Summary:
Acute myeloid leukemia is conventionally thought of as a medical emergency. However, several studies on the association of time from diagnosis to treatment with survival did not have concordant results. Here we analyze 55,985 AML patients from the National Cancer Database, and we show that in patients < 60 years-old a 5-day delay in chemotherapy initiation leads to worse long-term survival. The difference is small (HR 1.05, 95% CI 1.01–1.09 in multivariate analysis) but statistically significant. This study raises the issue of power to detect small differences in retrospective studies.
Keywords: TDT, Time from diagnosis to treatment, Time to treatment, acute myeloid leukemia, AML
Acute myeloid leukemia (AML) is devastating hematologic malignancy with dismal long-term survival.(1) Left untreated, it can be fatal within weeks.(2) The availability of molecular testing allowed for use of novel agents in some patients resulting in further improvement in survival rates.(3) However, results of such testing are often not available for days. This posed the question of whether induction treatment of AML should be delayed until molecular testing is available.
A few large studies investigated the relationship between time from diagnosis to treatment (TDT) and overall survival. A study from the USA showed that longer TDT was associated with worse overall survival in patients <60 years old, but two studies from Europe did not confirm the association.(4–6) Another study from Europe showed worse survival in patients after day 6 but this association was not significant in multivariate analysis.(7) Due to the conflicting results, we decided to investigate further.
We investigated the effect of TDT on overall survival in patients with newly diagnosed AML. We used the National Cancer Database (NCDB) to obtain records of patients diagnosed with AML. The NCDB is a clinical oncology database which obtains hospital data from community and academic settings.(8) The database records the date of initial diagnosis and the date that chemotherapy was started. The date of initial diagnosis relies on documentation of definitive diagnosis from the treating physician. The primary outcome of the study was overall survival, which was defined as the time from diagnosis to death or last live encounter. We excluded cases with acute promyelocytic leukemia, no histologic confirmation, < 18 years-old or >75 years-old, no overall survival data, no TDT data, no receipt of multi-agent chemotherapy and cases with TDT higher than 60 days. The study included data from 2004 to 2018, before the venetoclax FDA-approval in 2020. Thus, receipt of multi-agent chemotherapy limited the possibility of cases treated with non-intensive therapy. This study was approved by the University of Pittsburgh Institutional Review Board as non-human research study.
We first explored the impact of delay of each day using a univariate Cox model; with each day acting as a different categorical group. Because of a significant interaction of TDT with age, we analyzed patients <60 years old and ≥60 years-old separately. Although we hypothesized that the relationship would be linear, the following groups for patients <60 years-old had similar hazard ratios (HR): 0, 1–4, 5–8, 9 or more (Sup. Table 1). For ≥60 years-old the groups formed as follows: 0–1, 2–14, 15 or more (Sup. Table 2). The Aiken’s information criterion confirmed that these categories were a better fit than treating TDT as a continuous variable. Thus, we adopted this categorization for the analysis. Some variables such as race and insurance were reduced in broader categories to allow for better statistical modeling. The quartile of zip code income and high school education had three entries from 2004, 2012 and 2016. We used the entry closer to the year of diagnosis and we used the 2016 reference ranges for table 1.
Table 1.
Proportional hazards model for impact of TDT on overall survival in patients
<60 years old. The adjusted column represents hazard ratio (HR) values adjusted for year of diagnosis, age, sex, race, ethnicity, insurance, academic hospital, charlson-deyo score, income, education.
| TDT | Unadjusted HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| 0 | 1.07 | 1.02– 1.13 | .008 | 1.10 | 1.04–1.16 | < .001 |
| 1–4 | Ref | Ref | ||||
| 5–8 | 1.10 | 1.06 – 1.14 | < .001 | 1.05 | 1.01–1.09 | .026 |
| 9 or more | 1.17 | 1.13 – 1.22 | < .001 | 1.08 | 1.04–1.12 | < .001 |
The overall survival was graphed via the Kaplan-Meier method and the HRs were calculated using a Cox proportional hazards model. The non-parametric log-rank test was used to compare univariate survival distributions. To assess whether other variables could be confounding the effect of TDT on overall survival, we utilized multivariate Cox regression. For co-variates that were missing data we used a separate “unknown” category. The proportional hazards assumption was evaluated using Grambsch and Therneau’s method.(9) The power analysis was performed using an exponential test comparing two independent hazard rates. The number needed to treat (NNT) was calculated as detailed in Austin PC et al.(10) Statistical analysis was performed using STATA/SE 17.0, and the significance level was set to 5% with no adjustments for multiplicity.
From 2004 to 2018, we identified 153,405 cases of AML in the NCDB database, and 55,985 patients met the inclusion criteria. Sup. Table 3 summarizes the characteristics of included patients based on the TDT and age category. A total of 30,255 patients were below the age of 60. Most patients were treated in academic facilities (49.2%), were white (84.6%), not Hispanic (88.7%), insured (94.2%) and had no comorbidities (76.1%). Overall, there were no major differences observed in co-variates between the TDT groups apart from the Charlson-Deyo score (in patients <60 years-old, only ~4% of patients treated between days 0–4 had a score of 2 or 3 versus ~5% of patients treated after day 5) and the age (median increased by 1 year as TDT categories increased).
For patients <60 years-old, the median follow-up time was 23.23 months (interquartile range, 8.64 to 61.04 months). The univariate Kaplan-Meier in Figure 1A shows the survival curves for different TDT groups (log-rank P < .001). A univariate Cox regression model showed a significance difference between the TDT groups as well (Table 1). Apart from day 0, which is likely related to patients needing emergent treatment, every other category follows a time-dependent response. The longer the time was to chemotherapy start, the worse the long-term patient survival was. This relationship was also demonstrated in multivariate analysis including all the co-variates from Sup.Table 1 (Table 1). Using the adjusted Cox model we calculated the 5-year NNT 1 – 4 days versus 5 – 8 days at 61.61 (95% CI, 33.62 – 736.85). In other words, we estimate that 62 patients need to be treated within 1 – 4 days as opposed to 5 – 8 days to prevent one additional death. In contrast, patients ≥60 years-old (Figure 1B) did not have any long-term consequences from delay in treatment although there were differences observed in the first two-years in favor of longer TDT.
Figure 1.
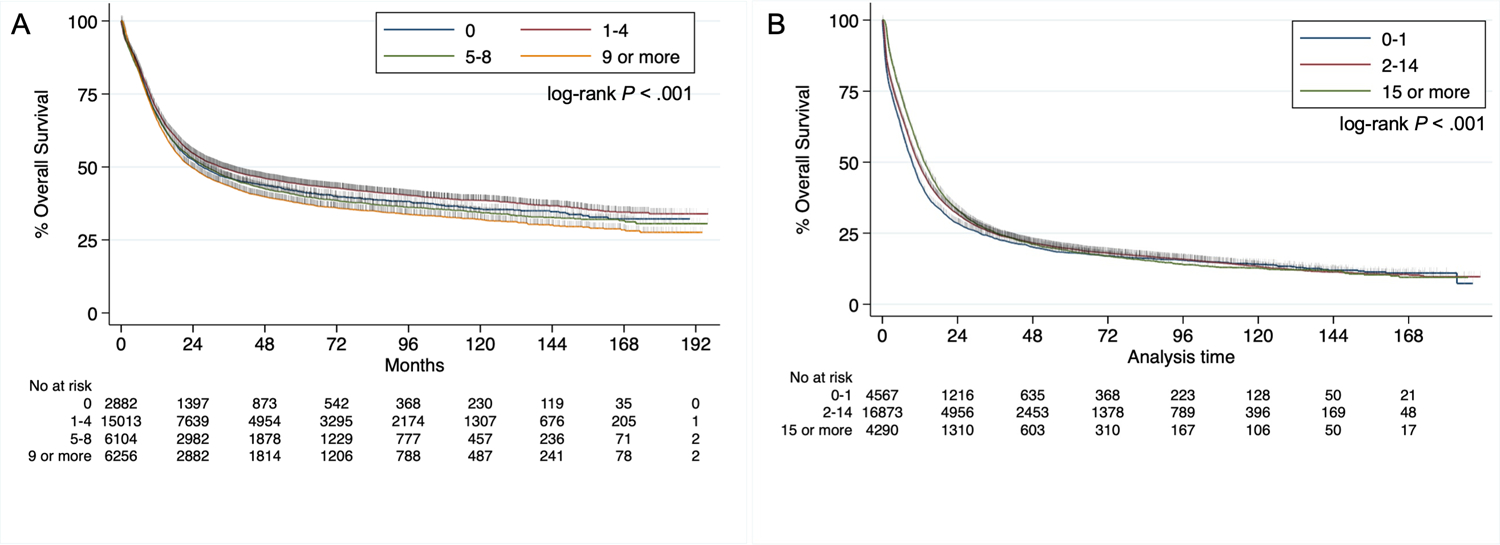
A. Kaplan-Meier for overall survival of patients <60 years-old based on TDT B. Kaplan-Meier for overall survival of patients ≥ 60 years-old based on TDT.
In this study, we found a time-response relationship between TDT and overall survival in younger patients with AML in both the univariate and multivariate analysis models. This finding is biologically plausible as AML in younger individuals tends to be a highly proliferating disease.(11) Thus, a few days delay of chemotherapy initiation may result in rise in leukemic burden, increasing the risk of residual disease post-induction. This in not observed in elderly AML, most likely because of overall poor prognosis and overall less proliferative disease.(11) The differences observed in the first two years in favor of longer TDT are likely reflective of slower proliferation rate of AML in patients that could afford a delay in treatment.
Our results most closely resemble the study from Sekkeres et al, which analyzed 1317 cases from the United States.(4) This study showed two time points that were important for survival: day 5 and day 10. Similar to our observation, their study reported a trend of worse survival for patients who started treatment on the first day of diagnosis. The patients who were treated with chemotherapy on the day of diagnosis likely had high risk disease prompting emergent treatment initiation and explaining their worse survival in comparison to patients who were able to tolerate a few days of delay. A Swedish study of 2374 cases showed worse survival if treatment was delayed for 5 days, but the survival did not worsen after that timepoint, and the relationship was not significant in multi-variate analysis.(7) In sharp contrast, a German study of 2263 patients and a French study of 599 patients did not find any association of TDT with overall survival.(5, 6)
Our study population had similar demographics to prior studies. The difference between our findings and other studies could be potentially explained by differences in induction treatment regimens between the United States and Europe. European countries often employ double induction strategies and/or higher doses of cytarabine, which might have compensated for the delay in treatment initiation. It is important to note that the impact of a 4-day delay in survival is small (HR 1.05). In Sup. Table 4, we detail the sample size needed to detect differences with adequate power with varying HR. With such small differences, the sample size needed is close to 15,000 patients. As a result, the difference in outcomes in previous studies is likely related to statistical power.
Our study has several strengths. The data span several treatment centers across the USA and are not confined to academic centers. The number of patients is 20-fold larger than the previous studies on this topic, allowing for detection of very small differences. The multi-variate analysis included many socioeconomic factors that can be associated with TDT and were not previously adjusted for in other studies.
Our analysis has several limitations. First, the data of the database cannot be independently verified by the investigators. The AML cases lack granular information like laboratory tests, cytogenetics and mutational status; thus, we could not adjust for these factors. Furthermore, there is no information available regarding cytoreductive treatment (ie leukapheresis) prior to chemotherapy initiation. Like previous studies on this question, we do not know the exact reasons that treatment was delayed in each case. It is possible that clinical factors like infections or intravenous access problems affected the decision of starting therapy. However, it is practically impossible to capture such decision-making details in any database of this size. Finally, it is obvious from the results of day 0, that there is a variable that the database is not capturing and predicts for worse outcomes. This variable is likely related to complications (ie diffuse intravascular coagulation) that necessitate emergent treatment.
In conclusion, in the largest study reported to date, longer TDT affected the overall survival of AML patients <60-year-old in a dose-dependent manner. The effect of treatment delay that we observed was small (HR 1.05 for a 5-day delay) and was likely detected due to including a large number of patients resulting in adequate power. These results should be applied in clinical practice with caution, by balancing the risks of delaying treatment in favor of tailored treatment with the potential benefit of improved survival with early treatment initiation. Often the tailored treatment offers much higher benefit than the small detriment of delaying therapy. Finally, we argue that retrospective studies should always be interpreted in the context of the power of the analysis to detect a specific difference.
Supplementary Material
Acknowledgements
Konstantinos Lontos is funded by the NIH TL1 TR001858.
Footnotes
Conflict of Interest
Dr. Michael Boyiadzis is an employee of Genentech and an adjust professor of medicine at the University of Pittsburgh
Data Availability Statement
The data analyzed in this manuscript are property of the National Cancer Database and can be obtained by Commission on Cancer-accredited facilities after a formal application.
References
- 1.Surveillance E, and End Results (SEER) Program Populations (1969–2019) (www.seer.cancer.gov/popdata), National Cancer Institute, DCCPS, Surveillance Research Program, released February 2021. [ [Google Scholar]
- 2.Southam CM, Craver LF, Dargeon HW, Burchenal JH. A study of the natural history of acute leukemia with special reference to the duration of the disease and the occurrence of remissions. Cancer. 1951;4(1):39–59. [DOI] [PubMed] [Google Scholar]
- 3.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med. 2017;377(5):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekeres MA, Elson P, Kalaycio ME, Advani AS, Copelan EA, Faderl S, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoli S, Berard E, Huguet F, Huynh A, Tavitian S, Vergez F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013;121(14):2618–26. [DOI] [PubMed] [Google Scholar]
- 6.Rollig C, Kramer M, Schliemann C, Mikesch JH, Steffen B, Kramer A, et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood. 2020;136(7):823–30. [DOI] [PubMed] [Google Scholar]
- 7.Juliusson G, Hagberg O, Lazarevic VL, Lehmann S, Hoglund M. Impact of treatment delay in acute myeloid leukemia revisited. Blood Adv. 2021;5(3):787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.[Available from: https://www.facs.org/quality-programs/cancer/ncdb.
- 9.Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81(3):515–26. [Google Scholar]
- 10.Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol. 2010;63(1):46–55. [DOI] [PubMed] [Google Scholar]
- 11.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this manuscript are property of the National Cancer Database and can be obtained by Commission on Cancer-accredited facilities after a formal application.


