NOR-CAYACS study). Supportive Care in Cancer, 2021. 29(6): p. 2947–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers Version 5.0. 2018, Children’s Oncology Group. [Google Scholar]
- 18.Adolescent and Young Adult (AYA) Oncology. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. 2021. [DOI] [PubMed]
- 19.Survivorship. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, 2021.
- 20.Armenian SH, et al. , Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol, 2017. 35(8): p. 893–911. [DOI] [PubMed] [Google Scholar]
- 21.Hypertension in Adults: Screening. U.S. Preventive Services Task Force, 2021. [Google Scholar]
- 22.COSTA LJM, VARELLA PCS, and DEL GIGLIO A, White coat effect in breast cancer patients undergoing chemotherapy. European Journal of Cancer Care, 2003. 12(4): p. 372–373. [DOI] [PubMed] [Google Scholar]
- 23.Whelton PK, et al. , 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Journal of the American College of Cardiology, 2018. 71(19): p. e127–e248. [DOI] [PubMed] [Google Scholar]
- 24.Li W, et al. , Vascular and Metabolic Implications of Novel Targeted Cancer Therapies: Focus on Kinase Inhibitors. J Am Coll Cardiol, 2015. 66(10): p. 1160–78. [DOI] [PubMed] [Google Scholar]
- 25.Maitland ML, et al. , Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res, 2009. 15(19): p. 6250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azizi M, Chedid A, and Oudard S, Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med, 2008. 358(1): p. 95–7. [DOI] [PubMed] [Google Scholar]
- 27.Eremina V, et al. , VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med, 2008. 358(11): p. 1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veronese ML, et al. , Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol, 2006. 24(9): p. 1363–9. [DOI] [PubMed] [Google Scholar]
- 29.Steeghs N, et al. , Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res, 2008. 14(11): p. 3470–6. [DOI] [PubMed] [Google Scholar]
- 30.Belcik JT, et al. , Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J Am Coll Cardiol, 2012. 60(7): p. 618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lankhorst S, et al. , Hypertension during vascular endothelial growth factor inhibition: focus on nitric oxide, endothelin-1, and oxidative stress. Antioxid Redox Signal, 2014. 20(1): p. 135–45. [DOI] [PubMed] [Google Scholar]
- 32.Maki-Petaja KM, et al. , Mechanisms Underlying Vascular Endothelial Growth Factor Receptor Inhibition-Induced Hypertension: The HYPAZ Trial. Hypertension, 2021. 77(5): p. 1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaherty KT, et al. , Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med, 2012. 367(2): p. 107–14. [DOI] [PubMed] [Google Scholar]
- 34.Long GV, et al. , Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol, 2017. 28(7): p. 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mincu RI, et al. , Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw Open, 2019. 2(8): p. e198890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caldeira D, et al. , Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS One, 2019. 14(2): p. e0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salem JE, et al. , Cardiovascular Toxicities Associated With Ibrutinib. J Am Coll Cardiol, 2019. 74(13): p. 1667–1678. [DOI] [PubMed] [Google Scholar]
- 38.Dickerson T, et al. , Hypertension and incident cardiovascular events following ibrutinib initiation. Blood, 2019. 134(22): p. 1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruppert AS, et al. , Adverse event burden in older patients with CLL receiving bendamustine plus rituximab or ibrutinib regimens: Alliance A041202. Leukemia, 2021. 35(10): p. 2854–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrd JC, et al. , Ibrutinib Treatment for First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia: Final Analysis of the Pivotal Phase Ib/II PCYC-1102 Study. Clin Cancer Res, 2020. 26(15): p. 3918–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pretorius L, et al. , Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am J Pathol, 2009. 175(3): p. 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natarajan G, et al. , Ibrutinib enhances IL-17 response by modulating the function of bone marrow derived dendritic cells. Oncoimmunology, 2016. 5(1): p. e1057385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valent P, et al. , Risk factors and mechanisms contributing to TKI-induced vascular events in patients with CML. Leuk Res, 2017. 59: p. 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tam CS, et al. , A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood, 2020. 136(18): p. 2038–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrd JC, et al. , Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J Clin Oncol, 2021. 39(31): p. 3441–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X and Wu S, Increased Risk of Hypertension with Enzalutamide in Prostate Cancer: A Meta-Analysis. Cancer Investigation, 2019. 37(9): p. 478–488. [DOI] [PubMed] [Google Scholar]
- 47.Iacovelli R, et al. , The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin Genitourin Cancer, 2018. 16(3): p. e645–e653. [DOI] [PubMed] [Google Scholar]
- 48.Attard G, et al. , Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab, 2012. 97(2): p. 507–16. [DOI] [PubMed] [Google Scholar]
- 49.Fizazi K, et al. , Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol, 2019. 20(5): p. 686–700. [DOI] [PubMed] [Google Scholar]
- 50.Zytiga. Package insert. Janssen Biotech, I.
- 51.Galán-Arriola C, et al. , Coronary microcirculation damage in anthracycline cardiotoxicity. Cardiovasc Res, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C, et al. , Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation, 2010. 121(5): p. 675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hader SN, et al. , Detrimental effects of chemotherapy on human coronary microvascular function. Am J Physiol Heart Circ Physiol, 2019. 317(4): p. H705–H710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gajalakshmi P, et al. , Breast cancer drugs dampen vascular functions by interfering with nitric oxide signaling in endothelium. Toxicol Appl Pharmacol, 2013. 269(2): p. 121–31. [DOI] [PubMed] [Google Scholar]
- 55.Finkelman BS, et al. , Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients With Breast Cancer. J Am Coll Cardiol, 2017. 70(2): p. 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McMahon KR, et al. , Long-term renal follow-up of children treated with cisplatin, carboplatin, or ifosfamide: a pilot study. Pediatric Nephrology, 2018. 33(12): p. 2311–2320. [DOI] [PubMed] [Google Scholar]
- 57.Knijnenburg SL, et al. , Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clinical journal of the American Society of Nephrology : CJASN, 2012. 7(9): p. 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campia U, et al. , Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement From the American Heart Association. Circulation, 2019. 139(13): p. e579–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Hashmi S, et al. , Busulphan-cyclophosphamide cause endothelial injury, remodeling of resistance arteries and enhanced expression of endothelial nitric oxide synthase. PLoS One, 2012. 7(1): p. e30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kooijmans EC, et al. , Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. The Cochrane database of systematic reviews, 2019. 3(3): p. CD008944–CD008944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herradón E, et al. , Characterization of Cardiovascular Alterations Induced by Different Chronic Cisplatin Treatments. Frontiers in Pharmacology, 2017. 8(196). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cameron AC, Touyz RM, and Lang NN, Vascular Complications of Cancer Chemotherapy. The Canadian journal of cardiology, 2016. 32(7): p. 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sagstuen H, et al. , Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol, 2005. 23(22): p. 4980–90. [DOI] [PubMed] [Google Scholar]
- 64.Boer H, et al. , Long-term exposure to circulating platinum is associated with late effects of treatment in testicular cancer survivors. Ann Oncol, 2015. 26(11): p. 2305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soultati A, et al. , Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev, 2012. 38(5): p. 473–83. [DOI] [PubMed] [Google Scholar]
- 66.Stoter G, et al. , Ten-year survival and late sequelae in testicular cancer patients treated with cisplatin, vinblastine, and bleomycin. J Clin Oncol, 1989. 7(8): p. 1099–104. [DOI] [PubMed] [Google Scholar]
- 67.Solimando DA, et al. , Hypertensive reactions associated with paclitaxel. Cancer Invest, 1996. 14(4): p. 340–2. [DOI] [PubMed] [Google Scholar]
- 68.Spano JP, et al. , Current targeted therapies in breast cancer: clinical applications in the elderly woman. Oncologist, 2011. 16(8): p. 1144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ekholm E, et al. , Paclitaxel changes sympathetic control of blood pressure. Eur J Cancer, 1997. 33(9): p. 1419–24. [DOI] [PubMed] [Google Scholar]
- 70.Izzedine H, et al. , Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant, 2006. 21(11): p. 3038–45. [DOI] [PubMed] [Google Scholar]
- 71.Baker KS, et al. , Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood, 2007. 109(4): p. 1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fakhouri F, et al. , Presentation and revascularization outcomes in patients with radiation-induced renal artery stenosis. Am J Kidney Dis, 2001. 38(2): p. 302–9. [DOI] [PubMed] [Google Scholar]
- 73.Sharabi Y, et al. , Baroreflex failure as a late sequela of neck irradiation. Hypertension, 2003. 42(1): p. 110–6. [DOI] [PubMed] [Google Scholar]
- 74.Farrugia MK and Mattes MD, Radiation-Association Hypertension in Patients Undergoing Treatment for Prostate Cancer. J Radiother Pract, 2020. 19(2): p. 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright JT Jr., et al. , A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med, 2015. 373(22): p. 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reboussin DM, et al. , Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2018. 138(17): p. e595–e616. [DOI] [PubMed] [Google Scholar]
- 77.Zamorano JL, et al. , 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J, 2016. 37(36): p. 2768–2801. [DOI] [PubMed] [Google Scholar]
- 78.Virani SA, et al. , Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can J Cardiol, 2016. 32(7): p. 831–41. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y, et al. , Traditional Cardiovascular Risk Factors and Individual Prediction of Cardiovascular Events in Childhood Cancer Survivors. J Natl Cancer Inst, 2020. 112(3): p. 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neilan TG, et al. , Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol, 2012. 110(11): p. 1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suominen A, et al. , Long-term renal prognosis and risk for hypertension after myeloablative therapies in survivors of childhood high-risk neuroblastoma: A nationwide study. Pediatr Blood Cancer, 2020. 67(8): p. e28209. [DOI] [PubMed] [Google Scholar]
- 82.Yancy CW, et al. , 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation, 2017. 136(6): p. e137–e161. [DOI] [PubMed] [Google Scholar]
- 83.Thavendiranathan P, et al. , Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol, 2014. 63(25 Pt A): p. 2751–68. [DOI] [PubMed] [Google Scholar]
- 84.Sawaya H, et al. , Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging, 2012. 5(5): p. 596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thavendiranathan P, et al. , Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J Am Coll Cardiol, 2021. 77(4): p. 392–401. [DOI] [PubMed] [Google Scholar]
- 86.Unger T, et al. , 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens, 2020. 38(6): p. 982–1004. [DOI] [PubMed] [Google Scholar]
- 87.Izzedine H, et al. , Hypertension and angiotensin system inhibitors: impact on outcome in sunitinib-treated patients for metastatic renal cell carcinoma. Ann Oncol, 2015. 26(6): p. 1128–1133. [DOI] [PubMed] [Google Scholar]
- 88.Becerril-Alarcon Y, et al. , Inulin Supplementation Reduces Systolic Blood Pressure in Women with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Cardiovasc Ther, 2019. 2019: p. 5707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strandberg TE and Salomaa V, White coat effect, blood pressure and mortality in men: prospective cohort study. European Heart Journal, 2000. 21(20): p. 1714–1718. [DOI] [PubMed] [Google Scholar]
- 90.Cohen JB, et al. , Cardiovascular Events and Mortality in White Coat Hypertension: A Systematic Review and Meta-analysis. Ann Intern Med, 2019. 170(12): p. 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen JB, et al. , Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC CardioOncol, 2019. 1(2): p. 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]


