Abstract
Background:
Young adults, age 18–39, are at a stage of life which may make them more vulnerable than older adults to impairments in health-related quality of life (HRQOL) during and after hematopoietic cell transplantation (HCT). Health self-efficacy (HSE) is the belief that one can implement strategies to produce a desired health outcome and has been associated with health outcomes in oncology research. Little is known about HRQOL or HSE in young adult HCT survivors compared to older HCT survivors.
Objective:
Given the age-specific psychosocial challenges facing young adult HCT recipients and research on non-transplant young adult cancer survivors, we hypothesized that young adult survivors would have worse post-HCT HRQOL compared to older adults and that among young adult HCT survivors, higher levels of HSE would be associated with higher HRQOL and lower levels of cancer-related distress.
Study Design:
This is a cross-sectional secondary analysis of two combined baseline datasets from multi-center studies of HCT survivors approached for participation in clinical trials of survivorship interventions. Participants from 20 transplant centers in the US were 1–10 years post-HCT, ≥18 years of age at the time of study enrollment, had no evidence of disease relapse/progression or subsequent malignancies, and read English adequate to consent and complete assessments. Medical record and patient-reported data were obtained for demographics and HCT-related clinical factors and complications (e.g. total body irradiation, chronic graft-versus-host disease). Participants completed surveys on HRQOL (Short-form [SF]-12), HSE, and Cancer and Treatment Distress (CTXD), which includes six subscales and an overall mean score. SF-12 was calculated for both mental (MCS) and physical (PCS) component scores. Participants were compared between two cohorts: young adults (age 18 to 39 at transplant) and older adults (age ≥40 at transplant). Multiple linear regression analyses determined factors associated with HSE, PCS, MCS and CTXD within young adults.
Results:
In this analysis of N=979 survivors, compared to older adults, young adult participants had lower mental health scores (SF-12 MCS: 48.40 vs. 50.23, P=0.04) and higher cancer-related distress (CTXD: 0.96 vs. 0.85, P=0.04), though better physical health (SF-12 PCS: 48.99 vs. 47.18, P=0.049). Greater overall cancer-related distress was driven by higher levels of uncertainty, financial concern, and medical demand subscales for young adults compared with older adults. Young adults also had lower HSE (2.93 vs. 3.08, P=0.0004). In a multivariate model, HSE was strongly associated with age group (p=0.0005) after adjusting for multiple other transplant related factors. Among young adults, HSE was associated with mental and physical components of the SF-12 and the CTXD, and HSE remained significant after adjusting for other transplant-related factors.
Conclusions:
Overall, young adult HCT survivors have lower mental health, increased cancer-related distress, and lower levels of HSE compared to older adults. Although the direction of these effects cannot be determined with these data, the strong association between HSE and HRQOL among young adults suggests that targeting interventions to improve HSE may have broad impact on health outcomes.
Keywords: Young Adult, HCT, Survivorship, HRQOL, HSE, self-efficacy
Introduction
Young adults (YA; age 18–39) are at a stage of life which may make them vulnerable to greater intensity and frequency of impairments in health-related quality of life (HRQOL) during and after hematopoietic cell transplantation (HCT) than older adults.1, 2 Adolescents and YAs are at a developmental stage marked by rapid changes in cognitive, social, and emotional growth and have historically experienced a gap in services relating to their psychosocial needs.3, 4 Many of these factors place YA patients at risk for poor HRQOL as they undergo treatment for cancer.5, 6
In the HCT setting, only a few studies have been performed analyzing HRQOL in YA patients. Long-term YA survivors of HCT report significant ongoing physical and psychosocial consequences of past illness and its treatments.7 Data from a previous, INternet-based Survivorship Program with Information and Resources randomized controlled trial (INSPIRE) demonstrated that adolescent and YAs and middle age adults have more financial and overall distress (CTXD) than older adults.8 Both YA and older adult HCT survivors have numerous unmet medical and psychosocial needs, though limited evidence suggests these groups differ on specific concerns.9 Health self-efficacy (HSE) is the belief that one can implement strategies to produce a desired health outcome and HSE is associated with both self-management and coping in cancer survivors.10, 11
In this large multicenter cross-sectional assessment, we focus on HRQOL as indicated by standardized, widely used measures of physical and mental health as well as cancer-related distress specifically developed for HCT survivors. Given previous findings about the age-specific psychosocial challenges to adolescent and YA HCT recipients, we hypothesized that YA survivors would have lower HRQOL and greater distress compared to older adults, that YA HCT survivors would have reduced HSE compared to older adults, and finally that among YA HCT survivors, higher levels of HSE would be associated with improved mental and physical health, and lower levels of cancer-related distress.
Methods
Participants
We analyzed baseline data from two randomized controlled trials enrolling at 20 transplant centers: INSPIRE and the individualized treatment summary and Survivorship Care Plans (SCP) randomized controlled trial.12, 13 Patients were eligible for the SCP cohort if they were one to five years post-HCT, ≥18 years at the time of study enrollment, with no evidence of disease relapse/progression or second cancers, and read English adequate to consent and complete assessments.13 Inclusion criteria for INSPIRE were similar, with the exception that survivors were 2–10 years post-HCT and needed to have internet and e-mail access for the intervention trial, but those who did not have internet could complete the baseline survey by mail.12 From the combined INSPIRE-SCP cohort we excluded cases with diagnoses or treatment that were predominantly only present in one age group: (multiple myeloma, solid tumors, HCT for a non-malignant conditions, non-myeloablative conditioning, or those who had more than one HCT). Participants were divided into two cohorts based on age at HCT: YA (age 18–39) and older adult (age ≥40).
Procedure
Participants from the INSPIRE and SCP studies were contacted and consented to participate as previously described.12, 13 The primary studies, INSPIRE and SCP, obtained clinical and demographic factors from research medical records as well as HRQOL measures in survivors of HCT. The variables used in this analysis matched across the two studies and were combined into a single cohort. This cross-sectional secondary analysis was approved by the Institutional Review Board at the Cleveland Clinic in addition to previous approvals at each of the participating institutions for the primary studies.
Measures
Research records included demographics (age at HCT, sex, race, ethnicity) and clinical variables (years from HCT to study entry, diagnosis, receipt of total body irradiation, allogeneic vs. autologous HCT, and a history of chronic graft versus host disease (cGVHD) for allogeneic recipients). Patient-reported outcome measures included Short Form-12 (SF-12), Cancer and Treatment Distress (CTXD) and Health Self-Efficacy (HSE).
The SF-12 health survey includes a mental and physical component score (MCS or PCS) based on differential weighting of each question.13–17 The SF-12 survey is standardized with norms so that the general population mean score is 50, with a standard deviation of 10, and higher scores indicate better functioning.18 The SF-12 has been previously used across multiple studies of patients who received HCT.13–17
The CTXD scale includes 23 items for which patients are asked to rate ‘how much distress or worry (such as feeling upset, tense, sad, frustrated) each item caused you in the past week, even if the event has not happened.’ Items address various aspects of the HCT experience (dealing with insurance, changes in appearance, thinking about relapse). The response scale is 0 (none) to 3 (severe).8, 19 A higher mean score indicates a higher level of distress, with scores of >0.90 indicating elevated distress in survivors a year or more after HCT. The CTXD also includes six subscales: health burden, uncertainty, family strain, finances, identity, and medical demands. Testing supports that CTXD captures >90% of cases with depression or anxiety symptoms, as well as post-traumatic stress symptoms, and its value as a predictor of health outcomes has been demonstrated in several HCT studies.8, 13, 17, 19, 20
HSE measures confidence that a person can manage health demands and was adapted from the general self-efficacy measure, for example, ‘It is easy for me to stick to my aims and accomplish my health goals.’21 The HSE includes 7 items with response options ranging from 1=“not at all true” to 4=“exactly true.” Higher mean scores indicate stronger belief in self-efficacy.21
Statistical Analyses
We described continuous variables as means and standard deviations and made comparisons between age groups with t-tests. We described categorical variables as frequency counts and percentages and compared groups with chi-square tests. We analyzed the association of HSE with the HRQOL measures in YA survivors using Pearson correlations. We then created multivariable linear regression models to determine clinical variables associated with HSE. The regression model contains a parameter estimate for each variable, with the P-value indicating whether the parameter estimate differs from 0. Finally, we created multivariable linear regression models among YA survivors to determine whether HSE was associated with CTXD, MCS or PCS, after controlling for age, sex, race, ethnicity, years since HCT, transplant group (autologous vs. allogeneic without cGVHD vs. allogeneic with cGVHD), and receipt of total body irradiation. All tests were two-sided and a P-value of 0.05 or less was considered statistically significant. Statistical analysis was carried out using SAS Studio version 3.7 (SAS Institute, Cary, NC).
Results
Patient and Transplant Characteristics
A total of 979 patients were included in the analyses, with 194 (19.8%) transplanted before age 40 and 785 (80.2%) transplanted at age 40 or older. The cohort included 489 allogeneic HCT recipients and 490 autologous recipients. Characteristics of survivors within the two age groups are shown in Table 1. The YA group had a greater proportion of female participants (54.1% vs. 41.8%, p=0.002), more frequently received allogeneic HCT (59.3% vs. 47.6%, p=0.004), and more frequently received total body irradiation (35.6% vs. 17.5%, p<0.0001). Diagnoses were different based on age group, with notably higher prevalence of acute lymphoblastic leukemia and Hodgkin lymphoma in YAs, and non-Hodgkin lymphoma in older adults. Race and ethnicity did not vary significantly between age groups, and the majority of study participants were White and non-Hispanic. Importantly, no differences between age groups were seen for the rates of cGVHD among those receiving allogeneic HCT.
Table 1.
Patient Characteristics and Study Instruments by Age Group
| Young Adult (n=194) | Older Adult (n=785) | ||||
|---|---|---|---|---|---|
| Variable | N | % | N | % | P-Value |
| Patient and Transplant Charecteristics | |||||
| Age at HCT, years (mean + SD, [range]) | 31 ± 6 (19.1–39.8) | 56 ± 8 (40.0–84.5) | - | ||
| Sex (Male) | 89 | 45.90% | 457 | 58.20% | 0.002 |
| Race | 0.28 | ||||
| Asian | 6 | 3.10% | 12 | 1.50% | |
| Black | 7 | 3.60% | 18 | 2.30% | |
| Multiple Races | 3 | 1.50% | 10 | 1.30% | |
| Native American | 3 | 1.50% | 5 | 0.60% | |
| White | 175 | 90.20% | 740 | 94.30% | |
| Ethnicity | 0.2 | ||||
| Hispanic | 12 | 6.20% | 32 | 4.10% | |
| Non-Hispanic | 179 | 92.30% | 743 | 94.60% | |
| Unknown | 3 | 1.50% | 10 | 1.30% | |
| Years from transplant to study entry (mean + SD, [range]) | 5.2 ± 2.2 , (1.2–10.7) | 5.1 ± 2.1 (1.0–10.9) | 0.59 | ||
| Diagnosis | <0.0001 | ||||
| Acute Lymphoblastic Leukemia | 32 | 16.50% | 32 | 4.10% | |
| Acute Myeloid Leukemia | 48 | 24.70% | 167 | 21.30% | |
| Chronic Lymphoblastic Leukemia | 3 | 1.50% | 17 | 2.20% | |
| Chronic Myeloid Leukemia | 7 | 3.60% | 21 | 2.70% | |
| Hodgkin Lymphoma | 43 | 22.20% | 43 | 5.50% | |
| Myelodysplastic Syndrome/ MPN | 17 | 8.80% | 98 | 12.50% | |
| Non-Hodgkin Lymphoma | 42 | 21.60% | 402 | 51.20% | |
| Other leukemia | 2 | 1.00% | 5 | 0.60% | |
| Total Body Irradiation | 69 | 35.60% | 137 | 17.50% | <0.0001 |
| Transplant Type | 0.004 | ||||
| Autologous | 79 | 40.70% | 411 | 52.40% | |
| Allogeneic | 115 | 59.30% | 374 | 47.60% | |
| History of cGVHD (allogeneic only) | 0.21 | ||||
| Yes | 97 | 84.30% | 332 | 80.80% | |
| No | 18 | 15.70% | 42 | 10.20% | |
| Study Instruments (mean +SD) | |||||
| Short Form-12 Physical Component Score | 48.99 | 11.09 | 47.18 | 10.8 | 0.049 |
| Short Form-12 Mental Component Score | 48.4 | 11.49 | 50.23 | 10.47 | 0.04 |
| Cancer and Treatment Distress Overall | 0.96 | 0.69 | 0.85 | 0.61 | 0.04 |
| Cancer and Treatment Distress Subscales | |||||
| Health Burden | 1.22 | 0.96 | 1.27 | 0.88 | 0.55 |
| Uncertainty | 1.08 | 0.74 | 0.95 | 0.72 | 0.03 |
| Family Strain | 0.88 | 0.87 | 0.79 | 0.81 | 0.18 |
| Finances | 0.98 | 0.97 | 0.77 | 0.82 | 0.003 |
| Identity | 0.92 | 0.8 | 0.8 | 0.73 | 0.051 |
| Medical Demands | 0.58 | 0.67 | 0.47 | 0.59 | 0.03 |
| Health Self-Efficacy | 2.93 | 0.47 | 3.08 | 0.5 | 0.0004 |
Note: YA: Young Adult; HCT: Hematopoietic Cell Transplant; SD: standard deviation; MPN: Myeloproliferative Neoplasm; cGVHD: chronic graft versus host disease; Short Form-12 (higher value is better functioning), Cancer and Treatment Distress (lower number indicates less distress)
Study Instruments and Age Cohorts
Study instrument overall and subscale scores are shown in Table 1. YA survivors had higher physical component scores (48.99 vs. 47.18, p=0.049), but lower mental component scores (48.40 vs. 50.23, p=0.04) and higher distress on the CTXD (0.96 vs. 0.85, p=0.04). For the CTXD subscales, YA patients reported greater distress related to uncertainty, finances, and medical demands than older adults, though no differences for health burden, family strain or identity. Mean HSE scores in YA was also lower than older adults (2.93 vs. 3.08, p=0.0004).
Health Self-Efficacy and Quality of Life
Noting the decreased HSE demonstrated by the YA age group, a multivariate model was created to determine associations of clinical and demographic factors with HSE (Table 2). After adjusting for years since HCT, sex, race, ethnicity, total body irradiation and transplant group (autologous, allogeneic without cGVHD, allogeneic with cGVHD), age group at transplant remained an independent predictor of HSE. Additionally, as time since transplant increased, HSE scores decreased. Interaction terms were not statistically significant.
Table 2.
Multivariable Linear Regression Model for Health Self-Efficacy
| Variable | Parameter Estimate | 95% CI | P-Value |
|---|---|---|---|
| Age Group | 0.0004 | ||
| Older (≥40 years) | 0.151 | (0.068,0.235) | |
| YA (18–39 years) | - | - | |
| Years since HCT (per 1 yr) | −0.017 | (−0.032,-.002) | 0.025 |
| Sex | |||
| Male | - | - | - |
| Female | −0.042 | (−0.107,0.024) | 0.21 |
| Race | |||
| White | - | - | - |
| Non-White* | −0.08 | (−0.217,0.057) | 0.25 |
| Ethnicity | |||
| Hispanic | |||
| Non-Hispanic | −0.037 | (−0.201,0.128) | 0.66 |
| Total Body Irradiation | |||
| No | −0.009 | (−0.095,0.077) | 0.84 |
| Yes | - | - | - |
| Transplant Group | |||
| Autologous | - | - | - |
| Allogeneic no cGVHD | 0.14 | (0.000,0.279) | 0.12 |
| Allogeneic cGVHD | −0.004 | (−0.072,0.071) |
Note: SE: standard error; CI: Confidence Interval; YA: Young Adult; HCT: Hematopoietic Cell Transplant; cGVHD: chronic graft versus host disease;
includes Asian, Black, Multiple Races, and Native American
We next analyzed the association of HSE with HRQOL measures in YA survivors using Pearson correlation. HSE was associated with the SF-12 MCS (r=0.56, p<0.0001), SF-12 PCS (r=0.39, p<0.0001), and overall CTXD (r=-0.48, p<0.0001) (Figure 1).
Figure 1.
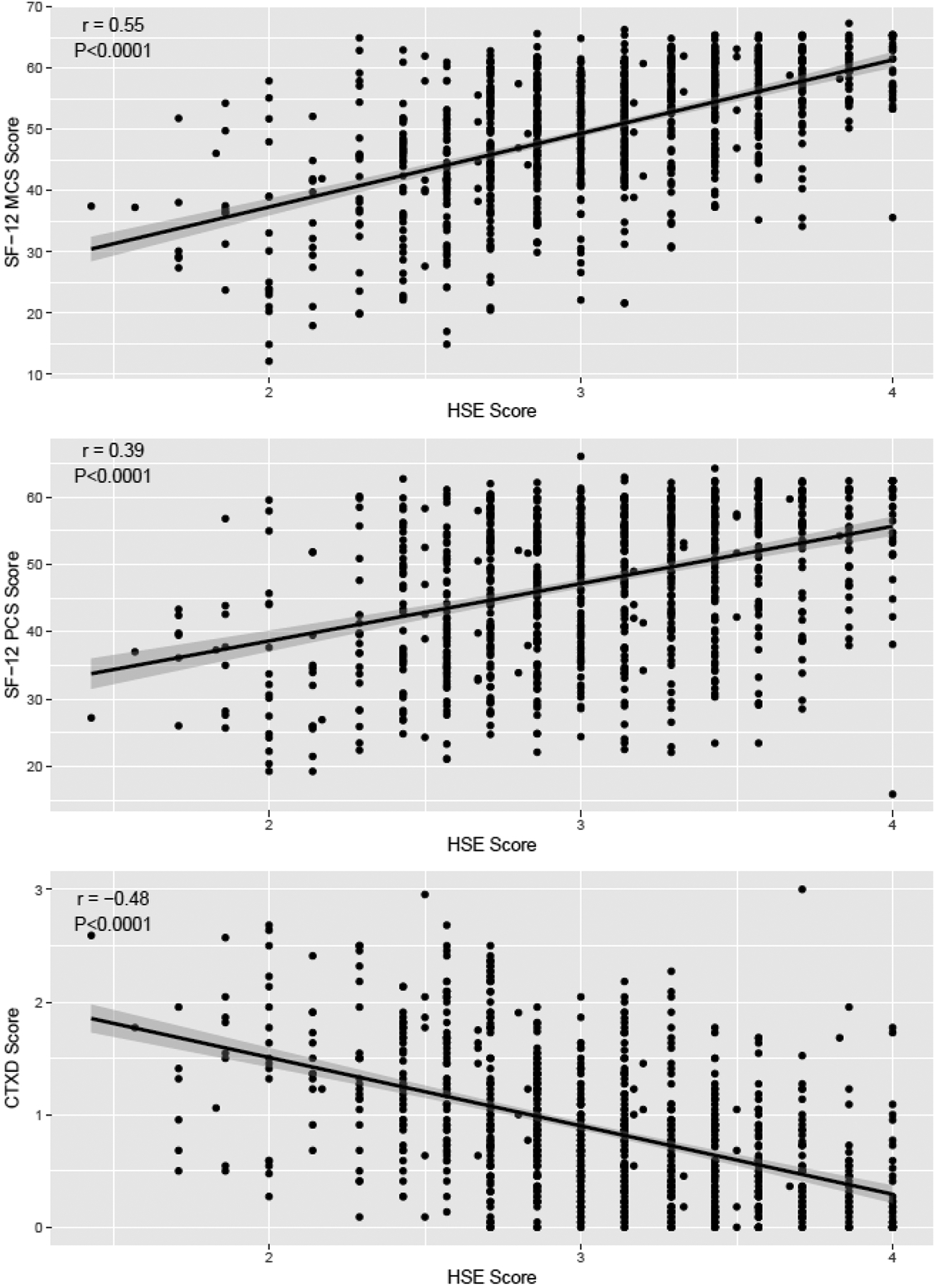
Pearson correlation of HSE score vs. multiple measures of quality of life in allogeneic YA HCT survivors. a. SF-12 Mental Component. B. SF-12 Physical Component. C. CTXD. Note: HSE=Health Self-Efficacy; SF=Short Form; CTXD=Cancer and Treatment Distress
In multivariable analysis of YA, after adjusting for patient age at baseline survey, years since HCT, sex, race, ethnicity, total body irradiation and transplant group (autologous, allogeneic without cGVHD, allogeneic with cGVHD), HSE remained an independent predictor of the SF-12 PCS and MCS, and the CTXD (Table 3). Interaction terms between variables were not statistically significant.
Table 3.
Multivariable Linear Regression Models for Short Form-12 Components and Cancer Treatment Distress in YA Patients
| Short Form-12 Physical Component Score | Short Form-12 Mental Component Score | Cancer and Treatment Distress | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Parameter Estimate | 95% CI | P-Value | Parameter Estimate | 95% CI | P-Value | Parameter Estimate | 95% CI | P-Value |
| Age at Baseline Survey (per 1 yr increase) | −0.107 | (−0.160,−0.053) | <0.0001 | 0.014 | (−0.034,0.062) | 0.56 | 0.00005 | (−0.003,0.003) | 0.98 |
| Sex (Femal e vs. Male) | −0.382 | (−1.702,0.939) | 0.57 | −0.686 | (−1.874,0.503) | 0.26 | 0.057 | (−0.016,0.129) | 0.13 |
| Race (NonWhite* vs. White) | 0.206 | (−2.566,2.977) | 0.88 | 1.435 | (−1.059,3.929) | 0.26 | 0.071 | (−0.081,0.224) | 0.36 |
| Ethnicity (Non-Hispanic vs. Hispanic) | −0.692 | (−4.006,2.622) | 0.68 | 0.793 | (−2.189,3.775) | 0.6 | −0.111 | (−0.296,0.075) | 0.24 |
| Total Body Irradiation (No vs. Yes) | −1.189 | (−2.913,0.535) | 0.18 | −0.374 | (−1.926,1.177) | 0.64 | 0.082 | (−0.013,0.177) | 0.09 |
| Transplant Group | |||||||||
| Allogeneic no cGVHD | - | - | - | - | - | - | - | - | - |
| Allogeneic with cGVHD | −3.157 | (−5.956,0.359) | 0.0271 | −2.791 | (−5.309,−0.273) | 0.0299 | −0.0004 | (−0.155, 0.154) | 0.996 |
| Autologous | 1.061 | (−1.775,3.898) | 0.46 | −0.378 | (−2.930,2.175) | 0.77 | −0.203 | (−0.360,−0.047) | 0.0107 |
| Years Since HCT (per 1 yr increase) | 0.181 | (−0.124,0.487) | 0.24 | 0.028 | (−0.247, 0303) | 0.84 | −0.023 | (0.040, 0.006) | 0.0082 |
| Health Self-Efficacy (per 1 point) | 9.018 | (7.679,10.358) | <0.0001 | 11.95 | (10.745,13.155) | <0.0001 | −0.615 | (−0.688,−0.541) | <0.0001 |
Note: CI: Confidence Interval; Short Form-12 (higher value is better functioning); Cancer and Treatment-Related (lower number indicates less distress); HCT: Hematopoetic Cell Transplant;
includes Asian, Black, Multiple Races, and Native American
Discussion
Overall, we found that YA survivors have worse mental HRQOL and increased cancer-related distress compared to older adults, though they also have slightly better physical HRQOL. Further, YA survivors had lower levels of HSE, even after adjusting for multiple potential confounding variables, suggesting they may feel less able to advocate for themselves, problem solve to overcome health challenges, and accomplish their health goals. Interestingly, the reduced mental quality of life in YA HCT survivors occurred despite slightly better physical quality of life. Importantly, among YA participants, HSE was strongly associated with mental and physical HRQOL and cancer-related distress while other demographic and clinical variables were not.
Previous work analyzing HRQOL in allogeneic HCT recipients did not show a difference between YAs and older adults in the Functional Assessment of Cancer Therapy-Bone Marrow Transplantation (FACT-BMT) questionnaire total score or any specific domain in the first year post-HCT.22 However, our study of long-term survivors did demonstrate increased cancer-related distress among YA recipients, which was driven by concerns regarding, finances, medical demands, and uncertainty, and with no subscales higher in the older cohort. Increased distress regarding financial concerns among YAs was previously demonstrated in HCT survivors.8 As well, adolescent and YA HCT survivors have previously reported significant need for information related to nutrition, body image and sexuality, and coping strategies compared to older adults,9 suggesting different drivers of distress between age groups and differing needs within their survivorship care.
Findings confirm our hypothesis that self-efficacy would be pivotal to higher levels of mental function and lower distress. Self-efficacy is the confidence that one can execute strategies to produce a desired outcome.10 Cancer survivor HSE can vary greatly between individual patients.10 HSE is particularly important for HCT survivors where management of late-effects requires coordination of care and initiative, particularly in care models where the HCT center does not continue to serve as the medical home. Among cancer patients with a broad range of diagnoses, self-efficacy improves quality of life by reducing perceived stress and increasing quality of life.23–25 Therefore, it is not surprising to see the significant relationship between HSE and multiple HRQOL scales demonstrated in our HCT survivors.
Interventions to target HSE may be beneficial in improving YA HCT survivors’ quality of life. YA HCT patients have higher levels of discontinuation of long-term follow-up than both pediatric patients and older adults.26 In childhood cancer survivors, attendance in a survivorship clinic, having health insurance, and having a regular care provider (oncologist or non-oncologist) were associated with greater HSE, suggesting that increased attendance to long-term follow up might lead to higher HSE in similar populations.25 Interventions such as nurse-led interviews, counseling, and internet-based interventions to promote HSE in cancer survivors have had some success,27–30 and similar strategies warrant further study in YA HCT survivors. YA survivors report particular information needs regarding nutrition, body shape/sexuality, and relaxation techniques and prefer advice communicated in a one-on-one setting.9, 31 Other survivorship randomized controlled trials in YA cancer survivors have found that they have low participation rates in face to face clinic visits and may respond better to digital modalities such as video sessions or mobile apps.32 Whether interventions to improve this information dissemination and skills training would improve HSE or HRQOL in this population is an area in need of further study.
Our study has both strengths and limitations. To our knowledge, this is the largest such study to examine QOL in YA HCT survivors, and the first to find an association between HSE and QOL in this population. This large, heterogeneous cohort represents patients across a multitude of hematologic diseases and includes a national representation of 20 U.S. transplant centers. The study includes both patients with autologous and allogeneic HCTs and examined the role of both total body irradiation and cGVHD, which are known to drive medical late-effects in HCT survivors. The National Cancer Institute defines adolescent and YA patients as 15–39 years of age, but we only included subjects over 18 at the time of study enrollment because centers participating in the INSPIRE and SCP studies were adult HCT programs. Hence our findings may not be generalizable to the experience and needs of those treated at a children’s hospital, or for younger adolescents.33 Young adult survivors of childhood cancer (i.e. a patient who underwent HCT at 10 years old and is now 25 years old) may have different concerns associated with HRQOL than the population we studied (those mainly experiencing HCT and survivorship as a young adult). Those treated at pediatric centers who require transfer to adult centers for survivorship care may also differ from our cohort. Additionally, our patient population was mostly comprised of White, non-Hispanic patients as they are over-represented in HCT populations. Our study did not detect a difference between race and HSE. However, given the small number of racial and ethnic minorities in our study, we did not have the power to detect a relationship within specific peoples from racial and ethnic minorities with HRQOL or HSE. Notably, we were not able to determine if there was a difference between patients from a racial or ethnic minority who chose to enroll or not enroll in this study, but in the primary INSPIRE study, no differences were seen.12 Finally, though previous studies have demonstrated that self-efficacy is a driver of HRQOL in patients with cancer,23 our study was not designed to determine causality. Accordingly, patients with lower HRQOL or higher levels of distress may feel that they have less ability to alter their health outcomes.
While this cross-sectional study cannot determine causality, this study clearly shows important findings that need to be considered by the HCT community. Overall, our data suggests that many YA HCT survivors will have impairments of HRQOL and that these needs exceed those in older adults. HSE is strongly related to HRQOL in YA and targeting interventions, such as one-on-one counseling or remote digital modalities specifically adapted to YA needs, to improve HSE in this population merits further investigation.
Highlights.
Young adult HCT survivors have impaired HRQOL compared to older adults
Cancer-related distress was driven by uncertainty, finances, and medical demands
Health self-efficacy is associated with favorable HRQOL outcomes in young adults.
Financial Disclosures
The authors report no conflicts of interest relevant to this work. The authors report the following financial relationships.
| Jim | consulted for Janssen Scientific Affairs and Merck and has grant funding from Kite Pharma. |
| Wingard | consulted for Celgene, Merck, Cidara, Takeda |
| Hashmi | honoraria from Novartis, Mallinckrodt, Pfizer, and Janssen; honoraria from Novartis, Mallinckrodt, Pfizer, and Janssen; Consulting for Novartis, Kite, Juno, Takeda, CRISPR |
| Cerny | Consultant/Ad Board Member Pfizer Inc, Amgen Inc., Jazz Pharmaceuticals; DSMB Member-Allovir Inc., Stocks- Actinium Pharmaceuticals, Bluebird Bio/SeventyFour Inc., Dynavax Pharma, Atyr Pharma, Gamida Cell, Miragen Therapeutics, Mustang Bio, Novavax, Ovid Therapeutics, Sorrento Therapeutics, TG Therapeutics, Vaxart Inc, and Veru Inc |
| Stiff | Consulting for CRISPR, Pfizer, Morphosys; grant receipts from Gamida Cell, Amgen, Pfizer, Gilead, Incyte, Karyopharm, Cellectar, Bristol- Myers Squibb, Johnson and Johnson, Alovir, Atara Seattle Genetics |
| Holten | Research funding from Incyte and Vitrac Therapeutics. Clinical trial adjudication for CSL Behring. Consultantion for Generon and Ossium. |
| Others | No COI to report |
Funding
Supported in part by a grant from PCORI (CD-12-11-4062 to National Marrow Donor Program, KSB, NSM); and grants from the National Cancer Institute [(R01 CA160684 to KLS), (R01 CA215134 to KLS, KSB, NSM.), (U01 CA246659 to KLS, KSB)], and NIH NCATS (2KL2TR002547 to SJR.).The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- HCT
Hematopoietic cell transplantation
- HRQOL
Health-Related Quality of Life
- YA
Young Adults
- INSPIRE
INternet-based Survivorship Program with Information and Resources randomized controlled trial
- SCP
Individualized treatment summary and Survivorship Care Plans randomized controlled trial
- CTXD
Cancer and Treatment Distress measure
- SF-12
Short Form-12 HRQOL measure
- cGVHD
Chronic graft-versus-host disease
- HSE
Health Self-Efficacy measure
- FACT-BMT
Functional Assessment of Cancer Therapy-Bone Marrow Transplantation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathanda RR, Hamilton BK, Rybicki L, et al. Quality-of-Life Trajectories in Adolescent and Young Adult versus Older Adult Allogeneic Hematopoietic Cell Transplantation Recipients. Biol Blood Marrow Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tremolada M, Bonichini S, Taverna L, Basso G, Pillon M. Health-related quality of life in AYA cancer survivors who underwent HSCT compared with healthy peers. European journal of cancer care. 2018;27:e12878. [DOI] [PubMed] [Google Scholar]
- 3.Mehta PA, Rotz SJ, Majhail NS. Unique Challenges of Hematopoietic Cell Transplantation in Adolescent and Young Adults with Hematologic Malignancies. Biol Blood Marrow Transplant. 2018;24:e11–e19. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RH. AYA in the USA. International Perspectives on AYAO, Part 5. J Adolesc Young Adul. 2013;2:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodergren SC, Husson O, Robinson J, et al. Systematic review of the health-related quality of life issues facing adolescents and young adults with cancer. Qual Life Res. 2017;26:1659–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sodergren SC, Husson O, Rohde GE, et al. Does age matter? A comparison of health-related quality of life issues of adolescents and young adults with cancer. Eur J Cancer Care (Engl). 2018;27:e12980. [DOI] [PubMed] [Google Scholar]
- 7.Lahaye M, Aujoulat I, Vermylen C, Brichard B. Long-Term Effects of Haematopoietic Stem Cell Transplantation after Pediatric Cancer: A Qualitative Analysis of Life Experiences and Adaptation Strategies. Front Psychol. 2017;8:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SMW, Yi JC, Jim HSL, et al. Age and gender differences in financial distress among hematopoietic cell transplant survivors. Support Care Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulewka K, Strauss B, Hochhaus A, Hilgendorf I. Clinical, social, and psycho-oncological needs of adolescents and young adults (AYA) versus older patients following hematopoietic stem cell transplantation. Journal of Cancer Research and Clinical Oncology. 2021;147:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster C, Breckons M, Cotterell P, et al. Cancer survivors’ self-efficacy to self-manage in the year following primary treatment. J Cancer Surviv. 2015;9:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinz A, Friedrich M, Kuhnt S, Zenger M, Schulte T. The influence of self-efficacy and resilient coping on cancer patients’ quality of life. European Journal of Cancer Care. 2019;28:e12952. [DOI] [PubMed] [Google Scholar]
- 12.Yi JC, Sullivan B, Leisenring WM, et al. Who enrolls in an online cancer survivorship program? Reach of the INSPIRE randomized controlled trial for hematopoietic cell transplantation survivors. Biology of Blood and Marrow Transplantation. 2020;26:1948–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majhail NS, Murphy E, Laud P, et al. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica. 2019;104:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basinski JR, Alfano CM, Katon WJ, Syrjala KL, Fann JR. Impact of delirium on distress, health-related quality of life, and cognition 6 months and 1 year after hematopoietic cell transplant. Biology of Blood and Marrow Transplantation. 2010;16:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera N, Storer B, Flowers ME, et al. Nonmalignant late effects and compromised functional status in survivors of hematopoietic cell transplantation. Journal of clinical oncology. 2012;30:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syrjala KL, Yi JC, Artherholt SB, et al. An online randomized controlled trial, with or without problem-solving treatment, for long-term cancer survivors after hematopoietic cell transplantation. J Cancer Surviv. 2018;12:560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 19.Syrjala KL, Sutton SK, Jim HS, et al. Cancer and treatment distress psychometric evaluation over time: A BMT CTN 0902 secondary analysis. Cancer. 2017;123:1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syrjala KL, Yi JC, Langer SL. Psychometric properties of the Cancer and Treatment Distress (CTXD) measure in hematopoietic cell transplantation patients. Psychooncology. 2016;25:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarzer R, Jerusalem M. Generalized self-efficacy scale. Measures in health psychology: A user’s portfolio. Causal and control beliefs. 1995;1:35–37. [Google Scholar]
- 22.Mathanda RR, Hamilton BK, Rybicki L, et al. Quality-of-Life Trajectories in Adolescent and Young Adult versus Older Adult Allogeneic Hematopoietic Cell Transplantation Recipients. Biol Blood Marrow Transplant. 2020;26:1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreitler S, Peleg D, Ehrenfeld M. Stress, self-efficacy and quality of life in cancer patients. Psycho‐Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer. 2007;16:329–341. [DOI] [PubMed] [Google Scholar]
- 24.Bradford N, Pitt E, Rumble S, Cashion C, Lockwood L, Alexander K. Persistent Symptoms, Quality of Life, and Correlates with Health Self-Efficacy in Adolescent and Young Adult Survivors of Childhood Cancer. J Adolesc Young Adul. 2021. [DOI] [PubMed] [Google Scholar]
- 25.Miller KA, Wojcik KY, Ramirez CN, et al. Supporting long-term follow-up of young adult survivors of childhood cancer: Correlates of healthcare self-efficacy. Pediatric blood & cancer. 2017;64:358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamura K, Yamashita T, Atsuta Y, et al. High probability of follow-up termination among AYA survivors after allogeneic hematopoietic cell transplantation. Blood Adv. 2019;3:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts AL, Fisher A, Smith L, Heinrich M, Potts HW. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta-analysis. Journal of Cancer Survivorship. 2017;11:704–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampic C, Ljungman L, Micaux Obol C, Eriksson L, Wettergren L. A web-based psycho-educational intervention (Fex-Can) targeting sexual dysfunction and fertility-related distress in young adults with cancer: study protocol of a randomized controlled trial. BMC cancer. 2019;19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Valle M, Martín-Payo R, Cuesta-Briand B, Lana A. Impact of two nurse-led interventions targeting diet among breast cancer survivors: Results from a randomized controlled trial. European Journal of Cancer Care. 2018;27:e12854. [DOI] [PubMed] [Google Scholar]
- 30.Wu LM, Chen CM, Hsu HT, Liu Y, Su HL. Tailored education enhances healthy behaviour self-efficacy in childhood cancer survivors: A randomised controlled study with a 4-month follow-up. European Journal of Cancer Care. 2019;28:e13063. [DOI] [PubMed] [Google Scholar]
- 31.Cooke L, Chung C, Grant M. Psychosocial care for adolescent and young adult hematopoietic cell transplant patients. Journal of psychosocial oncology. 2011;29:394–414. [PMC free article] [PubMed] [Google Scholar]
- 32.Syrjala KL, Walsh CA, Yi JC, et al. Cancer survivorship care for young adults: a risk-stratified, multicenter randomized controlled trial to improve symptoms. Journal of Cancer Survivorship. 2021:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood WA, Lee SJ, Brazauskas R, et al. Survival improvements in adolescents and young adults after myeloablative allogeneic transplantation for acute lymphoblastic leukemia. Biology of Blood and Marrow Transplantation. 2014;20:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]


